Abstract
Background
Magnesium sulphate has been used in some settings as a tocolytic agent to inhibit uterine activity in women in preterm labour with the aim of preventing preterm birth.
Objectives
To assess the effects of magnesium sulphate therapy given to women in threatened preterm labour with the aim of preventing preterm birth and its sequelae.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (last searched 31 January 2014).
Selection criteria
Randomised controlled trials of magnesium sulphate as the only tocolytic, administered by any route, compared with either placebo, no treatment or alternative tocolytic therapy (not magnesium sulphate) to women considered to be in preterm labour.
Data collection and analysis
At least two review authors assessed trial eligibility and risk of bias and undertook data extraction independently.
Main results
The 37 included trials (total of 3571 women and over 3600 babies) were generally of moderate to high risk of bias. Antenatal magnesium sulphate was compared with either placebo, no treatment, or a range of alternative tocolytic agents.
For the primary outcome of giving birth within 48 hours after trial entry, no significant differences were seen between women who received magnesium sulphate and women who did not (whether placebo/no alternative tocolytic drug, betamimetics, calcium channel blockers, cox inhibitors, prostaglandin inhibitors, or human chorionic gonadotropin) (19 trials, 1913 women). Similarly for the primary outcome of serious infant outcome, there were no significant differences between the infants exposed to magnesium sulphate and those not (whether placebo/no alternative tocolytic drug, betamimetics, calcium channel blockers, cox inhibitors, prostaglandin inhibitors, human chorionic gonadotropin or various tocolytic drugs) (18 trials; 2187 babies). No trials reported the outcome of extremely preterm birth. In the seven trials that reported serious maternal outcomes, no events were recorded.
In the group treated with magnesium sulphate compared with women receiving antenatal placebo or no alternative tocolytic drug, a borderline increased risk of total death (fetal, neonatal, infant) was seen (risk ratio (RR) 4.56, 95% confidence interval (CI) 1.00 to 20.86; two trials, 257 babies); none of the comparisons between magnesium sulphate and other classes of tocolytic drugs showed differences for this outcome (10 trials, 991 babies). The outcomes of neonatal and/or infant deaths and of fetal deaths did not show differences between magnesium sulphate and no magnesium sulphate, whether compared with placebo/no alternative tocolytic drug, or any specific class of tocolytic drug. For most of the other secondary outcomes, there were no significant differences between magnesium sulphate and the control groups for risk of preterm birth (except for a significantly lower risk with magnesium sulphate when compared with barbiturates in one trial of 65 women), gestational age at birth, interval between trial entry and birth, other neonatal morbidities, or neurodevelopmental outcomes. Duration of neonatal intensive care unit stay was significantly increased in the magnesium sulphate group compared with the calcium channel blocker group, but not when compared with cox inhibitors or prostaglandin inhibitors. No maternal deaths were reported in the four trials reporting this outcome. Significant differences between magnesium sulphate and controls were not seen for maternal adverse events severe enough to stop treatment, except for a significant benefit of magnesium sulphate compared with betamimetics in a single trial.
Authors' conclusions
Magnesium sulphate is ineffective at delaying birth or preventing preterm birth, has no apparent advantages for a range of neonatal and maternal outcomes as a tocolytic agent and its use for this indication may be associated with an increased risk of total fetal, neonatal or infant mortality (in contrast to its use in appropriate groups of women for maternal, fetal, neonatal and infant neuroprotection where beneficial effects have been demonstrated).
Keywords: Female; Humans; Pregnancy; Obstetric Labor, Premature; Fetal Death; Magnesium Sulfate; Magnesium Sulfate/adverse effects; Magnesium Sulfate/therapeutic use; Premature Birth; Premature Birth/prevention & control; Randomized Controlled Trials as Topic; Tocolytic Agents; Tocolytic Agents/adverse effects; Tocolytic Agents/therapeutic use; Treatment Outcome
Plain language summary
Magnesium sulphate for preventing preterm birth in threatened preterm labour
Even short‐term postponement of birth when labour begins early (before 37 weeks) can help improve outcomes for babies, as the woman can take corticosteroid drugs to help develop the baby's lungs in a short time. Magnesium sulphate is one of the drugs that has been used to try to stop the uterus contracting in women who go into labour too soon.
This review of 37 trials including 3571 women and their infants did not find that magnesium sulphate, given to women who go into labour too soon, prevented babies being born too soon or reduced the risks of the baby developing serious health problems. However, antenatal magnesium sulphate is effective in helping women who develop pre‐eclampsia (high blood pressure and protein in the urine) and for helping to protect babies' brains.
Background
Description of the condition
Preterm birth remains the principal cause of early neonatal death (March of Dimes 2012). Infants born preterm (before 37 weeks' gestation) often suffer significant immediate morbidity and need lengthy stays in neonatal intensive care units (Claas 2010; Darlow 2009). Moreover, there is a significant risk of long‐term neurological morbidity in a proportion of the survivors (Kugelman 2012). The more preterm the baby the greater are the risks, especially when birth occurs before 32 weeks (Boyle 2012). Parents are understandably worried and distressed when their baby is born preterm. Parents, health professionals and society share the burden of responsibility and costs, both personal and monetary, for preterm birth and its sequelae. The prevention of preterm birth therefore remains an important priority.
Description of the intervention
Tocolytic agents inhibit uterine contractions, and a variety have been used to inhibit uterine activity in women in preterm labour and so attempt to prevent preterm birth. Agents used include betamimetics, prostaglandin inhibitors, calcium channel blockers, ethanol, oxytocin receptor antagonists and magnesium sulphate. The ideal tocolytic agent should be easy to administer, inexpensive, without significant maternal, fetal or neonatal side effects, and effective at delaying preterm birth, at least long enough to permit the use of antenatal corticosteroids (Haas 2009; Roberts 2006).
There is considerable variation in the type of tocolytic agent used in different parts of the world. Magnesium sulphate has been widely used as a tocolytic in the United States of America (Besinger 1990; Grimes 2006), although there have been reports of an increase in infant mortality (Mittendorf 2002) and admission to neonatal intensive care (Greenberg 2011) with a suggestion that there is a dose‐response relationship (Greenberg 2011; Mittendorf 2002). In 2013 the US Food and Drug Administration advised against the use of antenatal magnesium sulphate for more than five to seven days when used to try to stop preterm labour, due to concerns about fetal and neonatal bone development; this led to the American College of Obstetricians and Gynecologists issuing a committee opinion supporting short‐term but not long‐term use (ACOG 2013).
How the intervention might work
Magnesium sulphate was described as having an effect on uterine contractility by increasing the duration of labour in the late 1950s (Hall 1959). The exact mechanism of magnesium sulphate as a tocolytic agent is only partially understood. Magnesium decreases the frequency of depolarisation of smooth muscle, by modulating calcium uptake, binding and distribution in smooth muscle cells. The net result is inhibition of uterine contractions. Magnesium sulphate is essential for cellular health including glycolysis, oxidative phosphorylation, protein synthesis and plasma membrane integrity (McIntosh 1989; Mildvan 1987).
Magnesium sulphate, by its peripheral vasodilator effects when infused intravenously, produces flushing, sweating, and a sensation of warmth. Reported maternal side effects relate to dosage and speed of infusion and include nausea, vomiting, headache, palpitations and rarely, pulmonary oedema. Administration to concentrations above the recommended therapeutic range can lead to respiratory depression, respiratory arrest and cardiac arrest (McDonnell 2009). For the neonate, hypermagnesaemia can lead to hyporeflexia, poor sucking, and, rarely, respiratory depression needing mechanical ventilation (Lipsitz 1971).
Why it is important to do this review
The previous version of this review concluded that magnesium sulphate was ineffective at delaying birth or preventing preterm birth and that its use was associated with an increased risk of infant mortality. Despite this evidence, using magnesium sulphate for tocolysis has remained a common practice in the USA in particular (Grimes 2006). It is therefore important to integrate evidence that has become available since the last update in order to see the impact on the review's previous conclusions.
Objectives
To assess the effects of magnesium sulphate therapy given to women in threatened preterm labour with the primary aim of preventing preterm birth and its sequelae.
Methods
Criteria for considering studies for this review
Types of studies
All published, unpublished and ongoing randomised trials that compared outcomes for women in threatened preterm labour given magnesium sulphate alone for tocolysis, with outcomes in controls, with or without placebo or alternative tocolytic drug therapy (not magnesium sulphate), reported as papers or abstracts. Quasi‐randomised trials were included.
Types of participants
Women considered to be in preterm labour given magnesium sulphate to reduce their risk of preterm birth.
Types of interventions
Magnesium sulphate as the only tocolytic, administered intravenously or orally, compared with either placebo, no placebo or alternative tocolytic therapy. Trials where magnesium sulphate was used as the primary tocolytic with an adjuvant tocolytic used in the case of failure, were included. Trials where the primary tocolytic was not magnesium sulphate but where magnesium sulphate was used as an adjuvant after treatment failure were excluded. Trials that assessed the use of magnesium sulphate as maintenance therapy after preterm labour were not included as they are covered in a separate review (Han 2013).
Types of outcome measures
Clinically relevant outcomes for trials of tocolysis for inhibiting preterm labour have been prespecified following consultation with the editors and authors of the individual reviews.
Consensus was reached on a set of ‘core’ outcomes, which are highlighted below. These will be included in all tocolysis reviews. In addition to these core outcomes, individual teams may include other outcomes as necessary.
Primary outcomes
Primary outcomes were chosen to be most representative of the clinically important measures of effectiveness and complications. Serious outcomes for the women and their infants are composite endpoints. All these events individually were expected to be rare and a modest change in their incidence more likely to be detected by using composite outcomes. The incidence of individual components were explored in the secondary outcomes.
Birth less than 48 hours after trial entry.
Extremely preterm birth (less than 28 weeks' gestation).
Serious infant outcome (defined as death or chronic lung disease [need for supplemental oxygen at 28 days of life or later], grade three or four intraventricular haemorrhage or periventricular leukomalacia, major neurosensory disability (defined as any of legal blindness, sensorineural deafness requiring hearing aids, moderate or severe cerebral palsy, or developmental delay/intellectual impairment [defined as developmental quotient (DQ) or intelligence quotient (IQ) less than two standard deviations below mean])).
Serious maternal outcome (defined as death, cardiac arrest, respiratory arrest, admission to intensive care unit).
Secondary outcomes
These include other measures of effectiveness, complications, satisfaction with care and health service use.
For the infant/child
Fetal death, neonatal and infant death;
preterm birth (less than 37 weeks);
very preterm birth (less than 34 weeks);
gestational age at birth;
birth less than 24 hours after trial entry;
interval between trial entry and birth;
Apgar score less than seven at five minutes;
respiratory distress syndrome (RDS);
use of assisted ventilation;
air leak syndrome;
chronic lung disease (need for supplemental oxygen at 28 days of life or later);
intraventricular haemorrhage (IVH);
grade three or four IVH;
periventricular leukomalacia (PVL);
necrotising enterocolitis (NEC);
proven neonatal infection;
cerebral palsy;
blindness;
deafness;
developmental delay or intellectual impairment.
For the woman
Maternal death;
cardiac arrest;
respiratory arrest;
admission to intensive care unit;
discontinuation of therapy because of maternal adverse effects;
adverse drug reaction;
other adverse effects of therapy (including nausea, vomiting, respiratory depression, hypotension, tachycardia);
women's satisfaction with the therapy;
bleeding episodes (antepartum haemorrhage, postpartum haemorrhage, need for transfusion);
mode of birth.
Use of health services
Length of postnatal stay;
admission to neonatal intensive care and length of stay.
Search methods for identification of studies
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Electronic searches
We contacted the Trials Search Co‐ordinator to search the Cochrane Pregnancy and Childbirth Group's Trials Register (last searched 31 January 2014).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
weekly searches of Embase;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and Embase, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searched the register for each review using the topic list rather than keywords.
We did not apply any language restrictions.
Data collection and analysis
For methods used in the previous version of this review, see 'Crowther 2002'.
For this update, the following methods were used for assessing the reports that were identified as a result of the updated search.
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Selection of studies
At least two review authors independently assessed for inclusion all potential studies identified as a result of the search strategy. We resolved any disagreements through discussion.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors extracted the data using the agreed form. We resolved any discrepancies through discussion. We entered data into Review Manager software (RevMan 2012) and checked for accuracy.
When information regarding any of the above was unclear, we attempted to contact authors of original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion.
(1) Random sequence generation (checking for possible selection bias)
For each included study we described the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
For each included study we described the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
For each included study we described the methods used, if any, to blind study participants and personnel from which intervention a participant received. We considered studies to be at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
For each included study we described the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received.
We assessed the methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
For each included study, we described the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusions where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, we re‐included missing data in the analyses which we undertook.
We assessed the methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; 'as treated' analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
For each included study we investigated the possibility of selective outcome reporting bias and described what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we planned to assess the likely magnitude and direction of the bias and whether we considered it is likely to impact on the findings. We explored the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Measures of treatment effect
For dichotomous data we used the numbers of events in the control and intervention groups of each study to calculate risk ratios (RRs) with 95% confidence intervals. For continuous data we calculated the mean difference (MDs) between treatment groups where outcomes were measured in the same way. Standardised mean differences would have been used if the outcomes from trials were the same but different methods had been used to collect the data. We reported 95% confidence intervals for all outcomes.
Unit of analysis issues
There were no major unit of analysis issues. Several trials had unit of analysis issues with reporting of interventions used or multiple births, which meant that not all of the outcome data could be included in the meta‐analysis (Parilla 1997; Pezzati 2001).
Dealing with missing data
Levels of attrition were noted. For all of the outcomes, we carried out analyses using an intention‐to‐treat basis, where possible. All participants were analysed, where possible in the treatment group to which they were randomised, regardless of the actual treatment received. The denominator for each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We considered whether the clinical and methodological characteristics of the included studies were sufficiently similar for meta‐analysis to provide a clinically meaningful summary. This was done by assessing statistical heterogeneity using the Tau², Chi² and I² statistics. An I² measurement greater than 30% was taken to indicate substantial heterogeneity (Higgins 2011), and either a Tau² greater than zero, or a low P value (less than 0.10) in the Chi² test for heterogeneity. Where substantial heterogeneity was detected, we explored possible explanations in sensitivity/subgroup analyses. Statistical heterogeneity was taken into account when interpreting the results, especially where there was any variation in the direction of effect.
Assessment of reporting biases
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results. Some types of reporting bias (e.g. publication bias, multiple publication bias, language bias etc) reduce the likelihood that all studies eligible for a review will be retrieved. If all eligible studies are not retrieved, the review may be biased. We conducted a comprehensive search for eligible studies and were alert for duplication of data. When there were 10 or more studies in analyses, we used funnel plots to explore the possibility of small study effects (a tendency for estimates of the intervention effect to be more beneficial in smaller studies).
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2012). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect. If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we used a random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary was treated as the average range of possible treatment effects and we discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not meaningful, we did not combine trials.
Where we used random‐effects analysis, the results were presented as the average treatment effect with 95% confidence intervals, and estimates of I².
Subgroup analysis and investigation of heterogeneity
We planned subgroup analyses to examine separately the primary outcomes, and fetal, neonatal and infant mortality, for women exposed to magnesium sulphate compared with no treatment or placebo and women given magnesium sulphate compared with type of alternative tocolytic therapy (such as betamimetics, prostaglandin inhibitors, calcium channel blockers, nitric oxide donors and sedatives).
Further analyses were planned to assess the primary outcomes, and fetal, neonatal and infant mortality, by dose of magnesium sulphate used. This was achieved by comparing trials with a magnesium maintenance protocol that recommended magnesium sulphate infusion rates of up to 2 g/hour with trials with a magnesium maintenance protocol of more than 2 g/hour.
We conducted subgroup interaction tests.
Sensitivity analysis
We conducted a sensitivity analysis by looking at the primary outcomes, and fetal, neonatal and infant mortality, only in those trials that demonstrated low risk of bias for allocation concealment.
We also conducted a sensitivity analysis restricted to trials that reported if it was possible for women to have been switched to an alternative drug or treatment.
Results
Description of studies
Also see Characteristics of included studies table.
Results of the search
Fifty‐four trials of magnesium sulphate in threatened preterm labour have been identified.
Thirty‐seven (with a total of 3571 women) of these 54 trials met our inclusion criteria (Aramayo 1990; Armson 1992; Asgharnia 2002; Beall 1985; Borna 2007; Chau 1992; Clavin 1996; Cotton 1984; Cox 1990; El‐Sayed 1999; Floyd 1992; Fox 1993; Glock 1993; Haghighi 1999; Hollander 1987; Klauser 2012; Larmon 1999; Lorzadeh 2007; Lyell 2007; Ma 1992; McWhorter 2004; Miller 1982; Mittendorf 2002; Morales 1993; Parilla 1997; Parsons 1987; Pezzati 2001; Sayin 2010; Schorr 1997; Sciscione 1993; Steer 1977; Surichamorn 2001; Taherian 2007; Tchilinguirian 1984; Wang 2000; Wilkins 1988; Zhu 1996).
Included studies
Three of the trials were conducted in China (Ma 1992; Wang 2000; Zhu 1996), one in Mexico (Aramayo 1990), five in Iran (Asgharnia 2002; Borna 2007; Haghighi 1999; Lorzadeh 2007; Taherian 2007), one from Italy (Pezzati 2001), one from Turkey (Sayin 2010), one from Thailand (Surichamorn 2001) and the remainder in the United States of America.
Gestational age
The gestational age at trial entry varied between the trials.
Women at less than 30 weeks' gestation (Parilla 1997);
women at less than 32 weeks' gestation (Asgharnia 2002; Klauser 2012; Morales 1993; Schorr 1997);
women at less than 34 weeks' gestation (Borna 2007; Cotton 1984; Cox 1990; Floyd 1992; Glock 1993; Larmon 1999; Lyell 2007; Mittendorf 2002; McWhorter 2004; Parsons 1987; Pezzati 2001);
women at less than 35 weeks' gestation (Chau 1992; El‐Sayed 1999; Hollander 1987; Lorzadeh 2007; Surichamorn 2001);
women at less than 36 weeks' gestation (Aramayo 1990; Armson 1992; Haghighi 1999; Ma 1992; Sayin 2010; Sciscione 1993; Taherian 2007; Tchilinguirian 1984; Wilkins 1988; Zhu 1996).
The remainder included women at greater gestational ages up to 37 weeks. The gestation of women was not reported by Clavin 1996 or Wang 2000.
Loading dose
The protocol for the amount of magnesium sulphate to be used varied. The following loading doses of magnesium sulphate were reported:
4 g/hour was used in 21 trials (Aramayo 1990; Asgharnia 2002; Beall 1985; Chau 1992; Cotton 1984; Cox 1990; El‐Sayed 1999; Floyd 1992; Fox 1993; Hollander 1987; Lorzadeh 2007; Lyell 2007; Miller 1982; Mittendorf 2002; Parsons 1987; Pezzati 2001; Steer 1977; Surichamorn 2001; Taherian 2007; Tchilinguirian 1984; Wilkins 1988);
4.5 g/hour was used in one trial (Sayin 2010);
2.5 g/hour to 5 g/hour used in one trial (Wang 2000);
6 g/hour was used in seven trials (Armson 1992; Glock 1993; Haghighi 1999; Klauser 2012; Larmon 1999; Morales 1993; Schorr 1997);
4 to 6 g/hour was used in two trials (Borna 2007; McWhorter 2004);
4 to 8 g/hour was used in one trial (Parilla 1997).
Two trials did not state the dose used (Clavin 1996; Sciscione 1993).
Maintenance dose
The protocol for the amount of magnesium sulphate used for maintenance varied between 1.5 to 6 g/hour in the trials. The following doses were reported for magnesium sulphate maintenance
2 g or less (14 trials)
1 g/hour was used in one trial (Sayin 2010);
1.5 g/hour was used in one trial (Zhu 1996);
1.5 to 2 g/hour was used in one trial (Wang 2000);
2 g/hour was used in 11 trials (Aramayo 1990; Asgharnia 2002; Cotton 1984; Lorzadeh 2007; Lyell 2007; Ma 1992; Miller 1982; Pezzati 2001; Steer 1977; Tchilinguirian 1984; Wilkins 1988).
More than 2 g (21 trials)
1.5 to 3.5 g/hour was used in one trial (Beall 1985);
2.5 g/hour was used in one trial (Parilla 1997);
2 to 3 g/hour was used in four trials (Cox 1990; Mittendorf 2002; Parsons 1987; Taherian 2007);
2 to 4 g/hour was used in 11 trials (Armson 1992; Borna 2007; Chau 1992; El‐Sayed 1999; Fox 1993; Glock 1993; Haghighi 1999; Hollander 1987; Larmon 1999; McWhorter 2004; Surichamorn 2001);
2 to 5 g/hour was used in one trial (Morales 1993);
2 to 6 g/hour was used in one trial (Schorr 1997);
4 to 6 g/hour was used in two trials (Floyd 1992; Klauser 2012).
Two trials did not specify the amount used (Clavin 1996; Sciscione 1993).
Comparisons
Magnesium was compared with nine other preparations or classes of drugs in the 37 included trials.
1) Magnesium versus no other tocolytic drugs
Cox 1990 (saline);
Cotton 1984 (dextrose);
Fox 1993 (hydration, sedation);
Ma 1992 (sedation).
2) Magnesium versus betamimetics
Aramayo 1990; Chau 1992; Cotton 1984; Miller 1982; Parsons 1987; Surichamorn 2001 (terbutaline);
Armson 1992; Hollander 1987; Pezzati 2001; Sayin 2010; Tchilinguirian 1984; Wang 2000; Wilkins 1988; Zhu 1996 (ritodrine);
Beall 1985; Sciscione 1993 (ritodrine and/or terbutaline).
3) Magnesium versus calcium channel blockers
Floyd 1992; Glock 1993; Haghighi 1999; Klauser 2012; Lyell 2007; Taherian 2007 (nifedipine);
Larmon 1999 (nicardipine).
4) Magnesium versus cox inhibitors
Borna 2007 (celecoxib);
McWhorter 2004 (rofecoxib).
5) Magnesium versus prostaglandin inhibitors
Asgharnia 2002; Klauser 2012; Morales 1993; Parilla 1997 (indomethacin);
Schorr 1997 (ketorolac).
6) Magnesium versus alcohol
Steer 1977 (alcohol; dextrose).
7) Magnesium versus human chorionic gonadotropin
8) Magnesium versus nitroglycerin
9) Magnesium versus obstetrician's preference
Excluded studies
All of the 17 excluded trials were excluded because they did not meet the inclusion criteria for study design or comparison (Behrad 2003; Di Renzo 2005; Kara 2009; Mittendorf 2000; Ogburn 1985; Pryde 2001; Soguk 2004; Terrone 2000; Wischnik 1989; Zygmunt 2003), were not randomised (Herschel 2001; Ieda 1991; Scudiero 2000), used magnesium as an adjuvant therapy (Ferguson 1984; Hatjis 1987), did not meet the criteria for included population (How 1998) or did not report on tocolysis (How 2006).
Risk of bias in included studies
Figure 1 and Figure 2 illustrate the risk of bias of the included trials in this review. Overall, we judged the included trials to be of moderate to high risk of bias.
1.
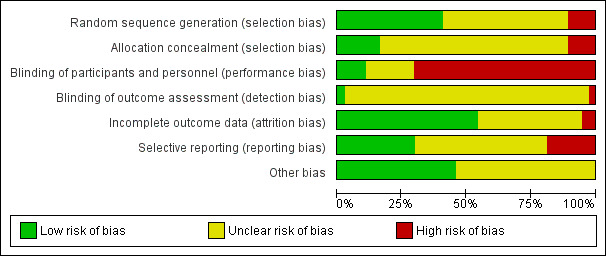
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
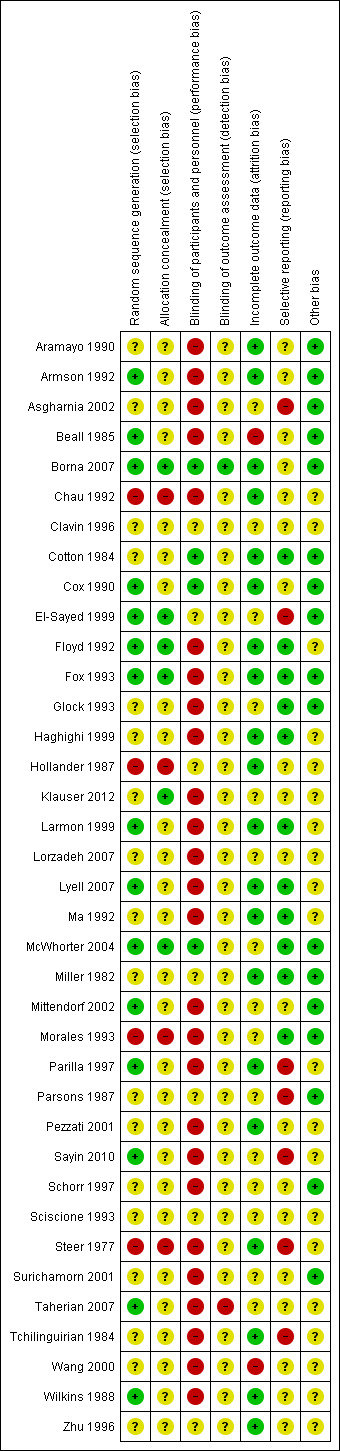
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Adequate allocation concealment was reported in six trials (Borna 2007; El‐Sayed 1999; Floyd 1992; Fox 1993; Klauser 2012; McWhorter 2004).
Four trials were quasi‐randomised (Chau 1992; Hollander 1987; Morales 1993; Steer 1977).
The remaining 27 trials provided insufficient detail to make a judgement on the adequacy of allocation concealment.
Reporting of methods of sequence generation was more common, with 15 trials reporting adequate methods such as computer‐generated random number tables.
Blinding
Use of a placebo and/or adequate blinding of participants and study personnel was reported in only four trials (Borna 2007; Cotton 1984; Cox 1990; McWhorter 2004). For most of the remaining trials, blinding of participants and study personnel would not have been feasible due to interventions being administered by different routes, for example.
Blinding of outcome assessors was mentioned in only one trial (Pezzati 2001), where some outcomes were reported to have been assessed in a blinded manner. None of the other 36 trials specifically mentioned blinded assessment of outcomes, although we judged that this may have been done in one trial (Borna 2007).
Incomplete outcome data
Most trials (n = 20) were judged to be at low risk of attrition bias. Only two trials (Beall 1985; Wang 2000) reported high and/or imbalanced losses to follow‐up. For the 15 remaining trials, attrition bias was judged to be unclear.
Selective reporting
Only 11 trials appeared to be free of selective reporting bias. The main reason for being judged to be at unclear (n = 19) or high (n = 7) risk of bias was lack of reporting of perinatal outcomes. Two of the seven trials judged to have a high selective reporting bias each reported a single outcome ‐ maternal temperature in Parsons 1987 and doppler flow in Sayin 2010.
Other potential sources of bias
The majority of trials (n = 20) were judged to have unclear risk of other sources of bias for reasons including baseline imbalances and unit of analysis issues.
Effects of interventions
1. Magnesium sulphate group versus placebo/no treatment or other tocolytic agent (all included trials)
Primary outcomes
Primary outcomes for the infant
'Birth in less than 48 hours from treatment' was reported in 19 trials that included 1913 women (Aramayo 1990; Asgharnia 2002; Borna 2007; Chau 1992; Cotton 1984; Fox 1993; Glock 1993; Haghighi 1999; Larmon 1999; Lorzadeh 2007; Lyell 2007; Ma 1992; McWhorter 2004; Morales 1993; Sayin 2010; Surichamorn 2001; Taherian 2007; Tchilinguirian 1984; Wilkins 1988). No significant differences were seen for the risk of birth within 48 hours of treatment for women given magnesium sulphate compared with women who did not receive magnesium sulphate (whether placebo/no alternative tocolytic drug: average risk ratio (RR) 0.56, 95% confidence interval (CI) 0.27 to 1.14, three trials, 182 women, I² = 80%; betamimetics: average RR 1.09, 95% CI 0.72 to 1.65, seven trials, 503 women, I² = 0%; calcium channel blockers: average RR 1.19, 95% CI 0.86 to 1.65, five trials, 588 women, I² = 0%; cox inhibitors: average RR 1.08, 95% CI 0.91 to 1.27, two trials, 318 women, I² = 0%: prostaglandin inhibitors: average RR 0.93, 95% CI 0.71 to 1.22, two trials, 221 women, I² = 0%; or human chorionic gonadotropin (HCG): average RR 1.37, 95% CI 0.47 to 4.04, one trial, 101 women, I² = 0%) ‐ Analysis 1.1. Significant heterogeneity was noted for the comparison of magnesium sulphate with placebo/no alternative tocolytic drugs and so a random‐effects model was used.
1.1. Analysis.
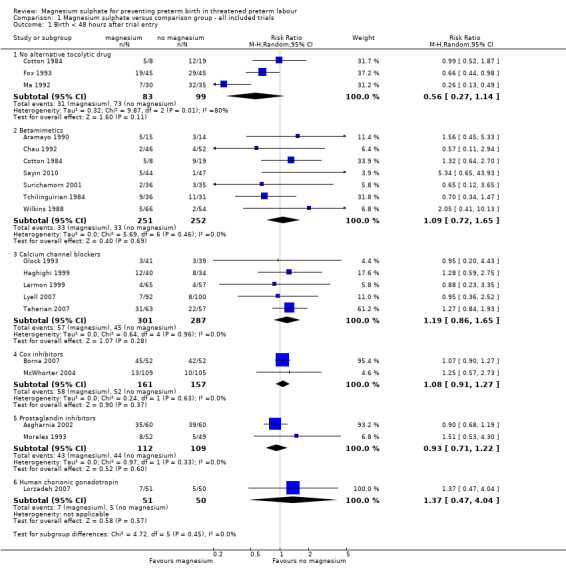
Comparison 1 Magnesium sulphate versus comparison group ‐ all included trials, Outcome 1 Birth < 48 hours after trial entry.
There were no data on extremely preterm birth (< 28 weeks' gestation).
'Serious infant outcome' was reported in 18 trials (Beall 1985; Borna 2007; Cotton 1984; Cox 1990; Floyd 1992; Fox 1993; Glock 1993; Klauser 2012; Larmon 1999; Lorzadeh 2007; Lyell 2007; McWhorter 2004; Mittendorf 2002; Morales 1993; Pezzati 2001; Sayin 2010; Schorr 1997; Surichamorn 2001). No significant differences were evident for the risk of serious infant outcome for women who received magnesium sulphate compared with women who did not (whether placebo/no alternative tocolytic drug: RR 2.34, 95% CI 0.78 to 7.01, three trials, 284 infants; betamimetics: RR 0.92, 95% CI 0.20 to 4.12, five trials, 344 infants; calcium channel blockers: RR 1.02, 95% CI 0.37 to 2.83, five trials, 675 infants; cox inhibitors: RR 8.19, 95% CI 0.45 to 150.22, two trials, 314 infants: prostaglandin inhibitors: RR 0.77, 95% CI 0.27 to 2.21, three trials, 355 infants; or various tocolytic drugs: RR 2.47, 95% CI 0.69 to 8.81, one trial, 106 infants) ‐ Analysis 1.3. The composition of this outcome varied between trials ‐ the Characteristics of included studies table describes the composite outcome to be used for each trial.
1.3. Analysis.
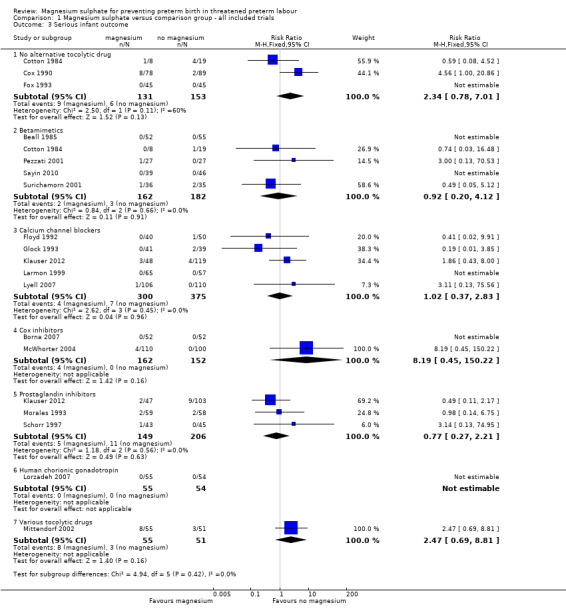
Comparison 1 Magnesium sulphate versus comparison group ‐ all included trials, Outcome 3 Serious infant outcome.
Primary outcomes for the mother
No serious maternal outcomes were reported in seven trials (n = 930 women ‐ Armson 1992; Asgharnia 2002; Beall 1985; Fox 1993; Klauser 2012; Lorzadeh 2007; Lyell 2007) ‐ Analysis 1.4.
1.4. Analysis.
Comparison 1 Magnesium sulphate versus comparison group ‐ all included trials, Outcome 4 Serious maternal outcome.
Secondary outcomes
Secondary outcomes for the infant
For 'birth in less than 24 hours from treatment' data were reported in four trials (Asgharnia 2002; Cox 1990; Wang 2000; Zhu 1996) that included 473 women. No difference was seen for the risk of birth within 24 hours of treatment for women given magnesium sulphate compared with no alternative tocolytic drug (RR 1.05, 95% CI 0.64 to 1.74, one trial, 156 women) ‐ Analysis 1.2. However, there was an increased risk for birth in less than 24 hours for women given magnesium sulphate compared with betamimetics (RR 4.39, 95% CI 1.75 to 11.05, two trials, 197 women), and a decreased risk for women given magnesium sulphate when compared with prostaglandin inhibitors (RR 0.56, 95% CI 0.37 to 0.84, one trial, 120 women).
1.2. Analysis.
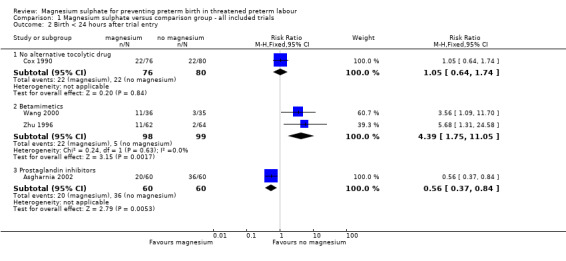
Comparison 1 Magnesium sulphate versus comparison group ‐ all included trials, Outcome 2 Birth < 24 hours after trial entry.
No benefit was seen for magnesium sulphate on the risk of preterm birth at less than 37 weeks for women given magnesium sulphate compared with betamimetics (average RR 1.03, 95% CI 0.77 to 1.39, six trials, 473 women, I² = 56%), calcium channel blockers (average 1.06, 95% CI 0.87 to 1.29, three trials, 362 women, I² = 0%) or prostaglandin inhibitors (average RR 1.83, 95% CI 0.58 to 5.81, one trial, 88 women). However, there was a decreased risk for women given magnesium sulphate when compared with no alternative tocolytic drug (average RR 0.62, 95% CI 0.46 to 0.83, one trial, 65 women) ‐ Analysis 1.5. Heterogeneity was noted for the comparison of magnesium sulphate with betamimetics drugs and so a random‐effects model was used. No benefit was seen for magnesium sulphate compared with calcium channel blockers on the risk of very preterm birth at less than 34 weeks (RR 0.89, 95% CI 0.55 to 1.45, two trials, 170 women) ‐ Analysis 1.6.
1.5. Analysis.
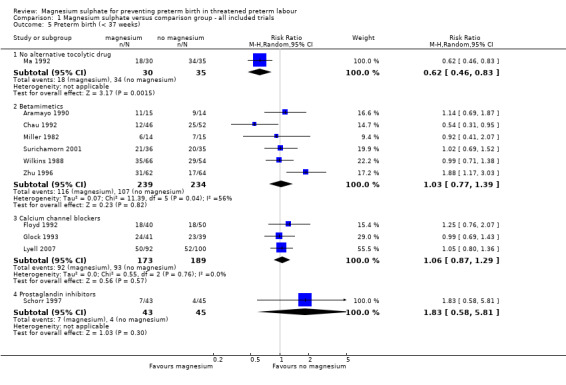
Comparison 1 Magnesium sulphate versus comparison group ‐ all included trials, Outcome 5 Preterm birth (< 37 weeks).
1.6. Analysis.
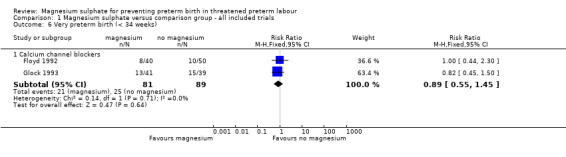
Comparison 1 Magnesium sulphate versus comparison group ‐ all included trials, Outcome 6 Very preterm birth (< 34 weeks).
No significant differences were seen for gestational age at birth for women who received magnesium sulphate compared with women who did not (whether placebo/no alternative tocolytic drug: average RR ‐0.50 weeks, 95% CI ‐1.85 to 0.85, three trials, 273 women, I² = 68%; betamimetics: average RR ‐0.36 weeks, 95% CI ‐1.61 to 0.89, three trials, 152 women, I² = 53%; calcium channel blockers: average RR ‐0.06 weeks, 95% CI ‐0.72 to 0.60, three trials, 439 women, I² = 1%; calcium channel blockers labour in first 48 hours: average RR ‐0.21 weeks, 95% CI ‐0.93 to 0.51, one trial, 53 women; calcium channel blockers labour in 2‐10 days: average RR ‐0.20 weeks, 95% CI ‐1.82 to 1.42, one trial, 12 women; cox inhibitors: average RR ‐0.17 weeks, 95% CI ‐0.95 to 0.61, two trials, 298 women, I² = 15%: or prostaglandin inhibitors: average RR ‐0.60 weeks, 95% CI ‐1.98 to 0.78, one trial, 150 women) ‐ Analysis 1.7. Heterogeneity was noted for the comparison of magnesium sulphate with no other tocolytics or betamimetic drugs and so a random‐effects model was used.
1.7. Analysis.
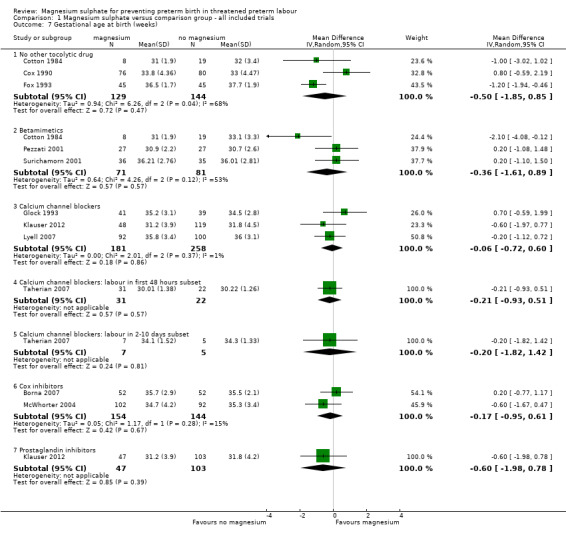
Comparison 1 Magnesium sulphate versus comparison group ‐ all included trials, Outcome 7 Gestational age at birth (weeks).
Interval between trial entry and birth (days) was reported in six trials including 556 women. No significant differences were seen between groups in time from trial entry to birth (magnesium sulphate versus no other tocolytic drug: mean difference (MD) 0.23 days, 95% CI ‐3.83 to 4.29, three trials, 273 women, I² = 9%; magnesium sulphate versus betamimetics: MD ‐1.72 days, 95% CI ‐14.89 to 11.45, three trials, 182 women, I² = 89%; magnesium sulphate versus prostaglandin inhibitors: MD ‐0.20 days, 95% CI ‐5.06 to 4.66, one trial, 101 women) ‐ Analysis 1.8. Significant heterogeneity was noted for the comparison of magnesium sulphate with betamimetic drugs and so a random‐effects model was used. The data for the interval between trial entry and birth show the mean to be smaller than the standard deviation in some trials, suggesting skew in these data. Caution is therefore recommended in interpretation of these data.
1.8. Analysis.
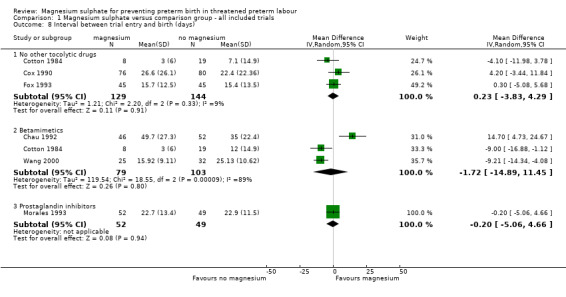
Comparison 1 Magnesium sulphate versus comparison group ‐ all included trials, Outcome 8 Interval between trial entry and birth (days).
In the group treated with magnesium sulphate compared with babies receiving antenatal placebo or no alternative tocolytic drug, a borderline increased risk of total death (fetal, neonatal and infant) was seen (RR 4.56, 95% CI 1.00 to 20.86; two trials, 257 babies); none of the comparisons between magnesium sulphate and other classes of tocolytic drugs showed differences for this outcome (10 trials, 991 babies) ‐ Analysis 1.9. The outcomes of neonatal and/or infant deaths and of fetal deaths did not show differences between magnesium sulphate and no magnesium sulphate, whether compared with placebo/no alternative tocolytic drug, or any specific class of tocolytic drug ‐ Analysis 1.11. Two fetal deaths occurred in the magnesium sulphate group in one trial (Cox 1990) and there was one fetal death in the calcium channel blocker group in Floyd 1992. In the other 11 trials that reported on fetal deaths there were none ‐ Analysis 1.10.
1.9. Analysis.
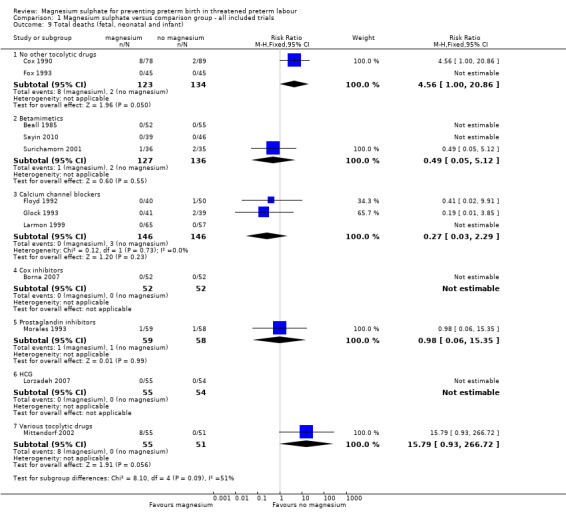
Comparison 1 Magnesium sulphate versus comparison group ‐ all included trials, Outcome 9 Total deaths (fetal, neonatal and infant).
1.11. Analysis.
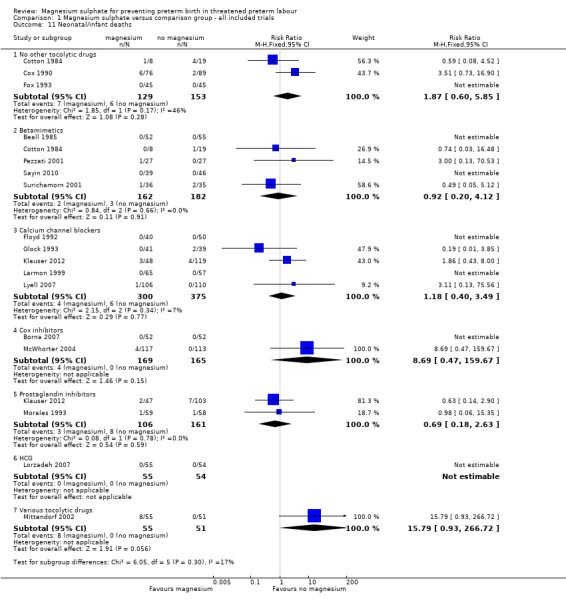
Comparison 1 Magnesium sulphate versus comparison group ‐ all included trials, Outcome 11 Neonatal/infant deaths.
1.10. Analysis.
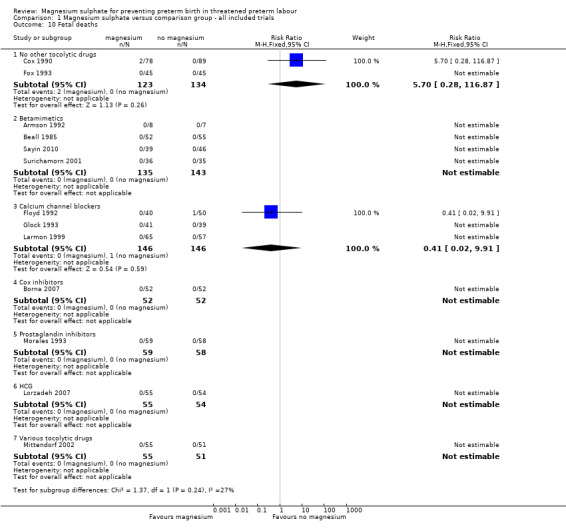
Comparison 1 Magnesium sulphate versus comparison group ‐ all included trials, Outcome 10 Fetal deaths.
We investigated the effects of magnesium sulphate on other neonatal morbidities (Analysis 1.12 to Analysis 1.20). In one trial of 90 infants, no significant difference was seen between magnesium and calcium channel blockers for Apgar less than seven at five minutes (RR 1.07, 95% CI 0.39 to 2.94) ‐ Analysis 1.12. No beneficial effect was seen for magnesium sulphate on the risk of other neonatal morbidity including risk of respiratory distress syndrome (Analysis 1.13), need for assisted ventilation (Analysis 1.14), chronic lung disease (Analysis 1.15), intraventricular haemorrhage (any) (Analysis 1.16), severe intraventricular haemorrhage (Analysis 1.17), periventricular leukomalacia (Analysis 1.18), necrotising enterocolitis (Analysis 1.19), or proven infection (Analysis 1.20).
1.12. Analysis.
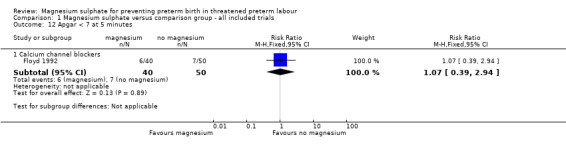
Comparison 1 Magnesium sulphate versus comparison group ‐ all included trials, Outcome 12 Apgar < 7 at 5 minutes.
1.20. Analysis.
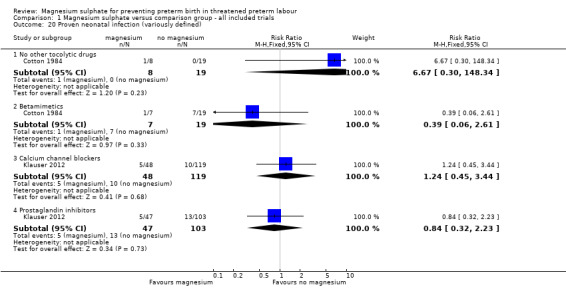
Comparison 1 Magnesium sulphate versus comparison group ‐ all included trials, Outcome 20 Proven neonatal infection (variously defined).
1.13. Analysis.
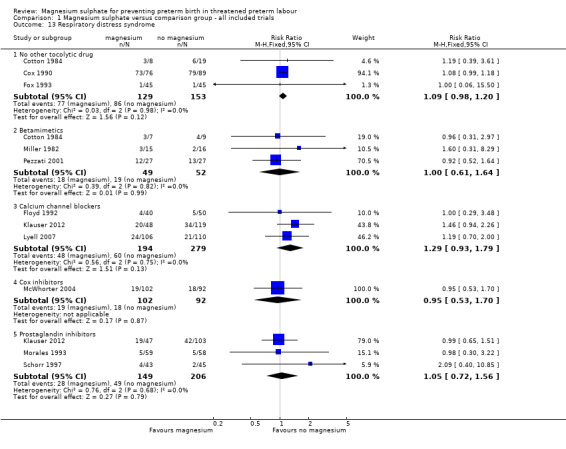
Comparison 1 Magnesium sulphate versus comparison group ‐ all included trials, Outcome 13 Respiratory distress syndrome.
1.14. Analysis.
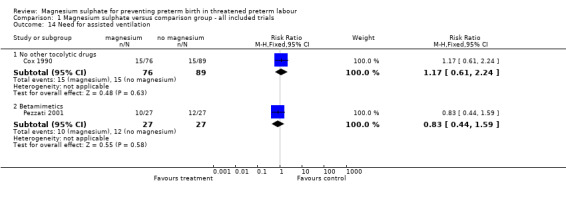
Comparison 1 Magnesium sulphate versus comparison group ‐ all included trials, Outcome 14 Need for assisted ventilation.
1.15. Analysis.
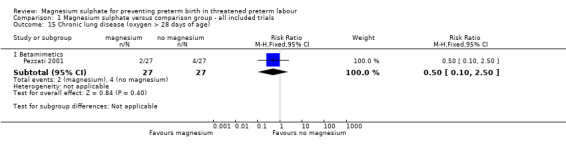
Comparison 1 Magnesium sulphate versus comparison group ‐ all included trials, Outcome 15 Chronic lung disease (oxygen > 28 days of age).
1.16. Analysis.
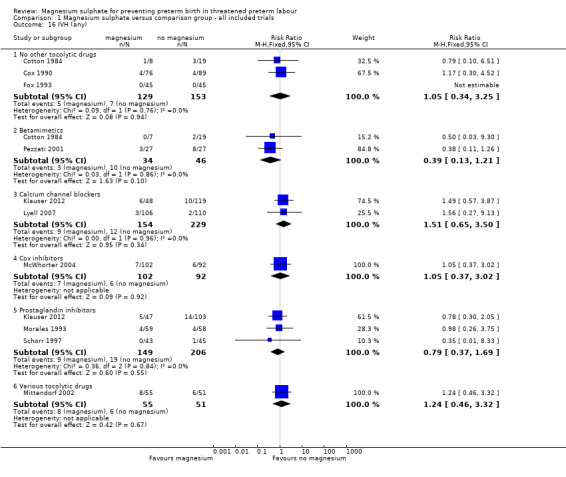
Comparison 1 Magnesium sulphate versus comparison group ‐ all included trials, Outcome 16 IVH (any).
1.17. Analysis.
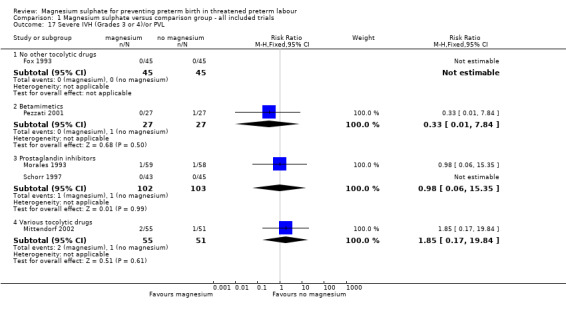
Comparison 1 Magnesium sulphate versus comparison group ‐ all included trials, Outcome 17 Severe IVH (Grades 3 or 4)/or PVL.
1.18. Analysis.
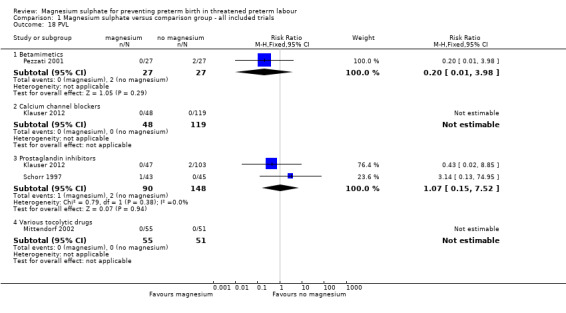
Comparison 1 Magnesium sulphate versus comparison group ‐ all included trials, Outcome 18 PVL.
1.19. Analysis.
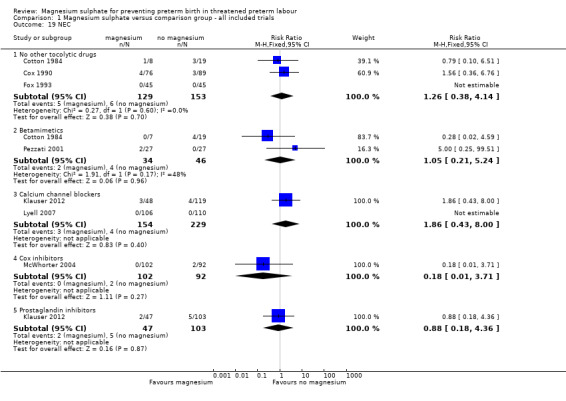
Comparison 1 Magnesium sulphate versus comparison group ‐ all included trials, Outcome 19 NEC.
We examined the effects of magnesium sulphate on neurodevelopment, although minimal data were reported. Data on cerebral palsy at childhood follow‐up at 18 months corrected age were available from one trial only (Mittendorf 2002). No significant reduction in the risk of cerebral palsy was reported (RR 0.13, 95% CI 0.01 to 2.51; 106 infants) ‐ Analysis 1.21.
1.21. Analysis.
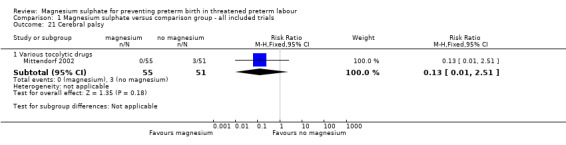
Comparison 1 Magnesium sulphate versus comparison group ‐ all included trials, Outcome 21 Cerebral palsy.
Secondary outcomes for the women
There was a lack of reporting of maternal outcomes. There were no maternal deaths in the four trials that reported this outcome (Armson 1992; Beall 1985; Borna 2007; Klauser 2012). No cardiac arrests were reported (Armson 1992; Klauser 2012; McWhorter 2004). Cox 1990 reported one respiratory arrest in the magnesium sulphate group (1/76) compared with no respiratory arrests in the control group (0/80). There were no admissions to intensive care in the single trial reporting this outcome (McWhorter 2004). Lyell 2007 reported significantly more maternal outcomes (a composite of shortness of breath, pulmonary oedema, hypotension and chest pain) in the women who were given magnesium (P = 0.03) compared with controls (20/92 versus 10/100, respectively). Klauser 2012 reported one case of pulmonary oedema in the magnesium sulphate group and one woman in the nifedipine group developed pleural effusion.
Fourteen trials that included 1134 women reported on maternal adverse effects sufficient to discontinue treatment. No significant differences were seen for any of the comparisons of magnesium sulphate, except for magnesium sulphate compared with betamimetics, which favoured magnesium sulphate (average RR 0.14 95% CI 0.03 to 0.75, five trials, 398 women). Significant heterogeneity was noted for the comparison of magnesium sulphate with no other tocolytic drugs and so a random‐effects model was used (average RR 1.31, 95% CI 0.01 to 221.68, four trials, 302 women, I² = 85%) ‐ Analysis 1.26.
1.26. Analysis.
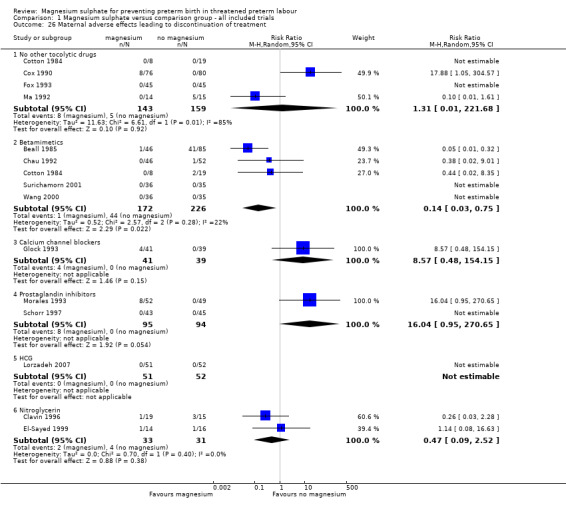
Comparison 1 Magnesium sulphate versus comparison group ‐ all included trials, Outcome 26 Maternal adverse effects leading to discontinuation of treatment.
Klauser 2012 reported only cessation of therapy due to adverse events or treatment failure; however, neither of these was recorded in the magnesium group.
More women who had been treated with magnesium sulphate experienced nausea compared with calcium channel blockers (average RR 5.25, 95% CI 2.29 to 12.07, I² = 32%, one trial, 192 women), although no differences were observed between magnesium sulphate and other tocolytics ‐ Analysis 1.27. More women who had been treated with magnesium sulphate experienced vomiting or nausea and/or vomiting when compared with calcium channel blockers or HCG (RR 5.22, 95% CI 2.08 to 13.10 one trial, 192 women; RR 63.75, 95% CI 4.01 to 1013.51, one trial, 101 women), although again no differences were observed between magnesium sulphate and other tocolytics ‐ Analysis 1.28 and Analysis 1.29 respectively.
1.27. Analysis.
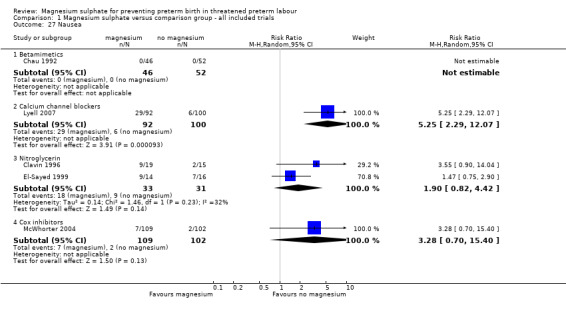
Comparison 1 Magnesium sulphate versus comparison group ‐ all included trials, Outcome 27 Nausea.
1.28. Analysis.
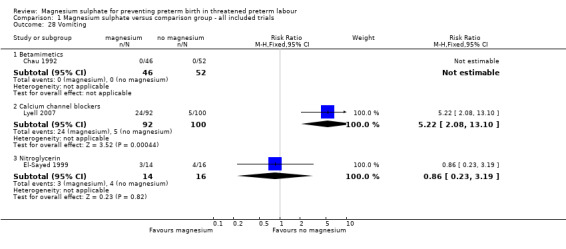
Comparison 1 Magnesium sulphate versus comparison group ‐ all included trials, Outcome 28 Vomiting.
1.29. Analysis.
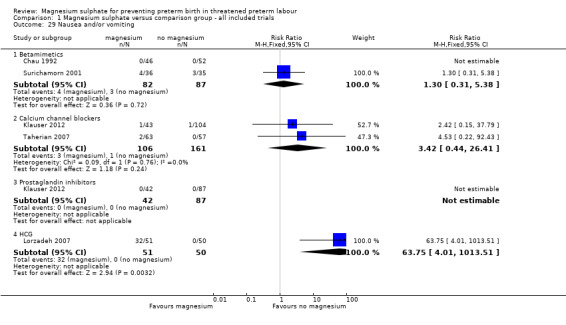
Comparison 1 Magnesium sulphate versus comparison group ‐ all included trials, Outcome 29 Nausea and/or vomiting.
Significantly fewer women in the magnesium sulphate groups experienced hypotension compared with nitroglycerin (RR 0.32, 95% CI 0.14 to 0.74, two trials of 64 women), although no differences were observed between magnesium sulphate and other tocolytics ‐ Analysis 1.30. No significant differences between groups were seen for tachycardia (Analysis 1.31) or in the rate of caesarean birth (Analysis 1.32).
1.30. Analysis.
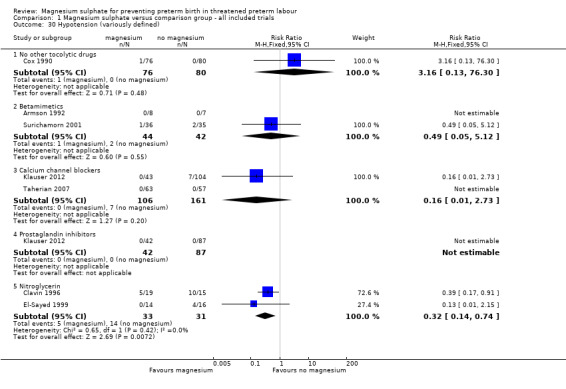
Comparison 1 Magnesium sulphate versus comparison group ‐ all included trials, Outcome 30 Hypotension (variously defined).
1.31. Analysis.
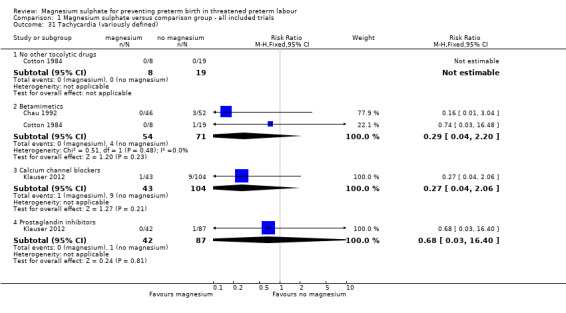
Comparison 1 Magnesium sulphate versus comparison group ‐ all included trials, Outcome 31 Tachycardia (variously defined).
1.32. Analysis.
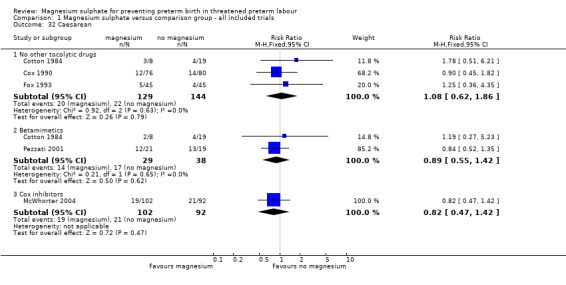
Comparison 1 Magnesium sulphate versus comparison group ‐ all included trials, Outcome 32 Caesarean.
With regards to health service usage, no differences were seen between the magnesium sulphate and control groups for neonatal intensive care unit admission ‐ Analysis 1.33. Heterogeneity was noted for the comparison of magnesium sulphate with calcium channel blockers and so a random‐effects model was used. Evidence from two trials (Klauser 2012; Lyell 2007) indicated a significant increase in length of neonatal intensive care unit stay in the group exposed to magnesium sulphate compared with calcium channel blockers (MD 4.55 days, 95% CI 0.96 to 8.15, 383 infants) ‐ Analysis 1.34.
1.33. Analysis.
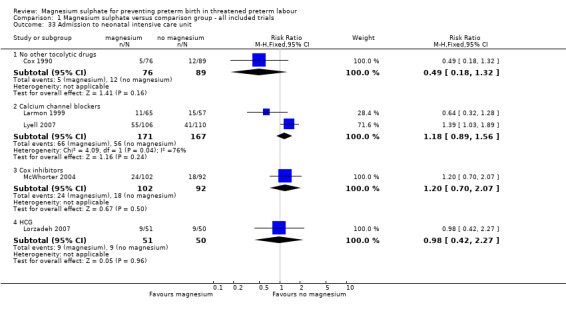
Comparison 1 Magnesium sulphate versus comparison group ‐ all included trials, Outcome 33 Admission to neonatal intensive care unit.
1.34. Analysis.
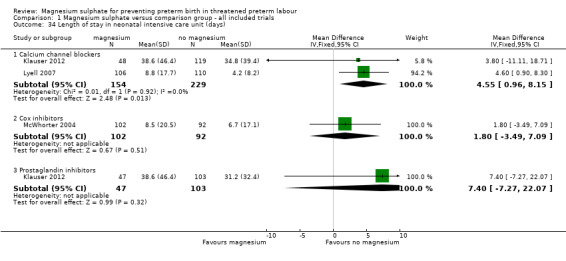
Comparison 1 Magnesium sulphate versus comparison group ‐ all included trials, Outcome 34 Length of stay in neonatal intensive care unit (days).
Data were available for many of the secondary outcomes, although little information was given for maternal outcomes and for use of health services. No outcome data were reported for: extremely preterm birth (less than 28 weeks), air leak syndrome, blindness, deafness, developmental delay/intellectual impairment, adverse drug reaction, respiratory depression, women's assessment of therapy/care, antepartum or postpartum haemorrhage, need for transfusion and length of postnatal stay.
2. Magnesium sulphate for tocolysis (subgrouped by dose of magnesium sulphate)
To explore the possible effect of magnesium sulphate dosage on outcomes, the trials were subgrouped where possible according to the maintenance of magnesium sulphate recommended in the trial reports as:
low dose (2 g/hour or less), 14 trials: Aramayo 1990; Asgharnia 2002; Cotton 1984; Lorzadeh 2007; Lyell 2007; Ma 1992; Miller 1982; Pezzati 2001; Sayin 2010; Steer 1977; Tchilinguirian 1984; Wang 2000; Wilkins 1988; Zhu 1996); and
higher dose (greater than 2 g/hour), 21 trials: Armson 1992; Beall 1985; Borna 2007; Chau 1992; Cox 1990; El‐Sayed 1999; Floyd 1992; Fox 1993; Glock 1993; Haghighi 1999; Hollander 1987; Klauser 2012; Larmon 1999; McWhorter 2004; Mittendorf 2002; Morales 1993; Parilla 1997; Parsons 1987; Schorr 1997; Surichamorn 2001; Taherian 2007).
Primary outcomes
Primary outcomes for the infant
Birth in less than 48 hours from treatment
Nine trials contributed data to the low‐dose subgroup (≤ 2 g/hr) (Aramayo 1990; Asgharnia 2002; Cotton 1984; Lorzadeh 2007; Lyell 2007; Ma 1992; Sayin 2010; Tchilinguirian 1984; Wilkins 1988) and 10 trials contributed to the higher‐dose subgroup (Borna 2007; Chau 1992; Fox 1993; Glock 1993; Haghighi 1999; Larmon 1999; McWhorter 2004; Morales 1993; Surichamorn 2001; Taherian 2007). Significant heterogeneity was found for the risk of birth within 48 hours of treatment for the low‐dose subgroup only (I² = 61%). No difference was seen for the risk of birth within 48 hours of treatment for women given 2 g/hour of magnesium sulphate or less (average RR 0.91, 95% CI 0.60 to 1.38, using a random‐effects model), or in women given more than 2 g/hour of magnesium sulphate (average RR 1.04, 95% CI 0.90 to 1.19, using a random‐effects model) compared with controls ‐ Analysis 2.1. The evidence indicates that neither a low dose nor a high dose of magnesium were effective in prolonging time to birth (with a nonsignificant subgroup interaction test of Chi² 0.35, I² = 0%, P = 0.55).
2.1. Analysis.
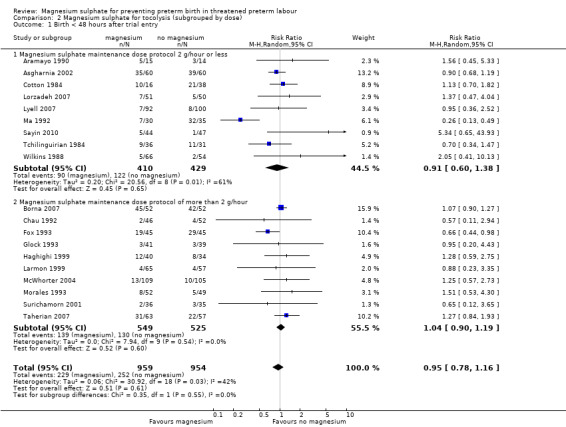
Comparison 2 Magnesium sulphate for tocolysis (subgrouped by dose), Outcome 1 Birth < 48 hours after trial entry.
No data were reported for extremely preterm birth (less than 28 weeks).
Serious infant outcomes were reported in four trials of low‐dose maintenance magnesium sulphate (Cotton 1984; Lorzadeh 2007; Pezzati 2001; Sayin 2010) and 14 trials of high dose (Beall 1985; Borna 2007; Cox 1990; Floyd 1992; Fox 1993; Glock 1993; Klauser 2012; Larmon 1999; Lyell 2007; McWhorter 2004; Mittendorf 2002; Morales 1993; Schorr 1997; Surichamorn 2001). There was no evidence of heterogeneity; or of a difference in serious infant outcomes between the low‐dose magnesium sulphate subgroup and the control group (RR 0.83, 95% CI 0.15 to 4.65); or for the higher‐dose magnesium group compared with the control group (RR 1.48, 95% CI 0.83 to 2.63), I² = 0%; 32/1014 compared with 30/1173, respectively). The subgroup interaction test was not significant (Chi² = 0.39, I² 0%, P = 0.53) ‐ Analysis 2.2.
2.2. Analysis.
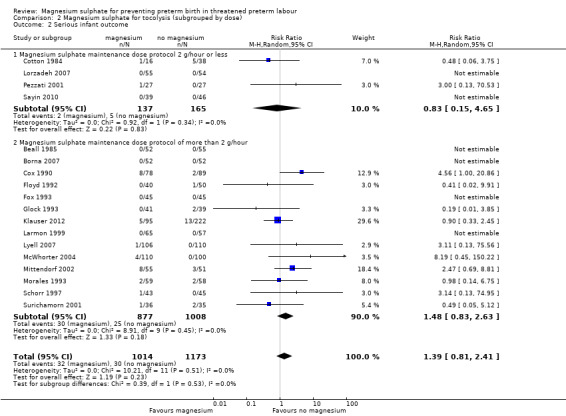
Comparison 2 Magnesium sulphate for tocolysis (subgrouped by dose), Outcome 2 Serious infant outcome.
Primary outcome for the women
Serious maternal outcomes were reported in seven trials (three in the low‐dose and four in the higher‐dose subgroup). No events were reported in any of these ‐ Analysis 2.3.
2.3. Analysis.
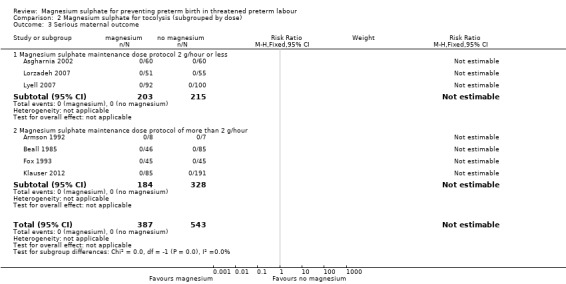
Comparison 2 Magnesium sulphate for tocolysis (subgrouped by dose), Outcome 3 Serious maternal outcome.
Secondary outcomes (selected)
For total deaths (Analysis 2.4) and fetal deaths (Analysis 2.5), subgroup interaction tests could not be calculated as no deaths were reported in the low‐dose subgroup. For neonatal and/or infant deaths (Analysis 2.6), the subgroup interaction test was not significant (ChiI² = 0.93, I² = 0%, P = 0.33).
2.4. Analysis.
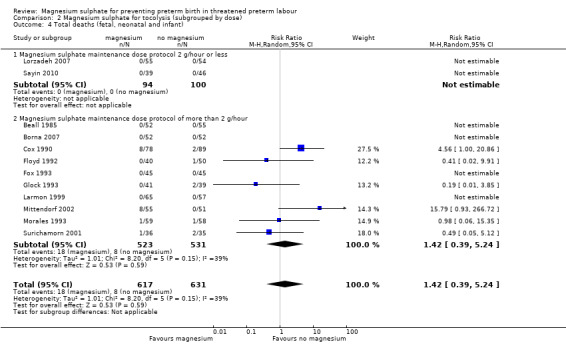
Comparison 2 Magnesium sulphate for tocolysis (subgrouped by dose), Outcome 4 Total deaths (fetal, neonatal and infant).
2.5. Analysis.
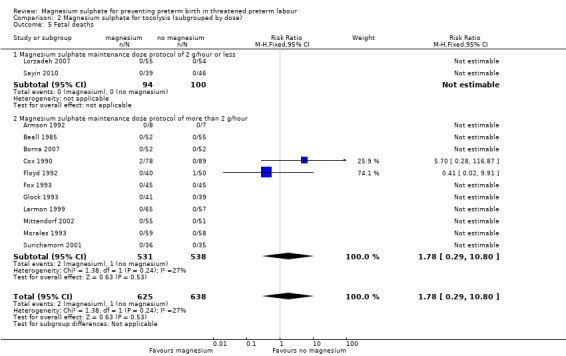
Comparison 2 Magnesium sulphate for tocolysis (subgrouped by dose), Outcome 5 Fetal deaths.
2.6. Analysis.
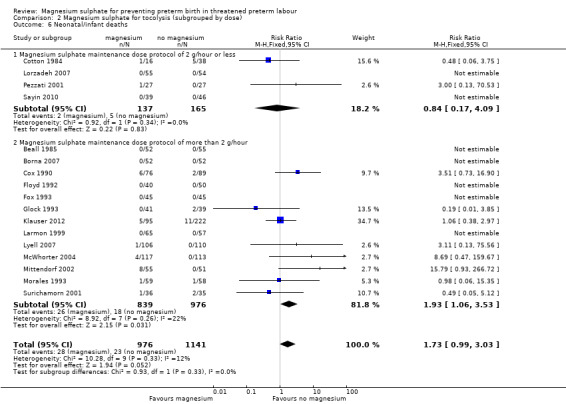
Comparison 2 Magnesium sulphate for tocolysis (subgrouped by dose), Outcome 6 Neonatal/infant deaths.
3. Magnesium sulphate versus placebo/no treatment or other tocolytic agent ‐ sensitivity analysis (using trials at low risk of bias)
Six trials including 846 women were rated as low risk of bias for allocation concealment (Borna 2007; El‐Sayed 1999; Floyd 1992; Fox 1993; Klauser 2012; McWhorter 2004).
Primary outcomes
Primary outcomes for the infant
Birth in less than 48 hours from treatment
Restricting the analysis to the three trials with adequate allocation concealment that reported this outcome (Borna 2007; Fox 1993; McWhorter 2004) made little material difference (RR 0.83, 95% CI 0.44 to 1.58).
Serious infant outcomes
Restricting the analysis to the five trials with adequate allocation concealment (Borna 2007; Floyd 1992; Fox 1993; Klauser 2012; McWhorter 2004) again made little difference to the result (RR 1.21, 95% CI 0.52 to 2.80).
Primary outcomes for the mother
Serious maternal outcomes
No serious maternal outcomes were reported in the two trials (Fox 1993; Klauser 2012) with adequate allocation concealment ‐ as was also the case for the other five trials.
Secondary outcomes
Fetal, neonatal and infant mortality
Restricting the analysis to the three trials with adequate allocation concealment (Borna 2007; Floyd 1992; Fox 1993) reporting total fetal, neonatal and infant deaths maintained a non‐significant result (RR 0.41, 95% CI 0.02 to 9.91). Note that this analysis now contains only one death (as does the corresponding sensitivity analysis for fetal death).
For neonatal and infant deaths, restricting the analysis to the five trials with adequate allocation concealment (Borna 2007; Floyd 1992; Fox 1993; Klauser 2012; McWhorter 2004) changed the effect from borderline increased mortality in the magnesium sulphate group compared with controls to a non‐significant finding (RR 1.59, 95% CI 0.64 to 3.95).
4. Magnesium sulphate for tocolysis (subgrouped by tocolytic agent)
None of the subgroup interaction tests indicated significant differences in effect of different tocolytic agents for the primary outcomes or for the outcomes of fetal, neonatal or infant death. See Analysis 1.1; Analysis 1.3; Analysis 1.4; Analysis 1.9 for data relating to these outcomes by type of tocolytic agent.
5. Switching/cross‐over from one tocolytic agent to another
Switching tocolytic agent in the event of treatment failure or adverse event was reported or suggested in 11 trials ‐ see Characteristics of included studies (but may have gone unreported in some of the other 26 trials). A sensitivity analysis omitting data for the primary outcomes and fetal/neonatal mortality from these 11 trials shows little impact for the primary outcomes of birth less than 48 hours after trial entry; serious infant composite outcome; serious maternal composite and secondary outcomes of total perinatal mortality or fetal death. However, omission of the 11 trials did shift the neonatal/infant mortality result into statistical significance (from RR 1.73, 95% CI 1.00 to 3.00 to RR 1.77, 95% CI 1.01 to 3.10).
Reporting bias (funnel plots)
We have constructed funnel plots for analyses with 10 or more trials (Figure 3; Figure 4; Figure 5; Figure 6; Figure 7; Figure 8; Figure 9; Figure 10; Figure 11; Figure 12).
3.
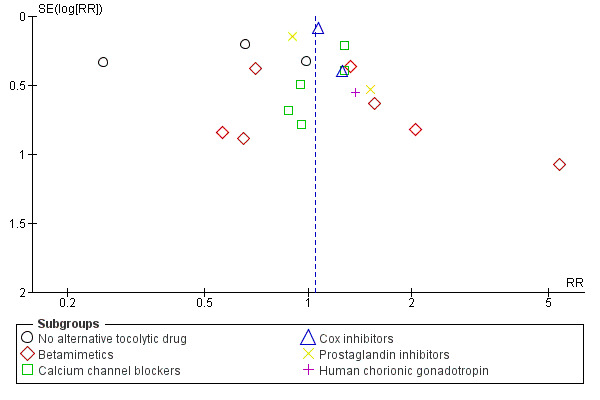
Funnel plot of comparison: 1 Magnesium sulphate versus comparison group ‐ all included trials, outcome: 1.1 Birth < 48 hours after trial entry.
4.
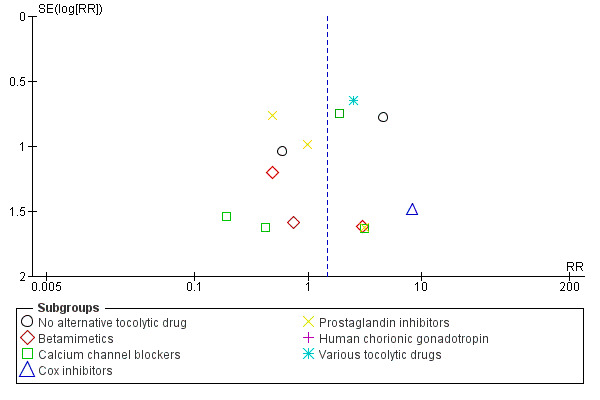
Funnel plot of comparison: 1 Magnesium sulphate versus comparison group ‐ all included trials, outcome: 1.3 Serious infant outcome.
5.
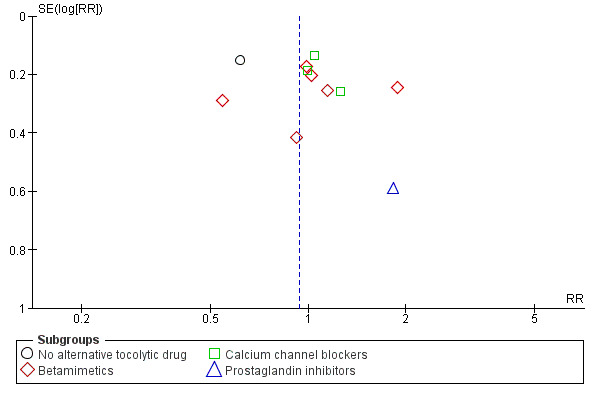
Funnel plot of comparison: 1 Magnesium sulphate versus comparison group ‐ all included trials, outcome: 1.5 Preterm birth (< 37 weeks).
6.
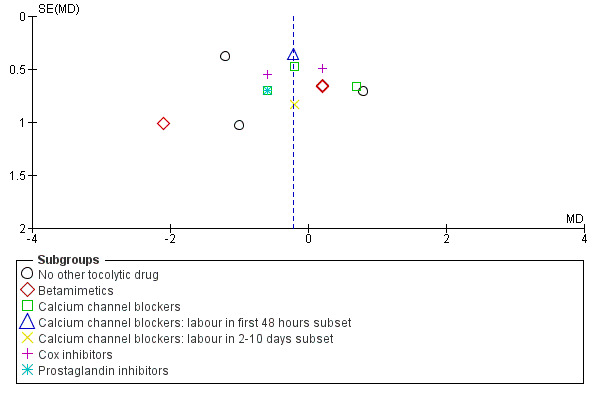
Funnel plot of comparison: 1 Magnesium sulphate versus comparison group ‐ all included trials, outcome: 1.7 Gestational age at birth (weeks).
7.
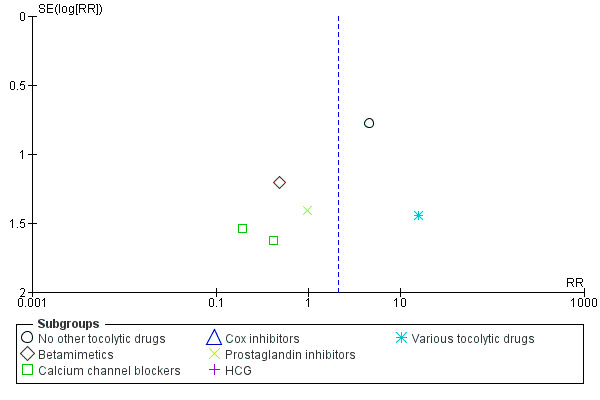
Funnel plot of comparison: 1 Magnesium sulphate versus comparison group ‐ all included trials, outcome: 1.9 Total deaths (fetal, neonatal and infant).
8.
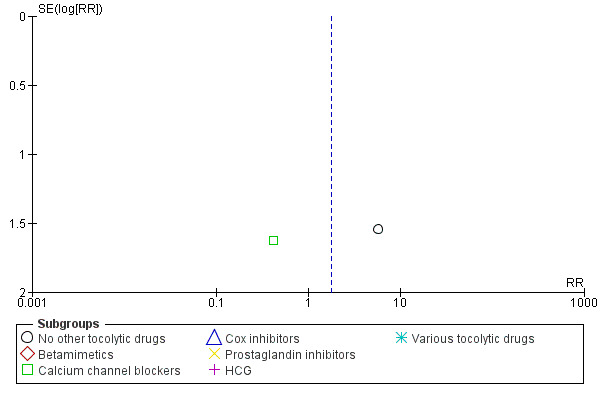
Funnel plot of comparison: 1 Magnesium sulphate versus comparison group ‐ all included trials, outcome: 1.10 Fetal deaths.
9.
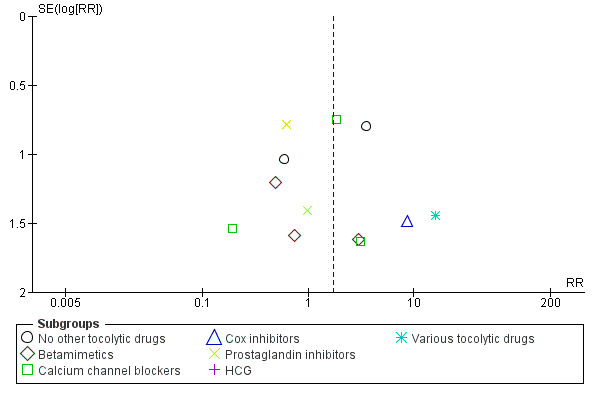
Funnel plot of comparison: 1 Magnesium sulphate versus comparison group ‐ all included trials, outcome: 1.11 Neonatal/infant deaths.
10.
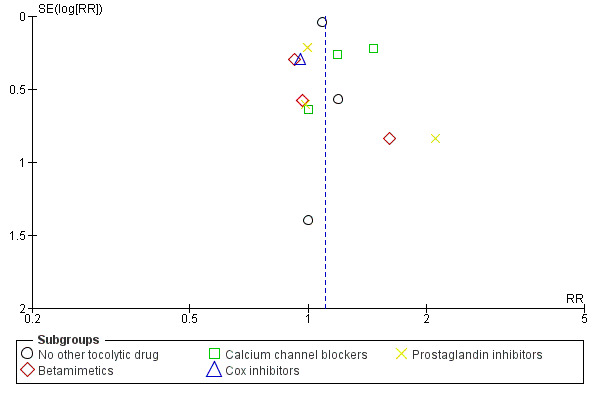
Funnel plot of comparison: 1 Magnesium sulphate versus comparison group ‐ all included trials, outcome: 1.13 Respiratory distress syndrome.
11.
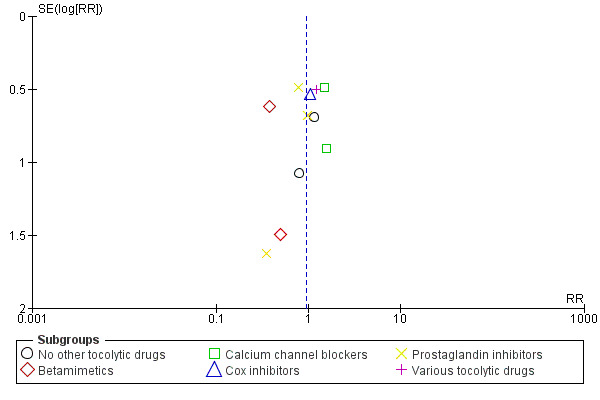
Funnel plot of comparison: 1 Magnesium sulphate versus comparison group ‐ all included trials, outcome: 1.16 IVH (any).
12.
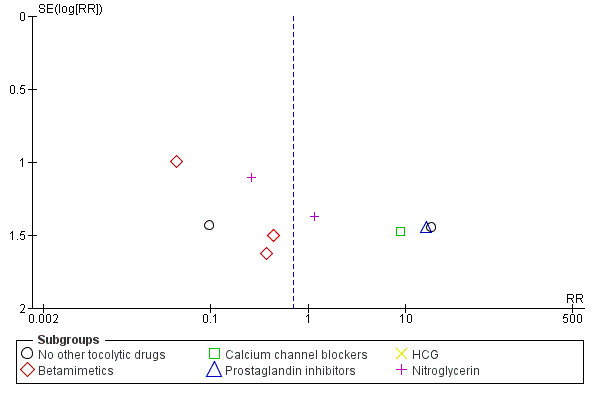
Funnel plot of comparison: 1 Magnesium sulphate versus comparison group ‐ all included trials, outcome: 1.26 Maternal adverse effects leading to discontinuation of treatment.
In Figure 8 (total deaths), there is some suggestion of asymmetry (missing small trials showing more deaths in the magnesium sulphate groups) which may be due to under‐reporting of fetal deaths. There is similar asymmetry evident for Figure 10 (respiratory distress syndrome) and Figure 11 (IVH) but none of these three instances of asymmetry are likely to be sufficient to reverse the findings of no significant differences between magnesium sulphate and control groups.
Figure 12 (maternal adverse effects severe enough for discontinuation) does not show a symmetrical funnel plot, perhaps due to differential influences of different tocolytic agents.
Discussion
Summary of main results
Given that magnesium sulphate has been widely used as a tocolytic for many years, it remains surprising that the evidence to support its use is generally unclear or with moderate to high risk of bias.
From the available data, there was no convincing evidence of a clinically important tocolytic effect for magnesium sulphate; it did not have any substantial effect on the proportion of women delivering within 48 hours, either overall, or in any of the subgroup analyses. A higher dose did not make any difference to the outcome. Moreover, there was no evidence of any substantial improvement in neonatal morbidity. Indeed, magnesium sulphate may be associated with a borderline increase in paediatric deaths, although this result was not seen for overall fetal, neonatal and/or infant mortality or when trials with a higher risk of bias were omitted in a sensitivity analysis. Babies exposed to magnesium sulphate were not more likely to be admitted to the neonatal intensive care, but if admitted they were more likely to stay longer than those babies not exposed to magnesium sulphate.
There was little evidence of either major benefit or harm to the mother from giving magnesium sulphate. Cessation of treatment due to maternal adverse events was not significantly different between the magnesium sulphate and control groups.
In many comparisons there was considerable heterogeneity. Some of this may have been caused by the different drugs used in the comparison groups, ranging from betamimetics, calcium channel blockers, prostaglandin inhibitors, to nitric oxide donors. However, even in some analyses restricted to subgroups of similar tocolytics, residual heterogeneity of unknown origin remained.
Overall completeness and applicability of evidence
Some funnel plot asymmetry suggests that several small negative trials may be missing although this is unlikely to have affected the overall findings of no significant differences between magnesium sulphate and control groups. If such trials exist, they may be unpublished as a comprehensive search was done for this review.
No long‐term outcomes were reported in any trial except for cerebral palsy in a single trial.
Quality of the evidence
Of the 37 included trials, adequate allocation concealment was reported in only six trials, mainly by the use of sealed envelopes. Four trials were quasi‐randomised. In the majority of trials magnesium sulphate was compared with other drugs with reputed tocolytic activity, which made blinding of women and investigators infeasible or very difficult. Only one trial made mention of blinding outcome assessors; and two‐thirds of trials were judged to be at some risk of selective outcome reporting bias. About one‐third of trials showed baseline imbalance in numbers randomised, probably reflecting the less than adequate randomisation in most trials. No trials were rated as being at low risk of bias on all accepted criteria.
Potential biases in the review process
Selective outcome reporting bias is likely to have been an influence in this review. Expected maternal and infant outcomes were often not reported in standard ways, such that we could not pool data for these outcomes. In particular, failure to report fetal deaths (or absence thereof) contributes to difficulty in interpreting the perinatal and infant death data.
Agreements and disagreements with other studies or reviews
The conclusions of this updated review have altered from previous versions by diluting the evidence for increased neonatal mortality when magnesium is used as a tocolytic, although findings for the primary outcomes do not differ between versions. Our findings are consistent with a recent network meta‐analysis of tocolytic therapy where prostaglandin inhibitors and calcium channel blockers (but not magnesium sulphate) showed the highest chance of delaying birth and improving neonatal and maternal outcomes (Haas 2012). The current American College of Obstetricians and Gynecology Practice Bulletin on 'Management of preterm labour' supports betamimetics, calcium channel blockers or nonsteroidal anti‐inflammatory drugs (NSAIDs) for short‐term treatment (up to 48 hours) as first‐line therapy to allow antenatal corticosteroids to be administered (ACOG 2012).
It is important to distinguish the evidence for magnesium sulphate used as a tocolytic from its use in pregnancy as an anticonvulsant (for preventing eclampsia ‐ Duley 2010) or use prior to very preterm birth for neuroprotection of the fetus and infant (Doyle 2009), where short‐term use of antenatal magnesium sulphate has been shown to be effective and safe.
Authors' conclusions
Implications for practice.
Evidence does not support the use of magnesium sulphate as an appropriate tocolytic agent to use for women in preterm labour. It is ineffective in delaying preterm birth and may be associated with a increased risk of death for the neonate (in contrast to beneficial effects of magnesium sulphate for maternal, fetal and infant neuroprotection in appropriate groups of women). Babies exposed to magnesium sulphate were not more likely to be admitted to the neonatal intensive care, but if admitted they were more likely to stay longer than those babies not exposed to magnesium sulphate.
Implications for research.
Follow‐up of children whose mothers were enrolled in the already completed randomised trials is warranted. This will enable neurodevelopmental status during childhood to be determined.
What's new
| Date | Event | Description |
|---|---|---|
| 31 January 2014 | New search has been performed | Search updated. |
| 31 January 2014 | New citation required and conclusions have changed | For this update we added 14 trials ‐ 12 new trials (Asgharnia 2002; Borna 2007; Clavin 1996; Klauser 2012; Lorzadeh 2007; Lyell 2007; McWhorter 2004; Pezzati 2001; Sayin 2010; Surichamorn 2001; Taherian 2007; Wang 2000) and two trials that were previously excluded (Parilla 1997; Parsons 1987). The conclusions of this updated review have altered from previous versions by diluting the evidence for increased neonatal mortality when magnesium is used as a tocolytic, although findings for the primary outcomes do not differ between versions. |
History
Protocol first published: Issue 2, 1998 Review first published: Issue 4, 2002
| Date | Event | Description |
|---|---|---|
| 17 September 2008 | Amended | Converted to new review format. |
Acknowledgements
The contribution of Professor Janet Hiller and Professor Lex Doyle to the original review and update in 2002 is acknowledged. We also acknowledge the assistance of Emily Bain in data extraction and for methodological support.
As part of the pre‐publication editorial process, this review has been commented on by two peers (an editor and referee who is external to the editorial team), a member of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
Data and analyses
Comparison 1. Magnesium sulphate versus comparison group ‐ all included trials.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Birth < 48 hours after trial entry | 19 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 No alternative tocolytic drug | 3 | 182 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.27, 1.14] |
| 1.2 Betamimetics | 7 | 503 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.72, 1.65] |
| 1.3 Calcium channel blockers | 5 | 588 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.86, 1.65] |
| 1.4 Cox inhibitors | 2 | 318 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.91, 1.27] |
| 1.5 Prostaglandin inhibitors | 2 | 221 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.71, 1.22] |
| 1.6 Human chorionic gonadotropin | 1 | 101 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.47, 4.04] |
| 2 Birth < 24 hours after trial entry | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 No alternative tocolytic drug | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.64, 1.74] |
| 2.2 Betamimetics | 2 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.39 [1.75, 11.05] |
| 2.3 Prostaglandin inhibitors | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.37, 0.84] |
| 3 Serious infant outcome | 18 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 No alternative tocolytic drug | 3 | 284 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.34 [0.78, 7.01] |
| 3.2 Betamimetics | 5 | 344 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.20, 4.12] |
| 3.3 Calcium channel blockers | 5 | 675 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.37, 2.83] |
| 3.4 Cox inhibitors | 2 | 314 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.19 [0.45, 150.22] |
| 3.5 Prostaglandin inhibitors | 3 | 355 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.27, 2.21] |
| 3.6 Human chorionic gonadotropin | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.7 Various tocolytic drugs | 1 | 106 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.47 [0.69, 8.81] |
| 4 Serious maternal outcome | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 No alternative tocolytic drug | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Betamimetics | 2 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Calcium channel blockers | 2 | 339 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.4 Prostaglandin inhibitors | 2 | 249 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.5 HCG | 1 | 106 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Preterm birth (< 37 weeks) | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 No alternative tocolytic drug | 1 | 65 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.46, 0.83] |
| 5.2 Betamimetics | 6 | 473 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.77, 1.39] |
| 5.3 Calcium channel blockers | 3 | 362 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.87, 1.29] |
| 5.4 Prostaglandin inhibitors | 1 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 1.83 [0.58, 5.81] |
| 6 Very preterm birth (< 34 weeks) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Calcium channel blockers | 2 | 170 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.55, 1.45] |
| 7 Gestational age at birth (weeks) | 11 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 No other tocolytic drug | 3 | 273 | Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐1.85, 0.85] |
| 7.2 Betamimetics | 3 | 152 | Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐1.61, 0.89] |
| 7.3 Calcium channel blockers | 3 | 439 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.72, 0.60] |
| 7.4 Calcium channel blockers: labour in first 48 hours subset | 1 | 53 | Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.93, 0.51] |
| 7.5 Calcium channel blockers: labour in 2‐10 days subset | 1 | 12 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐1.82, 1.42] |
| 7.6 Cox inhibitors | 2 | 298 | Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.95, 0.61] |
| 7.7 Prostaglandin inhibitors | 1 | 150 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐1.98, 0.78] |
| 8 Interval between trial entry and birth (days) | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 No other tocolytic drugs | 3 | 273 | Mean Difference (IV, Random, 95% CI) | 0.23 [‐3.83, 4.29] |
| 8.2 Betamimetics | 3 | 182 | Mean Difference (IV, Random, 95% CI) | ‐1.72 [‐14.89, 11.45] |
| 8.3 Prostaglandin inhibitors | 1 | 101 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐5.06, 4.66] |
| 9 Total deaths (fetal, neonatal and infant) | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 No other tocolytic drugs | 2 | 257 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.56 [1.00, 20.86] |
| 9.2 Betamimetics | 3 | 263 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.05, 5.12] |
| 9.3 Calcium channel blockers | 3 | 292 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.03, 2.29] |
| 9.4 Cox inhibitors | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.5 Prostaglandin inhibitors | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.06, 15.35] |
| 9.6 HCG | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.7 Various tocolytic drugs | 1 | 106 | Risk Ratio (M‐H, Fixed, 95% CI) | 15.79 [0.93, 266.72] |
| 10 Fetal deaths | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 No other tocolytic drugs | 2 | 257 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.70 [0.28, 116.87] |
| 10.2 Betamimetics | 4 | 278 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.3 Calcium channel blockers | 3 | 292 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.02, 9.91] |
| 10.4 Cox inhibitors | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.5 Prostaglandin inhibitors | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.6 HCG | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.7 Various tocolytic drugs | 1 | 106 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Neonatal/infant deaths | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 No other tocolytic drugs | 3 | 282 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.87 [0.60, 5.85] |
| 11.2 Betamimetics | 5 | 344 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.20, 4.12] |
| 11.3 Calcium channel blockers | 5 | 675 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.40, 3.49] |
| 11.4 Cox inhibitors | 2 | 334 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.69 [0.47, 159.67] |
| 11.5 Prostaglandin inhibitors | 2 | 267 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.18, 2.63] |
| 11.6 HCG | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.7 Various tocolytic drugs | 1 | 106 | Risk Ratio (M‐H, Fixed, 95% CI) | 15.79 [0.93, 266.72] |
| 12 Apgar < 7 at 5 minutes | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 Calcium channel blockers | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.39, 2.94] |
| 13 Respiratory distress syndrome | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 No other tocolytic drug | 3 | 282 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.98, 1.20] |
| 13.2 Betamimetics | 3 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.61, 1.64] |
| 13.3 Calcium channel blockers | 3 | 473 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.93, 1.79] |
| 13.4 Cox inhibitors | 1 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.53, 1.70] |
| 13.5 Prostaglandin inhibitors | 3 | 355 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.72, 1.56] |
| 14 Need for assisted ventilation | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 14.1 No other tocolytic drugs | 1 | 165 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.61, 2.24] |
| 14.2 Betamimetics | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.44, 1.59] |
| 15 Chronic lung disease (oxygen > 28 days of age) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 15.1 Betamimetics | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.10, 2.50] |
| 16 IVH (any) | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 16.1 No other tocolytic drugs | 3 | 282 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.34, 3.25] |
| 16.2 Betamimetics | 2 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.13, 1.21] |
| 16.3 Calcium channel blockers | 2 | 383 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.65, 3.50] |
| 16.4 Cox inhibitors | 1 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.37, 3.02] |
| 16.5 Prostaglandin inhibitors | 3 | 355 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.37, 1.69] |
| 16.6 Various tocolytic drugs | 1 | 106 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.46, 3.32] |
| 17 Severe IVH (Grades 3 or 4)/or PVL | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 17.1 No other tocolytic drugs | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Betamimetics | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.84] |
| 17.3 Prostaglandin inhibitors | 2 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.06, 15.35] |
| 17.4 Various tocolytic drugs | 1 | 106 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.85 [0.17, 19.84] |
| 18 PVL | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 18.1 Betamimetics | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 3.98] |
| 18.2 Calcium channel blockers | 1 | 167 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18.3 Prostaglandin inhibitors | 2 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.15, 7.52] |
| 18.4 Various tocolytic drugs | 1 | 106 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19 NEC | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 19.1 No other tocolytic drugs | 3 | 282 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.38, 4.14] |
| 19.2 Betamimetics | 2 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.21, 5.24] |
| 19.3 Calcium channel blockers | 2 | 383 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.86 [0.43, 8.00] |
| 19.4 Cox inhibitors | 1 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.01, 3.71] |
| 19.5 Prostaglandin inhibitors | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.18, 4.36] |
| 20 Proven neonatal infection (variously defined) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 20.1 No other tocolytic drugs | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.67 [0.30, 148.34] |
| 20.2 Betamimetics | 1 | 26 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.06, 2.61] |
| 20.3 Calcium channel blockers | 1 | 167 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.45, 3.44] |
| 20.4 Prostaglandin inhibitors | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.32, 2.23] |
| 21 Cerebral palsy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 21.1 Various tocolytic drugs | 1 | 106 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.01, 2.51] |
| 22 Maternal death | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 22.1 Betamimetics | 2 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22.2 Calcium channel blockers | 1 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22.3 Cox inhibitors | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22.4 Prostaglandin inhibitors | 1 | 129 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Cardiac arrest | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 23.1 Betamimetics | 1 | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23.2 Calcium channel blockers | 1 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23.3 Cox inhibitors | 1 | 214 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23.4 Prostaglandin inhibitors | 1 | 129 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24 Respiratory arrest | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 24.1 No other tocolytic drugs | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.16 [0.13, 76.30] |
| 24.2 Calcium channel blockers | 1 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24.3 Prostaglandin inhibitors | 1 | 129 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25 Admission to intensive care unit | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 25.1 Cox inhibitors | 1 | 214 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 26 Maternal adverse effects leading to discontinuation of treatment | 14 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 26.1 No other tocolytic drugs | 4 | 302 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.01, 221.68] |
| 26.2 Betamimetics | 5 | 398 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.03, 0.75] |
| 26.3 Calcium channel blockers | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 8.57 [0.48, 154.15] |
| 26.4 Prostaglandin inhibitors | 2 | 189 | Risk Ratio (M‐H, Random, 95% CI) | 16.04 [0.95, 270.65] |
| 26.5 HCG | 1 | 103 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 26.6 Nitroglycerin | 2 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.09, 2.52] |
| 27 Nausea | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 27.1 Betamimetics | 1 | 98 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 27.2 Calcium channel blockers | 1 | 192 | Risk Ratio (M‐H, Random, 95% CI) | 5.25 [2.29, 12.07] |
| 27.3 Nitroglycerin | 2 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 1.90 [0.82, 4.42] |
| 27.4 Cox inhibitors | 1 | 211 | Risk Ratio (M‐H, Random, 95% CI) | 3.28 [0.70, 15.40] |
| 28 Vomiting | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 28.1 Betamimetics | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 28.2 Calcium channel blockers | 1 | 192 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.22 [2.08, 13.10] |
| 28.3 Nitroglycerin | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.23, 3.19] |
| 29 Nausea and/or vomiting | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 29.1 Betamimetics | 2 | 169 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.31, 5.38] |
| 29.2 Calcium channel blockers | 2 | 267 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.42 [0.44, 26.41] |
| 29.3 Prostaglandin inhibitors | 1 | 129 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 29.4 HCG | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 63.75 [4.01, 1013.51] |
| 30 Hypotension (variously defined) | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 30.1 No other tocolytic drugs | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.16 [0.13, 76.30] |
| 30.2 Betamimetics | 2 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.05, 5.12] |
| 30.3 Calcium channel blockers | 2 | 267 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.01, 2.73] |
| 30.4 Prostaglandin inhibitors | 1 | 129 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 30.5 Nitroglycerin | 2 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.14, 0.74] |
| 31 Tachycardia (variously defined) | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 31.1 No other tocolytic drugs | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 31.2 Betamimetics | 2 | 125 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.04, 2.20] |
| 31.3 Calcium channel blockers | 1 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.04, 2.06] |
| 31.4 Prostaglandin inhibitors | 1 | 129 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.03, 16.40] |
| 32 Caesarean | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 32.1 No other tocolytic drugs | 3 | 273 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.62, 1.86] |
| 32.2 Betamimetics | 2 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.55, 1.42] |
| 32.3 Cox inhibitors | 1 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.47, 1.42] |
| 33 Admission to neonatal intensive care unit | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 33.1 No other tocolytic drugs | 1 | 165 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.18, 1.32] |
| 33.2 Calcium channel blockers | 2 | 338 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.89, 1.56] |
| 33.3 Cox inhibitors | 1 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.70, 2.07] |
| 33.4 HCG | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.42, 2.27] |
| 34 Length of stay in neonatal intensive care unit (days) | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 34.1 Calcium channel blockers | 2 | 383 | Mean Difference (IV, Fixed, 95% CI) | 4.55 [0.96, 8.15] |
| 34.2 Cox inhibitors | 1 | 194 | Mean Difference (IV, Fixed, 95% CI) | 1.80 [‐3.49, 7.09] |
| 34.3 Prostaglandin inhibitors | 1 | 150 | Mean Difference (IV, Fixed, 95% CI) | 7.40 [‐7.27, 22.07] |
1.22. Analysis.
Comparison 1 Magnesium sulphate versus comparison group ‐ all included trials, Outcome 22 Maternal death.
1.23. Analysis.
Comparison 1 Magnesium sulphate versus comparison group ‐ all included trials, Outcome 23 Cardiac arrest.
1.24. Analysis.
Comparison 1 Magnesium sulphate versus comparison group ‐ all included trials, Outcome 24 Respiratory arrest.
1.25. Analysis.
Comparison 1 Magnesium sulphate versus comparison group ‐ all included trials, Outcome 25 Admission to intensive care unit.
Comparison 2. Magnesium sulphate for tocolysis (subgrouped by dose).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Birth < 48 hours after trial entry | 19 | 1913 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.78, 1.16] |
| 1.1 Magnesium sulphate maintenance dose protocol 2 g/hour or less | 9 | 839 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.60, 1.38] |
| 1.2 Magnesium sulphate maintenance dose protocol of more than 2 g/hour | 10 | 1074 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.90, 1.19] |
| 2 Serious infant outcome | 18 | 2187 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.81, 2.41] |
| 2.1 Magnesium sulphate maintenance dose protocol 2 g/hour or less | 4 | 302 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.15, 4.65] |
| 2.2 Magnesium sulphate maintenance dose protocol of more than 2 g/hour | 14 | 1885 | Risk Ratio (M‐H, Random, 95% CI) | 1.48 [0.83, 2.63] |
| 3 Serious maternal outcome | 7 | 930 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Magnesium sulphate maintenance dose protocol 2 g/hour or less | 3 | 418 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Magnesium sulphate maintenance dose protocol of more than 2 g/hour | 4 | 512 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Total deaths (fetal, neonatal and infant) | 12 | 1248 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [0.39, 5.24] |
| 4.1 Magnesium sulphate maintenance dose protocol 2 g/hour or less | 2 | 194 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Magnesium sulphate maintenance dose protocol of more than 2 g/hour | 10 | 1054 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [0.39, 5.24] |
| 5 Fetal deaths | 13 | 1263 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.78 [0.29, 10.80] |
| 5.1 Magnesium sulphate maintenance dose protocol of 2 g/hour or less | 2 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Magnesium sulphate maintenance dose protocol of more than 2 g/hour | 11 | 1069 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.78 [0.29, 10.80] |
| 6 Neonatal/infant deaths | 17 | 2117 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.73 [0.99, 3.03] |
| 6.1 Magnesium sulphate maintenance dose protocol of 2 g/hour or less | 4 | 302 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.17, 4.09] |
| 6.2 Magnesium sulphate maintenance dose protocol of more than 2 g/hour | 13 | 1815 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.93 [1.06, 3.53] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aramayo 1990.
| Methods | Single‐centre RCT. | |
| Participants | 30 women. Inclusion criteria: in preterm labour between 28‐36 weeks' gestation with intact membranes. The diagnosis of labour was made if persistent uterine contractions occurred 3 times in 10 minutes and cervical examination suggested 'active labour'. Gestational age range: 28‐36 weeks. Exclusion criteria: cervical incompetence, congenital malformation, ruptured membranes, fetal death, maternal cardiac disease. Setting: Mexico, 1988‐1989. |
|
| Interventions |
MAGNESIUM VS BETAMIMETICS 1) magnesium sulphate (n = 15): loading dose 4 g IV; maintenance 2 g/hr; 2) terbutaline for labour inhibition (n = 15): 1.25 mg in 500 mL dextrose given at 10 drops per minute; followed by 5 mg oral tablet 8 hourly. |
|
| Outcomes | Postponement of birth for at least 48 hrs after initiation of therapy; 2‐6 days, 7‐12 days, > 12 days after treatment; preterm birth. | |
| Notes | Antenatal corticosteroid use: not stated.
Surfactant use: not stated. Sample size calculation: not stated. Funding: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomised study." |
| Allocation concealment (selection bias) | Unclear risk | "randomised study"; no further details reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible to blind the interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of outcome assessors being blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 woman in the terbutaline group was excluded because of severe fetal distress. |
| Selective reporting (reporting bias) | Unclear risk | Only infant outcome reported was preterm birth; no maternal health outcomes reported. |
| Other bias | Low risk | No apparent evidence of other bias. |
Armson 1992.
| Methods | 2‐centre RCT. | |
| Participants | 15 women.
Inclusion criteria: in preterm labour. Gestational age range: between 27‐36 weeks' gestation.
Exclusion criteria: women with obstetric or medical contraindications to tocolysis. Setting: Pennsylvania, USA (timeframe not stated). |
|
| Interventions | MAGNESIUM VS BETAMIMETICS 1) magnesium sulphate (n = 8): loading dose 6 g IV MgSO4 over 30 minutes. Maintenance at 2 g/hr. Increased by 0.5 g/hr every 30 minutes until tocolysis achieved, a maximum of 4 g/hr attained or unacceptable side effects; 2) ritodrine (n = 7): loading dose 50 μg/min. Maintenance: increased by 50 μg/min at 15 minute intervals until tocolysis attained, a maximum of 350 μg/min, or unacceptable side effects. Duration: if tocolysis was successful the infusion rate was maintained at the lowest effective dose for 12 hrs. | |
| Outcomes | Fetal and maternal deaths, birth within 12 hrs, and maternal cardiovascular and respiratory effects. | |
| Notes | Antenatal corticosteroid use: 'were not given'.
Surfactant use: not stated. Sample‐size calculation: not stated. Funding: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "patients were prospectively randomised with a random number table." |
| Allocation concealment (selection bias) | Unclear risk | No details provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No details provided, but blinding unlikely due to the nature of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Women were excluded if they progressed to preterm birth in spite of maximum therapy or who had adverse side effects necessitating discontinuation of therapy. 1 woman treated with MgSO4 progressed to birth in spite of maximum therapy and was therefore excluded from the study. |
| Selective reporting (reporting bias) | Unclear risk | Not all expected outcomes were reported. |
| Other bias | Low risk | No apparent evidence of other bias. |
Asgharnia 2002.
| Methods | RCT. | |
| Participants | 120 pregnant women. Inclusion criteria: pregnant women with intact membranes and preterm labour, with cervical dilatation of at least 2 cm. Exclusion criteria: premature rupture of membranes, GA < 24 or > 32 weeks, complete cervical dilatation, severe haemorrhage, chorioamnionitis and triple or higher order gestation. Setting: Gilan University, Iran. |
|
| Interventions |
MAGNESIUM VS PROSTAGLANDIN INHIBITORS 1) magnesium sulphate, loading dose 4 g IV followed by 2 g/hr until uterine activity diminished (assume n = 60); 2) indomethacin, 25 mg every 6 hrs for 4 doses (assume n = 60). |
|
| Outcomes | Delay in birth (24 and 48 hrs); maternal complications. | |
| Notes | Antenatal corticosteroid use: not stated.
Surfactant use: not stated. Sample‐size calculation: not stated. Funding: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly managed" ‐ no further details given. |
| Allocation concealment (selection bias) | Unclear risk | "randomly managed" ‐ no further details given. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported but not likely to have been feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported. |
| Selective reporting (reporting bias) | High risk | Numbers of women in each group were not reported; the only outcomes reported were delay in birth at 24 and 48 hrs; and complications (reported only that there were none but individual complications were not listed). |
| Other bias | Low risk | Similar baseline characteristics. |
Beall 1985.
| Methods | Single‐centre randomised trial. | |
| Participants | 176 women.
Inclusion criteria: in preterm labour > 36 weeks' gestation (persistent uterine contractions more than 1 in 10 after half an hr bed rest and hydration or cervical change). Gestational age > 36 weeks'.
Exclusion criteria: preterm prelabour rupture of membranes or contraindications to tocolysis. Setting: Los Angeles, USA, 1983‐1984. |
|
| Interventions |
MAGNESIUM VS BETAMIMETICS 1) Magnesium sulphate (n = 46) ‐ loading dose 4 g IV MgSO4. Maintenance at 1.5 g/hr. Increased by 0.5 g/hr every 30 minutes until tocolysis achieved, a maximum of 3.5 g/hr attained or unacceptable side effects. 2) Ritodrine (n = 45) ‐ loading dose 100 μg/min. Maintenance increased by 50% every 10 minutes until contractions ceased, a maximum of 350 μg/min attained or if unacceptable side effects. 3) Terbutaline (n = 40) ‐ loading dose 20 μg/min. Maintenance increased by 50% every 10 minutes until contractions ceased, a maximum of 70 μg/min or if unacceptable side effects. Duration: if tocolysis was successful, the infusion rate was maintained for 12 hrs. 1 half‐hour prior to completion of IV therapy, women commenced oral terbutaline and were discharged home 48 hrs later on 2.5 mg every 4 hrs. |
|
| Outcomes | Delivery delayed for at least 48 hrs; maternal events after randomisation including any adverse effects (maternal death; maternal adverse events leading to discontinuation of treatment); fetal death; neonatal/infant death. Serious infant outcome: able to be defined as perinatal/infant mortality. |
|
| Notes | Antenatal corticosteroid use: not directly stated as given.
Surfactant use: not available. Sample‐size calculation: not stated. Funding: not stated. For failure of tocolysis because of continued contractions or inability to tolerate an effective dose, women were given a course of therapy with the other class of drug. Successful tocolysis was attributed to the last used drug for each woman. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomly assigned to one of the three treatment groups by means of a random number table." |
| Allocation concealment (selection bias) | Unclear risk | No details provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of women and study personnel not feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High attrition, ITT analysis was not conducted. 37 women were excluded because of failure to observe the exclusion criteria on enrolment (n = 31) or failure to adhere to the study protocol (n = 6). 8 women were excluded as data were not available for their episode of preterm labour. |
| Selective reporting (reporting bias) | Unclear risk | Primary and secondary outcomes were not clearly stated; reported on adverse effects which were not prespecified. |
| Other bias | Low risk | No apparent evidence of other bias. |
Borna 2007.
| Methods | Single‐centre RCT. | |
| Participants | 104 women (GA 32.07 [0.3] weeks in magnesium group and 31.4 [0.3] weeks in the celecoxib group). Inclusion criteria: between 24 and 34 weeks' gestation; preterm labour; singleton pregnancy; intact amniotic membranes; cervical dilatation 4 cm or less. Exclusion criteria: cervical dilatation > 4 cm; a non‐reassuring fetal heart rate tracing; or any other contraindication to tocolysis (such as intra‐amnionitic infection, based on clinical presentation) or fetal anomaly; a baseline amniotic fluid index < 8; placenta praevia; renal or hepatic dysfunction; thrombocytopaenia; platelet dysfunction or coagulation disorder; known history of peptic ulcer disease; use of fluconazole. Setting: teaching hospital in Tehran, Iran. |
|
| Interventions |
MAGNESIUM SULPHATE VS COX INHIBITOR 1) Magnesium sulphate 4 to 6 g as a IV loading dose; maintenance ‐ continuous infusion rate of 2 to 4 g/hr with placebo capsules (n = 52). 2) Celecoxib 100 mg orally twice daily as a capsule; with placebo infusion IV physiologic saline 80 mL/h for the duration of the study (n = 52). All women: Medication was given for a maximum of 48 hrs; medication was discontinued if the woman gave birth; if preterm labour persisted and the medication was switched; or if significant adverse effects developed and the woman requested that the medication be stopped, or if any clinical condition occurred that required the discontinuation of tocolysis. All women had continuous fetal monitoring for the duration of the study. Other medication: no women received aspirin or other tocolytics other than the study agent during the study period. |
|
| Outcomes | Birth < 48 hrs after trial entry; fetal, neonatal or infant death; maternal death; GA at birth; birthweight; adverse effects of therapy (headache, lethargy, palpitations); caesarean; amniotic fluid index. Serious infant outcome: able to be shown as fetal, neonatal or infant death. Study reports that "There were no severe maternal and neonatal complications in either group believed to be related to the study medications". |
|
| Notes | Antenatal corticosteroid use: women were offered 12 mg of IM betamethasone every 24 hrs. Surfactant use: not stated. Sample size calculation: yes. Funding: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐number table. |
| Allocation concealment (selection bias) | Low risk | "randomly assigned by the pharmacy." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "investigators and patients were blinded as to which preparation the patient was taking. At no time before data analysis did any clinical investigator have access to or knowledge of the identity of the assigned drug." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Judged likely to have been done. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses reported. |
| Selective reporting (reporting bias) | Unclear risk | Limited range of neonatal outcomes reported; adverse events and mode of birth not reported completely. |
| Other bias | Low risk | No apparent evidence of other bias. |
Chau 1992.
| Methods | 2‐centre quasi‐RCT. | |
| Participants | 98 women.
Inclusion criteria: a clinical diagnosis of preterm labour. Gestational age range: 23‐35 weeks' and estimated fetal weight between 500‐2500 g.
Exclusion criteria: women with ruptured membranes, multiple gestation, various maternal diseases, fetal death, congenital abnormality, obstetric haemorrhage, advanced cervical dilatation (≥ 4 cm), maternal or fetal condition making delivery advisable. Setting: New Orleans, Louisiana, USA, 1989‐1991. |
|
| Interventions | MAGNESIUM VS BETAMIMETICS 1) magnesium sulphate (n = 46) ‐ loading dose 4g IV MgSO4 over 30 minutes. Maintenance at 2 g/hr. Continued for 24 hrs or until no contractions for 12 hrs. Maximum 4 g/hr; 2) terbutaline (n = 52) ‐ loading dose 0.25 mg subcutaneously every 30 minutes for 3 doses and then 4 hourly for 24 hrs, or until contractions were absent for at least 12 hrs. 30 minutes prior to completion of IV therapy women in the magnesium sulphate group commenced oral 2‐3 g magnesium gluconate, and women in the terbutaline group started 5 mg terbutaline every 4‐6 hrs. | |
| Outcomes | Delay in birth for 48 hrs, 1 week or until 37 weeks; time from tocolytic initiation to birth, gestational age at birth; birthweight, Apgar scores, infectious complications, adverse effects. | |
| Notes | Antenatal corticosteroid use: not stated.
Surfactant use: not stated. Sample‐size calculation: stated. Funding: not stated. In case of failure of tocolysis because of continued contractions or inability to tolerate an effective dose, women were switched to the other agent. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "patients received either magnesium or terbutaline depending upon the last digit of their assigned hospital number." |
| Allocation concealment (selection bias) | High risk | Inadequate allocation as last digit of hospital number was used for randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Women and researchers unlikely to be blinded as intervention was subcutaneous or IV; there was no placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided, but unlikely. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No reporting of losses and ITT analysis was conducted. |
| Selective reporting (reporting bias) | Unclear risk | Not all expected neonatal outcomes were reported. |
| Other bias | Unclear risk | Some imbalance ‐ numbers differ between groups (46 in magnesium group and 52 in the no magnesium group). |
Clavin 1996.
| Methods | RCT (number of sites not stated). | |
| Participants | 34 women. Inclusion criteria: not stated. Exclusion criteria: not stated. Setting: New Orleans, USA (timeframe not stated). |
|
| Interventions |
MAGNESIUM VS NITROGLYCERIN 1) Magnesium sulphate IV ‐ no further details. 2) Nitroglycerin IV ‐ no further details. |
|
| Outcomes | Delivery delay 48 hrs, 1 week, and until 37 weeks; change of medication due to initial tocolysis drug failure or severe adverse effects; gestational age at birth; total days to birth. | |
| Notes | Antenatal corticosteroid use: not stated.
Surfactant use: not stated. Sample size calculation: stated. Funding: not stated. For failure of tocolysis because of continued contractions or severe adverse effects, women were switched to the other agent. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "prospectively randomized', no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details provided but possible as both interventions were IV. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 7 of 15 women randomised to nitroglycerin were switched to magnesium. No details as to whether ITT analysis was conducted. |
| Selective reporting (reporting bias) | Unclear risk | Not all expected outcomes were reported. |
| Other bias | Unclear risk | Lack sufficient details in the abstract to be able to judge. |
Cotton 1984.
| Methods | Single‐centre RCT. | |
| Participants | 56 women.
Inclusion criteria: diagnosis of preterm labour was made. Gestational age range: 26‐34 weeks.
Exclusion criteria: patients with cervical dilatation > 4 cm. Setting: Los Angeles, USA (time frame not stated). |
|
| Interventions | MAGNESIUM VS BETAMIMETIC OR PLACEBO 1) Magnesium sulphate (n = 16) ‐ loading dose 4 g IV. Maintenance: 2 g/hr. 2) Terbutaline (n = 19) ‐ loading dose 9.2 μg/min IV. Maintenance: increased 5 μg/min to 25.3 μg/min. 3) Dextrose (n = 19) 125 mL/hr. Duration: therapy continued for 12 hrs after contractions stopped. Stopped if cervix > 7 cm, amnionitis or adverse effects. | |
| Outcomes | Postponement of birth for at least 48 hrs; time from initiation of tocolytic therapy until birth, gestational age at birth, birthweight, Apgar scores, RDS, PDA, neonatal mortality, IVH, infection, hypoglycaemia, maternal events after randomisation, NEC. Serious infant outcome: able to be shown as neonatal mortality. |
|
| Notes | Antenatal corticosteroid use: 14/54 women received corticosteroids.
Surfactant use: not available. Sample‐size calculation: not stated. Funding: National Institutes of Health and the Ariel Kaare Rosholt Weathers‐Lowin Medical Research Foundation. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "patients were randomised into one of three treatment groups", no other details provided. |
| Allocation concealment (selection bias) | Unclear risk | No details provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Women were blinded through use of a placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details reported of outcome assessors being blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 woman in the magnesium sulphate group was lost to follow‐up after 1 week. |
| Selective reporting (reporting bias) | Low risk | Relevant outcomes appear to have been reported. |
| Other bias | Low risk | No apparent evidence of other bias. |
Cox 1990.
| Methods | Single‐centre RCT. | |
| Participants | 156 women, 167 infants (11 sets of twins).
Inclusion criteria: in preterm labour between 24‐34 weeks', intact membranes and no maternal or fetal necessitating delivery. Gestational age range: 24‐34 weeks' gestation.
Exclusion criteria: ruptured membranes or maternal or fetal reason for delivery. Setting: Dallas, Texas, USA, 1987‐1989. |
|
| Interventions |
MAGNESIUM VS PLACEBO 1) Magnesium sulphate (n = 76 women, 78 infants): loading dose 4 g IV. Maintenance: 2 g/hr. Increasing to 3 g/hr if still contracting after > 1 hr. Duration: therapy continued for 24 hrs. 2) Saline (n = 80, 89 infants): 80 mL/hr for 24 hrs. |
|
| Outcomes | Gestational age at birth, birthweight, fetal, neonatal and infant mortality, IVH (any), RDS, NEC, preterm birth, need for assisted ventilation, NICU admission, birth < 24 hrs after trial entry, interval between trial entry and birth; maternal respiratory arrest, hypotension, maternal adverse events. Serious infant outcome: able to be defined as perinatal death. |
|
| Notes | Antenatal corticosteroid use: not stated.
Surfactant use: not stated. Sample‐size calculation: not stated. Funding: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were assigned to treatment and control groups by means of a random number table ...." |
| Allocation concealment (selection bias) | Unclear risk | "Patients were assigned to treatment and control groups by means of a random number table with group allocation predetermined and placed in consecutively numbered and sealed envelopes" ‐ no mention that the envelopes were opaque. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Women were blinded using saline as a placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up were stated and ITT analysis has been conducted. |
| Selective reporting (reporting bias) | Unclear risk | Primary outcomes were not stated a priori; most expected outcomes were reported. |
| Other bias | Low risk | No apparent evidence of other bias though no adjustment made for twins. |
El‐Sayed 1999.
| Methods | Single‐centre RCT. | |
| Participants | 31 women.
Inclusion criteria: 31 women with preterm labour. Gestational age range: less than 35 weeks.
Exclusion criteria: women with cervical dilatation > 4 cm, placenta praevia or placental abruption, hypertension, fetal growth restriction, fetal anomaly incompatible with life, non‐reassuring fetal testing. Setting: Standford, California, USA (time frame not stated). |
|
| Interventions |
MAGNESIUM VS NITROGLYCERIN 1) Magnesium sulphate (n = 15) ‐ loading dose 4 g IV. Maintenance: 2 g/hr. Increasing to 4 g/hr to control contractions. 2) Nitroglycerin (n = 16) ‐ 100 μg bolus then initial infusion rate 1 μg/kg/min increased to maximum 10 μg/kg/min. |
|
| Outcomes | 12 hrs or more of successful tocolysis; maternal side effects of therapy, fetal heart rate abnormalities; hypotension. | |
| Notes | Antenatal corticosteroid use: not stated.
Surfactant use: not stated. Sample size calculation: stated as 60 women, but no basis provided for this calculation. Funding: not stated. Trial stopped recruitment midway because of the clearly higher rate of successful tocolysis for magnesium sulphate and the observation that 25% women receiving nitroglycerin had persistent hypotension requiring discontinuation of therapy. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "assigned randomly to IV nitroglycerin or IV magnesium sulphate".."shuffled thoroughly.." |
| Allocation concealment (selection bias) | Low risk | Randomisation was performed by a third party not involved in the conduct of the analysis of the trial "who prepared 30 labels' for nitroglycerin and 30 labels for magnesium and placed them 'in 60 unmarked, opaque envelopes that were sealed, .....and numbered sequentially". |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details of blinding although it would have been possible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details of blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 1 woman in the magnesium sulphate group left the hospital against medical advice before receiving any therapy. 2 women, 1 in each group, discontinued therapy but appear to have been included in the ITT analysis. |
| Selective reporting (reporting bias) | High risk | Lacked fetal outcomes apart from heart rate. |
| Other bias | Low risk | No apparent evidence of other bias. |
Floyd 1992.
| Methods | Single‐centre RCT. | |
| Participants | 90 women.
Inclusion criteria: in preterm labour. No medical or obstetric complications precluding the continuation of pregnancy, singleton gestation, intact membranes, no previous tocolytic therapy in this pregnancy, no chorioamnionitis. Gestational age range: 20‐34 weeks' gestation.
Exclusion criteria: as above. Setting: Mississippi, USA (timeframe not stated). |
|
| Interventions |
MAGNESIUM VS CALCIUM CHANNEL BLOCKERS 1) Magnesium sulphate (n = 40) ‐ loading dose 4 g IV. Maintenance: 4‐6 g/hr as needed to keep the uterus quiescent. Duration: after 6 hrs of uterine quiescence women were started on oral magnesium gluconate, 2 g every 4 hrs. Continued until 37 weeks or birth whichever occurred first. 2) Nifedipine (n = 50) ‐ loading dose 30 mg orally followed by 20 mg 8 hourly until cessation of contractions. Duration: continued on 20 mg nifedipine 8 hourly until 37 weeks or birth whichever was the earliest. |
|
| Outcomes | Delivery < 34 weeks, 34‐37 weeks, > 37 weeks, number of days the gestation extended after treatment, maternal complications, neonatal death, fetal death, birthweight, Apgar score < 7 at 5 mins; hypoglycaemia, RDS, lethargy, depression at birth. Serious infant outcome: able to be defined as perinatal mortality. |
|
| Notes | Antenatal corticosteroid use: not stated.
Surfactant use: not stated. Sample size calculation: not stated. Funding: Vicksburg Hospital Medical Foundation. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "a random number table generated by computer.' |
| Allocation concealment (selection bias) | Low risk | "Assignment was by selection of consecutively numbered, opaque, sealed envelopes that designated one of the study drugs as determined by a random number table generated by computer." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Neither the patients nor the investigators were blinded as the interventions were administered via different routes (oral and IV). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details as to whether outcome assessors were blinded, but unlikely. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There are no reported losses and analysis by ITT was conducted. |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes reported and included both maternal and fetal outcomes. |
| Other bias | Unclear risk | Imbalance in numbers randomised to each group (40 and 50). |
Fox 1993.
| Methods | Single‐centre RCT. | |
| Participants | 101 women, 90 consented.
Inclusion criteria: in documented preterm labour eligible; gestational age range: between 34‐37 weeks.
Exclusion criteria: women with cervical dilatation ≥ 3 cm, ruptured membranes, medical or obstetric complications necessitating delivery, suspected anomalies, maternal allergy to magnesium sulphate.
Total recruited: 101 women, 45 to magnesium (vs) 45 to control group (conservative management). Setting: Jackson, Mississippi, USA (timeframe not stated). |
|
| Interventions | MAGNESIUM VS CONSERVATIVE MANAGEMENT 1) Magnesium sulphate (n = 45): loading dose 4 g IV as a bolus. Maintenance: 2‐4 g/hr until uterine quiescence obtained. Duration: after uterine quiescence oral magnesium until 37 weeks' gestation. 2) Control group (n = 45): 'conservative management with hydration, sedation and observation'. 'Underwent an identical evaluation' however labour was allowed to continue. | |
| Outcomes | Interval from diagnosis to delivery; gestational age at time of delivery; adverse effects of therapy; maternal infections; birthweight; neonatal morbidity and mortality. Serious infant outcome: able to be defined as perinatal mortality and IVH 3/4 and/or PVL. |
|
| Notes | Antenatal corticosteroid use: "not used in any patient".
Surfactant use: not stated. Sample size calculation: stated but not a priori. Funding: Vicksburg Hospital Medical Foundation. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "table of random numbers". |
| Allocation concealment (selection bias) | Low risk | "randomisation was performed by using the sealed‐envelope method in which the group selection was generated from a table of random numbers. A disinterested third party (the pharmacy) was in charge of selection of the envelope for each patient. The treating physicians did not have access to the envelopes". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Women were not blinded as interventions were IV drug vs usual management. Researchers would not be blinded to interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details but unlikely. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of loss after randomisation and ITT analysis conducted. |
| Selective reporting (reporting bias) | Low risk | Outcomes appear to be appropriate and all were reported on. |
| Other bias | Low risk | No apparent evidence of other bias. |
Glock 1993.
| Methods | Single‐centre RCT. | |
| Participants | 100 women, 20 excluded post randomisation.
Inclusion criteria: preterm labour and intact membranes. Gestational age range: between 20‐34 weeks.
Exclusion criteria: women with medical or obstetric complications, previous tocolytic therapy this pregnancy, fetal distress, severe growth restriction, fetal anomaly. Setting: Orlando, Florida, USA, 1991‐1992. |
|
| Interventions |
MAGNESIUM VS CALCIUM CHANNEL BLOCKERS 1) Magnesium sulphate (n = 41) ‐ loading dose 6 g IV over 30 minutes. Maintenance: 2‐4 g/hr as needed to keep the uterus quiescent for 24 hrs. Duration: after 24 hrs of arrest of contractions the woman was weaned at a rate of 0.5 g/hr every 4‐6 hrs. Women were started on 5 mg oral terbutaline every 6 hrs until 34 weeks' gestation completed. 2) Nifedipine (n = 39) ‐ loading dose 10 mg sublingually, repeated every 20 minutes up to a maximum of 40 mg in the first hr of treatment. If contractions stopped, given 20 mg oral nifedipine 4 hourly for 48 hrs. Continued on 10 mg nifedipine 8 hourly until 34 completed weeks. |
|
| Outcomes | Birth delayed 2 days, birth > 34 weeks' gestation; GA at birth; maternal adverse effects, birthweight, Apgar score, fetal, neonatal and infant mortality. Serious infant mortality: able to shown as perinatal mortality |
|
| Notes | Antenatal corticosteroid use: women at > 24 weeks' gestation were given betamethasone.
Surfactant use: not stated. Sample size calculation: not a priori. Funding: not stated. If progressive labour or serious adverse effects, IV ritodrine was added. All women received 2 g ampicillin pending results of cervical cultures. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | ''randomised"; no further details. |
| Allocation concealment (selection bias) | Unclear risk | "randomised to receive nifedipine or magnesium sulphate by means of sealed envelopes". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Women were not blinded and staff are unlikely to be blinded as this an oral vs IV intervention and there was no placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided but unlikely to have been blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Losses to follow‐up: 20/100 women excluded post randomisation for reasons of ineligibility. |
| Selective reporting (reporting bias) | Low risk | Most expected maternal and fetal outcomes were reported. |
| Other bias | Low risk | No apparent evidence of other bias. |
Haghighi 1999.
| Methods | Single‐centre randomised trial. | |
| Participants | 74 women. Inclusion criteria: primigravid women with singleton pregnancy in preterm labour. Gestational age range: 23‐36 weeks. Exclusion criteria: none stated. Setting: Tehran, Iran, 18 months duration. |
|
| Interventions |
MAGNESIUM VS CALCIUM CHANNEL BLOCKERS 1) Magnesium sulphate (n = 40): loading dose 6 g IV over 15 minutes. Maintenance: 2‐4 g/hr as needed to stop contractions for 48 hrs. Duration: after 12 hrs of arrest of contractions women were started on 5 mg oral terbutaline 6 hourly. Magnesium sulphate was discontinued if contractions persisted > 48 hrs or cervical dilatation > 4 cm. 2) Nifedipine (n = 34): loading dose 10 mg sublingually, repeated every 20 minutes up to a maximum of 40 mg in the first hr of treatment. If contractions stopped, given 20 mg oral nifedipine 6 hourly for 24 hrs then 8 hourly for 24 hrs. Nifedipine was discontinued if adverse effects occurred or the uterine contractions did not stop within the 2 hr period after the fourth dose of nifedipine. |
|
| Outcomes | Delaying birth > 48 hrs, maternal adverse effects, birthweight, Apgar score, and duration of admission to neonatal intensive care. | |
| Notes | Antenatal corticosteroid use: not stated.
Surfactant use: not stated. Sample‐size calculation: not stated a priori. Funding: none stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "were selected randomly to receive", no other details provided. |
| Allocation concealment (selection bias) | Unclear risk | No details provided on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Women and staff could not be blinded as the interventions were IV vs oral and there was no placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details as to whether outcome assessors were blinded but it is unlikely. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women appear to have been followed up and analysed. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported and include maternal and fetal outcomes. |
| Other bias | Unclear risk | Some baseline imbalance in numbers randomised to each group (40 and 34). |
Hollander 1987.
| Methods | Single‐centre quasi‐RCT. | |
| Participants | 70 women.
Inclusion criteria: in preterm labour. Gestational age range: between 20‐35 weeks.
Exclusion criteria: women with cervical dilatation > 4 cm, preterm ruptured membranes, maternal or fetal complications necessitating delivery or multiple gestation. Setting: Baltimore, Maryland, USA. 1984‐1985. |
|
| Interventions | MAGNESIUM VS BETAMIMETICS 1) Magnesium sulphate (n = 34) ‐ loading dose 4 g IV MgSO4 over 20 minutes. Maintenance at 2 g/hr. Increased by 1 g/hr every 30 minutes until tocolysis achieved, or a magnesium concentration of 6‐8 mg/dL was obtained. 2) Ritodrine (n = 36) ‐ loading dose 100 μg/min. Maintenance increased by 50 μg/min every 10‐15 minutes until tocolysis attained, a maximum of 350 μg/min obtained or if unacceptable side effects. Duration: if tocolysis was successful the infusion rate was maintained at the lowest effective dose for 12 hrs. Oral tocolytic therapy given to all patients 30 minutes before discontinuation of IV therapy. Either ritodrine 10 mg orally every 2 hrs or terbutaline 5 mg orally every 4‐6 hrs. Terbutaline was often chosen for patients without third party health coverage. | |
| Outcomes | Delay in birth > 72 hrs; adverse effects, gestational age at birth ‐ but unable to use any outcome data due to cross‐overs between groups and the way these were reported. | |
| Notes | Antenatal corticosteroid use: not stated.
Surfactant use: not stated. Sample‐size calculation: not given. Funding: not stated. Women with failure to achieve tocolysis or had adverse side effects were placed on the alternative tocolytic. Women with failure of both regimens had no further tocolytic therapy. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "patients were prospectively randomised"..."Randomisation was generated through the use of random number tables with ritodrine hydrochloride being given for odd integers and magnesium sulphate for even integers". Although a random number table was used to generate numbers the authors then proceeded to randomise by odd and even numbers. |
| Allocation concealment (selection bias) | High risk | "Randomisation was generated through the use of random number tables with ritodrine hydrochloride being given for odd integers and magnesium sulphate for even integers". There was no allocation concealment as interventions were administered based on odd or even numbers. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details of blinding taking place, no placebo given. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No evidence that outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There do not appear to be any losses and data are provided on an ITT basis with results of first‐line therapy. |
| Selective reporting (reporting bias) | Unclear risk | Not all expected outcomes were reported. |
| Other bias | Unclear risk | There is a suggestion that failures in the ritodrine group were given magnesium sulphate in a cross‐over fashion and likewise with magnesium sulphate failures. |
Klauser 2012.
| Methods | Multi‐arm RCT (NCT00811057). | |
| Participants | 301 women (90 magnesium sulphate; 114 nifedipine; 97 indomethacin). Inclusion criteria: women between 20 and 32 weeks' gestation, confirmed to be in preterm labour (regular uterine contractions, 5 mins apart or less, cervical dilatation of at least 1 cm or a change from the previous vaginal examination); with intact membranes; singleton or twin pregnancy, vertex presentation, with cervical dilatation from 1 ‐ 6 cm and sufficient effacement and decrease in station. Only women with 'idiopathic' preterm labour were included. Exclusion criteria: women with triplet or quad pregnancies, women with significant medical and surgical reasons for early delivery (severe pre‐eclampsia, placental abruption, fetal malformations inconsistent with life, chorioamnionitis, IUGR, nonreassuring fetal heart tracing. Setting: Obstetrics Dept, University of Mississippi Medical Center, USA. |
|
| Interventions |
MAGNESIUM VS CALCIUM CHANNEL BLOCKERS VS PROSTAGLANDIN INHIBITORS 1) Magnesium sulphate (n = 85 women; 95 infants (10 twins)): 6 g IV loading dose of magnesium sulphate over 20 mins followed by 4‐6 g/hr until contractions had ceased for 1‐2 hr, and then discontinued. 2) Nifedipine (n = 104 women; 119 infants (15 twins)): 30 mg loading dose orally followed by 20‐30 mg every 4‐6 hrs until contractions stopped. 3) Indomethacin (n = 87 women; 103 infants (103 twins)): women received 100 mg rectal suppository which could be repeated once, 2 hrs after the initial dose if contractions continued. This was followed by 50 mg indomethacin by mouth every 6 hrs until contractions had ceased for 12 hrs. Only used for 48 hrs as a total treatment cycle. (Pepcid 20 mg was given orally twice a day to minimise GI irritation.) No tocolytics were given as maintenance therapy. |
|
| Outcomes | Women who had not given birth after 48 hrs; neonatal mortality; gestational age at birth; nausea/vomiting; hypotension; tachycardia; fetal ductal constriction; oligohydramnios; birthweight; cord pH; neonatal morbidity (RDS, PDA, sepsis, NEC, IVH, PVL), days on ventilation, days in NICU. Serious infant outcome (able to compile a composite of death and PVL). |
|
| Notes | Antenatal corticosteroid use: all women.
Surfactant use: not reported. Sample‐size calculation: yes. Funding: no external sources; "no declarations of interest". No antibiotics were given. Drugs could be switched for treatment failure or adverse effects, but women were analysed according to their original group allocation. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only reported as "generated by random generation". |
| Allocation concealment (selection bias) | Low risk | Not entirely clear, but probably secure third party "After enrolment a disinterested third party (UMC Pharmacy Service) ..... selected the next in a series of opaque envelopes, containing a card generated by random selection, assigning the patient to one of the three study groups.. and the appropriate medication was sent to the labor/delivery suite." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not possible for women or study personnel given the 3 different regimens. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 25/301 (8%) women lost to follow‐up or results not analysed:
|
| Selective reporting (reporting bias) | Unclear risk | Events were not reported for the neonatal morbidity composite (only reported as P = 0.504). |
| Other bias | Unclear risk | No other obvious sources of bias identified, although more women were randomised to nifedipine (114 vs 90 magnesium sulphate vs 97 indomethacin) and no adjustment made for twins. |
Larmon 1999.
| Methods | Single‐centre RCT. | |
| Participants | 122 women. Inclusion criteria: women with singleton pregnancy and intact membranes, in documented preterm labour. Gestational age range: between 24‐34 weeks. Exclusion criteria: women with medical or obstetric complications needing delivery, previous tocolytic therapy this pregnancy, cervical dilatation ≥ 4 cm, non‐reassuring fetal status, growth restriction, fetal anomaly. Setting: Jackson, Mississippi, USA. 1996‐1997. |
|
| Interventions |
MAGNESIUM VS CALCIUM CHANNEL BLOCKERS 1) Magnesium sulphate (n = 65): loading dose 6 g IV. Maintenance: 2‐4 g/hr as needed to keep the uterus quiescent. Duration: after preterm arrested, started on oral magnesium lactate (as Mag‐Tab, 4 tablets every 12 hrs) 1 hr before discontinuation of IV therapy. Continued until 37 weeks or birth. 2) Nicardipine (n = 57): initial treatment 40 mg orally followed by 20 mg after 2 hrs if still contracting. Continued up to a maximum of 80 mg. Maintenance: 45 mg sustained release nicardipine every 12 hrs once contractions stopped until 37 weeks or birth. |
|
| Outcomes | Time to uterine quiescence; time gained in utero; recurrence of preterm labour; failure of tocolysis; fetal and neonatal mortality. Serious infant outcome: able to be defined as perinatal mortality. |
|
| Notes | Antenatal corticosteroid use: all women received betamethasone. Surfactant use: not stated. Sample‐size calculation: yes. Funding: Vicksburg Hospital Medical Foundation. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomisation envelopes were prepared by means of a random number table". |
| Allocation concealment (selection bias) | Unclear risk | "The next numbered opaque envelope was opened to assign each patient" ‐ no mention of envelopes being sealed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Because of the different routes of administration, neither women or physicians were blinded to treatment allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No evidence that outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There do not appear to be any losses to follow‐up and ITT analysis has been conducted. |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes were reported and included both maternal and baby outcomes. |
| Other bias | Unclear risk | Baseline imbalance in numbers randomised to each group (65 and 57). |
Lorzadeh 2007.
| Methods | RCT. | |
| Participants | 101 women (109 babies), at 22 to 35 weeks' GA. Inclusion criteria: intact membranes and cervical dilatation less than 4 cm in preterm labour; singleton and twin pregnancies. Exclusion criteria: premature rupture of membrane, cervical dilatation > 4 cm, placental abruption, diabetes mellitus, cardiorespiratory disease, genitourinary infection, pelvic anatomical anomalies, fetal anomalies. Setting: university maternity hospital, Iran (February 2000 to February 2002). |
|
| Interventions |
MAGNESIUM VS HUMAN CHORIONIC GONADOTROPIN 1) magnesium sulphate ‐ 4 g loading dose IV (1 g/min (?)) followed by continuous infusion of 2 g/hr until 12 hrs of uterine quiescence achieved (n = 51 women, 55 babies); 2) human chorionic gonadotropin ‐ single dose of 5000 IU and 1000 units in 500 mL dextrose IV (n = 50 women, 54 babies). |
|
| Outcomes | Delay of labour, complications, NICU admission (for respiratory distress). Serious infant outcome: text mentions no serious outcomes. |
|
| Notes | Antenatal corticosteroid use: all women received steroids.
Surfactant use: not reported. Sample‐size calculation: not reported. Funding: not reported. If contractions increased or dilatation progressed, the woman was then excluded from the study and was able to be switched to another tocolytic regimen if she continued to have contractions after 6 hrs therapy. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly allocated", no further details reported. |
| Allocation concealment (selection bias) | Unclear risk | "randomly allocated", no further details reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported but blinding not feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported but judged to be unlikely to have occurred. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | None reported ‐ although methods state that women were excluded if their contractions increased. |
| Selective reporting (reporting bias) | Unclear risk | Most expected outcomes reported except preterm birth; some lack of detail about specific neonatal complications. |
| Other bias | Unclear risk | No apparent evidence of other sources of bias. |
Lyell 2007.
| Methods | Multicentre RCT: NCT00185900. | |
| Participants | 196 women (229 babies). Inclusion criteria: in active preterm labour (2 or more contractions every 10 minutes with cervical change, ruptured membranes, or 2 cm or more dilatation and 80% effacement) at 24 to 33 6/7 weeks' GA. Exclusion criteria: placental abruption, placenta praevia, non‐reassuring fetal status, IUGR, chorioamnionitis and maternal medical disease. Setting: Stanford University Medical Center and Santa Clara Valley Medical Center, California, USA. |
|
| Interventions |
MAGNESIUM VS CALCIUM CHANNEL BLOCKERS 1) magnesium sulphate ‐ 4 g bolus followed by 2 g/hr infusion (with discretion to give additional 2 g magnesium sulphate boluses as needed for persistent labour): n = 92 (106 babies)*; 2) nifedipine ‐ 10 mg sublingually every 20 minutes for 3 doses total, followed by 20 mg orally every 4 to 6 hrs, based on physician judgment: n = 100 (110 babies)*. Physicians were instructed to continue treatment with magnesium sulphate or nifedipine until at least 12 hrs of uterine quiescence (defined as 6 or fewer contractions per hr) occurred within the first 48 hrs. After study treatment, physicians were instructed to manage women according to their usual routine, which may or may not have included oral maintenance tocolysis with nifedipine. |
|
| Outcomes | Prevention of birth for 48 hrs with uterine quiescence; birth before 48 hrs; hrs to quiescence, gestational age at birth; preterm birth (< 37 weeks; < 32 weeks); episodes of recurrent preterm labour; maternal adverse effects; a composite of serious maternal adverse events (chest pain, pulmonary oedema, shortness of breath, hypotension); birthweight; birthweight < 2500 g; neonatal morbidities (composite of RDS, IVH, NEC, sepsis and fetal or neonatal death); perinatal death; neonatal death; birthweight; birthweight < 2500 g; RDS; IVH; NEC; sepsis; NICU admission; GA at NICU admission; birthweight at NICU admission; NICU length of stay. Serious infant outcome: able to be shown as perinatal mortality. |
|
| Notes | Antenatal corticosteroid use: all women received betamethasone.
Surfactant use: not reported. Sample‐size calculation: yes. Funding: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "random numbers table". |
| Allocation concealment (selection bias) | Unclear risk | "sequentially numbered opaque envelopes" ‐ but no mention that the envelopes were sealed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Different routes of administration were used so blinding was not feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4/196 postrandomisation exclusions ("not meeting study entry criteria" ‐ 1 woman 35 weeks' GA who had not yet received tocolysis; 3 women did not meet criteria for preterm labour, 2 had cervical dilatation but no contractions, and 1 had frequent uterine contractions but no cervical change) *unclear if 106 or 105 babies in the magnesium sulphate group and 110 or 111 in the nifedipine group are evaluable. |
| Selective reporting (reporting bias) | Low risk | Most expected outcomes were reported. |
| Other bias | Unclear risk | Slight imbalance in numbers of women randomised to each group (92 and 100). |
Ma 1992.
| Methods | Single‐centre RCT. | |
| Participants | 65 women.
Inclusion criteria: women with uncomplicated preterm labour. Gestational age range: between 28‐36 weeks.
Exclusion criteria: complicated preterm labour. Setting: Beijing, China.1988‐1991. |
|
| Interventions | MAGNESIUM VS NO ALTERNATIVE TOCOLYTIC AGENT 1) Magnesium sulphate (n = 30): loading dose 5 g IV MgSO4 Maintenance 2 g/hr. 2) Barbiturate and bed rest (n = 35). | |
| Outcomes | Delay in birth > 48 hrs; adverse effects for women and neonate. | |
| Notes | Antenatal corticosteroid use: not stated.
Surfactant use: not stated. Sample size calculation: not given. Funding: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "they were divided into two groups randomly". |
| Allocation concealment (selection bias) | Unclear risk | No details provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Intervention via different routes, no placebo. Women and staff were not likely to be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided on whether outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up evident and ITT analysis conducted. |
| Selective reporting (reporting bias) | Low risk | Most prespecified outcomes were reported and included maternal and fetal outcomes, but perinatal death was not reported. |
| Other bias | Unclear risk | Some baseline imbalance in numbers randomised to each group (30 and 35). |
McWhorter 2004.
| Methods | RCT. | |
| Participants | 214 women (8 women in each group had twins). Inclusion criteria: 22 to 34 weeks' gestation with preterm labour, intact amniotic membranes, cervical dilatation of 4 cm or less. Exclusion criteria: medical complications contraindicating tocolysis, nonreassuring fetal surveillance, evidence of fetal growth restriction, allergy to nonsteroidal anti‐inflammatory drugs, sonographic evidence of lethal congenital abnormalities. Setting: Orlando, USA. Timeframe not specified. |
|
| Interventions |
MAGNESIUM VS COX INHIBITORS 1) magnesium sulphate (n = 109): IV 4‐6 g in 20% solution as a loading dose followed by a continuous infusion at 2‐4 g/hr + placebo pill; 2) rofecoxib (n = 105) 50 mg orally once a day + saline infusion at a rate of 80 mL/hr for the duration of the study. |
|
| Outcomes | Delay in birth for 48 hrs; success (arrest of labour and no birth within 48 hrs in women who received only their randomised medication); neonatal mortality. Serious infant outcome: able to be shown as neonatal mortality. |
|
| Notes | Antenatal corticosteroid use: at least 1 injection of betamethasone in 98% of rofecoxib and 96% of magnesium groups.
Surfactant use: not stated. Sample size calculation: yes. Funding: not stated. Some switching of medication was allowed. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐number table. |
| Allocation concealment (selection bias) | Low risk | Randomised by the pharmacy. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both investigators and women were blinded throughout the trial; there was placebo control. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details as to whether outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 214 women randomised, 4/105 in the rofecoxib group did not receive assigned medication and 3/109 did not receive assigned medication in the magnesium group. 76% of the rofexicob and 84% of the magnesium group stopped medication before 48 hrs due to treatment failure with medication stopped or switched, delivery of infant or adverse effects. The authors state that ITT analysis was conducted. 88% of the rofecoxib group and 94% of the magnesium group had birth outcome data. There was no explanation of the missing data or if the missing women differed from those women who completed the study. |
| Selective reporting (reporting bias) | Low risk | Outcomes were comprehensive and included maternal and fetal outcomes. |
| Other bias | Low risk | No apparent evidence of other bias. |
Miller 1982.
| Methods | Single‐centre RCT. | |
| Participants | 29 women. Inclusion criteria: 29 women in preterm labour of unknown aetiology. Gestational age range: before 37 weeks. Exclusion criteria: women with medical or obstetric complications. Setting: Charleston, South Carolina, USA. 1979‐1980. | |
| Interventions | MAGNESIUM VS BETAMIMETIC 1) Magnesium sulphate (n = 14): loading dose 4 g IV MgSO4. Maintenance at 2 g/hr. MgSO4 for 2 hrs then 1 g/hr MgSo4 for 22 hrs. 2) Terbutaline (n = 15): loading dose 0.25 mg over 20 minutes followed by 10 μg/min increased by 5 μg/min increments up to a maximum of 25 μg/min or contractions ceased. Maintenance 5 mg oral tablet terbutaline 20 hrs after initial therapy. | |
| Outcomes | Contractions ceased for at least 24 hrs; maternal and neonatal adverse effects and other outcomes. | |
| Notes | Antenatal corticosteroid use: not stated.
Surfactant use: not stated. Sample size calculation: not given. Funding: not stated. Possibility of cross‐over/switching to the alternative tocolytic. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized treatment" ‐ no further details. |
| Allocation concealment (selection bias) | Unclear risk | "a sealed numbered envelope was opened to begin the previously randomised treatment". |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No evidence of blinding although it could have been feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details reported on whether outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2/14 women in the magnesium group had treatment failure and switched to terbutaline, 1/15 women in the terbutaline group had treatment failure and switched to magnesium. |
| Selective reporting (reporting bias) | Low risk | Outcomes included both maternal and fetal outcomes. |
| Other bias | Low risk | No apparent evidence of other bias. |
Mittendorf 2002.
| Methods | Single‐centre RCT. | |
| Participants | 92 women. Inclusion criteria: women in preterm labour at > 24 weeks and < than 34 weeks' gestation and dilated 4 cm or less. Gestational age range: > 24 weeks, < 34 weeks. Exclusion criteria: cervical dilatation > 4 cm, pre‐eclampsia. Total recruited: 92 women into the tocolytic arms, 46 in magnesium group (vs) 46 in other tocolytic group. Setting: Chicago, USA. October 1995 ‐ January 1997. |
|
| Interventions | MAGNESIUM VS OTHER TOCOLYTIC 1) Magnesium sulphate loading dose IV 4 g bolus. Maintenance: 2‐3 g/hr infusion: (n = 46 mothers, 55 babies). 2) Other tocolytic drug treatment schedule not given. ('unblinded obstetrician's choice of ritodrine, terbutaline, indomethacin or nifedipine') (n = 46 mothers, 51 babies). | |
| Outcomes | Total paediatric mortality (sum of fetal, neonatal, and post neonatal mortality) and cerebral palsy; IVH, childhood outcomes. Serious infant outcome: able to be defined as total perinatal and infant mortality; IVH 3/4 or PVL; cerebral palsy. |
|
| Notes | Antenatal corticosteroid use: 89% of women given.
Surfactant use: as clinically indicated. Sample size calculation: based on anticipated reductions in the occurrence of neonatal IVH. Funding: United Cerebral Palsy Research and Educational Foundation. Recruitment to the trial was stopped because of an excess of deaths in the magnesium arm at interim data safety monitoring. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computerised program was used "to ensure balance in the randomisation process". Blocks of 6 randomised by race (black vs other), length of gestation (≤ 28 weeks vs > 28 weeks), and singleton or twin pregnancy. |
| Allocation concealment (selection bias) | Unclear risk | No details provided on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Investigators were not blinded as they selected the other tocolytic agent. Unlikely that women were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided as to whether outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT analysis conducted. Losses to follow‐up not reported apart from death. |
| Selective reporting (reporting bias) | Unclear risk | Outcomes were primarily fetal and lacked maternal outcome data including adverse effects. |
| Other bias | Low risk | No apparent evidence of other bias. |
Morales 1993.
| Methods | Single‐centre quasi‐RCT. | |
| Participants | 114 women. Inclusion criteria: women in preterm labour with intact membranes. Gestational age range: less than 32 weeks. Exclusion criteria: women with cervical dilatation > 4 cm, fetal growth restriction, congenital anomaly incompatible with life. Setting: Orlando, Florida, USA. 1988‐1989. |
|
| Interventions | MAGNESIUM VS PROSTAGLANDIN INHIBITOR 1) Magnesium sulphate (n = 52 women; 59 babies); Loading dose 6 g IV bolus over 30 minutes. Maintenance: 2‐5 g/hr. 2) Indomethacin (n = 49 women; 58 babies): loading dose 100 mg rectal suppository. Maintenance: 25 mg orally every 4 hrs for 48 hrs. If regular contractions persisted 1‐2 hrs after the initial 100 mg suppository this was repeated. After cessation of contractions for 12 hrs all women received oral terbutaline 5 mg every 6 hrs for prophylaxis against recurrent preterm labour. | |
| Outcomes | Delaying birth > 48 hrs, extending gestation, neonatal death; maternal adverse effects, RDS, birthweight, pulmonary hypertension, IVH. Serious infant outcome: able to be shown as neonatal mortality and IVH 3/4. |
|
| Notes | Antenatal corticosteroid use: betamethasone was used.
Surfactant use: not stated. All women received vitamin K 10 mg IM. If < 30 weeks women received 780 mg IV phenobarbital. Sample‐size calculation: not stated. Funding: not stated. In the event of failure of the primary tocolytic agent, women were treated with the alternative tocolytic. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "women were randomised by sealed envelopes to receive either indomethacin or magnesium sulphate for tocolysis by means of random number tables. Subjects assigned odd integers were allocated to the magnesium sulphate group". |
| Allocation concealment (selection bias) | High risk | "women were randomised by sealed envelopes to receive either indomethacin or magnesium sulphate for tocolysis by means of random number tables. Subjects assigned odd integers were allocated to the magnesium sulphate group". Although the authors state that the women were randomised by sealed envelopes they were allocated based on whether the randomised numbers were odd or even. There was therefore no allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | There was no evidence of blinding, and medication was administered via different routes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unlikely that the outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 13/114 women were excluded because of failure to adhere to study protocol. ITT analysis was conducted. |
| Selective reporting (reporting bias) | Low risk | Both maternal and fetal outcomes are reported. |
| Other bias | Low risk | No apparent evidence of other bias. |
Parilla 1997.
| Methods | Single‐centre RCT. | |
| Participants | 24 women. Inclusion criteria: having regular uterine contractions, less than 30 weeks' gestation, progressive cervical dilatation and effacement. Exclusion criteria: ruptured membranes, uterine bleeding, intrauterine growth restriction, chorioamnionitis, pre‐eclampsia, abnormal fetal heart tracing. Setting: Chicago, USA. September 1993 to September 1996. |
|
| Interventions |
MAGNESIUM VS PROSTAGLANDIN INHIBITORS 1) Magnesium sulphate (n = 12): loading dose 8 g IV over first hr and 4 g over second hr, maintenance dose at 2.5 g/hr continued for 12 hrs after the cessation of contractions. 2) Indomethacin (n = 12): loading dose 50‐100 mg orally/rectally. Maintenance dose 25‐50 mg orally every 4 to 6 hrs for 24‐48 hrs. |
|
| Outcomes | Cerebral blood flow and IVH, NEC, and RDS ‐ unable to be used as reported by exposure, not as randomised. |
|
| Notes | Antenatal corticosteroid use: betamethasone was used in all women.
Surfactant use: not stated. Sample‐size calculation: not stated. Funding: not stated. If there was tocolysis failure then a second agent was substituted. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer generated series of random numbers." |
| Allocation concealment (selection bias) | Unclear risk | "Opaque sealed envelopes." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding is not reported and is not likely to have occurred as the route of administration differed between the interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There are no details as to whether outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Doppler data were available for 21 of the 24 neonates. |
| Selective reporting (reporting bias) | High risk | There are no maternal outcomes reported and neonatal death is reported in the results but is not listed a priori in the methods section. |
| Other bias | Unclear risk | There are unit of analysis issues for some of the outcomes (some women received both magnesium sulphate and indomethacin). |
Parsons 1987.
| Methods | Single‐centre RCT. | |
| Participants | 72 women. Inclusion criteria: in preterm labour (no other details) and between 25 to 34 weeks' gestation. Exclusion criteria: ruptured membranes, initial temperature 99.8F or higher, treated with antibiotics or tocolytic infusion time less than 12 hrs. Setting: Chicago, USA. September 1983 to July 1984. |
|
| Interventions |
MAGNESIUM VS BETAMIMETICS 1) Magnesium sulphate (n not stated) Loading dose 4 g IV over 10 minutes. Maintenance 2 g/hr increasing 0.5 g/hr every 20 minutes to a maximum of 3 g/hr. 2) Terbutaline (n not stated) Loading dose 0.25 mg IV. Maintenance 10 micrograms/minute increased by 5 micrograms per minute every 10 minutes until contractions decreased to less than every 30 minutes or woman had excessive adverse effects. IV therapy was continued for 12 hrs after the cessation of contractions. |
|
| Outcomes | Maternal oral temperature. | |
| Notes | Antenatal corticosteroid use: not stated.
Surfactant use: not stated. Sample‐size calculation: not stated. Funding: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized", no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | There were no details as to whether women or investigators were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There were no details as to whether the outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Data were available for analysis for 26 women in each group although it is not reported if this is due to exclusion criteria after randomisation or some other factor. |
| Selective reporting (reporting bias) | High risk | The only outcome reported is maternal oral temperature. There are no neonatal outcomes and no reporting of adverse effects. |
| Other bias | Low risk | No apparent evidence of other bias. |
Pezzati 2001.
| Methods | RCT. | |
| Participants | 40 women recruited at 24 to 34 weeks' GA resulting in 54 babies (6 sets of twins in the magnesium group and 6 sets of twins and 1 set of triplets in the ritodrine group). Inclusion criteria: risk of preterm labour (defined as regular uterine contractions associated with cervical dilatation of at least 1 cm but < 5 cm); intact fetal membranes, no maternal or fetal complications necessitating delivery. Exclusion criteria: mothers with pre‐eclampsia or eclampsia and with any significant complications during pregnancy or birth; mothers who had finished 1 complete cycle of betamethasone in the last week of gestation; infants with malformations; IUGR, perinatal asphyxia, infection, anaemia, polycythaemia, PDA. Setting: Firenze, Italy. |
|
| Interventions |
MAGNESIUM VS BETAMIMETIC 1) magnesium sulphate (n = 21 women; 27 babies): IV infusion, loading dose of 4 g over 20‐30 mins followed by 2 g/hr. 2) ritodrine (n = 19 women; 27 babies): loading dose of 50 μg/min to a maximum dose of 250 μg/min until uterine contractions stopped or maternal heart rate was 140 beats/min or more. |
|
| Outcomes | Various measures of cerebral flow; gestational age; birthweight; 5 minute Apgar score; mode of birth; surfactant and inotrope drug administration; RDS; need for mechanical ventilation; PVL, IVH (latter 2 by cerebral ultrasounds at least twice during the first week of life and weekly until hospital discharge), mortality, NEC, ROP, BPD. Serious infant outcome only able to be reported as neonatal mortality (not able to establish which babies had more than 1 event). |
|
| Notes | Antenatal corticosteroid use: all women received betamethasone.
Surfactant use: 13% of babies received surfactant. Sample‐size calculation: not reported. Funding: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly" ‐ no further details reported. |
| Allocation concealment (selection bias) | Unclear risk | "by means of sealed envelopes." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible to blind the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Partial: "newborns were examined by the same investigator, blinded to the group assignment of the infant". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up reported. |
| Selective reporting (reporting bias) | Unclear risk | Primary effects of tocolytic drugs not reported, i.e. timing of birth (e.g. birth within 48 hrs); few maternal outcomes reported. |
| Other bias | Unclear risk | No apparent risk of other bias although outcomes not adjusted for high proportion of multiple births (risk of some lack of independence). |
Sayin 2010.
| Methods | Single‐centre RCT. | |
| Participants | 85 women (and 83 healthy pregnant controls, not included in this review). Inclusion criteria: women in confirmed preterm labour, 4 or more contractions per hr with documented cervical changes; 26 to 36 weeks' gestational age. Exclusion criteria: multiple pregnancy, fetal congenital abnormality, IUGR, cervical dilatation > 4 cm or effacement ≥ 80%, pre‐eclampsia, eclampsia, abrupto or placenta previa, chorioamnionitis, diabetes mellitus, maternal heart disease, previous preterm episode in index pregnancy or delivered before 48 hrs after initiating therapy. Setting: Turkey. No details of timeframe. |
|
| Interventions |
MAGNESIUM VS BETAMIMETIC (PLUS CALCIUM ANTAGONIST) 1) Magnesium sulphate (n = 39): loading dose 4.5 g IV over 20 minutes. Maintenance 1 g/hr. 2) Ritodrine + verapamil (n = 46): ritodrine IV loading dose 0.05 mg/min and verapamil 0.0025 mg/min followed by incremental increases of ritodrine 0.05 mg/min and verapamil 0.0025 mg/min every 20 minutes until contractions stopped. Maximum dose ritodrine 0.35 mg and verapamil 0.03 mg. |
|
| Outcomes | Birth before 48 hrs, blood flow through umbilical artery, middle cerebral artery, bilateral uterine arteries and ductus venosus, perinatal mortality. Serious infant outcome: able to be shown as perinatal mortality. |
|
| Notes | Antenatal corticosteroid use: all women 34 weeks' GA or less received betamethasone 12 mg IM in 2 doses 12 hrs apart (74% ritodrine and 54% in magnesium group).
Surfactant use: not stated. Sample size calculation: not reported. Funding: not reported. Focus of study was maternal and fetal Doppler flow. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomly assigned", "computer based programme". |
| Allocation concealment (selection bias) | Unclear risk | No details of allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No evidence of blinding (which could have been possible as both drugs were administered IV). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details as to whether the outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 5 women in the magnesium group and 1 in the ritodrine group were excluded as they delivered before 48 hrs after medication given. ITT analysis was conducted on all 85 women. |
| Selective reporting (reporting bias) | High risk | The only outcomes of relevance to this review was birth before 48 hrs and perinatal mortality ‐ the primary study outcomes were doppler blood flow; there were no maternal outcomes such as side effects reported and no other fetal outcomes. |
| Other bias | Unclear risk | Some baseline imbalance in numbers randomised (39 and 45). |
Schorr 1997.
| Methods | Single‐centre RCT. | |
| Participants | 100 women. Inclusion criteria: women in confirmed preterm labour with intact membranes. Gestational age range: between 20‐32 weeks. Setting: Jackson, Mississippi, USA. No details of timeframe. |
|
| Interventions | MAGNESIUM VS PROSTAGLANDIN INHIBITORS 1) Magnesium sulphate (n = 43) ‐ Loading dose 6 g IV bolus. Maintenance: 2‐6 g/hr. 2) Ketorolac (n = 45) ‐ loading dose 60 mg intramuscularly. Maintenance: 30 mg intramuscularly every 6 hrs for a maximum of 24 hrs. All women received oral magnesium sulphate for continued tocolysis and until 37 weeks. | |
| Outcomes | Arrest of preterm labour; maternal side effects, neonatal morbidity, PVL, IVH. Serious infant outcome: able to be shown as PVL or IVH 3/4 (assumes no perinatal mortality). |
|
| Notes | Antenatal corticosteroid use: not stated.
Surfactant use: not stated. Sample‐size calculation: not stated. Funding: Vicksburg Hospital Medical Foundation. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Women were randomised. No further details. |
| Allocation concealment (selection bias) | Unclear risk | No details regarding allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No evidence of blinding which was unlikely as medication delivered via different routes (IV and IM). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details as to whether outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Losses to follow‐up: 12/100. ITT analysis was conducted. |
| Selective reporting (reporting bias) | Unclear risk | Limited number of fetal outcomes reported. |
| Other bias | Low risk | No apparent evidence of other bias. |
Sciscione 1993.
| Methods | Single‐centre RCT. | |
| Participants | 132 women.
Inclusion criteria: women in preterm labour with intact membranes. Gestational age range: between 20‐36 weeks. Setting: Newark, Delaware, USA. Timeframe not stated. |
|
| Interventions |
MAGNESIUM VS BETAMIMETICS 1) magnesium sulphate; 2) ritodrine and terbutaline. Numbers per group not reported. Dose: treatment schedules were not stated in the abstract. |
|
| Outcomes | Delay in birth > 48 hrs, failure of tocolysis; maternal adverse effects ‐ reported only as P values. |
|
| Notes | Antenatal corticosteroid use: not stated.
Surfactant use: not stated. Sample size calculation: not stated. Funding: not stated. Women with failed tocolysis on 1 agent were switched to the other agent. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Women "were randomised". |
| Allocation concealment (selection bias) | Unclear risk | No details provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details in abstract. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details in abstract. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details in abstract. |
| Selective reporting (reporting bias) | Unclear risk | Limited fetal outcomes; outcomes only reported as P values. |
| Other bias | Unclear risk | Women who had failure with betamimetics were switched to magnesium sulphate and those who failed magnesium sulphate were switched to betamimetics. |
Steer 1977.
| Methods | Single‐centre quasi‐RCT. | |
| Participants | 71 women.
Inclusion criteria: gestational age range: less than 37 weeks, intact membranes, < 5 cm cervical dilatation.
Exclusion criteria: women with amnionitis or 'bleeding greater than show'. Setting: New York, USA. Time frame not stated. |
|
| Interventions | MAGNESIUM VS NO ALTERNATIVE TOCOLYTIC AGENT 1) Magnesium sulphate (n = 31): loading dose 4 g IV bolus. Maintenance: 2 g/hr continued until labour subsided or labour had progressed to an irreversible stage. 2) Alcohol (n = 31): loading dose 9.5% v/v ethanol in 5% dextrose in water prepared. 15 mL/kg body weight over the first 2‐hr period. Maintenance: 1.5 mL/kg body weight/hr until contractions stopped or labour progressed. 3) Dextrose in water (n = 9): 100 mL/hr infused IV. | |
| Outcomes | delivery delayed > 1 week, contractions ceased > 24 hrs. | |
| Notes | Antenatal corticosteroid use: not stated.
Surfactant use: not stated. Sample size calculation: not stated. Funding: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Patients received intravenous magnesium sulphate or alcohol according to the last digit of their hospital number. A small group of patients were chosen at random to receive an infusion of 5% dextrose in water for a control group". |
| Allocation concealment (selection bias) | High risk | See above ‐ there was no allocation concealment in this trial. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No details but unlikely to be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details as to whether outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women appear to have been followed with no losses and ITT analysis conducted. |
| Selective reporting (reporting bias) | High risk | The outcomes reported are very limited for both maternal and fetal outcomes, reporting only on delivery delayed for more than 1 week and cessation of contractions > 24 hrs. |
| Other bias | Unclear risk | No apparent evidence of other bias. |
Surichamorn 2001.
| Methods | Single‐centre RCT. | |
| Participants | 96 women eligible (71 analysed). Inclusion criteria: preterm labour, 28 to 35 weeks' GA, regular and painful contractions at intervals of < 10 minutes (observed for at least 30 mins), cervix effaced or almost effaced, dilatation ≤ 3 cm, singleton pregnancy. Exclusion criteria: fever, placenta praevia, abruptio placentae, fetal abnormality, hydramnios, incompetent cervix, premature rupture of membranes, hypertension, hyperthyroidism, diabetes mellitus, received prior tocolytic agent, contraindication to the tocolytic agents used in trial. Setting: Sing Buri, Thailand. January 1995 to December 1998. |
|
| Interventions |
MAGNESIUM VS BETAMIMETIC 1) Magnesium sulphate (n = 49): loading dose 4 g IV over 20 minutes. Maintenance 2 g/hr increasing to a max of 4 g/hr as needed to arrest labour for 24 hrs. After 24 hrs weaned to 0.5 mg every 4 to 6 hrs. Then oral terbutaline treatment given (10 mg/day) until 36 weeks' GA. 2) terbutaline (n = 47): loading dose 0.25 mg IV bolus. Maintenance 10 μg/min increasing by 5 μg/min every 10 minutes up to a maximum of 25 μg/minute or until contractions stopped. Once contractions stopped then infusion maintained for 2 hrs. Then subcutaneous 0.25 mg terbutaline 4 hourly for 24 hrs. Then oral terbutaline treatment given (10 mg/day) until 36 weeks' GA. |
|
| Outcomes | Stopping labour for 24 hrs, treatment failure, delayed delivery for 2 days, 3 days, 7 days and up to 37 weeks' GA, admission for recurrent labour, maternal side effects, birthweight, Apgar scores, survival of neonate. Serious infant outcome: able to be shown as perinatal mortality. |
|
| Notes | Antenatal corticosteroid use: dexamethasone 12 mg administered in 2 doses 24 hrs apart and then weekly until 32 weeks' GA.
Surfactant use: not stated. Sample size calculation: not stated. Funding: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized". No further details supplied. |
| Allocation concealment (selection bias) | Unclear risk | "sealed envelopes." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention that women or staff were blinded; and the regimens differed making it very unlikely that either were blinded. There was no placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There are no details as to whether the outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 25 women were excluded after randomisation due to not meeting entry criteria (12 in the terbutaline group and 13 in the magnesium group). Overall analysis was conducted on 35 terbutaline and 36 magnesium participants. |
| Selective reporting (reporting bias) | Unclear risk | Although the outcomes reported covered both maternal and fetal outcomes they were not prespecified or listed a priori in the methods section and are therefore potentially subject to selective reporting. |
| Other bias | Low risk | No apparent evidence of other bias. |
Taherian 2007.
| Methods | 2‐centre RCT. | |
| Participants | 120 women. Inclusion criteria: pregnant women experiencing preterm labour at 26‐36 weeks within intact membranes. Exclusion criteria: taking other tocolytic agents, cervical dilatation ≥ 5 cm or obstetrical contraindication for tocolysis use. Setting: Isfahan, Iran. 2005‐2006. |
|
| Interventions |
MAGNESIUM VS CALCIUM CHANNEL BLOCKERS 1) magnesium sulphate (n = 63) loading dose 4 g IV; maintenance dose 2‐3 g/hr iv. 2) nifedipine (n = 57) loading dose 10 mg orally every 20 minutes (max 40 mg in first hr). Maintenance dose 10‐20 mg every 6 hrs. All women were initially on bedrest and were hydrated with 500 mL of Ringers solution. |
|
| Outcomes | Primary tocolytic effect (labour in first 48 hrs); secondary tocolytic effect (labour in 2‐10 days), maternal adverse effects (hypotension, tachycardia, palpitation, flushing, headaches, dizziness and nausea, drowsiness, blurred vision); gestational age at birth; birthweight; Apgar score at 1 and 5 minutes. | |
| Notes | Antenatal corticosteroid use: all women < 34 weeks' gestation received betamethasone.
Surfactant use: not stated. Sample‐size calculation: not stated. Funding: not stated. If tocolysis failed within 24‐48 hrs, other medication was given such as isoxsuprine or indomethacin. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported but judged as unlikely to have been done. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 8/63 women in the magnesium group and 10/57 women in the nifedipine group were classed as treatment failures (as they required other tocolytic agents); these women were included in the analyses. |
| Selective reporting (reporting bias) | Unclear risk | Tocolytic effect was reported only as labour within 48 hrs; or within 10 days ‐ actual timing of birth was not reported. |
| Other bias | Unclear risk | Some possible randomisation imbalance (63 vs 57). |
Tchilinguirian 1984.
| Methods | Single‐centre RCT. | |
| Participants | 67 women.
Inclusion criteria: 67 women in preterm labour with and without ruptured membranes.
Gestational age range: between 24‐36 weeks.
Exclusion criteria: women with cervical dilatation > 4 cm, medical or obstetrical complications necessitating delivery. Setting: New Jersey, USA. 1981‐1983. |
|
| Interventions |
MAGNESIUM VS BETAMIMETICS
1) Magnesium sulphate (n = 37): loading dose 4 g IV MgSO4. Maintenance at 2 g/hr. Reduced to 1 g/hr when tocolysis achieved.
2) Ritodrine (n = 30): used "according to the widely adapted protocol of the manufacturers".
In both groups medication was continued for 12 hrs after cessation of contractions or until unacceptable adverse effects necessitated stopping the therapy. Both groups of women received oral ritodrine for prophylaxis. |
|
| Outcomes | delivery delayed > 48 hrs; delivery > 34 weeks' GA. | |
| Notes | Antenatal corticosteroid use: not used.
Surfactant use: not used. Sample size calculation: not stated. Funding: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "were randomly assigned". No other details provided. |
| Allocation concealment (selection bias) | Unclear risk | No details provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Women and staff were not blinded and there was no placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There were no details as to whether the outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up are evident and ITT analysis was conducted. |
| Selective reporting (reporting bias) | High risk | Limited outcomes reported, no fetal outcomes. No adverse effects reported; tocolytic effects only reported as labour within 48 hrs, 2‐7 days, > 7 days ‐ actual timing not reported. |
| Other bias | Unclear risk | Some baseline imbalance in numbers randomised to each group (37 and 30). |
Wang 2000.
| Methods | RCT. | |
| Participants | 71 women, meeting criteria for preterm labour including premature rupture of membranes, placenta praevia, twin pregnancy, uterine abnormality. Exclusion criteria: significant antepartum haemorrhage, chorioamnionitis, diabetes mellitus, heart disease, maternal or fetal complications necessitating delivery, contraindications to ß2‐adrenergic agonist. Setting: Department of Obstetrics and Gynecology, The Second Affiliated Hospital, West China University of Medical Sciences (WCUMS), Chengdu, PR China. |
|
| Interventions |
MAGNESIUM VS BETAMIMETICS 1) magnesium sulphate (n = 36): 2.5 to 5 g loading dose IV over 5 minutes, followed by 15 to 20 g in 5% glucose 1000 mL IV until contractions were arrested; daily dose < 25 g. 2) ritodrine (n = 35): initial 50 μg/min IV, increasing at 50 μg/min every 10 to 30 minutes until contractions ceased or maternal. Heart rate > 130 beats/min, then the dosage was reduced by 50 μg and maintained for 12 to 18 hrs, then gradual reduction in dose until the rate was 50 μg/min. 30 minutes before cessation of the infusion, 10 mg oral ritodrine was given every 4 to 6 hrs for 3 days, then 10 mg every 8‐12 hrs until 36 weeks' GA. All women: if contractions recommenced, the infusion could be repeated. |
|
| Outcomes | Time for arrest of contractions; delivery in 24 hrs; prolongation of pregnancy; PPH; birthweight; maternal adverse effects. | |
| Notes | Antenatal corticosteroid use: not stated.
Surfactant use: not stated. Sample‐size calculation: not stated. Funding: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "patients were randomly divided into two groups"; no further details reported. |
| Allocation concealment (selection bias) | Unclear risk | "patients were randomly divided into two groups"; no further details reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported but not likely to be feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 14/71 (19.7%) lost to follow‐up; unequal between groups ‐ 11/36 in the magnesium group and 3/35 in the ritodrine group. |
| Selective reporting (reporting bias) | Unclear risk | Not all expected outcomes were reported, e.g. perinatal mortality and a number of neonatal morbidities. |
| Other bias | Unclear risk | No apparent source of other bias. |
Wilkins 1988.
| Methods | Single‐centre RCT. | |
| Participants | 120 women.
Inclusion criteria: 120 women. Gestational age range: between 25‐36 weeks.
Exclusion criteria: women with ruptured membranes, > 4 cm cervical dilatation, medical or obstetric complications necessitating delivery, fetal anomaly, growth restriction. Setting: New York, USA.1985‐1987. |
|
| Interventions | MAGNESIUM VS BETAMIMETICS 1) MgSO4 (n = 66): loading dose 4 g IV MgSO4 over 15 minutes. Maintenance at 2 g/hr. Rate increased if contractions continued and the serum level was below 5‐8 mg/dl, and it was decreased if serum levels exceeded this range. Infusion continued for 24 hrs after contractions abated before oral ritodrine was used as maintenance. 2) Ritodrine (n = 54): loading dose infusion 0.1 mg/min, increasing by 0.05 mg/min every 15‐20 minutes until contractions abated or maternal pulse > 140 beats/min, or maximum 0.35 mg/min. Oral ritodrine was used for maintenance. For both groups ritodrine 20 mg every 2 hrs tapering to every 4 hrs before discharge. Continued until 37 weeks. Outpatient management included weekly visits to a high‐risk clinic with weekly cervical examinations and uterine contraction monitoring if symptoms, and liberal use of modified bed rest at home. | |
| Outcomes | Delay in birth > 48 hrs; maternal adverse effects, delay in birth > 7 days, delay in birth > 37 weeks. | |
| Notes | Antenatal corticosteroid use: not stated.
Surfactant use: not stated. Sample‐size calculation: not stated. Funding: not stated. Second‐line therapy with the alternative tocolytic agent was necessary (due to tocolysis failure) in 18% (10) of women receiving ritodrine and 30% (22) of women receiving magnesium sulphate as first‐line therapy. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "as determined by random numbers table". |
| Allocation concealment (selection bias) | Unclear risk | "patients were assigned to ritodrine or magnesium sulphate by selection of the next consecutively numbered sealed envelope that designated the drug treatment to use" ‐ no mention that the envelopes were opaque. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Women and staff not blinded; no placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details as to whether outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was no evidence of losses to follow‐up and data were analysed by ITT. |
| Selective reporting (reporting bias) | Unclear risk | There were a limited number of baby outcomes reported. |
| Other bias | Unclear risk | Some baseline imbalance in numbers randomised to each group (66 and 54). |
Zhu 1996.
| Methods | Single‐centre RCT. | |
| Participants | 126 women
Inclusion criteria: in preterm labour. Gestational age range: between 28‐36 weeks. Setting: Guangzhou, China. 1993‐1995. |
|
| Interventions | MAGNESIUM VS BETAMIMETICS 1) Magnesium sulphate (n = 62) ‐ loading dose 5 g IV. Maintenance at 1.5‐2 g/hr. 2) Ritodrine (n = 64) ‐ loading dose 0.05 mg/min IV increasing to 0.1 mg/min. Maintenance 0.05‐0.1 mg/min infusion. | |
| Outcomes | Treatment to delivery interval, delay in delivery; maternal adverse effects. | |
| Notes | Antenatal corticosteroid use: not stated.
Surfactant use: not stated. Sample‐size calculation: not stated. Funding: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned". |
| Allocation concealment (selection bias) | Unclear risk | No details provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No evidence of blinding staff or women. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up stated and ITT analysis conducted. |
| Selective reporting (reporting bias) | Unclear risk | Lacked fetal and neonatal outcomes. |
| Other bias | Unclear risk | No evidence of other bias. |
BPD: bronchopulmonary dysplasia GA: gestational age GI: gastrointestinal hr: hour IM: intramuscular ITT: intention‐to‐treat IUGR: intrauterine growth restriction IV: intravenous IVH: intraventricular haemorrhage MgS04: magnesium sulphate min: minute NEC: necrotising enterocolitis NICU: neonatal intensive care unit PDA: patent ductus arteriosus PPH: postpartum Haemorrhage PVL: periventricular leukomalacia RCT: randomised controlled trial RDS: respiratory distress syndrome ROP: retinopathy of prematurity vs: versus
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Behrad 2003 | Compared 2 different doses of magnesium sulphate. |
| Di Renzo 2005 | A combination of magnesium sulphate and aminophylline was compared with ritodrine. |
| Ferguson 1984 | Magnesium sulphate was only used as an adjuvant therapy to ritodrine. |
| Hatjis 1987 | Magnesium sulphate was only used as an adjuvant therapy to ritodrine. |
| Herschel 2001 | Case report not a randomised trial. |
| How 1998 | Not all women were in preterm labour at entry into the trial. All women had preterm premature rupture of the membranes. |
| How 2006 | Compared a combination of magnesium and nifedipine (used as maintenance) with no tocolysis. |
| Ieda 1991 | Magnesium and ritodrine were compared with controls; not clear what the controls were and not clear if these comparisons were randomised. |
| Kara 2009 | Trial of magnesium sulphate versus nifedipine, but magnesium group 'crossed over' to terbutaline in the event of tocolytic failure. |
| Mittendorf 2000 | A review article, not a randomised trial. |
| Ogburn 1985 | Magnesium sulphate use assessed after single tocolytic failure. |
| Pryde 2001 | Review article, not a randomised trial. |
| Scudiero 2000 | Case‐control study. |
| Soguk 2004 | Compared 2 doses of magnesium. |
| Terrone 2000 | Compared 2 doses of magnesium. |
| Wischnik 1989 | Dual therapy, magnesium was used in combination with another tocolytic. |
| Zygmunt 2003 | Compares 2 different regimens of magnesium. |
Characteristics of studies awaiting assessment [ordered by study ID]
Lotfalizadeh 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | In Farsi, needs to be translated. |
Characteristics of ongoing studies [ordered by study ID]
Briery 2011.
| Trial name or title | Magnesium sulfate versus placebo for tocolysis in PPROM. |
| Methods | Randomised controlled trial. |
| Participants | English/Spanish speaking women with PPROM, 18‐45 years, 26 to 32.6 weeks' gestation, cervical dilatation ≤ 4 cm. |
| Interventions | Magnesium sulfate x 48 hours versus placebo saline IV x 48 hours. |
| Outcomes | Primary: hours of latency to delivery. Secondary: maternal postpartum length of stay; maternal infection rates; neonatal ventilator days; neonatal early onset infection; neonatal length of stay. |
| Starting date | April 2007. |
| Contact information | Regional Obstetric Consultants, USA. Lorrie Mason, email: lorrie@rocob.com |
| Notes |
Sooky 2010.
| Trial name or title | Comparison of delaying in premature labour by indomethacin and Mg sulfate in pregnant women referred to maternity hospital. |
| Methods | Randomised controlled trial. |
| Participants | Inclusion criteria: gestational age 24‐32 based on LMP and sonography during first trimester, single pregnancy, intact membrane, lack of GI or kidney disease, myasthenia gravis, 15‐45 years Exclusion criteria: rupture membrane, vaginal bleeding, dilatation ≥ 5 centimetre, placenta and fetal abnormalities, allergy to indomethacin, tocolytic therapy. |
| Interventions | Mg sulfate 4 g %20, IV, during 20 minutes and then 2 g until decreasing or stop of uterine contraction in IV infusion. If there was no decreasing in uterine contraction with 2 g then 3 g Mg sulfate was used versus Indomethacin, 50 mg, rectally, and then 50 mg every 6 hours until 24 hours. |
| Outcomes | Primary outcome: uterine contraction every 15 minutes until 1 hour palpation of uterine contraction via abdomen. Secondary outcomes: vaginal bleeding; rupture of membrane; duration of delivery delaying; fetal heart rate. |
| Starting date | 2008. |
| Contact information | Zahra Sooky Kashan University of Medical Sciences and Health Services, Ravand street, Kashan Isfahan Iran, Islamic Republic Of Phone: 00983615550021‐5 Fax: 00983615556633 soki_z@kaums.ac.ir |
| Notes |
GI: gastrointestinal IV: intravenous LMP: last menstrual period PPROM: preterm premature rupture of membranes
Differences between protocol and review
In this update, we have conducted a sensitivity analysis to assess the influence of excluding cross‐over trials where another tocolytic was used in case of tocolytic failure with magnesium.
Clinically relevant outcomes for trials of tocolysis for inhibiting preterm labour have been prespecified following consultation with the editors and authors of the individual reviews. Consensus was reached on six ‘core’ outcomes. These have now been highlighted in the methods section of the review, August 2014.
Contributions of authors
Caroline Crowther (CAC) wrote the original protocol, considered trials for inclusion, extracted data and the review with contributions from Professors Janet Hiller and Lex Doyle. For this update, Philippa Middleton (PM) and Julie Brown (JB) were involved in the identification of trials, data extraction, risk of bias judgements, and updating the text of the review. PM and JB wrote drafts of the updated review. All authors have commented on the drafts of the update and the final version.
Sources of support
Internal sources
ARCH, Robinson Institute/School of Paediatrics and Reproductive health, The University of Adelaide, Australia.
Department of Obstetrics and Gynaecology, University of Melbourne, Australia.
Liggins Institute, University of Auckland, New Zealand.
External sources
National Health and Medical Research Council, (NHMRC), Australia.
Declarations of interest
Caroline Crowther was the chief investigator for the Australasian multicentred randomised trial of magnesium sulphate given as a cerebroprotective agent to women at risk of very preterm birth (less than 30 weeks' gestation) within 24 hours of expected delivery for the prevention of cerebral palsy and mortality in infants (ACTOMgSO4). CAC and PM are principal investigators on the MAGENTA trial (ACTRN 12611000491965).
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Aramayo 1990 {published data only}
- Aramayo FJF, Martinez FJ, Rosales CL. Tocolytic therapy with magnesium sulfate and terbutaline for inhibition of premature labour [Terapia tocolitica con sulfato de magnesio y terbutalina para la inhibicion del trabajo de parto pretermino]. Ginecologia y Obstetricia de Mexico 1990;58:265‐9. [PubMed] [Google Scholar]
Armson 1992 {published data only}
- Armson BA, Samuels P, Miller F, Verbalis J, Main EK. Evaluation of maternal fluid dynamics during tocolytic therapy with ritodrine hydrochloride and magnesium sulfate. American Journal of Obstetrics and Gynecology 1992;167:758‐65. [DOI] [PubMed] [Google Scholar]
Asgharnia 2002 {published data only}
- Asgharnia M, Sobhani A, Omidvar‐Jalali Z. Comparison of Mg‐sulfate and indomethacin in management of preterm labor. Journal of Gorgan University of Medical Sciences 2002;4(10):7‐12. [Google Scholar]
Beall 1985 {published data only}
- Beall MH, Edgar BW, Paul RM, Smith‐Wallace T. A comparison of ritodrine, terbutaline, and magnesium sulfate for the suppression of preterm labor. American Journal of Obstetrics and Gynecology 1985;153:854‐9. [DOI] [PubMed] [Google Scholar]
Borna 2007 {published data only}
- Borna S, Saeidi FM. Celecoxib versus magnesium sulfate to arrest preterm labor: randomized trial. Journal of Obstetrics and Gynaecology Research 2007;33(5):631‐4. [DOI] [PubMed] [Google Scholar]
Chau 1992 {published data only}
- Chau AC, Gabert HA, Miller JM. A prospective comparison of terbutaline and magnesium for tocolysis. Obstetrics and Gynecology 1992;80:847‐51. [PubMed] [Google Scholar]
Clavin 1996 {published data only}
- Clavin DK, Bayhi DA, Nolan TE, Rigby FB, Cork RC, Miller JM. Comparison of intravenous magnesium sulfate and nitroglycerin for preterm labour: preliminary data [abstract]. American Journal of Obsterics and Gynecology 1996;174(1 Pt 2):307. [Google Scholar]
Cotton 1984 {published data only}
- Cotton DB, Strassner HT, Hill LM, Schifrin BS, Paul RH. Comparison of magnesium sulfate, terbutaline and a placebo for inhibition of preterm labor. A randomized study. Journal of Reproductive Medicine 1984;29:92‐7. [PubMed] [Google Scholar]
Cox 1990 {published data only}
- Cox SM, Sherman ML, Leveno KJ. Randomized investigation of magnesium sulfate for prevention of preterm birth. American Journal of Obstetrics and Gynecology 1990;163:767‐72. [DOI] [PubMed] [Google Scholar]
- Cox SM, Sherman ML, Leveno KJ. Single‐center randomized trial of magnesium sulfate for inhibition of uterine contractions in preterm labor. Proceedings of 10th Annual Meeting of Society of Perinatal Obstetricians; 1990 Jan 23‐27; Houston, Texas, USA. 1990:39.
El‐Sayed 1999 {published data only}
- El‐Sayed YY, Riley ET, Holbrook RH, Cohen SE, Chitkara U, Druzin ML. Randomized comparison of intravenous nitroglycerin and magnesium sulfate for treatment of preterm labour. Obstetrics and Gynecology 1999;93:79‐83. [DOI] [PubMed] [Google Scholar]
Floyd 1992 {published data only}
- Floyd RC, McLaughlin BN, Martin RW, Roberts WE, Wiser WL, Morrison JC. Comparison of magnesium and nifedipine for primary tocolysis and idiopathic preterm labor [abstract]. American Journal of Obstetrics and Gynecology 1992;166:446 [SPO Abstract 662]. [Google Scholar]
- Floyd RC, McLaughlin BN, Perry KG, Martin RW, Sullivan CA, Morrison JC. Magnesium sulfate or nifedipine hydrochloride for acute tocolysis of preterm labor: efficacy and side effects. Journal of Maternal‐Fetal Investigation 1995;5:25‐9. [Google Scholar]
Fox 1993 {published data only}
- Fox MD, Allbert JR, McCaul JF, Martin RW, McLaughlin BN, Morrison JC. Neonatal morbidity between 34 and 37 weeks' gestation. Journal of Perinatology 1993;XIII:349‐63. [PubMed] [Google Scholar]
- Fox MD, McCaul JF, Martin RW, Roberts WE, McLaughlin B, Morrison JC. Neonatal morbidity between 34‐37 weeks' gestation. American Journal of Obstetrics and Gynecology 1992;166:360. [Google Scholar]
Glock 1993 {published data only}
- Glock JL, Morales WJ. Efficacy and safety of nifedipine versus magnesium sulfate in the management of preterm labor: a randomized study. American Journal of Obstetrics and Gynecology 1993;169:960‐4. [DOI] [PubMed] [Google Scholar]
- Morales WJ, Glock JL. Efficacy and safety of nifedipine vs magnesium sulfate in the management of preterm labor: a randomized study. American Journal of Obstetrics and Gynecology 1993;168:375 [SPO Abstract 119]. [DOI] [PubMed] [Google Scholar]
Haghighi 1999 {published data only}
- Haghighi L. Prevention of preterm delivery: nifedipine or magnesium sulfate. International Journal of Gynecology and Obstetrics 1999;66(3):297‐8. [DOI] [PubMed] [Google Scholar]
Hollander 1987 {published data only}
- Hollander DI, Nagey DA, Pupkin MJ. Magnesium sulfate and ritodrine hydrochloride: a randomized comparison. American Journal of Obstetrics and Gynecology 1987;156:631‐7. [DOI] [PubMed] [Google Scholar]
Klauser 2012 {published data only}
- Klauser CK, Briery CM, Keiser SD, Martin RW, Kosek MA, Morrison JC. Effect of antenatal tocolysis on neonatal outcomes. Journal of Maternal‐Fetal and Neonatal Medicine 2012;25(12):2778‐81. [DOI] [PubMed] [Google Scholar]
- Klauser CK, Briery CM, Martin RW, Langston L, Magann EF, Morrison JC. A comparison of three tocolytics for preterm labor: a randomized clinical trial. Journal of Maternal‐Fetal and Neonatal Medicine 2014;27(8):801‐6. [DOI] [PubMed] [Google Scholar]
Larmon 1999 {published data only}
- Larmon JE, Ross BS, May WL, Dickerson GA, Fischer RG, Morrison JC. Oral nicardipine versus intravenous magnesium sulfate for the treatment of preterm labor. American Journal of Obstetrics and Gynecology 1999;181:1432‐7. [DOI] [PubMed] [Google Scholar]
Lorzadeh 2007 {published data only}
- Lorzadeh N, Kazemirad S, Lorzadrh M, Dehnori A. A comparison of human chorionic gonadotropin with magnesium sulphate in inhibition of preterm labor. Journal of Medical Science 2007;7(4):640‐4. [Google Scholar]
Lyell 2007 {published data only}
- Lyell D, Penn A, Caughey A, Kogut E, McLellan L, Adams B, et al. Neonatal outcomes following antenatal magnesium sulfate exposure: follow up from a magnesium vs. nifedipine tocolysis RCT. American Journal of Obstetrics and Gynecology 2009;201(6 Suppl 1):S180‐S181. [Google Scholar]
- Lyell D, Pullen K, Campbell L, Ching S, Burrs D, Chitkara U, et al. Magnesium sulfate versus nifedipine for acute tocolysis of preterm labor [abstract]. American Journal of Obstetrics and Gynecology 2005;193(6 Suppl):S18. [DOI] [PubMed] [Google Scholar]
- Lyell DJ, Pullen K, Campbell L, Ching S, Druzin ML, Chitkara U, et al. Magnesium sulfate compared with nifedipine for acute tocolysis of preterm labor ‐ a randomized controlled trial. Obstetrics and Gynecology 2007;110(1):61‐7. [DOI] [PubMed] [Google Scholar]
Ma 1992 {published data only}
- Ma L. Magnesium sulfate in prevention of preterm labor. Chung Hua I Hsueh Tsa Chih Taipei 1992;72(3):158‐61. [PubMed] [Google Scholar]
McWhorter 2004 {published data only}
- McWhorter J, Carlan SJ, O'Leary TD. Rofecoxib versus magnesium sulfate to arrest preterm labor: a randomized double‐blind trial. Obstetrics and Gynecology 2002;99(4 Suppl):2S. [DOI] [PubMed] [Google Scholar]
- McWhorter J, Carlan SJ, O'Leary TD, Richichi K, O'Brien WF. Rofecoxib versus magnesium sulfate to arrest preterm labor: a randomized trial. Obstetrics and Gynecology 2004;103:923‐30. [DOI] [PubMed] [Google Scholar]
Miller 1982 {published data only}
- Miller JM, Keane MWD, Horger EO. A comparison of magnesium sulfate and terbutaline for the arrest of premature labor. International Journal of Gynecology and Obstetrics 1984;22:117‐23. [Google Scholar]
- Miller JM, Keane MWD, Horger EO. Comparison of magnesium sulfate and terbutaline for the arrest of premature labor. Journal of Reproductive Medicine 1982;27(6):348‐51. [PubMed] [Google Scholar]
Mittendorf 2002 {published data only}
- Lee K‐S, Mittendorf R, Besinger R, Gianopoulos J, Pryde P. Mechanisms of mortality in the magnesium and neurologic endpoints trial (Magnet Trial): Papile grade III neonatal intraventricular hemorrhage (IVH). Pediatric Research 2002;51(2 of 2):411A. [Google Scholar]
- Lee K‐S, Mittendorf R, Besinger R, Gianopoulus J, Pryde P. Correlation between fetal‐fetal and maternal‐fetal ionized magnesium levels. Pediatric Research 2002;51(2 of 2):314A. [Google Scholar]
- Mittendorf R, Bentz L, Borg M, Roizen N. Does exposure to antenatal magnesium sulfate prevent cerebral palsy?. American Journal of Obstetrics and Gynecology 2000;182(1 Pt 2):S20. [Google Scholar]
- Mittendorf R, Bentz L, Kohn J, Covert R. Use of antenatal magnesium sulfate does not seem to prevent intraventricular haemorrhage. American Journal of Obstetrics and Gynecology 2000;182(1 Pt 2):S34. [Google Scholar]
- Mittendorf R, Covert R, Boman J, Khoshnood B, Lee K‐S, Seigler M. Is tocolytic magnesium sulphate associated with increased total paediatric mortality?. Lancet 1997;350:1517‐8. [DOI] [PubMed] [Google Scholar]
- Mittendorf R, Covert R, Elin R, Pryde PG, Khoshnood B, Lee K. Umbilical cord serum ionized magnesium level and total pediatric mortality. Obstetrics and Gynecology 2001;98:75‐8. [DOI] [PubMed] [Google Scholar]
- Mittendorf R, Dambrosia J, Dammann O, Pryde P, Lee K‐S, Ben‐Ami T, et al. Association between maternal serum ionized magnesium levels at delivery and neonatal intraventricular hemorrhage. Journal of Pediatrics 2002;140:540‐6. [DOI] [PubMed] [Google Scholar]
- Mittendorf R, Dambrosia J, Khoshnood B, Lee K‐S, Pryde P, Yousefzadeh D. Association between magnesium and intraventricular haemorrhage. American Journal of Obstetrics and Gynecology 2001;184(1):S188. [Google Scholar]
- Mittendorf R, Dambrosia J, Khoshnood B, Lee K‐S, Pryde P, Yousefzadeh D. Magnesium sulfate is no more efficacious than other tocolytic agents. American Journal of Obstetrics and Gynecology 2001;184(1):S188. [Google Scholar]
- Mittendorf R, Dambrosia J, Pryde PG, Lee KS, Gianopoulos JG, Besinger RE, et al. Association between the use of antenatal magnesium sulfate in preterm labor and adverse health outcomes in infants. American Journal of Obstetrics and Gynecology 2002;186(6):1111‐8. [DOI] [PubMed] [Google Scholar]
- Mittendorf R, Janeczek S, Macmillan W, Gianopoulos J, Besinger R, Karlman R, et al. Mechanisms of mortality in the magnesium and neurologic endpoints trial (MAGnet trial): fetal inflammatory response syndrome (FIRS). American Journal of Obstetrics and Gynecology 2001;185(6 Suppl):S151. [Google Scholar]
- Mittendorf R, Khoshmood B, Pryde P, Lee K‐S, Sriram S. Predicting neonatal serum ionized magnesium levels at delivery. Pediatric Research 2001;49(2 of 2):407A. [Google Scholar]
- Mittendorf R, Kuban K, Pryde PG, Gianopoulos JG, Yousefzadeh D. Antenatal risk factors associated with the development of lenticulostriate vasculopathy (lsv) in neonates. Journal of Perinatology 2005;25(2):101‐7. [DOI] [PubMed] [Google Scholar]
- Mittendorf R, Pryde P. Tocolytic magnesium sulfate: are the epidemiologic data reassuring?. American Journal of Obstetrics and Gynaecology 1998;179(1):280. [DOI] [PubMed] [Google Scholar]
- Mittendorf R, Pryde P, Khoshnood B, Lee K‐S. If tocolytic magnesium sulfate is associated with excess total pediatric mortality, what is its impact?. Obstetrics and Gynecology 1998;92(2):308‐11. [DOI] [PubMed] [Google Scholar]
- Mittendorf R, Roizen N, Moawad A, Khoshnood B, Lee K‐S. Association between cerebral palsy and coagulase‐negative staphylococci. Lancet 1999;354:1875‐6. [DOI] [PubMed] [Google Scholar]
- Mittendorf R, Stratford R, Khoshnood B, Lee K‐S, Pryde P. Persistence of elevated serum magnesium levels in the neonate. American Journal of Obstetrics and Gynaecology 2001;184(1):S50. [Google Scholar]
Morales 1993 {published data only}
- Morales WJ, Madhav H. Efficacy and safety of indomethacin compared with magnesium sulfate in the management of preterm labor: A randomized study. JAMA 1993;270(22):2676. [DOI] [PubMed] [Google Scholar]
- Morales WJ, Madhav H. Efficacy and safety of indomethacin compared with magnesium sulfate in the management of preterm labor: a randomized study. American Journal of Obstetrics and Gynecology 1993;169:97‐102. [DOI] [PubMed] [Google Scholar]
- Morales WJ, Madhav H. Efficacy and safety of indomethacin vs magnesium sulfate in the management of preterm labor: A randomized study. American Journal of Obstetrics and Gynecology 1991;164:280 [SPO Abstract 119]. [DOI] [PubMed] [Google Scholar]
Parilla 1997 {published data only}
- Parilla BV, Tamura RK, Cohen LS, Clark E. Lack of effect of antenatal indomethacin on fetal cerebral blood flow. American Journal of Obstetrics and Gynecology 1997;176(6):1166‐9. [DOI] [PubMed] [Google Scholar]
Parsons 1987 {published data only}
- Parsons MT, Owens CA, Spellacy WN. Thermic effects of tocolytic agents: decreased temperature with magnesium sulfate. Obstetrics and Gynecology 1987;69:88‐90. [PubMed] [Google Scholar]
Pezzati 2001 {published data only}
- Pezzati M, Giani T, Gambi B, Dani C, Bertini G, Biagiotti R, et al. Influence of maternal magnesium sulphate and ritodrine treatment on cerebral blood flow velocity of the preterm newborn. Acta Obstetricia et Gynecologica Scandinavica 2001;80:818‐23. [DOI] [PubMed] [Google Scholar]
Sayin 2010 {published data only}
- Arda S, Sayin NC, Sut N, Varol FG. The effect of tocolytic agents on maternal and fetal doppler flow patterns in women with preterm labor. Journal of Maternal‐Fetal and Neonatal Medicine 2008;21(Suppl 1):22. [Google Scholar]
- Sayin NC, Arda S, Varol FG, Sut N. The effects of ritodrine and magnesium sulfate on maternal and fetal Doppler blood flow patterns in women with preterm labor. European Journal of Obstetrics & Gynecology and Reproductive Biology 2010;152:50‐4. [DOI] [PubMed] [Google Scholar]
Schorr 1997 {published data only}
- Schorr SJ, Ascarelli MH, Rust OA, Ross EL, Calfee EF, Perry KG, et al. Ketorolac is a safe and effective drug for acute tocolysis. American Journal of Obstetrics and Gynecology 1997;176(1 Pt 2):S7 [SPO Abstract 16]. [Google Scholar]
- Schorr SJ, Ascarelli MH, Rust OA, Ross EL, Calfee EL, Perry KG, et al. Comparative study of ketorolac (toradol) and magnesium sulfate for arrest of preterm labor. Southern Medical Journal 1998;91(11):1028‐31. [DOI] [PubMed] [Google Scholar]
Sciscione 1993 {published data only}
- Sciscione A, Gorman R, Schlossman P, Colmorgen G. A randomized prospective study of intravenous magnesium sulfate, ritodrine, and subcutaneous terbutaline as treatments for preterm labor. American Journal of Obstetrics and Gynecology 1993;168:376 [SPO Abstract 281]. [Google Scholar]
Steer 1977 {published data only}
- Steer CM, Petrie RH. A comparison of magnesium sulfate and alcohol for the prevention of premature labor. American Journal of Obstetrics and Gynecology 1977;129:1‐4. [DOI] [PubMed] [Google Scholar]
Surichamorn 2001 {published data only}
- Surichamorn P. The efficacy of terbutaline and magnesium sulfate in the management of preterm labor. Journal of the Medical Association of Thailand 2001;84:98‐104. [PubMed] [Google Scholar]
Taherian 2007 {published data only}
- Taherian AA, Dehdar P. Comparison of efficacy and safety of nifedipine versus magnesium sulfate in treatment of preterm labour. Journal of Research in Medical Sciences 2007;12(3):136‐42. [Google Scholar]
Tchilinguirian 1984 {published data only}
- Tchilinguirian NG, Najem R, Sullivan GB, Craparo FJ. The use of ritodrine and magnesium sulfate in the arrest of premature labor. International Journal of Gynaecology and Obstetrics 1984;22:117‐23. [DOI] [PubMed] [Google Scholar]
Wang 2000 {published data only}
- Wang H, Zeng W, Liu H, Ou Y. A randomized controlled trial on the treatment of preterm labour with ritodrine hydrochloride and magnesium sulfate. West China University of Medical Sciences 2000;31(4):515‐7. [Google Scholar]
Wilkins 1988 {published data only}
- Wilkins IA, Lynch L, Mehalek KE, Berkowitz GS, Berkowitz RL. Efficacy and side effects of magnesium sulfate and ritodrine as tocolytic agents. American Journal of Obstetrics and Gynecology 1988;159:685‐9. [DOI] [PubMed] [Google Scholar]
Zhu 1996 {published data only}
- Zhu B, Fu Y. Treatment of preterm labor with ritodrine. Chung Hua Fu Chan Ko Tsa Chih 1996;31(12):721‐3. [PubMed] [Google Scholar]
References to studies excluded from this review
Behrad 2003 {published data only}
- Behrad V, Moossavifar N, Mojtahedzaden M, Esmalli H, Moghtadeii P. A prospective, randomized, controlled trial of high and low doses of magnesium sulfate for acute tocolysis. Acta Medica Iranica 2003;41(2):126‐31. [Google Scholar]
Di Renzo 2005 {published data only}
- Renzo GC, Mignosa M, Gerli S, Burnelli L, Luzi G, Clerici G, et al. The combined maternal administration of magnesium sulfate and aminophylline reduces intraventricular hemorrhage in very preterm neonates. American Journal of Obstetrics and Gynecology 2005;192:433‐8. [DOI] [PubMed] [Google Scholar]
Ferguson 1984 {published data only}
- Ferguson JE, Hensleigh PA, Kredenster D. Adjunctive use of magnesium sulfate with ritodrine for preterm labour. American Journal of Obstetrics and Gynecology 1984;148:166‐71. [DOI] [PubMed] [Google Scholar]
- Ferguson JE, Holbrook RH, Stevenson DK, Hensleigh PA, Kredentser D. Adjunctive magnesium sulfate infusion does not alter metabolic changes associated with ritodrine tocolysis. American Journal of Obstetrics and Gynecology 1987;156:103‐7. [DOI] [PubMed] [Google Scholar]
Hatjis 1987 {published data only}
- Hatjis CG, Swain M, Nelson LH, Meis PJ, Ernest JM. Efficacy of combined administration of magnesium sulfate and ritodrine in the treatment of premature labor. Obstetrics and Gynecology 1987;69:317‐22. [PubMed] [Google Scholar]
Herschel 2001 {published data only}
- Herschel M, Mittendorf R. Case report: Tocolytic magnesium sulfate toxicity and unexpected neonatal death. Journal of Perinatology 2001;21:261‐3. [DOI] [PubMed] [Google Scholar]
How 1998 {published data only}
- How H, Cook C, Cook V, Spinnato J. Preterm premature rupture of membranes: aggressive tocolysis versus expectant management. American Journal of Obstetrics and Gynecology 1996;174:306. [DOI] [PubMed] [Google Scholar]
- How HY, Cook CR, Cook VD, Miles DE, Spinnato JA. Preterm premature rupture of membranes: aggressive tocolysis versus expectant management. Journal of Maternal‐Fetal Medicine 1998;7:8‐12. [DOI] [PubMed] [Google Scholar]
How 2006 {published data only}
- How HY, Zafaranchi L, Stella C, Recht K, Maxwell R, Sibai B, et al. Magnesium sulfate (MGSO4) tocolysis versus no tocolysis in women with preterm labor between 32 0/7 and 34 6/7 weeks of gestation: a randomized controlled trial [abstract]. American Journal of Obstetrics and Gynecology 2005;193(6 Suppl):S6. [DOI] [PubMed] [Google Scholar]
- How HY, Zafaranchi L, Stella CL, Recht K, Maxwell RA, Sibai BM, et al. Tocolysis in women with preterm labor between 32 0/7 and 34 6/7 weeks of gestation: a randomized controlled pilot study. American Journal of Obstetrics and Gynecology 2006;194:976‐81. [DOI] [PubMed] [Google Scholar]
Ieda 1991 {published data only}
- Ieda K, Sasaki J, Mizuno S, Mimura S, Suzuki C, Miyazaki T, et al. The clinical effects of maternally administered magnesium sulfate on the neonate. Journal of Perinatal Medicine 1991;2:225S. [Google Scholar]
Kara 2009 {published data only}
- Kara M, Yilmaz E, Avci I, Öge T. Comparison of nifedipine with magnesium sulphate plus terbutaline for the treatment of preterm labor. Journal of the Turkish Society of Obstetrics and Gynecology 2009;6(4):250‐6. [Google Scholar]
Mittendorf 2000 {published data only}
- Mittendorf R, Pryde PG. An overview of the possible relationship between antenatal pharmacologic magnesium and cerebral palsy. Journal of Perinatal Medicine 2000;28:286‐93. [DOI] [PubMed] [Google Scholar]
Ogburn 1985 {published data only}
- Ogburn PL, Hansen CA, Williams PP, Butler JC, Joseph MS, Julian TM. Magnesium sulfate and B‐mimetic dual‐agent tocolysis in preterm labor after single‐agent failure. Journal of Reproductive Medicine 1985;30(8):583‐7. [PubMed] [Google Scholar]
Pryde 2001 {published data only}
- Pryde PG, Besinger RE, Gianopoulos JG, Mittendorf R. Adverse and beneficial effects of tocolytic therapy. Seminars in Perinatology 2001;25:316‐40. [DOI] [PubMed] [Google Scholar]
Scudiero 2000 {published data only}
- Scudiero R, Khoshnood B, Pryde PG, Lee KS, Mittendorf R. Perinatal death and tocolytic magnesium sulfate. Obstetrics and Gynecology 2000;96:178‐82. [DOI] [PubMed] [Google Scholar]
Soguk 2004 {published data only}
- Soguk C, Taoisiz OL, Mungan T. Low dose treatment protocol in magnesium sulfate tocolysis. International Journal of Gynecology and Obstetrics 2004;86:37‐8. [DOI] [PubMed] [Google Scholar]
Terrone 2000 {published data only}
- Terrone DA, Rinehart BK, Kimmel ES, May WL, Larmon JE, Morrison JC. A prospective, randomized, controlled trial of high and low maintenance doses of magnesium sulfate for acute tocolysis. American Journal of Obstetrics and Gynecology 2000;182(6):1477‐82. [DOI] [PubMed] [Google Scholar]
Wischnik 1989 {published data only}
- Wischnik A, Hettenbach A, Schmidt R, Zieger W, Hug G, Melchert F. The influence of magnesium sulphate on the fluid balance during tocolysis with the betamimetic agent fenoterol. Gynakologische Rundschau 1989;29(2):188‐91. [PubMed] [Google Scholar]
Zygmunt 2003 {published data only}
- Zygmunt M, Heilmann L, Berg C, Wallwiener D, Grischke E, Munstedt K, et al. Local and systemic tolerability of magnesium sulphate for tocolysis. European Journal of Obstetrics & Gynecology and Reproductive Biology 2003;107(2):168‐75. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Lotfalizadeh 2010 {published data only}
- Lotfalizadeh M, Teymoori M. Comparison of nifedipine and magnesium sulfate in the treatment of preterm. Iranian Journal of Obstetrics, Gynecology and Infertility 2010; Vol. 13, issue 2.
References to ongoing studies
Briery 2011 {published data only}
- Briery CM. Magnesium sulfate versus placebo for tocolysis in PPROM. ClinicalTrials.gov (http://clinicaltrials.gov) (accessed 8 July 2011) 2011.
Sooky 2010 {published data only}
- Sooky Z. Comparison of delaying in premature labour by indomethacin and Mg sulphate in pregnant women referred to maternity hospital. IRCT Iranian Registry of Clinical Trials (www.irct.ir) (accessed 6 December 2010) 2010.
Additional references
ACOG 2012
- American College of Obstetrics and Gynecology. Management of preterm labour: practice bulletin. Obstetrics & Gynecology 2012;119(6):1308‐16. [DOI] [PubMed] [Google Scholar]
ACOG 2013
- American College of Obstetrics and Gynecology. Magnesium sulfate use in obstetrics: committee opinion. Obstetrics & Gynecology 2013;122(3):727‐8. [DOI] [PubMed] [Google Scholar]
Besinger 1990
- Besinger R, Niebyl J. The safety and efficacy of tocolytic agents for the treatment of preterm labour. Obstetrical and Gynecological Survey 1990;45:415‐39. [DOI] [PubMed] [Google Scholar]
Boyle 2012
- Boyle E, Poulsen G, Field D, Kurinczuk J, Wolke D, Alfirevic Z, et al. Effects of gestational age at birth on health outcomes at 3 and 5 years of age: Population based cohort. BMJ 2012;344:e896. [DOI] [PMC free article] [PubMed] [Google Scholar]
Claas 2010
- Claas MJ, Bruinse HW, Heide‐Jalving M, Termote J, Vries LS. Changes in survival and neonatal morbidity in infants with a birthweight of 750g or less. Neonatology 2010;98:278‐88. [DOI] [PubMed] [Google Scholar]
Darlow 2009
- Darlow BA, Mogridge N, Harwood LJ, Wynn‐Williams MB, Austin NC. Admisson of all gestations to a regional neonatal unit versus controls: Neonatal morbidity. Journal of Paediatrics and Child Health 2009;45:181‐6. [DOI] [PubMed] [Google Scholar]
Doyle 2009
- Doyle LW, Crowther CA, Middleton P, Marret S, Rouse D. Magnesium sulphate for women at risk of preterm birth for neuroprotection of the fetus. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD004661.pub3] [DOI] [PubMed] [Google Scholar]
Duley 2010
- Duley L, Gülmezoglu AM, Henderson‐Smart DJ, Chou D. Magnesium sulphate and other anticonvulsants for women with pre‐eclampsia. Cochrane Database of Systematic Reviews 2010, Issue 11. [DOI: 10.1002/14651858.CD000025.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Greenberg 2011
- Greenberg M, Penn A, Thomas L, El‐Sayed Y, Caughey A, Lyell D. Neonatal medical admission in a term and late preterm cohort exposed to magnesium sulphate. American Journal of Obstetrics and Gynecology 2011;204(515):e1‐7. [DOI] [PubMed] [Google Scholar]
Grimes 2006
- Grimes DA, Nanda K. Magnesium sulfate tocolysis: time to quit. Obstetrics and Gynecology 2006;108(4):986‐9. [DOI] [PubMed] [Google Scholar]
Haas 2009
- Haas D, Imperiale T, Kirkpatrick P, Klein R, Zollinger T, Gowchowski A. Tocolytic therapy. A meta‐analysis and decision analysis. Obstetrics and Gynecology 2009;113:585‐94. [DOI] [PubMed] [Google Scholar]
Haas 2012
- Haas DM, Caldwell DM, Kirkparick P, McIntosh JJ, Welton NJ. Tocolytic therapy for preterm delivery: systematic review and network meta‐analysis. BMJ 2012;345:e6226. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hall 1959
- Hall D, McGaughery H Jr, Corey E, Thornton W. The effects of magnesium therapy on the duration of labour. American Journal of Obstetrics and Gynecology 1959;78:27‐32. [DOI] [PubMed] [Google Scholar]
Han 2013
- Han S, Crowther CA, Moore V. Magnesium maintenance therapy for preventing preterm birth after threatened preterm labour. Cochrane Database of Systematic Reviews 2013, Issue 5. [DOI: 10.1002/14651858.CD000940.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration 2011.
Kugelman 2012
- Kugelman A, Bader D, Lerner‐Geva L, Boyko V, Levitzki O, Riskin A, et al. Poor outcomes at discharge among extremely premature infants: a national population based study. Archives of Pediatrics & Adolescent Medicine 2012;166(6):543‐50. [DOI] [PubMed] [Google Scholar]
Lipsitz 1971
- Lipsitz PJ. The clinical and biochemical effects of excess magnesium in the newborn. Pediatrics 1971;47:501‐9. [PubMed] [Google Scholar]
March of Dimes 2012
- Howson CP, Kinney MV, Lawn JE (editors). Born Too Soon: The Global Action Report on Preterm Birth. Geneva: World Health Organization, 2012. [Google Scholar]
McDonnell 2009
- McDonnell NJ. Cardiopulmonary arrest in pregnancy: two case reports of successful outcomes in association with perimortem caesarian delivery. British Journal of Anaesthesia 2009;103(3):406‐9. [DOI] [PubMed] [Google Scholar]
McIntosh 1989
- McIntosh T, Vink R, Yamakami I, Faden A. Magnesium protects against neurological deficit after brain injury. Brain Research 1989;482:252‐60. [DOI] [PubMed] [Google Scholar]
Mildvan 1987
- Mildvan AS. Role of magnesium and other divalent cations in ATP‐utilizing enzymes. Magnesium 1987;6(1):28‐33. [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Roberts 2006
- Roberts D, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth.. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD004454.pub2] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Crowther 2002
- Crowther CA, Hiller JE, Doyle LW. Magnesium sulphate for preventing preterm birth in threatened preterm labour. Cochrane Database of Systematic Reviews 2002, Issue 4. [DOI: 10.1002/14651858.CD001060] [DOI] [PubMed] [Google Scholar]
Keirse 1995a
- Keirse M. Magnesium sulphate in preterm labour. [revised 07 April 1994]. In: Enkin MW, Keirse MJNC, Renfrew MJ, Neilson JP, Crowther C (eds.) Pregnancy and Childbirth Module. In: The Cochrane Pregnancy and Childbirth Database [database on disk and CDROM]. The Cochrane Collaboration; Issue 2, Oxford: Update Software; 1995.
Keirse 1995b
- Keirse M. Magnesium sulphate + betamimetics for tocolysis in preterm labour. [revised 07 April 1994]. In: Enkin MW, Keirse MJNC, Renfrew MJ, Neilson JP, Crowther C (eds.) Pregnancy and Childbirth Module. In: The Cochrane Pregnancy and Childbirth Database [database on disk and CDROM]. The Cochrane Collaboration; Issue 2, Oxford: Update Software; 1995.
Keirse 1995c
- Keirse M. Magnesium sulphate vs betamimetics for tocolysis in preterm labour. [revised 07 April 1994]. In: Enkin MW, Keirse MJNC, Renfrew MJ, Neilson JP, Crowther C (eds.) Pregnancy and Childbirth Module. In: The Cochrane Pregnancy and Childbirth Database [database on disk and CDROM]. The Cochrane Collaboration; Issue 2, Oxford: Update Software; 1995.
Keirse 1995d
- Keirse M. Magnesium sulphate vs ethanol for tocolysis in preterm labour. [revised 07 April 1994]. In: Enkin MW, Keirse MJNC, Renfrew MJ, Neilson JP, Crowther C (eds.) Pregnancy and Childbirth Module. In: The Cochrane Pregnancy and Childbirth Database [database on disk and CDROM]. The Cochrane Collaboration; Issue 2, Oxford: Update Software; 1995.


