Abstract
Apoptosis signal-regulating kinase (ASK) 1 is activated in response to various cytotoxic stresses including TNF, Fas and reactive oxygen species (ROS) such as H2O2, and activates c-Jun NH2-terminal kinase (JNK) and p38. However, the roles of JNK and p38 signaling pathways during apoptosis have been controversial. Here we show that by deleting ASK1 in mice, TNF- and H2O2-induced sustained activations of JNK and p38 are lost in ASK1–/– embryonic fibroblasts, and that ASK1–/– cells are resistant to TNF- and H2O2-induced apoptosis. TNF- but not Fas-induced apoptosis requires ROS-dependent activation of ASK1–JNK/p38 pathways. Thus, ASK1 is selectively required for TNF- and oxidative stress-induced sustained activations of JNK/p38 and apoptosis.
INTRODUCTION
Apoptosis plays an essential role in normal development and tissue homeostasis, and when dysregulated it contributes to various diseases including cancer, autoimmunity and neurodegenerative disorders (Thompson, 1995; Los et al., 1999; Yuan and Yankner, 2000). Apoptosis signal-regulating kinase (ASK) 1 is a mitogen-activated protein kinase kinase kinase (MAPKKK) family member (Ichijo et al., 1997) and is activated in response to H2O2, TNF and Fas through distinct mechanisms and relays those signals to stress-activated MAP kinases such as JNK and p38 (Ichijo et al., 1997; Chang et al., 1998; Nishitoh et al., 1998; Saitoh et al., 1998; Liu et al., 2000). Overexpression of wild-type or activated allele of ASK1 activates JNK and p38 and induces apoptosis in various cells through mitochondria-dependent caspase activation (Ichijo et al., 1997; Saitoh et al., 1998; Hatai et al., 2000). Eleven different mammalian MAPKKKs have been identified to date as upstream activators of the JNK and/or p38 pathway (Ichijo, 1999; Davis, 2000). However, the role of the JNK/p38 signaling pathways during apoptosis, as assayed by expression of dominant-negative proteins in vitro (Xia et al., 1995; Verheij et al., 1996) or by targeting of some of those genes (Nishina et al., 1997; Yang et al., 1997; Swat et al., 1998; Tournier et al., 2000), has been controversial. Here we show by deleting ASK1 in mice that TNF- and H2O2-induced activations of JNK and p38, especially sustained activations, are dramatically suppressed in ASK1–/– embryonic fibroblasts (MEFs), and that ASK1–/– cells are less sensitive than ASK1+/+ cells to death-inducing activities resulting from these types of stresses. Fas-induced apoptosis was intact, however, indicating that a clear difference exists between TNF and Fas in ASK1-dependency in cell killing. Furthermore, we show that TNF- but not Fas-induced apoptosis requires reactive oxygen species (ROS)-dependent activation of ASK1–JNK/p38 pathways.
RESULTS AND DISCUSSION
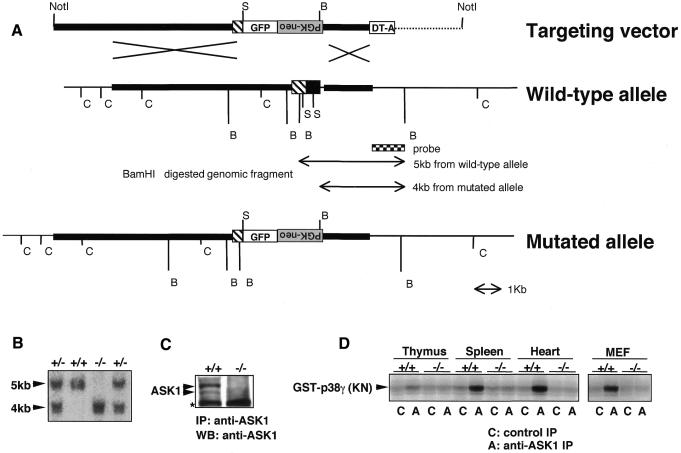
To determine the specific stimuli that require ASK1 for activation of specific MAPK pathways and to assess the roles played by ASK1–JNK/p38 pathways during apoptosis, we disrupted the ASK1 gene in murine 129/SvJ embryonic stem (ES) cells using a targeting vector in which the first coding exon was deleted (Figure 1A). Chimeric mice derived from two independent heterozygous ES clones were back-crossed to C57BL/6J mice, and homozygous mutant mice were obtained by F1 heterozygous intercrosses. Null mutation of ASK1 was confirmed by Southern blot of tail DNA (Figure 1B), western blot of MEFs lysate (Figure 1C) and immune complex kinase assay of various tissue samples (Figure 1D). ASK1–/– mice were born at the expected Mendelian frequency and were indistinguishable in appearance from wild-type littermates. Histological analysis of ASK1–/– mice detected no developmental abnormalities (data not shown).
Fig. 1. Generation of ASK1–/– mice by gene targeting. (A) Restriction map around the first coding exon of ASK1 gene and structure of the targeting vector. Homologous regions for recombination are drawn by thick lines in the restriction map. Coding and non-coding regions of ASK1 gene are represented by filled and hatched boxes, respectively. The genomic fragment used as a probe for Southern blot analysis for precise determination of recombination in ES cells and genotypes of mice is indicated below the map. The expected mutant allele and polymorphic fragments for genotyping from BamHI-digested genomic DNA are also indicated. GFP, coding sequence for green fluorescence protein followed by bovine growth hormone poly(A) signal; PGK-neo, coding sequence for neomycin phosphotransferase flanked by the phosphoglycerate kinase promoter and poly(A) signal; DT-A, diphtheria toxin A gene driven by MC1 promoter; C, SacI; S, SmaI; B, BamHI. Inverted characters for ‘PGK-neo’ indicate an insertion of the PGK-neo cassette in a reverse orientation. (B) Southern blot determining homologous recombination within the ASK1 locus of genomic DNA extracted from tails of F2 mice. BamHI digestion generated hybridized bands of the expected sizes for wild-type (5 kb) and mutant (4 kb). (C) Western blot demonstrating the absence of ASK1 protein in ASK1–/– MEFs. MEFs were immunoprecipitated with anti-ASK1 antiserum (DAV) and subsequently subjected to immunoblotting with anti-ASK1. Asterisk indicates non-specific reactions. (D) Immune complex kinase assay confirming the absence of ASK1 kinase activity in ASK1–/– mice. Extracts from indicated tissues and cells were immunoprecipitated with anti-ASK1 or control serum. Immune complex was incubated with GST–MKK6, and a kinase activity was then measured with the substrate GST–p38 γ(KN) which resulted in a phosphorylated image of GST–p38 γ (KN).
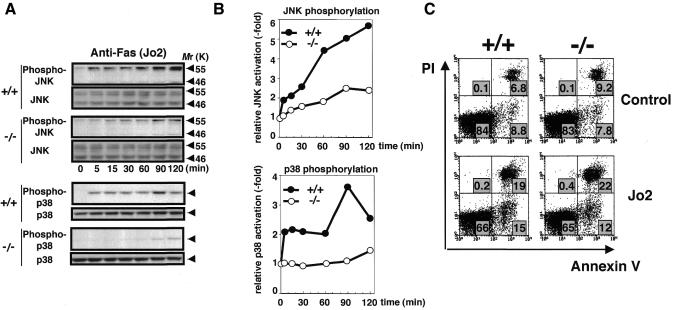
Death receptor-signaling represented by Fas and TNF receptors is known to share many common mechanisms (Gross et al., 1999; Nagata, 1999; Wallach et al., 1999), in that engagement of Fas or TNF receptors by their cognate ligands leads to the formation of a protein complex known as death-inducing signaling complex (DISC) and to the activation of caspase 8, which cleaves death effector substrates such as caspase 3 and Bid. Upon Fas and TNF receptor activation, alternate pathways involving, respectively, the Fas-binding protein Daxx and TNF receptor-associated factor 2 (TRAF2) are also activated, culminating in the activation of JNK/p38 through binding and activating ASK1 (Chang et al., 1998; Nishitoh et al., 1998). Consistent with our previous finding that ASK1 mediates Fas-induced JNK activation (Chang et al., 1998), Fas-induced JNK and p38 activities were decreased in ASK1–/– thymocytes (Figure 2A and B). However, Fas activation yielded comparable apoptotic changes in ASK1+/+ and ASK1–/– thymocytes (Figure 2C) and MEFs (Figure 4M), as determined by various assays including PI/Annexin V staining (Figure 2C), TUNEL (Figure 4M), DNA ladder and 3′-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay (data not shown). These findings indicate that ASK1–JNK/p38 pathways are not required for Fas-induced apoptosis, at least in these primary cells.
Fig. 2. Lack of JNK and p38 activation but not apoptotic alteration in ASK1–/– cells in response to anti-Fas (Jo2) treatment. (A) JNK and p38 activation in thymocytes. Cells were isolated from 6-week-old mice, preincubated in serum-free RPMI medium for 4 h and treated with 5 µg/ml Jo2 for the indicated period. Cell lysates were subjected to western blot analysis with antibodies specifically recognizing the activated forms of JNK (phospho-JNK) or p38 (phospho-p38). Membranes were reprobed with antibodies to total JNK or p38 as loading controls. (B) Graphic representation of (A). Fold activation was calculated by dividing the intensity of phosphorylated p55 JNK or p38 at different time points by that at time zero. Essentially similar results were obtained in three independent experiments. (C) Fluorescence analysis of apoptosis of Jo2-treated thymocytes from ASK1+/+ (left panels) or ASK1–/– mice (right panels). Cells were isolated from 6-week-old mice, suspended in serum-free RPMI medium, and cultured in the presence (lower panels) or absence (upper panels) of 5 µg/ml Jo2 for 8 h. Cells were analyzed by FACS after staining with Annexin V and propidium iodide (PI). Percentages of positive cells within a quadrant are indicated. Data are from one of more than five representative experiments.
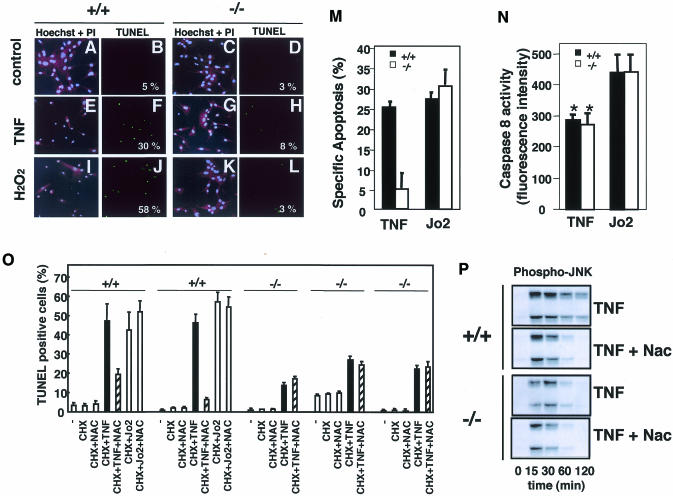
Fig. 4. Lack of TNF- and H2O2-induced apoptosis in ASK1–/– MEFs and differential requirement of ROS-ASK1 pathway in TNF- and Fas-induced apoptosis. Cell morphology (A, C, E, G, I, K) was analyzed by fluorescence double-staining with Hoechst 33258 (blue) and PI (red) of MEFs that were untreated (A and C), treated with 100 ng/ml of TNF for 8 h (E and G) or with 0.2 mM H2O2 plus 25 mM aminotriazole (catalase inhibitor) for 2h (I and K). TUNEL staining (green; B, D, F, H, J, L) of the same fields is shown with numbers which represent percentages of TUNEL-positive cells per total cell count. At least 100 cells from three random fields were counted in each experiment, and data are from one of more than three representative experiments. (M) Quantitative analysis of apoptosis of TNF- or Jo2-treated MEFs from ASK1+/+ or ASK1–/– mice. ASK1+/+ MEFs (black columns) and ASK1–/– MEFs (white columns) were treated with 100 ng/ml TNF plus 1 µg/ml cycloheximide (CHX), 5 µg/ml Jo2 plus 1 µg/ml CHX or 1 µg/ml CHX alone for 16 h. Apoptotic cells were scored by TUNEL staining as described above. Specific apoptosis was calculated as the percentage of TUNEL-positive cells in each experimental condition minus the percentage of TUNEL-positive cells with treatment with CHX alone. Data are from one of three representative experiments. (N) Quantitative analysis of caspase 8 activity in TNF- or Jo2-treated MEFs. ASK1+/+ MEFs (black columns) and ASK1–/– MEFs (white columns) were treated with 100 ng/ml TNF plus 1 µg/ml CHX or 5 µg/ml Jo2 plus 1 µg/ml CHX for 6 h. Caspase 8 activity was measured by a Caspase-8/FLICE Fluorometric Protease Assay Kit (MBL) using fluorogenic synthetic peptide IETD-7-amino-4-trifluoromethyl coumarin (AFC) as a substrate. The fluorescence intensity of the released AFC as caspase 8 activity was measured with an exitation wavelength of 360 nm and emission wavelength of 530 nm. Values are means (± SE) of four independent experiments. *, P <0.05 compared with Jo2-treated cells. (O) ASK1+/+ and ASK1–/– MEFs were treated with indicated combinations of CHX (1 µg/ml), Nac (1 mM), TNF (100 ng/ml) and Jo2 (5 µg/ml) for 24 h. TUNEL staining was performed, and the percentage of TUNEL-positive cells per total cell count in each experimental condition were calculated. Values are means (± SD) of three random fields (at least 100 cells per field counted). Results of MEFs prepared from independent embryos are shown. (P) Inhibition of TNF-induced sustained activation of JNK by Nac. ASK1+/+ and ASK1–/– MEFs were treated with 50 ng/ml of TNF in the presence or absence of Nac (1 mM), and cell lysates were subjected to western blot analysis with phospho-JNK antibody.
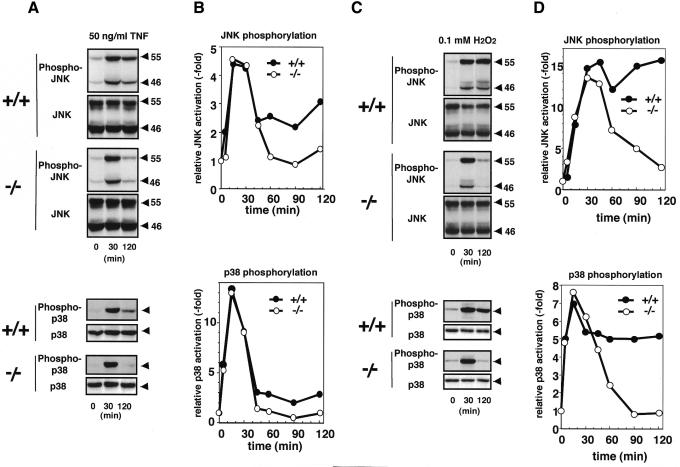
TNF- or H2O2-induced activations of JNK and p38 were indistinguishable between ASK1+/+ and ASK1–/– MEFs within 30 min after treatment (Figure 3). In contrast, however, dramatic differences were observed after 45 min; TNF- and H2O2-induced sustained activations of JNK and p38 were preserved only in ASK1+/+ but not in ASK1–/– MEFs (Figure 3). This is consistent with our previous observation that increased activity of ASK1 was maintained until 120 min after TNF addition (Ichijo et al., 1997). Early, i.e. within 30 min, activations of JNK/p38 by TNF and H2O2 in ASK1–/– MEFs appear to be compensated for by the other MAPKKKs, e.g. MEKK1 (Xia et al., 2000; Yujiri et al., 2000). Transient and persistent activations of ERK MAPK are known to lead to different cell fates (Marshall, 1995), in that early and transient activation of ERK causes proliferation of PC12 cells, whereas prolonged activation induces neuronal differentiation. Early/transient and late/sustained activations of JNK/p38 induced by TNF (Guo et al., 1998; Roulston et al., 1998), UV-C (Chen et al., 1996), γ-radiation (Chen et al., 1996) or growth factor withdrawal (Xia et al., 1995) have been implicated in cell survival and apoptosis, respectively. Different durations of specific MAPKs activation have thus been suggested as a general theme to control cell fate (Xia et al., 1995); however, genetic evidence has not been provided because of the difficulty in inactivating certain transiently or persistently activated MAPKs. In this regard, it was of interest to determine whether ASK1–/– cells have different sensitivity to TNF- and H2O2-induced apoptosis. Clear differences in apoptotic features were observed by cell morphology and TUNEL staining (Figure 4A–L); the population of cells undergoing typical apoptosis was much higher for ASK1+/+ than for ASK1–/– MEFs. Cell viability determined by MTT after treatment with TNF, H2O2 or other oxidative stressors such as diamide and tertiary-butyl hydroperoxidase was also higher in ASK1–/– than in ASK1+/+ MEFs (data not shown). These results indicate that ASK1 is required for TNF- and H2O2-induced prolonged activations of JNK and p38 as well as apoptosis.
Fig. 3. Lack of TNF- and H2O2-induced sustained activations of JNK and p38 in ASK1–/– MEFs. MEFs were treated with 50 ng/ml of TNF (A and B) or with 0.1 mM of H2O2 (C and D), and JNK and p38 activation was evaluated as described in Figure 2.
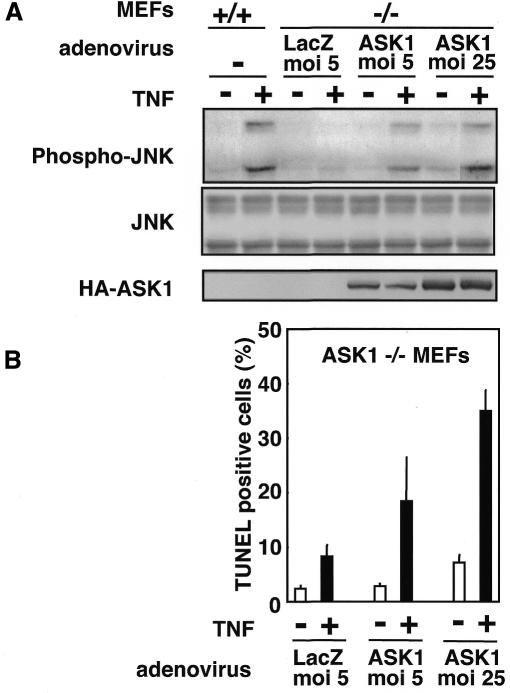
Next, we investigated why TNF but not Fas requires ASK1 to induce apoptosis. No difference was observed between ASK1+/+ and ASK1–/– MEFs in the expression levels of DISC proteins including caspase-8, Fas, FADD, TNFR1, TRADD and RIP as determined by RNAse protection analysis (data not shown). Because of the structural similarity between Fas and TNF receptors and the absolute requirement of FADD (Yeh et al., 1998) and caspase 8 (Varfolomeev et al., 1998) for induction of apoptosis by these death receptors, it is tempting to suggest that Fas and TNF kill cells by the same mechanism. However, several differences exist in the manner of cell death induced by Fas or TNF receptors, which cannot be explained if apoptotic cell fate is determined solely by the FADD-caspase 8 axis. For example, although Fas-induced apoptosis can occur in low oxygen and does not appear to require the generation of ROS (Jacobson and Raff, 1995), TNF-induced apoptosis can be inhibited by antioxidants such as N-acetyl-l-cysteine (Nac) (Schuize-Osthoff et al., 1994; Saitoh et al., 1998). In fact, TNF- but not Fas-induced apoptosis was partially inhibited by Nac in ASK1+/+ MEFs (Figure 4O). Moreover, caspase 8 activity induced by TNF was significantly lower than that induced by Fas (Figure 4N), although the same treatment resulted in a similar magnitude of apoptosis in ASK1+/+ MEFs (Figure 4M; black columns). These differences between TNF and Fas suggest that in parallel with FADD-caspase 8 axis, TNF activates and requires an antioxidant-sensitive, i.e. oxidative stress (or ROS)-dependent, signaling pathway to execute apoptosis. The ASK1–JNK/p38 pathway may thus be such a strong candidate due to the following reasons: (i) ASK1 is selectively required for TNF- but not Fas-induced apoptosis in the same cells (Figure 4M); (ii) TNF and TRAF2 can stimulate the production of ROS within cells (Liu et al., 2000), and TNF- but not Fas-induced activation of ASK1 requires ROS-dependent dissociation of an ASK1 inhibitor, thioredoxin (Saitoh et al., 1998; data not shown); (iii) activation of caspase 8 by TNF was not imparied in ASK1–/– MEFs (Figure 4N), indicating that the ASK1–JNK/p38 pathway is independent of the FADD-caspase 8 pathway; (iv) TNF-induced apoptosis in ASK1+/+ MEFs was inhibited by Nac to the similar extent of apoptosis observed in TNF-treated ASK1–/– MEFs (Figure 4O: compare hatched columns of +/+ with black columns of –/–); (v) TNF-induced apoptosis (Figure 4O: compare hatched columns and black columns of –/–) and sustained activation of JNK (Figure 4P) in ASK1–/– MEFs were no more sensitive to Nac; and (vi) Nac effectively inhibited only the sustained phases of TNF-induced JNK/p38 activations in ASK1+/+ MEFs (Figure 4P; data not shown), which followed the same time course of those in ASK1–/– MEFs. Finally, we examined whether re-introduction of ASK1 to ASK1–/– cells restores sensitivity to TNF (Figure 5). Expression of wild-type ASK1 in ASK1–/– MEFs using an adenovirus vector (Saitoh et al., 1998) restored not only TNF-induced JNK/p38 activations at a sustained phase (Figure 5A and data not shown) but also TNF-induced apoptosis in a dose-dependent manner of ASK1 (Figure 5B). Taken together, these results indicate that the ROS-dependent ASK1–JNK/p38 pathway and the ROS-independent FADD-caspase 8 pathway are both required for TNF-induced apoptosis.
Fig. 5. Restoration of TNF sensitivity in ASK1-re-introduced ASK1–/– MEFs. (A) Restoration of TNF-induced sustained activation of JNK. ASK1–/– MEFs were infected with adenoviruses encoding β-galactosidase (LacZ) or HA-tagged wild-type ASK1 (Saitoh et al. 1998) for 24 h. Cells were treated with TNF (50 ng/ml for 120 min), and cell lysates were subjected to westen blot analysis with phospho-JNK antibody. Essentially the same relsults were obtained for H2O2-treatment. Appropriate expression of exogenous ASK1 is shown by anti-HA blotting. (B) Restoration of TNF-induced apoptosis. ASK1–/– MEFs were infected as described above, treated with TNF (50 ng/ml in the presence of 1 µg/ml CHX) for 6 h, and the percentage of TUNEL-positive cells was calculated as described in Figure 4O. Values are means (± SD) of three random fields (at least 100 cells per field counted).
Thus, we have shown that ASK1 selectively mediates TNF- and oxidative stress-induced sustained activation of JNK/p38 and apoptosis. However, it is still unclear whether sustained activations of JNK and/or p38 are essential for ASK1-dependent apoptosis. How sustained activation of JNK/p38 can be molecularly converted to a pro-apoptotic signal will be an important issue to be addressed. We have also demonstrated for the first time that Fas and TNF differentially utilize JNK/p38 signaling pathways for regulation of apoptosis. With sufficient activation of the FADD-caspase 8 pathway by Fas, an alternate pathway may not be required to kill cells. Weaker activation of the FADD-caspase 8 pathway by TNF may be compensated for by simultaneous activation of the ROS-dependent ASK1–JNK/p38 death pathway.
METHODS
Generation of ASK1–/– mice. Several independent genomic fragments including the first coding exon of ASK1 gene were cloned from a 129/SvJ mouse genomic library (Stratagene). A 10 kb NotI (cloning site from phage vector)–SmaI fragment and a 2 kb HindIII–XbaI fragment were used as homologous regions for recombination. The first coding exon and a part of the intronic sequence of the ASK1 gene were replaced by green fluorescence protein gene and reverse-oriented neomycin-phosphotransferase cassette. pBluescript SK (Stratagene) was used as a backbone to construct the targeting vector with DT-A cassette for negative selection. The targeting construct linearized by NotI digestion was electroporated into J1 ES cells. G418-resistant clones of ES cells with intended recombination were screened by Southern blot analysis. Heterozygous mutant ES cells were injected into C57BL/6J blastocysts. Germline transmission of mutated alleles to F1 mice obtained by intercross of resultant male chimeras and female C57BL/6J was confirmed by Southern blot analysis. Homozygous mutant mice were obtained by F1 heterozygous intercrosses. Northern blot and RT–PCR analysis of mRNA derived from homozygous mutant mice revealed faint expression of a truncated mutant transcript (data not shown). Deficiency of ASK1 polypeptide was confirmed by western blot analysis and in vitro immune complex kinase assay.
MEFs cell culture. MEFs were prepared from day 12.5 embryos and cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum and 100 units/ml penicillin G in a 5% CO2 atmosphere at 37°C. All experiments with MEFs were performed with cells between passage 2 and 5 and repeated at least three times with three or four independent embryo-derived cultures for each genotype.
Immune complex kinase assay. The immune complex kinase assay has been described (Nishitoh et al., 1998). Briefly, cells were lysed in lysis buffer containing 20 mM Tris-HCl pH 7.5, 12 mM β-glycerophosphate, 150 mM NaCl, 5 mM EGTA, 10 mM NaF, 1% Triton X-100, 1% deoxycholate, 1 mM DTT, 1 mM sodium orthovanadate, 1 mM PMSF, and 1.5% aprotinin. Cell extracts were clarified by centrifugation, and the supernatants were immunoprecipitated with antiserum to ASK1 (DAV; Saitoh et al., 1998) using protein A–Sepharose (Zymed). The beads were washed twice with a buffer containing 150 mM NaCl, 20 mM Tris-HCl pH 7.5, 5 mM EGTA and 1 mM DTT, and subjected to kinase assays. A 0.2 µg portion of GST–MKK6 was first incubated with immune complexes for 10 min at 30°C in a final volume of 10 µl of a solution containing 20 mM Tris-HCl, pH 7.5, 20 mM MgCl2, and 100 µM ATP. Thereafter, activated complexes were incubated with 0.3 µCi of [γ-32P] ATP and 1 µg of GST–p38 γ (kinase negative mutant; KN) in the same solution (final volume 20 µl) for 10 min at room temperature. Kinase reactions were stopped by adding SDS sample buffer and analyzed by SDS–PAGE and a Fuji BAS2000 image analyzer.
Western blot analysis. Cells were lysed on ice in a buffer containing 50 mM Tris-HCl pH 8.0, 150 mM NaCl, 1% Nonidet P-40, 0.5% deoxycholate, 0.1% SDS, 1 mM PMSF and 150 units/ml aprotinin. After centrifugation, cell extracts were resolved on SDS–PAGE and electroblotted onto polyvinylidine difluoride membranes. After blocking with 5% skim milk in TBS-T (50 mM Tris-HCl pH 8.0, 150 mM NaCl and 0.05% Tween 20), the membranes were probed with antibodies to phospho-JNK or phospho-p38 (New England Biolabs) and reprobed with total-JNK and total-p38 (Santa Cruz), respectively. Blots were developed with ECL (Amersham). Phosphorylation of JNK (p55) and p38 was quantitated by Quantity OneR (PDI), and results are presented in fold activation compared with untreated cells. Plotting of p46 JNK (data not shown) yielded curves essentially the same as that for p55, and thus only p55 values are presented as graphs throughout this study.
TUNEL assay. MEFs were plated onto coverslips and cultured overnight. Cells were preincubated in serum-free medium for 2 h and then treated with various stress stimuli. Cells were fixed in 4% paraformaldehyde for 30 min at room temperature and permeabilized in 0.1% Triton X-100 for 2 min at 4°C. Cells were then stained with fluorescein isothiocyanate (FITC)-conjugated terminal deoxynucleotidyl TUNEL reaction mixture (Roche) for 1 h at room temperature and incubated with Hoechst 33258 (Sigma; 1 µg/ml) and propidium iodide (Sigma; 10 µg/ml) for 2 min. All dilutions were performed in PBS. Coverslips were mounted in Mowiol (Wako), and samples were analyzed and recorded by an OLYMPUS FLUOVIEW microscope.
Acknowledgments
ACKNOWLEDGEMENTS
We thank Y. Takahama and A. Kakizuka for valuable discussions. We also thank all the members of Cell Signaling Laboratory for their critical comments. This work was supported in part by Grants-in-Aid for scientific Research from the Ministry of Education, Science and Culture in Japan, CREST of Japan Science and Technology.
REFERENCES
- Chang H., Nishitoh, H., Yang, X., Ichijo, H. and Baltimore D. (1998) Activation of apoptosis signal-regulating kinase 1 (ASK1) by the death adaptor Daxx. Science, 281, 1860–1863. [DOI] [PubMed] [Google Scholar]
- Chen Y.-R., Wang, X., Templeton, D., Davis, R. and Tan, T.-H. (1996) The role of c-Jun N-terminal kinase (JNK) in apoptosis induced by ultraviolet C and γ-radiation. J. Biol. Chem., 50, 31929–31936. [DOI] [PubMed] [Google Scholar]
- Davis R.J. (2000) Signal transduction by the JNK group of MAP kinases. Cell, 103, 239–252. [DOI] [PubMed] [Google Scholar]
- Gross, A, McDonnell, J.M. and Korsmeyer, S.J. (1999) BCL-2 family members and the mitochondria in apoptosis. Genes Dev., 13, 1899–1911. [DOI] [PubMed] [Google Scholar]
- Guo Y.-L., Baysal, K., Kang, B., Yang, L.-J. and Williamson, J.R. (1998) Correlation between sustained c-Jun N-terminal protein kinase activation and apoptosis induced by tumor necrosis factor-α in rat mesangial cells. J. Biol. Chem., 273, 4027–4034. [DOI] [PubMed] [Google Scholar]
- Hatai T. et al. (2000) Execution of ASK1-induced apoptosis by the mitochondria-dependent caspase activation. J. Biol. Chem., 275, 26576–26581. [DOI] [PubMed] [Google Scholar]
- Ichijo H. (1999) From receptors to stress-activated MAP kinases. Oncogene, 18, 6084–6093. [DOI] [PubMed] [Google Scholar]
- Ichijo H. et al. (1997) Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science, 275, 90–94. [DOI] [PubMed] [Google Scholar]
- Jacobson M.D. and Raff, M. (1995) Programmed cell death and Bcl-2 protection in very low oxygen. Nature, 374, 814–816. [DOI] [PubMed] [Google Scholar]
- Liu H., Nishitoh, H., Ichijo, H. and Kyriakis, J.M. (2000) Activation of apoptosis signal-regulating kinase 1 (ASK1) by TNF receptor-associated factor-2 (TRAF2) requires prior dissociation of the ASK1 inhibitor thioredoxin. Mol. Cell. Biol., 20, 2198–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Los M., Wesselborg, S. and Schuize-Osthoff, K. (1999) The role of caspases in development, immunity, and apoptotic signal transduction: lessons from knockout mice. Immunity, 10, 629–639. [DOI] [PubMed] [Google Scholar]
- Marshall C.J. (1995) Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell, 80, 179–185. [DOI] [PubMed] [Google Scholar]
- Nagata S. (1999) Fas ligand-induced apoptosis. Annu. Rev. Genet., 33, 29–55. [DOI] [PubMed] [Google Scholar]
- Nishina H. et al. (1997) Stress-signalling kinase Sek1 protects thymocytes from apoptosis mediated by CD95 and CD3. Nature, 385, 350–353. [DOI] [PubMed] [Google Scholar]
- Nishitoh H., Saitoh, M., Mochida, Y., Takeda, K., Nakano, H., Rothe, M., Miyazono, K. and Ichijo, H. (1998) ASK1 is essential for JNK/SAPK activation by TRAF2. Mol. Cell, 2, 389–395. [DOI] [PubMed] [Google Scholar]
- Roulston A., Reinhard, C., Aimiri, P. and Williams, L.T. (1998) Early activation of c-Jun N-terminal kinase and p38 kinase regulate cell survival in response to tumor necrosis factor α. J. Biol. Chem., 273, 10232–10239. [DOI] [PubMed] [Google Scholar]
- Saitoh M., Nishitoh, H., Fujii, M., Takeda, K., Tobiume, K., Sawada, Y., Kawabata, M., Miyazono K. and Ichijo, H. (1998) Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J., 17, 2596–2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuize-Osthoff K., Krammer, P.H. and Dröge, W. (1994) Divergent signalling via APO-1/Fas and the TNF receptor, two homologous molecules involved in physiological cell death. EMBO J., 13, 4587–4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swat W. et al. (1998) SEK1/MKK4 is required for maintenance of a normal peripheral lymphoid compartment but not for lymphocyte development. Immunity, 8, 625–634. [DOI] [PubMed] [Google Scholar]
- Thompson C.B. (1995) Apoptosis in the pathogenesis and treatment of disease. Science, 267, 1456–1462. [DOI] [PubMed] [Google Scholar]
- Tournier C. et al. (2000) Requirement of JNK for stress-induced activation of the cytochrome c-mediated death pathway. Science, 288, 870–874. [DOI] [PubMed] [Google Scholar]
- Varfolomeev E.E. et al. (1998) Targeted disruption of the mouse Caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity, 9, 267–276. [DOI] [PubMed] [Google Scholar]
- Verheij M. et al. (1996) Requirement for ceramide-initiated SAPK/JNK signalling in stress-induced apoptosis. Nature, 380, 75–79. [DOI] [PubMed] [Google Scholar]
- Wallach D., Varfolomeev, E.E., Malinin, N.L., Goltsev, Y.V., Kovalenko, A.V. and Boldin, M.P. (1999) Tumor necrosis factor receptor and Fas signaling mechanisms. Annu. Rev. Immunol., 17, 331–367. [DOI] [PubMed] [Google Scholar]
- Xia Y., Makris, C., Su, B., Li, E., Yang, J., Nemerow, G.R. and Karin, M. (2000) MEK kinase 1 is critically required for c-jun N-terminal kinase activation by proinflammatory stimuli and growth factor-induced cell migration. Proc. Natl Acad. Sci. USA, 97, 5243–5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Z., Dickens, M., Raingeaud, J., Davis, R.J. and Greenberg, M.E. (1995) Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science, 270, 1326–1331. [DOI] [PubMed] [Google Scholar]
- Yang D.D. Kuan, C.Y., Whitmarsh, A.J., Rincon, M., Zheng, T.S., Davis, R.J., Rakic, P. and Flavell, R.A. (1997) Absence of excitotoxicity-induced apoptosis in the hippocampus of mice lacking the JNK3 gene. Nature, 389, 865–870. [DOI] [PubMed] [Google Scholar]
- Yeh W.-C. et al. (1998) FADD: essential for embryo development and signaling from some, but not all, inducers of apoptosis. Science, 279, 1954–1958. [DOI] [PubMed] [Google Scholar]
- Yuan J. and Yankner B.A. (2000) Apoptosis in the nervous system. Nature, 407, 802–809. [DOI] [PubMed] [Google Scholar]
- Yujiri T., Sather, S., Fanger, G.R. and Johnson, G.L. (2000) Role of MEKK1 in cell survival and activation of JNK and ERK pathways defined by targeted gene disruption. Science, 282, 1911–1914. [DOI] [PubMed] [Google Scholar]