Abstract
Importance.
Nirmatrelvir/ritonavir has potential to interact with many drugs.
Objective.
To estimate the prevalence of potential moderate to severe drug-drug interactions (DDIs) involving nirmatrelvir/ritonavir, identify interacting medications, and evaluate risk factors associated with potential DDIs.
Design, Setting, and Participants.
Cross-sectional study using electronic health records from the National COVID Cohort Collaborative Enclave, one of the largest COVID-19 data resources in the United States. Study participants were outpatients aged ≥18 years and started nirmatrelvir/ritonavir between December 23, 2021 and March 31, 2022.
Main Outcome and Measures.
Potential moderate to severe DDIs, defined as starting interacting medications reported by National Institutes of Health 30 days before or 10 days after starting nirmatrelvir/ritonavir.
Results.
Of 3214 outpatients who started nirmatrelvir/ritonavir, the mean age was 56.8±17.1 years, 39.5% were male, and 65.8% were non-Hispanic white. Overall, 521 (16.2%) were potentially exposed to at least one moderate to severe DDI, most commonly to atorvastatin (19.2% of all DDIs), hydrocodone (14.0%), or oxycodone (14.0%). After adjustment for covariates, potential DDIs were more likely among individuals who were older (odds ratio [OR] 1.16 per 10-year increase, 95% confidence interval [CI] 1.08-1.25), male (OR 1.36, CI 1.09-1.71), smokers (OR 1.38, CI 1.10-1.73), on more co-medications (OR 1.35, CI 1.31-1.39), and with a history of solid organ transplant (OR 3.63, CI 2.05-6.45).
Conclusions and Relevance.
One in six of individuals receiving nirmatrelvir/ritonavir were at risk of a potential moderate or severe DDI, underscoring the importance of clinical and pharmacy systems to mitigate such risks.
Keywords: Drug interactions, drug safety, SARS-CoV-2, antivirals, patient safety, nirmatrelvir/ritonavir
1. INTRODUCTION
As of April 3, 2023, the COVID-19 pandemic has caused more than 104 million infections and 1,125,366 deaths in the United States.[1] Fortunately, a variety of effective antiviral therapies have been developed and brought to market since the pandemic started, including nirmatrelvir/ritonavir, an oral antiviral medication, approved through a U.S. Food and Drug Administration Emergency Use Authorization (EUA) for the treatment of mild to moderate COVID-19 disease on Dec 22, 2021.[2]
Despite its demonstrated efficacy at reducing COVID-19-related hospitalization or death among unvaccinated individuals, nirmatrelvir/ritonavir has well characterized drug-drug interactions (DDIs) with dozens of medicines because of its effect on drug metabolizing enzymes and drug transporters.[3 -9] Specifically, both nirmatrelvir and ritonavir are CYP3A (Cytochrome P450, family 3, subfamily A)substrates, so use of nirmatrelvir/ritonavir with drugs that are potent CYP3A inducers may reduce the efficacy of nirmatrelvir/ritonavir, such as with phenobarbital or carbamazepine. On the other hand, due to the strong CYP3A inhibition effect of ritonavir, taking nirmatrelvir/ritonavir with drugs that are metabolized by CYP3A enzymes may result in dangerously elevated levels of interacting drugs, such as with cyclosporine, quinidine, or atorvastatin. [5] Despite the theoretical risks of such events, there is relatively little known about how commonly potential moderate or severe DDI may occur with nirmatrelvir/ritonavir in the United States. A review, which synthesized the potential DDIs of various COVID-19 treatments from six drug interaction databases, claimed that taking nirmatrelvir/ritonavir and cardioprotective drugs together may increase the risk of bleeding, rhabdomyolysis, and myopathy. [10]
We conducted a cross-sectional study using electronic medical records from a large national cohort of individuals with COVID-19 to characterize the prevalence of potential moderate to severe DDIs among individuals receiving nirmatrelvir/ritonavir in the United States. In addition, we examined the sociodemographic and clinical characteristics of such individuals to understand who may be at higher risk of these potential adverse events.
2. METHODS
2.1. Data and Subjects
We used the National Covid Cohort Collaborative (N3C) Data Enclave, one of the largest COVID-19 clinical data resources supported by National Institutes of Health (NIH) in the United States.[11] Its detailed design and development are described in other places.[12] Briefly, N3C regularly collects up-to-date individual-level information from electronic health records of both COVID-19 patients and non-COVID controls. Original data including sociodemographic characteristics, clinical observations, drug prescription, and laboratory tests are then transferred into the Observational Medical Outcomes Partnership (OMOP) common data model and stored in this central, secured enclave.[12-13] More than 70 sites have contributed data to this enclave and 5.8 million COVID-19 cases have been captured.[14] Individual patient-level data in the limited data set was used to conduct our analyses. Adults (aged ≥18 years) from 28 sites who were prescribed nirmatrelvir/ritonavir once between Dec 23, 2021 and March 31, 2022 were included. Because nirmatrelvir/ritonavir is authorized for outpatient use and because of our interest in the prevalence and prevention of DDIs in ambulatory care, we excluded persons who were hospitalized 30 days before nirmatrelvir/ritonavir initiation (Figure 1)[15].
Figure 1.
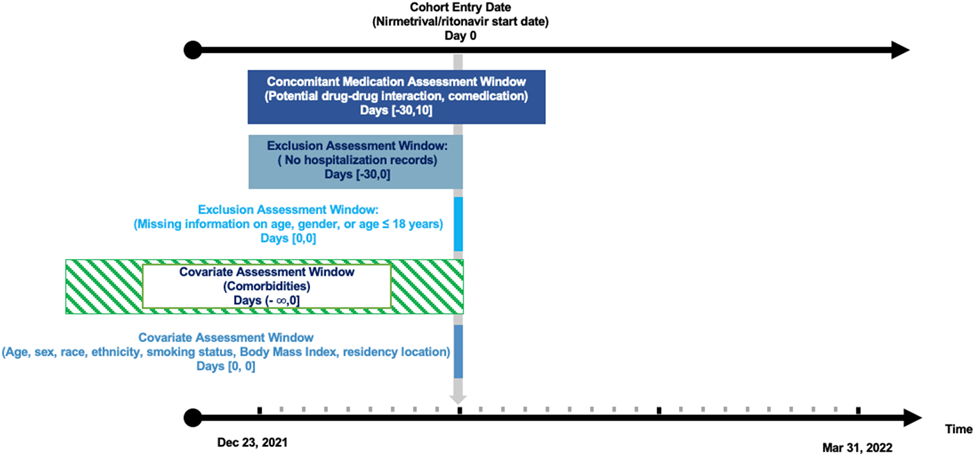
Study implementation
2.2. Definition of Drug-Drug Interaction
The N3C data includes information regarding when a given drug product was started, although the “start date” does not differentiate new prescriptions from refills, nor necessarily include every refill for a given medicine. [16] Using this information, we conservatively considered individuals who started or refilled an interacting medication, as identified by the NIH COVID 19 treatment guidelines, 30 days before or 10 days after starting nirmatrelvir/ritonavir (concomitant medication use period) as at risk for potential DDIs (eTable 1).[4] We added an extra 5 days after the 5-day nirmatrelvir/ritonavir treatment course to account for the remaining nirmatrelvir/ritonavir inhibiting effects.[4,17] In N3C, normalized medication identifiers and medication start date were clearly recorded while quantities, days of supply, or drug exposure end date were unavailable. Without information on treatment course, we were unable to tell if prescribed medications were taken simultaneously. Also, whether patients were asked to temporarily withhold or stop interacting drugs while taking nirmatrelvir/ritonavir was unknown. Therefore, we assumed that patients took prescribed medications continuously in the pre-specified concomitant medication use period and were potentially exposed to DDIs if offending medications were prescribed in this period.
2.3. Outcomes
Our main outcome was potential moderate to severe DDIs listed in the NIH COVID-19 treatment guideline.[4] The NIH categorized outpatient medications into five groups based on the likelihood and severity of the interaction with nirmatrelvir/ritonavir: (i) Medications Without Clinically Relevant Interactions, (ii) Continue Concomitant Medication and Monitor for Adverse Effects, (iii) Adjust Concomitant Medication Dose and Monitor for Adverse Effects, (iv) Temporarily Withhold Concomitant Medication If Clinically Appropriate, and (v) Prescribe Alternative COVID-19 Therapy. Nirmatrelvir/ritonavir users who were prescribed non-topical medications from one of the following categories were potentially at risk for moderate to severe DDIs: (i) Adjust Concomitant Medication Dose and Monitor for Adverse Effects; (ii) Temporarily Withhold Concomitant Medication If Clinically Appropriate; and (iii) Prescribe Alternative COVID-19 Therapy (eTable 1).
2.4. Covariates
We included sociodemographic factors (age, gender, race and ethnicity, residency location) and clinical factors (BMI (Body Mass Index), smoking status, number of co-medications, disease diagnosis, Charlson comorbidity index) in our analysis. We categorized race and ethnicity into five levels: non-Hispanic white, non-Hispanic black, Hispanic and Latino, Other (American Indian and Alaska Native, non-Hispanic; Asian, non-Hispanic; Native Hawaiian and Other Pacific Islander, non-Hispanic; Other race, non-Hispanic), and missing race and ethnicity to avoid small number of participants in specific race and ethnicity group. We extracted all the disease diagnosis history before and on the day of nirmatrelvir/ritonavir prescription to characterize comorbidity. We calculated the Charlson comorbidity index, a validated summary measure of 17 diseases for each participant and then categorized the index into five mutually exclusive groups: score of 0, 1, 2, 3, and 4 or more[18]. We also included diagnosis of cardiovascular diseases, diabetes, and pulmonary disease as dichotomous covariates. We included all medications started in the concomitant medication use period except nirmatrelvir/ritonavir as co-medications. A medicine in its salt form or ester form was regarded as the same as the medication without salt or ester. An exceedingly small number of patients were prescribed prodrug and its metabolites at the same time, and we counted them separately.
2.5. Statistical Analyses
We characterized the overall cohort using percentage for categorical variables and mean with standard deviation (SD), or median with interquartile range (IQR) when appropriate, for continuous variables. In the primary analysis, we calculated prevalence of potential moderate to severe DDIs in the overall cohort. To identify commonly prescribed interacting medications among people who were potentially at risk for DDIs, we collapsed medication and its combination products. Next, we performed logistic regression to evaluate the risk factors associated with potential moderate to severe DDIs. A univariable logistic regression model was used first to explore the independent associations between different covariates and potential DDIs. Basic sociodemographic factors including age, gender, race and ethnicity as well as factors with a p-value < 0.1 in the univariable model were examined in the final multivariable model. We rescaled age by dividing the original age by 10 and modelled it as continuous variables in our analysis. We included a dummy variable to capture individuals with missing information about race or ethnicity. BMI and residency location were excluded because of substantial missing data. The crude and adjusted odds ratio (OR) and their 95% confidence intervals (CI) were calculated to describe the associations.
2.6. Sensitivity Analysis
We performed two sensitivity analyses to examine whether the results vary based on how we defined the length of concomitant medication use. First, we applied a stricter definition of concomitant use by considering medications prescribed 15 days before or 10 days after nirmatrelvir/ritonavir start date as concomitant medications. Second, we extended the concomitant use period to 60 days before or 10 days after nirmatrelvir/ritonavir start date to do a less conservative analysis. In each sensitivity analysis, the period for non-hospitalization varies with the concomitant medication use period (eFigure 1). We fitted the final multivariable logistic model from the primary analysis to assess the association between risk factors and potential DDIs.
We followed Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines to conduct and report our study [19]. Data extraction was conducted using Spark SQL and statistical analysis was performed using R (version 3.5) in the N3C Data Enclave. The Johns Hopkins Medicine Institutional Review Boards waived the requirement for informed consent and deemed work in the N3C Data Enclave to be exempt research.
3. RESULTS
3.1. Characteristics of the Study Population
Of 3574 people who started nirmatrelvir/ritonavir between December 23, 2021 and March 31, 2022, 3351 received nirmatrelvir/ritonavir only once in the study period. After we further excluded hospitalized individuals (n=49) and individuals with missing age,gender, or age <18 years (n=88), we identified 3214 people eligible for analysis (Figure 2). Overall, the mean age was 56.8 years (SD 17.1), 39.5% were male, 65.8% were non-Hispanic White, 59.7% were non-smokers, and 70.4% were urban residents. The most common comorbidities were cardiovascular disease (53.8%), pulmonary disease (30.1%), and diabetes (26.2%). The median number of co-medications was two (IQR 0-3.0) (Table 1).
Figure 2.
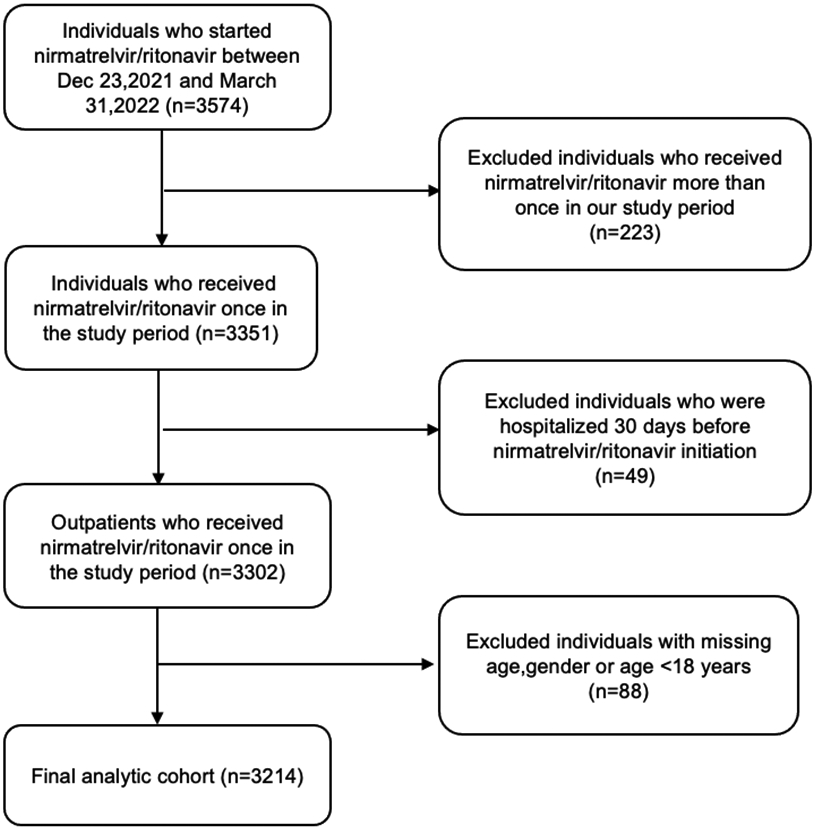
Study participants flow diagram.
Table 1.
Characteristics of the study population (n=3214 individuals)
| Characteristics | Total nirmatrelvir/ritonavir Users N= 3214 |
Individuals without potential DDIs N= 2693 (83.8%) |
Individuals with potential DDIs N=521 (16.2%) |
|---|---|---|---|
| Age, years, mean (SD)* | 56.8 (17.1) | 56.3 (17.3) | 59.4 (15.3) |
| Male gender (%) * | 39.5 | 38.2 | 46.1 |
| Race/ethnicity (%)* | |||
| Non-Hispanic White | 65.8 | 65.7 | 66.6 |
| Non-Hispanic Black | 9.6 | 9.7 | 9.0 |
| Hispanic | 8.0 | 7.6 | 9.8 |
| †Other or missing | 16.6 | 17 | 14.6 |
| Body Mass Index (%) | |||
| ≤25.0 kg/m2 | 19.3 | 19.5 | 18.7 |
| 25.1-30.0 kg/m2 | 20.6 | 21.0 | 18.4 |
| 30.1-40 kg/m2 | 22.6 | 23.1 | 20.0 |
| ≥40.1 kg/m2 | 9.5 | 9.5 | 9.4 |
| Missing | 28.0 | 27.0 | 33.6 |
| Smoking status (%) * | |||
| Current or former smoker | 40.3 | 38.5 | 49.5 |
| Nonsmoker | 59.7 | 61.5 | 50.5 |
| Number of comedications, median (IQR)* | 2 (0-3.0) | 1.0 (0-3.0) | 5.0 (3.0-9.0) |
| Comorbidity (%) | |||
| ‡Cardiovascular disease* | 53.8 | 51.2 | 67.0 |
| Diabetes* | 26.2 | 24.2 | 36.7 |
| Cancer* | 15.7 | 14.7 | 20.9 |
| Liver disease* | 10.4 | 9.2 | 16.5 |
| Peripheral vascular disease * | 9.7 | 8.6 | 15.4 |
| Pulmonary disease* | 30.1 | 27.9 | 41.3 |
| Rheumatic disease | 10.9 | 10.5 | 12.9 |
| Renal disease* | 8.8 | 7.7 | 14.4 |
| §Solid organ transplant * | 2.7 | 1.8 | 7.5 |
| Charlson Comorbidity Index,(%)* | |||
| 0 | 31.3 | 34.1 | 16.9 |
| 1 | 24.8 | 25.0 | 23.8 |
| 2 | 15.6 | 15.5 | 15.7 |
| 3 | 8.5 | 8.1 | 10.4 |
| >= 4 | 19.8 | 17.3 | 33.2 |
| Residency location, (%) * | |||
| Urban | 70.4 | 71.3 | 66.0 |
| Rural | 9.6 | 9.9 | 8.3 |
| Missing | 19.9 | 18.8 | 25.7 |
DDIs drug-drug interactions,SD standard deviation, IQR interquartile range
Other race and ethnicity includes American Indian and Alaska Native, non-Hispanic; Asian, non-Hispanic; Native Hawaiian and Other Pacific Islander, non-Hispanic; Other race, non-Hispanic.
Cardiovascular diseases include: coronary artery disease without myocardial infarction; myocardial infarction; congestive heart failure; hypertension; arrhythmia; stroke
Solid organ transplant includes: lung transplant; heart transplant; liver transplant; kidney transplant; pancreas transplant
Percentage may not add up to 100% due to rounding error.
p<0.05
3.2. Medications Involved in Potential Moderate to Severe DDIs
Approximately one in six (16.2%) people were potentially exposed to moderate to severe DDIs. Among the 521 patients with potential DDIs, statins and opioids were two major drug classes implicated in such DDIs. Atorvastatin was found to be the most frequently prescribed co-medication among nirmatrelvir/ritonavir users (19.2%), followed by hydrocodone (14.0%), oxycodone (14.0%), rosuvastatin (8.6%), and trazodone (6.3%). Additionally, 5.4% of nirmatrelvir/ritonavir users with potential DDIs received tacrolimus (Figure 3).
Figure 3.
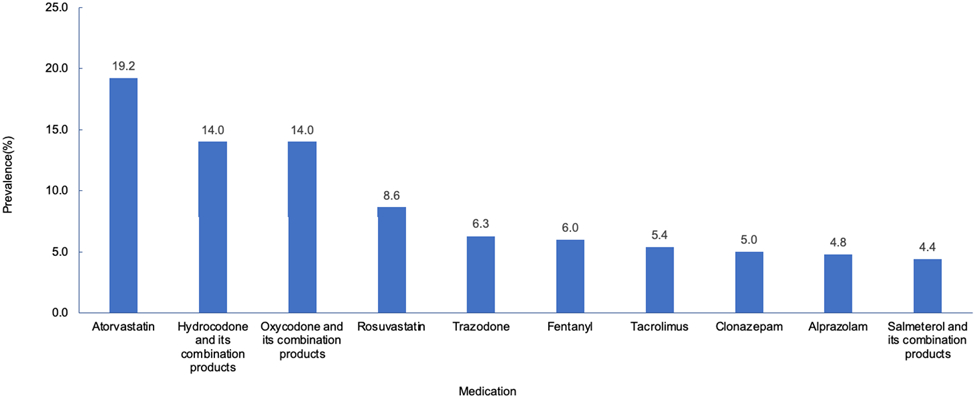
Prevalence of most frequently prescribed co-medications among 521 individuals with potential moderate to severe DDIs
3.3. Risk Factors Associated with Potential DDIs
Our multivariable regression model identified patient age, gender, smoking status, number of comedications, and history of solid organ transplant as independent risk factors of potential moderate to severe DDIs among outpatient nirmatrelvir/ritonavir users. Older adults had slightly higher odds of being potentially exposed to DDIs (OR 1.16, 95% CI 1.08-1.25 per 10-year increase). Males (OR 1.36, 95% CI 1.09-1.71), smokers (OR 1.38, 95% CI 1.10-1.73), and those on more co-medications (OR 1.35, 95% CI 1.31-1.39) were significantly more likely to be exposed to DDIs. In addition, individuals with a history of solid organ transplant had 3-fold higher odds (OR 3.63, 95% CI 2.05-6.45). However, compared with non-Hispanic whites, non-Hispanic blacks (OR 0.59, 95% CI 0.40-0.89) and individuals from other race and ethnicity (OR 0.37, 95% CI 0.18-0.78) had significantly lower odds of developing potential DDIs (Figure 4).
Figure 4.
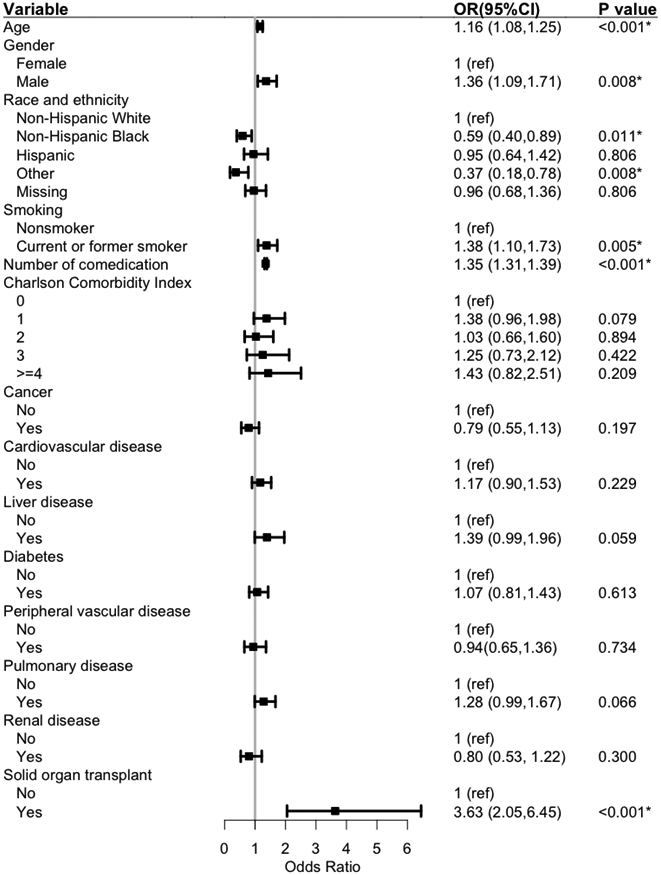
Adjusted association between risk factors and potential moderate to severe DDIs
DDIs drug-drug interactions, OR odds ratio, 95%CI 95% confidence interval
The p Value of rheumatic disease (p=0.115) was larger than 0.1 in univariable analysis and therefore not included in the multivariable model
*p Value is less than 0.05
3.4. Sensitivity Analyses
Our sensitivity analyses varying the definitions of concomitant medication use period (ie, shortened or extended) generated substantively similar findings as the primary analysis (eTable 2). The prevalence of potential occurrence of DDIs was 12.2% in the analysis with shortened concomitant medication use period and 22.2% after relaxing the co-medication definition (extended concomitant medication use period). In both analyses, older age, male gender, smoking, increasing number of co-medications, and history of solid organ transplant remained statistically significant. Participants with Charlson Comorbidity Index equal to 1 were more likely to be exposed to potential DDIs compared to those with an index of 0 in the extended concomitant medication use period, but not in the shortened concomitant medication use period.
4. DISCUSSION
We characterized potential DDIs and their risk factors among ambulatory U.S. adults treated with nirmatrelvir/ritonavir. About one in six (16.2%) had at least one moderate to severe potential DDI, usually due to co-prescribed statins or opioids. Advanced age, male gender, increasing number of co-medications, and history of solid organ transplant were associated with a greater risk of potential DDIs. Our results persisted in sensitivity analyses varying definitions of the concomitant medication use period. These findings are important because of how commonly nirmatrelvir/ritonavir is used to treat COVID-19 among ambulatory adults in the United States.
Our work extends other assessments of the risk of potential nirmatrelvir/ritonavir DDIs, which arise from the rapid and potent inhibition effect of ritonavir instead of induction effect. [9] For example, in an analysis of national prescription data from Denmark, the authors estimated the proportion of older adults at risk of DDIs with nirmatrelvir/ritonavir ranged from 0.7% to 32% across age and interacting drug groups.[20] In another assessment that theoretically exposed the trial cohort which included older, hospitalized adults with polypharmacy to nirmatrelvir/ritonavir, more than two-thirds of individuals (68%) were subject to potential DDIs with nirmatrelvir/ritonavir.[21] Since both studies reported the proportion of interacting drugs among all older people rather than real nirmatrelvir/ritonavir users to evaluate the prevalence of potential DDIs involving nirmatrelvir/ritonavir, the prevalence was likely underestimated.
We identified several sociodemographic and clinical characteristics that were significantly associated with potential DDIs including individuals who are older, male, and on multiple medications. Interestingly, tobacco use was also an independent risk factor of potential DDIs, in contrast with prior work that did not find such an association.[22,23] Such differences may arise in part from the nature of the DDIs of interest, including triggering drugs for nirmatrelvir/ritonavir DDIs, such as salmeterol, statins, and oxycodone, that may be more commonly used among smokers with comorbidities.[24] Similarly, our finding of a greater risk of potential DDIs among those with solid organ transplant likely reflects the more common use of immunomodulating medications, such as tacrolimus, among these individuals.
Our results highlight the importance of educational efforts targeting patients and providers to ensure that both are aware of the non-trivial prevalence of potential nirmatrelvir/ritonavir DDIs. Since the risk of DDIs varies considerably across different socioeconomic and clinical subpopulations, such information can be used to target educational initiatives. In addition, the non-trivial risk of nirmatrelvir/ritonavir DDIs speaks to the value of automated systems, including drug interaction websites and software to forestall potential DDIs,[25] and contemporary electronic medical record platforms with functionalities to screen for potential DDIs and to advise prescribers of such at the point of prescribing.[26,27] Although a variety of algorithms have been developed to perform such reviews, accuracy and comprehensiveness of these automated systems varies.[10,28]
Though we relied on NIH COVID 19 guidelines to identify potential DDIs involving nirmatrelvir/ritonavir, these guidelines provide an incomplete list of potential offending drugs, since the interacting medications listed by NIH are mainly CYP3A4 substrates. Ritonavir is a weak inhibitor of CYP2D6 at boosting doses of 100 mg/d to 200 mg/d and also inhibits transporters P-glycoprotein (P-gp), organic anion transporting polypeptides (OATP), and breast cancer resistance protein (BCRP), which may result in increasing concentration of medications whose metabolism relies on those enzymes and transporters.[9] Such inhibition effects should not be ignored. Additionally, drug classes such as calcium channel blockers and kinase inhibitors are not included in the NIH COVID-19 treatment guideline and information regarding some drug classes, such as chemotherapeutic agents, is imprecise. Moreover, there may be misclassification of the severity of potential DDIs. For example, oral midazolam and alprazolam have similar pharmacokinetic properties but belong to different potential DDI severity groups according to the NIH guideline.
Our study also has other limitations and generates new questions. First, our data do not include information as to whether clinicians or pharmacists may have counselled patients to temporarily withhold or stop potentially interacting drugs, and thus we may overestimate the prevalence of potential DDIs. On the other hand, many individuals take over-the-counter drugs and dietary supplements that may potentially interact with nirmatrelvir/ritonavir and lead to underestimates of the DDIs in question.[29] Second, our estimates of potential DDIs are conservative, since we don’t capture medicines prescribed outside of the participating N3C institutions, nor do we capture medicines prescribed more than 30 days prior to nirmatrelvir/ritonavir receipt unless they were refilled during our defined exposure window. Third, the N3C, while the one of the largest COVID cohorts in the United States, represents the experience of academic health systems. Fourth, we examined potential DDIs; future work is needed to examine the prevalence and risk factors for actual adverse events arising from these potential DDIs. Fifth, our analyses reflect early experience with nirmatrelvir/ritonavir and there may be important secular trends in its use, and potential DDIs, over time.[13] Finally, we analysed the association between a limited set of patient-level characteristics and potential nirmatrelvir/ritonavir DDIs; further work might examine other factors including prescriber training and experience.
5. CONCLUSION
In a large and diverse cohort of outpatient U.S. adults treated with nirmatrelvir/ritonavir, one in six were at risk of a potential moderate or severe DDI, underscoring the importance of clinical and pharmacy systems to mitigate such risks.
Supplementary Material
eTable 1. Potential moderate to severe DDIs of nirmatrelvir/ritonavir examined in the current study
eTable 2. Adjusted association between risk factors and potential moderate to severe DDIs with a shortened/ extended concomitant medication use period
eFigure 1. Study implementation for sensitivity analysis
ACKNOWLEDGEMENTS
N3C Attribution: The analyses described in this publication were conducted with data or tools accessed through the NCATS N3C Data Enclave covid.cd2h.org/enclave and supported by CD2H - The National COVID Cohort Collaborative (N3C) IDeA CTR Collaboration 3U24TR002306-04S2 NCATS U24 TR002306. This research was possible because of the patients whose information is included within the data from participating organizations (covid.cd2h.org/dtas) and the organizations and scientists (covid.cd2h.org/duas) who have contributed to the on-going development of this community resource (cite this https://doi.org/10.1093/jamia/ocaa196).
IRB: The N3C data transfer to NCATS is performed under a Johns Hopkins University Reliance Protocol # IRB00249128 or individual site agreements with NIH. The N3C Data Enclave is managed under the authority of the NIH; information can be found at https://ncats.nih.gov/n3c/resources.
Individual Acknowledgements For Core Contributors:
We gratefully acknowledge the following core contributors to N3C:
Adam B. Wilcox, Adam M. Lee, Alexis Graves, Alfred (Jerrod) Anzalone, Amin Manna, Amit Saha, Amy Olex, Andrea Zhou, Andrew E. Williams, Andrew Southerland, Andrew T. Girvin, Anita Walden, Anjali A. Sharathkumar, Benjamin Amor, Benjamin Bates, Brian Hendricks, Brijesh Patel, Caleb Alexander, Carolyn Bramante, Cavin Ward-Caviness, Charisse Madlock-Brown, Christine Suver, Christopher Chute, Christopher Dillon, Chunlei Wu, Clare Schmitt, Cliff Takemoto, Dan Housman, Davera Gabriel, David A. Eichmann, Diego Mazzotti, Don Brown, Eilis Boudreau, Elaine Hill, Elizabeth Zampino, Emily Carlson Marti, Emily R. Pfaff, Evan French, Farrukh M Koraishy, Federico Mariona, Fred Prior, George Sokos, Greg Martin, Harold Lehmann, Heidi Spratt, Hemalkumar Mehta, Hongfang Liu, Hythem Sidky, J.W. Awori Hayanga, Jami Pincavitch, Jaylyn Clark, Jeremy Richard Harper, Jessica Islam, Jin Ge, Joel Gagnier, Joel H. Saltz, Joel Saltz, Johanna Loomba, John Buse, Jomol Mathew, Joni L. Rutter, Julie A. McMurry, Justin Guinney, Justin Starren, Karen Crowley, Katie Rebecca Bradwell, Kellie M. Walters, Ken Wilkins, Kenneth R. Gersing, Kenrick Dwain Cato, Kimberly Murray, Kristin Kostka, Lavance Northington, Lee Allan Pyles, Leonie Misquitta, Lesley Cottrell, Lili Portilla, Mariam Deacy, Mark M. Bissell, Marshall Clark, Mary Emmett, Mary Morrison Saltz, Matvey B. Palchuk, Melissa A. Haendel, Meredith Adams, Meredith Temple-O'Connor, Michael G. Kurilla, Michele Morris, Nabeel Qureshi, Nasia Safdar, Nicole Garbarini, Noha Sharafeldin, Ofer Sadan, Patricia A. Francis, Penny Wung Burgoon, Peter Robinson, Philip R.O. Payne, Rafael Fuentes, Randeep Jawa, Rebecca Erwin-Cohen, Rena Patel, Richard A. Moffitt, Richard L. Zhu, Rishi Kamaleswaran, Robert Hurley, Robert T. Miller, Saiju Pyarajan, Sam G. Michael, Samuel Bozzette, Sandeep Mallipattu, Satyanarayana Vedula, Scott Chapman, Shawn T. O'Neil, Soko Setoguchi, Stephanie S. Hong, Steve Johnson, Tellen D. Bennett, Tiffany Callahan, Umit Topaloglu, Usman Sheikh, Valery Gordon, Vignesh Subbian, Warren A. Kibbe, Wenndy Hernandez, Will Beasley, Will Cooper, William Hillegass, Xiaohan Tanner Zhang. Details of contributions available at covid.cd2h.org/core-contributors
Data Partners with Released Data
The following institutions whose data is released or pending:
Available: Advocate Health Care Network — UL1TR002389: The Institute for Translational Medicine (ITM) • Boston University Medical Campus — UL1TR001430: Boston University Clinical and Translational Science Institute • Brown University — U54GM115677: Advance Clinical Translational Research (Advance-CTR) • Carilion Clinic — UL1TR003015: iTHRIV Integrated Translational health Research Institute of Virginia • Charleston Area Medical Center — U54GM104942: West Virginia Clinical and Translational Science Institute (WVCTSI) • Children’s Hospital Colorado — UL1TR002535: Colorado Clinical and Translational Sciences Institute • Columbia University Irving Medical Center — UL1TR001873: Irving Institute for Clinical and Translational Research • Duke University — UL1TR002553: Duke Clinical and Translational Science Institute • George Washington Children’s Research Institute — UL1TR001876: Clinical and Translational Science Institute at Children’s National (CTSA-CN) • George Washington University — UL1TR001876: Clinical and Translational Science Institute at Children’s National (CTSA-CN) • Indiana University School of Medicine — UL1TR002529: Indiana Clinical and Translational Science Institute • Johns Hopkins University — UL1TR003098: Johns Hopkins Institute for Clinical and Translational Research • Loyola Medicine — Loyola University Medical Center • Loyola University Medical Center — UL1TR002389: The Institute for Translational Medicine (ITM) • Maine Medical Center — U54GM115516: Northern New England Clinical & Translational Research (NNE-CTR) Network • Massachusetts General Brigham — UL1TR002541: Harvard Catalyst • Mayo Clinic Rochester — UL1TR002377: Mayo Clinic Center for Clinical and Translational Science (CCaTS) • Medical University of South Carolina — UL1TR001450: South Carolina Clinical & Translational Research Institute (SCTR) • Montefiore Medical Center — UL1TR002556: Institute for Clinical and Translational Research at Einstein and Montefiore • Nemours — U54GM104941: Delaware CTR ACCEL Program • NorthShore University HealthSystem — UL1TR002389: The Institute for Translational Medicine (ITM) • Northwestern University at Chicago — UL1TR001422: Northwestern University Clinical and Translational Science Institute (NUCATS) • OCHIN — INV-018455: Bill and Melinda Gates Foundation grant to Sage Bionetworks • Oregon Health & Science University — UL1TR002369: Oregon Clinical and Translational Research Institute • Penn State Health Milton S. Hershey Medical Center — UL1TR002014: Penn State Clinical and Translational Science Institute • Rush University Medical Center — UL1TR002389: The Institute for Translational Medicine (ITM) • Rutgers, The State University of New Jersey — UL1TR003017: New Jersey Alliance for Clinical and Translational Science • Stony Brook University — U24TR002306 • The Ohio State University — UL1TR002733: Center for Clinical and Translational Science • The State University of New York at Buffalo — UL1TR001412: Clinical and Translational Science Institute • The University of Chicago — UL1TR002389: The Institute for Translational Medicine (ITM) • The University of Iowa — UL1TR002537: Institute for Clinical and Translational Science • The University of Miami Leonard M. Miller School of Medicine — UL1TR002736: University of Miami Clinical and Translational Science Institute • The University of Michigan at Ann Arbor — UL1TR002240: Michigan Institute for Clinical and Health Research • The University of Texas Health Science Center at Houston — UL1TR003167: Center for Clinical and Translational Sciences (CCTS) • The University of Texas Medical Branch at Galveston — UL1TR001439: The Institute for Translational Sciences • The University of Utah — UL1TR002538: Uhealth Center for Clinical and Translational Science • Tufts Medical Center — UL1TR002544: Tufts Clinical and Translational Science Institute • Tulane University — UL1TR003096: Center for Clinical and Translational Science • University Medical Center New Orleans — U54GM104940: Louisiana Clinical and Translational Science (LA CaTS) Center • University of Alabama at Birmingham — UL1TR003096: Center for Clinical and Translational Science • University of Arkansas for Medical Sciences — UL1TR003107: UAMS Translational Research Institute • University of Cincinnati — UL1TR001425: Center for Clinical and Translational Science and Training • University of Colorado Denver, Anschutz Medical Campus — UL1TR002535: Colorado Clinical and Translational Sciences Institute • University of Illinois at Chicago — UL1TR002003: UIC Center for Clinical and Translational Science • University of Kansas Medical Center — UL1TR002366: Frontiers: University of Kansas Clinical and Translational Science Institute • University of Kentucky — UL1TR001998: UK Center for Clinical and Translational Science • University of Massachusetts Medical School Worcester — UL1TR001453: The UMass Center for Clinical and Translational Science (UMCCTS) • University of Minnesota — UL1TR002494: Clinical and Translational Science Institute • University of Mississippi Medical Center — U54GM115428: Mississippi Center for Clinical and Translational Research (CCTR) • University of Nebraska Medical Center — U54GM115458: Great Plains IDeA-Clinical & Translational Research • University of North Carolina at Chapel Hill — UL1TR002489: North Carolina Translational and Clinical Science Institute • University of Oklahoma Health Sciences Center — U54GM104938: Oklahoma Clinical and Translational Science Institute (OCTSI) • University of Rochester — UL1TR002001: UR Clinical & Translational Science Institute • University of Southern California — UL1TR001855: The Southern California Clinical and Translational Science Institute (SC CTSI) • University of Vermont — U54GM115516: Northern New England Clinical & Translational Research (NNE-CTR) Network • University of Virginia — UL1TR003015: iTHRIV Integrated Translational health Research Institute of Virginia • University of Washington — UL1TR002319: Institute of Translational Health Sciences • University of Wisconsin-Madison — UL1TR002373: UW Institute for Clinical and Translational Research • Vanderbilt University Medical Center — UL1TR002243: Vanderbilt Institute for Clinical and Translational Research • Virginia Commonwealth University — UL1TR002649: C. Kenneth and Dianne Wright Center for Clinical and Translational Research • Wake Forest University Health Sciences — UL1TR001420: Wake Forest Clinical and Translational Science Institute • Washington University in St. Louis — UL1TR002345: Institute of Clinical and Translational Sciences • Weill Medical College of Cornell University — UL1TR002384: Weill Cornell Medicine Clinical and Translational Science Center • West Virginia University — U54GM104942: West Virginia Clinical and Translational Science Institute (WVCTSI)
Submitted: Icahn School of Medicine at Mount Sinai — UL1TR001433: ConduITS Institute for Translational Sciences • The University of Texas Health Science Center at Tyler — UL1TR003167: Center for Clinical and Translational Sciences (CCTS) • University of California, Davis — UL1TR001860: UCDavis Health Clinical and Translational Science Center • University of California, Irvine — UL1TR001414: The UC Irvine Institute for Clinical and Translational Science (ICTS) • University of California, Los Angeles — UL1TR001881: UCLA Clinical Translational Science Institute • University of California, San Diego — UL1TR001442: Altman Clinical and Translational Research Institute • University of California, San Francisco — UL1TR001872: UCSF Clinical and Translational Science Institute
Pending: Arkansas Children’s Hospital — UL1TR003107: UAMS Translational Research Institute • Baylor College of Medicine — None (Voluntary) • Children’s Hospital of Philadelphia — UL1TR001878: Institute for Translational Medicine and Therapeutics • Cincinnati Children’s Hospital Medical Center — UL1TR001425: Center for Clinical and Translational Science and Training • Emory University — UL1TR002378: Georgia Clinical and Translational Science Alliance • HonorHealth — None (Voluntary) • Loyola University Chicago — UL1TR002389: The Institute for Translational Medicine (ITM) • Medical College of Wisconsin — UL1TR001436: Clinical and Translational Science Institute of Southeast Wisconsin • MedStar Health Research Institute — UL1TR001409: The Georgetown-Howard Universities Center for Clinical and Translational Science (GHUCCTS) • MetroHealth — None (Voluntary) • Montana State University — U54GM115371: American Indian/Alaska Native CTR • NYU Langone Medical Center — UL1TR001445: Langone Health’s Clinical and Translational Science Institute • Ochsner Medical Center — U54GM104940: Louisiana Clinical and Translational Science (LA CaTS) Center • Regenstrief Institute — UL1TR002529: Indiana Clinical and Translational Science Institute • Sanford Research — None (Voluntary) • Stanford University — UL1TR003142: Spectrum: The Stanford Center for Clinical and Translational Research and Education • The Rockefeller University — UL1TR001866: Center for Clinical and Translational Science • The Scripps Research Institute — UL1TR002550: Scripps Research Translational Institute • University of Florida — UL1TR001427: UF Clinical and Translational Science Institute • University of New Mexico Health Sciences Center — UL1TR001449: University of New Mexico Clinical and Translational Science Center • University of Texas Health Science Center at San Antonio — UL1TR002645: Institute for Integration of Medicine and Science • Yale New Haven Hospital — UL1TR001863: Yale Center for Clinical Investigation
Funding:
Dr. Mehta is supported by the National Institute for Aging (K01AG070329)
Footnotes
Disclosures: Dr. Alexander is past Chair and a current member of FDA’s Peripheral and Central Nervous System Advisory Committee; is a co-founding Principal and equity holder in Monument Analytics, a health care consultancy whose clients include the life sciences industry as well as plaintiffs in opioid litigation, for whom he has served as a paid expert witness; and is a past member of OptumRx’s National P&T Committee. Dr. Garibaldi is a member of the FDA Pulmonary-Asthma Drug Advisory Committee and has received consulting fees from Janssen Research and Development LLC, Gilead Sciences and Atea Pharmaceuticals. These arrangements have been reviewed and approved by Johns Hopkins University in accordance with its conflict of interest policies.
Ethics approval: The Johns Hopkins Medicine Institutional Review Boards waived the requirement for informed consent and deemed work in the N3C Data Enclave to be exempt research.
Data availability:
The datasets generated during and/or analysed during the current study are available in the NCATS N3C Data Enclave, https://covid.cd2h.org/
Code availability:
Code is available from Xuya Xiao
REFERENCES
- 1.Centers for Disease Control and Prevention. COVID Data Tracker. Atlanta, GA: US Department of Health and Human Services, CDC; 2023, April 03. https://covid.cdc.gov/covid-data-tracker [Google Scholar]
- 2.Coronavirus (COVID-19) Update: FDA Authorizes First Oral Antiviral for Treatment of COVID-19 [Internet]. U.S.Food & Drug Administration; c2021[cited 2023 Mar 22]. Available from: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-first-oral-antiviral-treatment-covid-19 [Google Scholar]
- 3.Pfizer’s Novel COVID-19 Oral Antiviral Treatment Candidate Reduced Risk of Hospitalization or Death by 89% in Interim Analysis of Phase 2/3 EPIC-HR Study [Internet]. Pfizer; c2021. [cited 2022 July 23]. Available from: https://www.pfizer.com/news/press-release/press-release-detail/pfizers-novel-covid-19-oral-antiviral-treatment-candidate. [Google Scholar]
- 4.COVID-19 Treatment Guidelines [Internet]. National Institutes of Health; c2022. [cited 2022 May 29]. Available from: https://www.covid19treatmentguidelines.nih.gov/. [Google Scholar]
- 5.COVID-19 Drug Interactions [Internet]. University of Liverpool; c2022. [cited 2022 May 10]. Available from: https://www.covid19-druginteractions.org/checker. [Google Scholar]
- 6.Ontario COVID-19 Drugs and Biologics Clinical Practice Guidelines Working Group, University of Waterloo School of Pharmacy. Nirmatrelvir/Ritonavir (Nirmatrelvir/ritonavir): What prescribers and pharmacists need to know. Ontario COVID-19 Science Advisory Table. 2022;3(58). 10.47326/ocsat.2022.03.58.3.0 [DOI] [Google Scholar]
- 7.Hong E, Almond LM, Chung PS, Rao AP, Beringer PM. Physiologically-Based Pharmacokinetic-Led Guidance for Patients With Cystic Fibrosis Taking Elexacaftor-Tezacaftor-Ivacaftor With Nirmatrelvir-Ritonavir for the Treatment of COVID-19. Clin Pharmacol Ther. 2022. Jun;111(6):1324–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stifani BM, Madden T, Micks E, Moayedi G, Tarleton J, Benson LS. Society of Family Planning Clinical Recommendations: Contraceptive Care in the Context of Pandemic Response. Contraception. 2022. Sep;113:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marzolini C, Kuritzkes DR, Marra F, Boyle A, Gibbons S, Flexner C, et al. Recommendations for the Management of Drug-Drug Interactions Between the COVID-19 Antiviral Nirmatrelvir/Ritonavir (Nirmatrelvir/ritonavir) and Comedications. Clin Pharmacol Ther. 2022. Dec;112(6):1191–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.S K SR, P AA, B S, Kalala KP, Pm A, Sabarathinam S. Drug interaction risk between cardioprotective drugs and drugs used in treatment of COVID-19: A evidence-based review from six databases. Diabetes Metab Syndr. 2022. Mar;16(3):102451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Institutes of Health (NIH). National Center for Advancing Translational Sciences (NCATS). National COVID Cohort Collaborative Data Enclave Repository [Internet]. Bethesda, Maryland: U.S. Department of Health and Human Services, National Institutes of Health; c2022. [cited: 2022 July 24]. Available from: https://covid.cd2h.org/. [Google Scholar]
- 12.Haendel MA, Chute CG, Bennett TD, Eichmann DA, Guinney J, Kibbe WA, et al. The National COVID Cohort Collaborative (N3C): Rationale, design, infrastructure, and deployment. J Am Med Inform Assoc. 2021. Mar 1;28(3):427–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehta HB, An H, Andersen KM, Mansour O, Madhira V, Rashidi ES, et al. Use of Hydroxychloroquine, Remdesivir, and Dexamethasone Among Adults Hospitalized With COVID-19 in the United States : A Retrospective Cohort Study. Ann Intern Med. 2021. Oct;174(10):1395–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.N3C Dashboards [Internet]. National Institutes of Health; c2022. [cited 2022 August 4]. Available from: https://covid.cd2h.org/dashboard. [Google Scholar]
- 15.Schneeweiss S, Rassen JA, Brown JS, Rothman KJ, Happe L, Arlett P, et al. Graphical Depiction of Longitudinal Study Designs in Health Care Databases. Ann Intern Med. 2019. Mar 19;170(6):398–406. [DOI] [PubMed] [Google Scholar]
- 16.N3C Data Overview [Internet]. National Institutes of Health; c2022. [cited 2023 May 15]. Available from: https://ncats.nih.gov/n3c/about/data-overview#data-stewardship-and-protection [Google Scholar]
- 17.Stader F, Khoo S, Stoeckle M, Back D, Hirsch HH, Battegay M, et al. Stopping lopinavir/ritonavir in COVID-19 patients: duration of the drug interacting effect. J Antimicrob Chemother. 2020. Oct 1;75(10):3084–3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. [DOI] [PubMed] [Google Scholar]
- 19.von Elm Erik et al. “The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies.” Annals of internal medicine vol. 147, 8 (2007): 573–7. [DOI] [PubMed] [Google Scholar]
- 20.Larsen CS. Assessing the proportion of the Danish population at risk of clinically significant drug-drug interactions with new oral antivirals for early treatment of COVID-19. Int J Infect Dis. 2022. Sep;122:599–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ross SB, Bortolussi-Courval É, Hanula R, Lee TC, Goodwin Wilson M, McDonald EG. Drug Interactions With Nirmatrelvir-Ritonavir in Older Adults Using Multiple Medications. JAMA Netw Open. 2022. Jul 1;5(7):e2220184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diksis N, Melaku T, Assefa D, Tesfaye A. Potential drug-drug interactions and associated factors among hospitalized cardiac patients at Jimma University Medical Center, Southwest Ethiopia. SAGE Open Med. 2019. Jun 11;7:2050312119857353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hughes JE, Russo V, Walsh C, Menditto E, Bennett K, Cahir C. Prevalence and Factors Associated with Potential Drug-Drug Interactions in Older Community-Dwelling Adults: A Prospective Cohort Study. Drugs Aging. 2021. Nov;38(11):1025–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smoking & Tobacco Use [Internet]. Atlanta, GA: US Department of Health and Human Services, CDC; c2022. [cited 2022 August 10]. Available from: https://www.cdc.gov/tobacco/basic_information/health_effects/index.htm. [Google Scholar]
- 25.Conti V, Sellitto C, Torsiello M, Manzo V, De Bellis E, Stefanelli B, et al. Identification of Drug Interaction Adverse Events in Patients With COVID-19: A Systematic Review. JAMA Netw Open. 2022. Apr 1;5(4):e227970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Porterfield A, Engelbert K, Coustasse A. Electronic prescribing: improving the efficiency and accuracy of prescribing in the ambulatory care setting. Perspect Health Inf Manag. 2014. Apr 1;11(Spring):1g. [PMC free article] [PubMed] [Google Scholar]
- 27.Numerous wrong dose errors with Nirmatrelvir/ritonavir. ISMP Medication Safety Alert! Acute Care [Internet]. PA: Institute for Safe Medication Practices; c2022. [cited 2022 August 1]. Available from: https://www.ismp.org/resources/numerous-wrong-dose-errors-Nirmatrelvir/ritonavir. [Google Scholar]
- 28.Kheshti R, Aalipour M, Namazi S. A comparison of five common drug-drug interaction software programs regarding accuracy and comprehensiveness. J Res Pharm Pract. 2016. Oct-Dec;5(4):257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qato DM, Wilder J, Schumm LP, Gillet V, Alexander GC. Changes in Prescription and Over-the-Counter Medication and Dietary Supplement Use Among Older Adults in the United States, 2005 vs 2011. JAMA Intern Med. 2016. Apr;176(4):473–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Potential moderate to severe DDIs of nirmatrelvir/ritonavir examined in the current study
eTable 2. Adjusted association between risk factors and potential moderate to severe DDIs with a shortened/ extended concomitant medication use period
eFigure 1. Study implementation for sensitivity analysis
Data Availability Statement
The datasets generated during and/or analysed during the current study are available in the NCATS N3C Data Enclave, https://covid.cd2h.org/
Code is available from Xuya Xiao


