Abstract
We report the case of a 4-month-old boy diagnosed with DiGeorge syndrome with novel ocular features. The patient was diagnosed through genetic testing, with a noted 22q11.2 deletion, and had the additional clinical findings of cardiac anomalies, Hirschsprung’s disease, and intracranial microhemorrhages. Eye findings included bilateral microphthalmia, persistent fetal vasculature, chorioretinal coloboma, and a unilateral orbital cyst. Given no known additional inciting exposures, a dysgenic mechanism resulting in failed closure of developmental fissures associated with the chromosomal deletion likely gave rise to these combined pathologies.
Case Report
A 4-month-old ex-term boy presented to the Pediatric Neuro-Ophthalmology clinic at the Byers Eye Institute, Stanford University, for evaluation of a left orbital mass with unidentifiable intraconal optic nerve on initial magnetic resonance imaging (MRI). Past medical history was significant for DiGeorge syndrome (22q11.2 deletion), confirmed through genetic testing soon after birth, with developmental abnormalities of large ventricular septal defect, interrupted aortic arch, Hirschsprung’s disease, and noted scattered intracranial hemorrhages on imaging at birth. There was no family history of ocular abnormalities.
On examination, the patient’s right eye could fix and follow light, with continuous pendular horizontal nystagmus and a reactive pupil. No light response was noted in the left eye. External examination showed bilateral microphthalmia. The left eye was noted to have restricted extraocular movements and was obscured by an inferior orbital mass protruding into the lower eyelid, causing mechanical ectropion and eyelid imbrication (Figure 1). Ophthalmic examination of the right eye revealed microcornea, partial aniridia, and a slightly subluxed lens; the left eye was noted to have microcornea. The posterior segment of the right eye was notable for a large chorioretinal coloboma involving the optic nerve and macula. The posterior segment of the left eye revealed complete chorioretinal coloboma, with visible retrolental persistent fetal vasculature (PFV) stalk. Although the PFV was not easily visualized on dilated examination of the right eye, imaging revealed bilateral PFV stalks.1 MRI also showed a 31 mm × 20 mm × 15 mm left intraconal cyst extending from the optic nerve head and causing superior displacement of the left globe (Figure 2), consistent with a congenital orbital cyst.
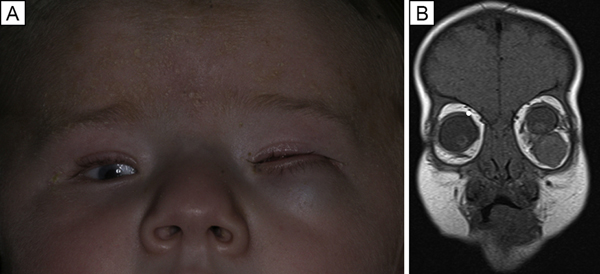
FIG 1.
A, External photograph at 4 months of age, notable for microphthalmia of both eyes; the left eye was immobile with an orbital mass displacing the globe superiorly. B, Coronal T1-weighted magnetic resonance image showing left superior globe displacement and microphthalmia.
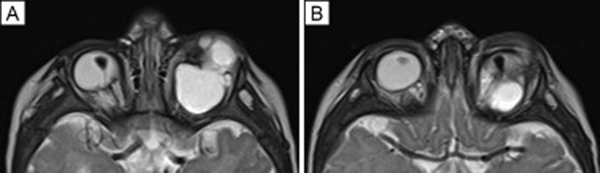
FIG 2.
A-B, Axial T2-weighted MRI images demonstrating bilateral persistent fetal vasculature as triangular-shaped retrolenticular tissue with low T2 signal against the normal high T2 signal of the globe. In addition, there is left greater than right microphthalmia, left chorioretinal coloboma and orbital intraconal cyst with superior globe displacement, shown by these vertically displaced cuts through both orbits.
The patient was initially managed conservatively with close observation. There was no indication for surgical removal of the cyst, given the patient’s age, limited visual potential of the left eye, and the desire to preserve orbital expansion with growth. The patient was referred to low-vision services. Two years later, he developed recurrent conjunctivitis from mechanical ectropion and eyelid imbrication caused by the enlarging orbital cyst. He subsequently underwent cyst excision.
Discussion
DiGeorge syndrome is caused by a partial deletion of the long arm of chromosome 22 (deletion 22q11.2), resulting in a myriad of phenotypic expressions with multiorgan dysgenesis. The majority of patients experience de novo mutations in this chromosomal segment, resulting most commonly in cardiac, craniofacial, thymic, and parathyroid abnormalities, though renal, endocrine, immunologic, and psychiatric complications are also known to arise.2
Ocular manifestations are common in DiGeorge syndrome but tend to be less severe.3,4 With respect to the orbit, cohort studies have demonstrated that eyelid abnormalities (ie, hooding, ptosis, distichiasis) are the most common manifestation of velocardiofacial syndrome.5,6 In the anterior segment, posterior embryotoxon, sclerocornea, Peters anomaly, iris remnants, cataracts, uveitis, and colobomas have all been reported.5–7 Tortuous retinal vessels are the most commonly associated posterior segment abnormalities, and a recent fluorescence in situ hybridization study identified a microdeletion of 22q11.2 as a possible additional genetic locus for familial exudative vitreoretinopathy.5 Microphthalmia and anterior segment dysgenesis have similarly been described in a few patients with DiGeorge syndrome and these findings are hypothesized to be associated with systemic developmental growth deficiency.8 However, PFV has not previously been described in DiGeorge syndrome.
To our knowledge, this is the first reported case of microphthalmia and PFV with orbital cyst in a patient with DiGeorge syndrome. Microphthalmia with orbital cyst is a rare colobomatous manifestation of failed closure of the orbital fissure. Colobomatous orbital cysts are usually unilateral and can occur as isolated malformations or in association with systemic findings or syndromes with a broad etiologic classification system that includes genetic, prenatally acquired, and associations (ie, coloboma, heart defects, choanal atresia, mental retardation, genitourinary abnormalities, ear abnormalities [CHARGE] syndrome).9 Our patient did have cardiac and renal findings, but thorough genetic evaluation did not reveal any choanae or ear abnormalities or a systemic CHARGE diagnosis. Phenotypic manifestations also fall along a spectrum from a relatively anophthalmic socket to large orbital cysts prolapsing through the palpebral fissure. Management depends on both age of the patient and size of the cyst. In a series of 34 patients treated for orbital cysts associated with anophthalmos or microphthalmos, none carried a diagnosis of DiGeorge syndrome, although 7 (20.6%) had some form of cardiac or craniofacial abnormality.10 Although colobomatous microphthalmia can be related to embryonic axis formation errors that can be a part of DiGeorge syndrome, it is also possible that this patient’s findings are due to an independent single gene defect. Our patient did undergo genetic and clinical evaluation to rule out additional findings often associated with alternative microphthalmia-associated mutations (ie, septo-optic dysplasia, pituitary hormone deficiencies, spinal abnormalities, or nonsyndromic holoproencephaly); however, explicit genetic testing for these mutations was not recommended by the genetics team, because the family was not planning to have additional children.
In the case of our patient, there was no indication for immediate surgery, because the current standard of care is observation of orbital cysts to allow the orbit to reach approximate adult volume. A compromise between concerns for cosmesis in early school-aged children and orbital expansion supports orbital cyst removal at the age of 5 years, because orbital growth at this stage is about 90% that of an adult.11 In cases of significant anophthalmos without a sizeable cyst, socket expansion is achieved with the use of conformers and implants.12 Exceedingly large cysts can cause asymmetric orbital expansion or lid complications, as was the case with our patient. Cyst debulking, excision, or even early enucleation and conformer placement is considered appropriate in these situations.
The ocular findings in DiGeorge syndrome, including eyelid abnormalities, posterior embryotoxon, Peters anomaly, and colobomas, are largely due to early developmental dysgenesis. Microphthalmia with orbital cysts, a presentation on the spectrum of colobomatous eye disorders, should be noted as a possible manifestation of DiGeorge syndrome–related ocular dysgenesis. Although DiGeorge syndrome is normally associated with mild ocular complications, more extreme forms of ocular and orbital malformation are possible.
Literature Search
The Ovid database was searched for English-language articles from 1970 to 2021 using the following search terms; DiGeorge syndrome, congenital microphthalmia, congenital anophthalmia, orbital cyst, persistent fetal vasculature, and coloboma.
References
- 1.Koontz NA, Seltman TA, Kralik SF, Mosier KM, Harnsberger HR. Classic signs in head and neck imaging. Clin Radiol 2016;71:1211–22. [DOI] [PubMed] [Google Scholar]
- 2.Swillen A, Vogels A, Devriendt K, Fryns JP. Chromosome 22q11 deletion syndrome: update and review of the clinical features, cognitive-behavioral spectrum, and psychiatric complications. Am J Med Genet 2000;97:128–35. [DOI] [PubMed] [Google Scholar]
- 3.Campbell IM, Sheppard SE, Crowley TB, et al. What is new with 22q? An update from the 22q and You Center at the Children’s Hospital of Philadelphia. Am J Med Genet A 2018;176:2058–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gokturk B, Topcu-Yilmaz P, Bozkurt B, et al. Ocular findings in children with 22q11.2 Deletion syndrome. J Pediatr Ophthalmol Strabismus 2016;53:218–22. [DOI] [PubMed] [Google Scholar]
- 5.Casteels I, Casaer P, Gewillig M, Swillen A, Devriendt K. Ocular findings in children with a microdeletion in chromosome 22q11.2. Eur J Pediatr 2008;167:751–5. [DOI] [PubMed] [Google Scholar]
- 6.Forbes BJ, Binenbaum G, Edmond JC, DeLarato N, McDonald-McGinn DM, Zackai EH. Ocular findings in the chromosome 22q11.2 deletion syndrome. J AAPOS 2007;11:179–82. [DOI] [PubMed] [Google Scholar]
- 7.Gottlieb C, Li Z, Uzel G, Nussenblatt RB, Sen HN. Uveitis in DiGeorge syndrome: a case of autoimmune ocular inflammation in a patient with deletion 22q11.2. Ophthalmic Genet 2010;31: 24–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tarlan B, Kiratli H, Kılıç E, Utine E, Boduroğlu K. A case of 22q11.2 deletion syndrome with right microphthalmia and left corneal staphyloma. Ophthalmic Genet 2014;35:248–51. [DOI] [PubMed] [Google Scholar]
- 9.Shields JA, Shields CL. Orbital cysts of childhood—classification, clinical features, and management. Surv Ophthalmol 2004;49: 281–99. [DOI] [PubMed] [Google Scholar]
- 10.McLean CJ, Ragge NK, Jones RB, Collin JRO. The management of orbital cysts associated with congenital microphthalmos and anophthalmos. Br J Ophthalmol 2003;87:860–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bentley RP, Sgouros S, Natarajan K, Dover MS, Hockley AD. Normal changes in orbital volume during childhood. J Neurosurg 2002;96:742–6. [DOI] [PubMed] [Google Scholar]
- 12.Helmuth Gundlach KK, Guthoff RF, Martin Hingst VH, Schittkowski MP, Bier UC. Expansion of the socket and orbit for congenital clinical anophthalmia. Plast Reconstr Surg 2005;116:1214–22. [DOI] [PubMed] [Google Scholar]