Abstract
Background
Surgical treatment for renal cell carcinoma (RCC) and inferior vena cava (IVC) tumor thrombus (TT) is difficult, and the postoperative complication rate is high. This study aimed to explore the safety and oncologic outcomes of neoadjuvant stereotactic ablative body radiotherapy (SABR) combined with surgical treatment for RCC and IVC-TT.
Methods
Patients with RCC and IVC-TTs were enrolled in this study. All patients received neoadjuvant SABR focused on the IVC at a dose of 30 Gy in 5 fractions, followed by 2 ~ 4 weeks of rest. Then, radical nephrectomy and IVC tumor thrombectomy were performed for each patient. Adverse effects, perioperative outcomes, and long-term prognoses were recorded.
Results
From June 2018 to January 2019, 8 patients were enrolled—4 with Mayo grade II TT and 4 with Mayo grade III TT. Four (50%) patients had complicated IVC wall invasion according to CT/MRI. All patients received neoadjuvant SABR as planned. Short-term local control was observed in all 8 patients. Only Grade 1–2 adverse events were reported. In total, 3 (37.5%) laparoscopic surgeries and 5 (62.5%) open surgeries were performed. The median operation time was 359 (IQR: 279–446) min, with a median intraoperative bleeding volume of 750 (IQR: 275–2175) ml. The median postoperative hospital stay was 7 (5–10) days. With a 26-month (range: 5–41) follow-up period, the estimated mean overall survival was 30.67 ± 5.38 months.
Conclusions
This is the first preoperative radiotherapy study in Asia that focused on patients with TT. This study revealed the considerable safety of neoadjuvant SABR for RCC with IVC-TT.
Trial registration
This study was registered in the Chinese Clinical Trials Registry on 2018-03-08 (ChiCTR1800015118). For more information, please see the direct link (https://www.chictr.org.cn/showproj.html?proj=25747).
Supplementary Information
The online version contains supplementary material available at 10.1186/s12894-024-01405-y.
Keywords: Renal cell carcinoma, Radiotherapy, Neoadjuvant therapy, Venous tumor thrombus
Background
Renal cell carcinoma (RCC) is a malignant tumor of the urinary system that accounts for 3–5% of all adult cancers [1]. RCC is characterized by macrovascular invasion, which results in inferior vena cava (IVC) tumor thrombus (TT) in 4-10% of locally advanced RCC patients and is associated with progressive clinical symptoms and poor prognosis [2–4]. Surgical treatment, namely, radical nephrectomy and IVC tumor thrombectomy, is the standard treatment for RCC with IVC-TT. However, compared to RCC without IVC-TT, for RCC with IVC-TT, the surgical difficulty and postoperative complication rate is significantly increased, especially for complex cases, including those with high Mayo-level TT or TT with IVC wall invasion [5]. The latter is also associated with a greater possibility of IVC segmental resection and a higher risk of IVC-TT recurrence [4].
To decrease the surgical difficulty mentioned above, many attempts have been made to reduce IVC-TT complexity before surgical treatment, including through neoadjuvant target therapy and immunological therapy [6–8]. Recently, the resurgence of radiotherapy for the preoperative treatment of RCC has also been highlighted with the development of newer technologies. Stereotactic ablative body radiotherapy (SABR), which allows for high-dose delivery to focal lesions, was reported to have a high local control rate and favorable safety profile for primary tumors and RCC metastasis in clinical trials [9].
Therefore, we propose that SABR can be used as a neoadjuvant therapy for RCC with IVC-TT to potentially reduce surgical complexity by downgrading the IVC-TT and reversing IVC wall invasion. To date, few studies have reported the use of neoadjuvant SABR for IVC-TT treatment in RCC patients, and its safety and effectiveness have not been well demonstrated [10]. Here, we report the results from a prospective cohort of RCC patients with IVC-TTs who received neoadjuvant SABR and underwent surgical treatment to provide insights into the safety of neoadjuvant SABR for renal tumor thrombus and its effectiveness in decreasing surgical difficulty.
Methods
Patients
RCC patients diagnosed with Mayo grade II ~ IV IVC-TTs according to imaging examinations who were eligible for radical nephrectomy and IVC tumor thrombectomy were enrolled, as described in registered and published protocols (ChiCTR1800015118) [11]. The trial protocol and informed consent form were approved by the Peking University Biomedical Ethics Committee. Patients who could not tolerate the treatment or who had a previous history of systemic therapy or radiotherapy in the area of RCC or IVC-TT were excluded. This study was reported according to the CONSORT guidelines.
The overall preoperative evaluation included demographic information, clinical symptoms, physical examination data, and CT/MRI data. The IVC-TT stage and IVC wall invasion were re-evaluated after 2–4 weeks of rest following SABR treatment. Then, each patient underwent radical nephrectomy and IVC tumor thrombectomy, which were performed by two experienced urologic surgeons. Perioperative parameters, including the operative time, surgical bleeding, and postoperative complication rate, were recorded to assess surgical difficulty.
The primary endpoint of this study was safety, which was assessed by the occurrence of postoperative complications and SABR-related adverse events (AEs) 4–6 weeks after the radiotherapy course. The Clavien‒Dindo classification system was used to assess the severity of postoperative complications. SABR-related AEs were evaluated using the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. Severe toxicity was defined as Grade III-IV toxicity.
The follow-up time was defined as the time from the date of surgery to the latest documented date of telephone or clinical follow-up.
Treatment plan
SABR
Only the IVC-TT was selected for the SABR treatment target. Target sections were delineated before SABR on the unenhanced CT phase. The IVC-TT was delineated according to the gross tumor volume (GTV), and the stomach, duodenum, jejunum, ileum, colon, spinal cord, liver, and esophagus were considered the organs at risk. The planning target volume (PTV) was generated by adding a 3-mm margin around the GTV. Patients were asked to lie on their backs with their hands at their sides to receive SABR. A total dose of 30 Gy in 5 fractions was delivered over 1 week. Further details are described in the published protocol [11].
Surgery
Open surgery and laparoscopic surgery were performed in this study, and the retroperitoneal approach is preferred for laparoscopy at our center. The patient position and trocar placement were described in previous studies [12, 13]. For right RCC with Mayo grade II IVC-TT, the right renal artery and right ureter were severed, and the distal end of the IVC, the left renal vein, and the proximal end of the IVC were dissected and blocked with vessel tourniquets successively. For right RCC with Mayo grade III IVC-TT, the hepatic ligament was severed to dissect the liver from the diaphragm, thus exposing and blocking the IVC under the diaphragm. The first hepatic portal was also dissected and blocked before IVC resection. For left RCC, the surgical process was similar to that for right RCC. However, the transperitoneal approach was applied for long and thick IVC-TTs, and the distal end of the IVC, right renal vein, and proximal end of the IVC were blocked. The right renal artery was also blocked if necessary. For patients with IVC wall invasion, IVC wall resection or segmental resection was used.
Statistical analysis
The Shapiro‒Wilk (S‒W) test was used to determine the normality of continuous variables. Continuous variables with a normal distribution are presented as the mean ± standard deviation (SD); otherwise, they are presented as medians and interquartile ranges (IQRs). The Kaplan‒Meier method was used to calculate the overall survival rate.
Results
From June 2018 to January 2019, a total of 8 RCC patients with Mayo grade II ~ III IVC-TTs were recruited for the study. The baseline characteristics are depicted in Table 1. Of the patients, 6 (75%) were male, and 2 (25%) were female. According to ECOG performance status, 2 (25%) patients received a score of 0, and 6 (75%) received a score of 1. Six patients had right-sided primary tumors, and 2 had left-sided tumors. Seven (87.5%) patients had grade cT1 tumors. One (12.5%) patient had a grade cM1 tumor. For the IVC-TT, 4 (50%) were Mayo grade II, and 4 (50%) were Mayo grade III. The average tumor thrombus length was 7.5 ± 3.6 cm. Four (50%) patients had complicated IVC wall invasion.
Table 1.
Baseline characteristics of the patients who received neoadjuvant SABR
| Variables | Case No. | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| Gender | Male | Female | Male | Male | Male | Female | Male | Male |
| Age | 60 | 61 | 63 | 50 | 46 | 60 | 76 | 34 |
| BMI | 21.5 | 20.6 | 21.2 | 19.4 | 25.3 | 25.4 | 20.5 | 25.9 |
| ECOG performance status | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 |
| ASA classification | I | III | III | II | III | II | II | II |
| Tumor side | Right | Left | Right | Right | Right | Left | Right | Right |
| Tumor diameter/cm | 10.4 | 5.6 | 7.5 | 8.3 | 12 | 5.6 | 14.2 | 12.2 |
| Clinical T stage | 3b | 3b | 4 | 4 | 3c | 4 | 4 | 3b |
| Clinical N stage | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 |
| Clinical M stage | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Mayo grade | II | III | III | II | III | II | III | II |
| Tumor thrombus length/cm | 4.6 | 5.5 | 8.9 | 3.5 | 14.4 | 5.7 | 10.1 | 6.5 |
| Vascular wall invasion | No | No | No | Yes | Yes | Yes | Yes | No |
BMI, body mass index; ECOG, Eastern Cooperative Oncology Group; ASA, American Society of Anesthesiologists
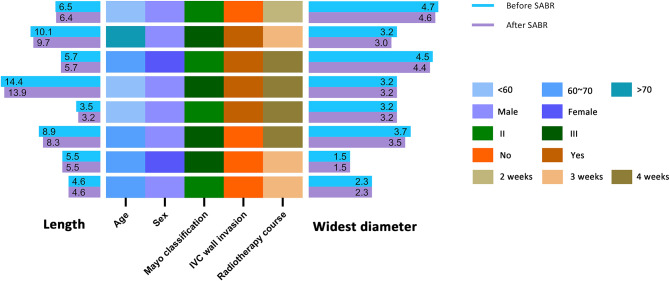
Figure 1 shows the clinical features of the IVC-TTs before and after SABR treatment. Three (37.5%) patients received a 4-week course of radiotherapy, 4 (50%) patients received a 3-week course, and 1 (12.5%) patient received a 2-week course. The average tumor thrombus length after SABR was 7.2 ± 3.4 cm, which slightly reduced compared with baseline, and no new occurrence of IVC wall invasion was observed after SABR, therefore short-term local control was observed for all 8 patients. MRI showed decreased enhancement of IVC-TTs after SABR, which is consistent with the findings of previous case reports, probably because of local edema and vascular reduction in the IVC-TT (Fig. 2).
Fig. 1.
The clinical features of patients with IVC-TTs before and after neoadjuvant SABR. Left histogram, the length of IVC-TTs before and after SABR; heatmap, clinical profiles; right histogram, the widest diameter of IVC-TTs before and after SABR
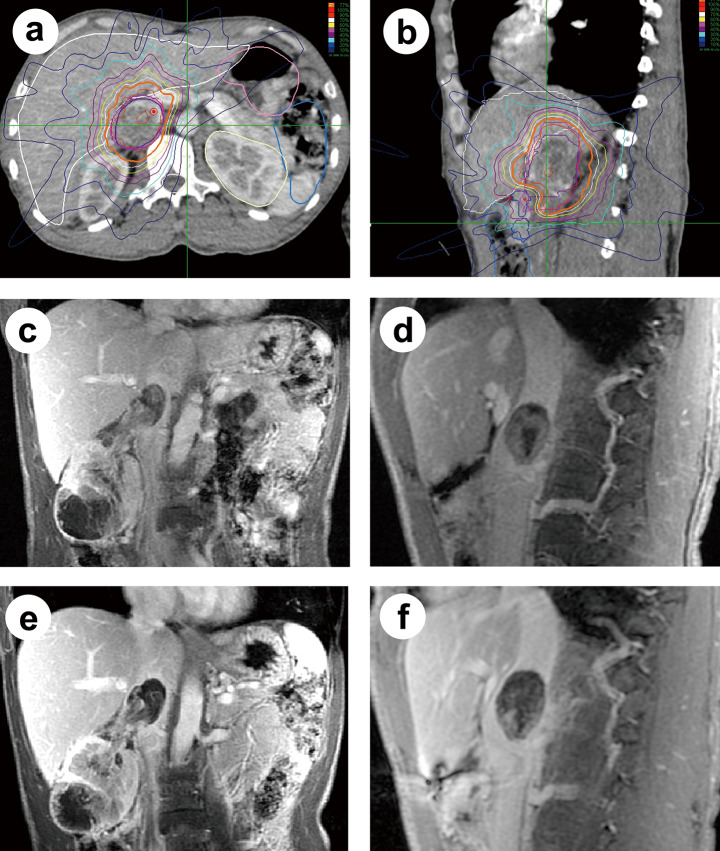
Fig. 2.
CT images showing the SABR treatment plan (a, b) and comparisons of IVC-TTs before (c, d) and after SABR (e, f). (a, b) The PTV of SABR on the cross-section and coronal planes. (c, d) Coronal views of the primary RCC tumor and Mayo grade II IVC-TT from a single patient before SABR. (e, f) CT images of the same primary tumor and IVC-TT after SABR, compared to those in c and d, respectively
The perioperative results and SABR-related AEs are reported in Additional file 1. The operation was successful in all patients. Three patients (37.5%) underwent laparoscopic surgery, and 5 (62.5%) underwent open surgery. The median operation time was 359 (IQR: 279–446) min, with a median intraoperative bleeding volume of 750 (IQR: 275–2175) ml. The median postoperative hospital stay was 7 [5–10] days. No severe intraoperative adhesion or fragile tissue were observed in all patients. According to the Clavien‒Dindo classification system, level I postoperative complications included 1 case of hypokalemia, and level II complications included 3 cases of anemia and 1 case of lymphatic fistula. No severe postoperative complications (defined as Clavien‒Dindo classification ≥ III) were reported. Four to six weeks after the SABR treatment, no patient had anorexia, diarrhea, enterocolitis, gastritis, dermatitis radiation, bone marrow hypocellular, or hepatic failure. Only grade 1–2 AEs were reported.
The median follow-up time was 26 (range: 5–41) months, and the estimated mean overall survival was 30.67 ± 5.38 months. The Kaplan‒Meier curve was plotted to depict the overall survival curve, as shown in Fig. 3.
Fig. 3.
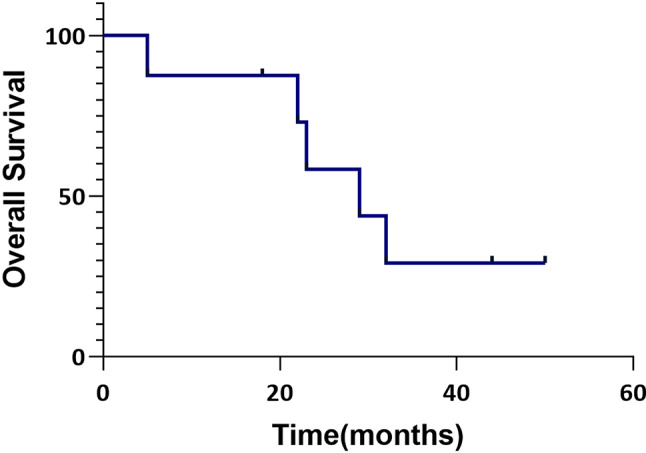
Kaplan‒Meier plot showing the overall survival of patients who received neoadjuvant SABR and underwent radical surgical treatment
Discussion
To the best of our knowledge, this is the first preoperative radiotherapy study in Asia that focused on TTs. This study aimed to determine the safety of neoadjuvant SABR for RCC IVC-TTs in terms of surgical complexity and adverse effects. 75% of the enrolled patients had Mayo grade III IVC-TTs or IVC wall invasion. Nevertheless, the surgical duration, intraoperative bleeding volume, and postoperative hospital stay were in line with our expectations when compared with previously reported experiences from our center [14]. In addition, there was no occurrence of severe postoperative complications or AEs; therefore, these patients met the primary endpoint of the study. The estimated mean survival also complied with the results presented previously for patients with IVC wall invasion.
To date, few studies have reported the application of SABR for RCC with IVC-TT, and most of the published studies are case reports and studies with a small sample size [10, 15–18]. The major studies are presented in Additional file 2. Three studies used SABR as an alternative therapy for surgery, and only one study focused on the safety of SABR as neoadjuvant therapy. Hannan et al. and Freifeld et al. were the earliest reporters to use SABRs for IVC-TT and metastasis treatment, with no occurrence of severe AEs [17, 18]. Additionally, one patient showed an excellent oncologic prognosis of 54 months. A larger cohort was reported by Freifeld et al. in 2022, in which a radiographic rate of 58% was reached and a significant symptom palliation effect was observed [16]. Recently, Castelnau-Marchand et al. reported a patient with IVC-TT and distant metastasis [15]. The patient received SABR and systemic therapy and achieved complete remission at the last follow-up of 54 months. Margulis et al. enrolled 6 patients who received neoadjuvant SABR (40 Gy in 5 fractions) and underwent radical nephrectomy and IVC thrombectomy after 4–14 days of rest [10]. No grade 4 or 5 AEs occurred, and all patients were alive after a median follow-up of 24 months. Exploratory analysis revealed decreased Ki-67 and increased PD-L1 expression in the IVC-TT, indicating activation of the immune system after SABR.
In comparison, our study enrolled more complex patients with higher IVC-TT grades, and half of them had IVC wall invasion. The results from these patients supported the conclusion of Margulis et al., indicating the safety of neoadjuvant SABR [10]. However, the radiographic response rate was not adequately reported in previous neoadjuvant studies and thus remains unclear. According to our results, no significant downgrading of the IVC-TT or reversal of IVC wall invasion was observed 2–4 weeks after SABR, but the radiographic results showed a decrease in enhancement, consistent with the observations of Freifeld et al. and Hannan et al., which probably resulted from local edema and vascular reduction in the IVC-TT [17, 18].
Few studies reported the adverse effect of neoadjuvant SABR on surgical difficulty. Although radiotherapy is associated with addition intraoperative adhesions and fragile tissues, no severe intraoperative adhesion or fragile tissue were observed in all patients. We suppose the absence of these adverse event resulted in the limited dosage and short SABR treatment interval, and the potential effect may require revaluation in higher dosage.
In theory, it is not easy to observe prominent changes in IVC-TT volume within a few weeks, especially during safety lead-in studies. Typically, several months are needed to observe a remarkable radiographic response, and a continual decrease in tumor volume can be observed within 1–2 years due to immune activation [19, 20]. In previous studies, although short-term changes were rarely reported, apparent reductions in IVC-TT size or even complete remission were recorded after several months, which indicated the potential effectiveness of SABR [15–18].
In addition, the growth rate of IVC-TTs is significantly faster than that of primary tumors, and even several-week intervals can lead to apparent progression [6, 21]. Froehner et al. reported a case of RCC with IVC-TT that progressed to Mayo grade III from Mayo grade I in one month [22]. In our study, although the IVC-TT volume did not decrease significantly within a few weeks, it remained stable or was slightly reduced. Therefore, we believe that these results can reveal the potential curative effect of SABR, as we used a relatively low dose of 30 Gy in 5 fractions. In the future, we will conduct a dose-escalation study to verify the effectiveness of neoadjuvant SABR for RCC patients with IVC-TTs.
Our study has several limitations. First, this was a single-arm cohort with a small sample size and some heterogeneity. Second, because of the patients’ strong demands for earlier surgery, the rest time following SABR was reduced to 2–4 weeks after providing informed consent, which is shorter than the planned period reported in the protocol. Thus, we recorded only the radiographic changes over a short-term interval. Finally, we enrolled only patients with Mayo grade II-III IVC-TTs, and further study is needed for Mayo grade IV IVC-TTs.
Conclusions
This is the first preoperative radiotherapy study in Asia that focused on TT. Neoadjuvant SABR for RCC with IVC-TT has considerable safety and excellent oncologic outcomes. A further dose-escalation study, which we will be conducting, is needed to verify the potential effectiveness of this treatment.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1: Perioperative outcomes and AEs of patients who received radical nephrectomy and IVC thrombectomy after SABR
Supplementary Material 2: Published studies that reported SABR for RCC with IVC-TT
Acknowledgements
The authors acknowledge the staff of the Department of Radiation Oncology and Department of Radiology of Peking University Third Hospital and the patients who participated in this study.
Abbreviations
- SABR
stereotactic ablative body radiotherapy
- RCC
renal cell carcinoma
- IVC
inferior vena cava
- TT
tumor thrombus
- AE
adverse effect
- CTCAE
Common Terminology Criteria for Adverse Events
- GTV
gross tumor volume
- PTV
planning target volume
- BMI
body mass index
- ECOG
Eastern Cooperative Oncology Group
- ASA
American Society of Anesthesiologists
- OS
overall survival
Author contributions
All the authors have read and approved the final manuscript. Conception and design: LM, HW, JW, JC, ZL, RP, YL, XP; Administrative support: LM, HW, SZ, JW; Acquisition of data: YL, RP, HZ, GW, XT; Data analysis and interpretation: YL, RP, XP, JC. Manuscript writing: All authors.
Funding
This study was supported by the National Natural Science Foundation of China (No: 81972381).
Data availability
All data are presented in the article and Supplementary Materials.
Declarations
Ethics approval and consent to participate
This study was performed in accordance with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of the Peking University Biomedical Ethics Committee. Informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jiyuan Chen, Zhuo Liu and Ran Peng contributed equally to this work.
Contributor Information
Shudong Zhang, Email: zhangshudong@bjmu.edu.cn.
Hao Wang, Email: hhbysy@126.com.
Lulin Ma, Email: malulinpku@163.com.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1). [DOI] [PubMed]
- 2.Brookman-May S, May M, Shariat SF, Xylinas E, Stief C, Zigeuner R, et al. Features associated with recurrence beyond 5 years after nephrectomy and nephron-sparing surgery for renal cell carcinoma: development and internal validation of a risk model (PRELANE score) to predict late recurrence based on a large multicenter database (CORONA/SATURN Project) Eur Urol. 2013;64(3):472–7. doi: 10.1016/j.eururo.2012.06.030. [DOI] [PubMed] [Google Scholar]
- 3.Brookman-May SD, May M, Shariat SF, Novara G, Zigeuner R, Cindolo L, et al. Time to recurrence is a significant predictor of cancer-specific survival after recurrence in patients with recurrent renal cell carcinoma–results from a comprehensive multi-centre database (CORONA/SATURN-Project) BJU Int. 2013;112(7):909–16. doi: 10.1111/bju.12246. [DOI] [PubMed] [Google Scholar]
- 4.Martinez-Salamanca JI, Huang WC, Millan I, Bertini R, Bianco FJ, Carballido JA, et al. Prognostic impact of the 2009 UICC/AJCC TNM staging system for renal cell carcinoma with venous extension. Eur Urol. 2011;59(1):120–7. doi: 10.1016/j.eururo.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Zhang X, Li YS, Xiao B, Li T, Zhang P, Chen YH, et al. Pure laparoscopic radical nephrectomy and inferior vena caval tumor thrombus removal in patients with complicated renal tumor. Chin Med J-Peking. 2019;132(19):2384–5. doi: 10.1097/CM9.0000000000000436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cost NG, Delacroix SE, Jr, Sleeper JP, Smith PJ, Youssef RF, Chapin BF, et al. The impact of targeted molecular therapies on the level of renal cell carcinoma vena caval tumor thrombus. Eur Urol. 2011;59(6):912–8. doi: 10.1016/j.eururo.2011.02.032. [DOI] [PubMed] [Google Scholar]
- 7.Labbate C, Hatogai K, Werntz R, Stadler WM, Steinberg GD, Eggener S, Sweis RF. Complete response of renal cell carcinoma vena cava tumor thrombus to neoadjuvant immunotherapy. J Immunother Cancer. 2019;7(1):66. doi: 10.1186/s40425-019-0546-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bigot P, Fardoun T, Bernhard JC, Xylinas E, Berger J, Rouprêt M, et al. Neoadjuvant targeted molecular therapies in patients undergoing nephrectomy and inferior vena cava thrombectomy: is it useful? World J Urol. 2014;32(1):109–14. doi: 10.1007/s00345-013-1088-1. [DOI] [PubMed] [Google Scholar]
- 9.Ali M, Mooi J, Lawrentschuk N, McKay RR, Hannan R, Lo SS, et al. The role of stereotactic ablative body Radiotherapy in Renal Cell Carcinoma. Eur Urol. 2022;82(6):613–22. doi: 10.1016/j.eururo.2022.06.017. [DOI] [PubMed] [Google Scholar]
- 10.Margulis V, Freifeld Y, Pop LM, Manna S, Kapur P, Pedrosa I, et al. Neoadjuvant SABR for Renal Cell Carcinoma Inferior Vena Cava Tumor Thrombus-safety lead-in results of a phase 2 trial. Int J Radiat Oncol Biol Phys. 2021;110(4):1135–42. doi: 10.1016/j.ijrobp.2021.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Y, Liu Z, Peng R, Xiao R, Wang J, Wang H, Ma L. Preoperative stereotactic body radiotherapy combined with surgical treatment for renal cell carcinoma and inferior vena cava tumour thrombus: study protocol for a single-arm cohort trial. BMJ Open. 2022;12(1):e055364. doi: 10.1136/bmjopen-2021-055364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tian X, Hong P, Liu Z, Huang Y, Wang G, Hou X, et al. En bloc retroperitoneal laparoscopic radical nephrectomy with inferior vena cava thrombectomy for renal cell carcinoma with level 0 to II venous tumor thrombus: a single-center experience. Cancer. 2020;126(S9):2073–8. doi: 10.1002/cncr.32747. [DOI] [PubMed] [Google Scholar]
- 13.Liu Z, Zhao X, Ge L, Wu B, Tang S, Hong P, et al. Completely laparoscopic versus open radical nephrectomy and infrahepatic tumor thrombectomy: comparison of surgical complexity and prognosis. Asian J Surg. 2021;44(4):641–8. doi: 10.1016/j.asjsur.2020.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Liu Z, Zhang Q, Zhao X, Zhu G, Tang S, Hong P, et al. Inferior vena cava interruption in renal cell carcinoma with tumor thrombus: surgical strategy and perioperative results. BMC Surg. 2021;21(1):402. doi: 10.1186/s12893-021-01400-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castelnau-Marchand P, Scher N, Bollet M, Chargari C, Toledano A. Stereotactic ablative radiotherapy for unresectable inferior vena cava tumor thrombus in a patient with renal cell carcinoma: a case report. Strahlenther Onkol. 2023;199(4):420–4. doi: 10.1007/s00066-023-02054-0. [DOI] [PubMed] [Google Scholar]
- 16.Freifeld Y, Pedrosa I, McLaughlin M, Correa RM, Louie AV, Maldonado JA et al. Stereotactic ablative radiation therapy for renal cell carcinoma with inferior vena cava tumor thrombus. Urol Oncol. 2022;40(4). [DOI] [PMC free article] [PubMed]
- 17.Freifeld Y, Margulis V, Woldu SL, Timmerman R, Brugarolas J, Hannan R. Stereotactic body Radiation Therapy for Renal Cell Carcinoma with Inferior Vena Cava Thrombus - initial experience Report and Literature Review. Kidney Cancer. 2019;3(1):71–7. doi: 10.3233/KCA-180044. [DOI] [Google Scholar]
- 18.Hannan R, Margulis V, Chun SG, Cannon N, Kim DWN, Abdulrahman RE, et al. Stereotactic radiation therapy of renal cancer inferior vena cava tumor thrombus. Cancer Biol Ther. 2015;16(5):657–61. doi: 10.1080/15384047.2015.1026506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song CW, Glatstein E, Marks LB, Emami B, Grimm J, Sperduto PW, et al. Biological principles of Stereotactic Body Radiation Therapy (SBRT) and Stereotactic Radiation surgery (SRS): Indirect Cell Death. Int J Radiat Oncol Biol Phys. 2021;110(1):21–34. [DOI] [PubMed]
- 20.Li M, Cai H, Deng R, Cheng J, Shi Y. Effects of exosomes on tumor immunomodulation and their potential clinical applications (review). Int J Oncol. 2022;61(6). [DOI] [PubMed]
- 21.Bex A, Van der Veldt AAM, Blank C, Meijerink MR, Boven E, Haanen JBAG. Progression of a caval vein thrombus in two patients with primary renal cell carcinoma on pretreatment with sunitinib. Acta Oncol. 2010;49(4):520–3. doi: 10.3109/02841860903521111. [DOI] [PubMed] [Google Scholar]
- 22.Froehner M, Heberling U, Zastrow S, Toma M, Wirth MP. Growth of a level III Vena Cava Tumor Thrombus within 1 Month. Urology. 2016;90:e1–e2. doi: 10.1016/j.urology.2015.12.043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1: Perioperative outcomes and AEs of patients who received radical nephrectomy and IVC thrombectomy after SABR
Supplementary Material 2: Published studies that reported SABR for RCC with IVC-TT
Data Availability Statement
All data are presented in the article and Supplementary Materials.