Abstract
Surfaces of higher eukaryotes are normally covered with microorganisms but are usually not infected by them. Innate immunity and the expression of gene-encoded antimicrobial peptides play important roles in the first line of defence in higher animals. The immune response in Drosophila promotes systemic expression of antimicrobial peptides in response to microbial infection. We now demonstrate that the epidermal cells underlying the cuticle of larvae respond to infected wounds by local expression of the genes for the antimicrobial peptide cecropin A. Thus, the Drosophila epidermis plays an active role in the innate defence against microorganisms. The immune deficiency (imd) gene was found to be a crucial component of the signal-induced epidermal expression in both embryos and larvae. In contrast, melanization, which is part of the wound healing process, is not dependent on the imd gene, indicating that the signalling pathways promoting melanization and antimicrobial peptide gene expression can be uncoupled.
INTRODUCTION
Surfaces of higher eukaryotes are naturally covered with microorganisms but are usually not infected by them. In mammals, recent discoveries have highlighted the importance of antimicrobial peptides produced by barrier epithelia (Schröder, 1999). Innate immunity and the expression of gene-encoded antimicrobial peptides have shown to play an important role as a first line of defence in higher animals, including mice and humans. Insects have been proven to be very useful models for the study of innate immunity as they are notably resistant to microorganisms and can be subjected to biochemical and genetic analyses (Boman et al., 1972; Hultmark, 1993). Recently, the molecular basis of pathogen recognition, signal transduction and effector mechanisms have been found to share considerable similarities between insects and mammals, suggesting the existence of an evolutionary relationship in the immune defence (Medzhitov and Janeway, 1998; Engström, 1999; Hoffmann et al., 1999).
Insects have successfully inhabited the terrestrial part of the biosphere and often lead their lives in habitats infested with microorganisms. When infected, insects mount a potent humoral immune response by the activation of proteolytic cascades resulting in blood clotting, melanin formation and production of an array of antimicrobial peptides with broad and overlapping specificity (reviewed in Bulet et al., 1999). Experimental infection with bacteria, or injections of microbial substances such as lipopolysaccharide (LPS), lead to induction of antimicrobial peptide genes in the fat body of third instar larvae, pupae and adults of Drosophila and other insects (reviewed in Engström, 1998). In the Drosophila embryo, we recently demonstrated the presence of an inducible immune response in the embryonic yolk and epidermis, but surprisingly not in the embryonic fat body (Önfelt Tingvall et al., 2001). It has thus been an open question as to when the fat body becomes immune-competent. Here we show that an immune response in the fat body can be induced during the first larval instar, and that this response persists through all larval stages.
In insects, and other arthropods, the integument is composed of an epicuticle and a procuticle that are secreted by a single cell layer of epidermis. Immune responses in the epithelia underlying the larval cuticle of insects have been reported in Bombyx mori and Hyalophora cecropia (Brey et al., 1993; Ashida and Brey, 1995), and Ceratitis capitata (Marmaras et al., 1993). Recently, it was described that a drosomycin promoter–green fluorescent protein (GFP) fusion was expressed in epithelial tissues covering the trachea of Drosophila in response to fungi. This local expression of drosomycin was not dependent on the Toll pathway, suggesting that local and systemic expression of antimicrobial peptides might be regulated by different signalling pathways (Ferrandon et al., 1998).
The present study demonstrates that cuticular abrasion of Drosophila larvae in the presence of bacteria induced expression of the Cecropin A1 (CecA1) gene in the cuticular epithelium. Analysis in mutant background revealed that the immune deficiency (imd) gene product (Lemaitre et al., 1995; Corbo and Levine, 1996) is necessary to mount an immune response in embryonic and larval epidermis in response to microbial challenge. Interestingly, the formation of melanin in the wound area was not dependent on the imd gene, suggesting that different signalling pathways are involved in activating expression of antimicrobial peptides and the pro-phenoloxidase cascade.
RESULTS
CecA1 gene expression is inducible in fat body of first and second instar larvae
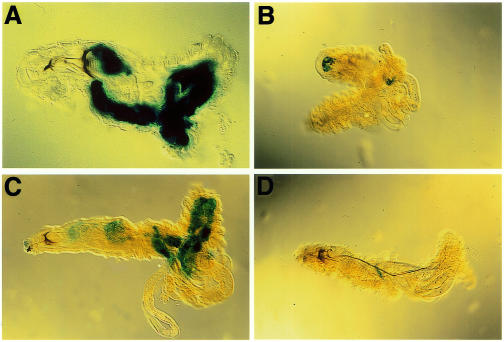
Although the fat body is the major site of CecA1 expression in flies and third instar larvae (Samakovlis et al., 1990; Roos et al., 1998), no expression could be induced in the embryonic fat body (Önfelt Tingvall et al., 2001). To determine the onset of inducible CecA1 expression in the fat body we used transgenic fly strains carrying the A10 construct, which contains 760 bp of the CecA1 upstream region fused to the Escherichia coli lacZ gene. These constructs were previously shown to mimic the induction of the CecA1 gene in terms of tissue-specificity and inducibility by infectious agents in embryos, third instar larvae and adults (Engström et al., 1993; Roos et al., 1998; Petersen et al., 1999; Önfelt Tingvall et al., 2001). All LPS-injected first and second instar A10 larvae displayed strong reporter gene expression in the fat body (Figure 1A and C). The uninjected control larvae did not promote any reporter gene expression (Figure 1B and D), while injection of sterile phosphate-buffered saline (PBS) promoted faint fat body staining in 20–25% of the larvae (data not shown), suggesting that wounding may trigger a weak response. In conclusion, a maturation of the fat body had occurred during the first larval instar, rendering it immune-competent. Analysis of deletion constructs in the CecA1 upstream region showed that inducible expression in the fat body of first and second instar larvae required the presence of a conserved region including a κB-like motif, a GATA motif and another putative regulatory element called R1 (data not shown). These results are the same as those found for CecA1 regulation in third instar larvae (Roos et al., 1998; Petersen et al., 1999) indicating that LPS-inducible CecA1 expression in the fat body is controlled via the same regulatory promoter elements in all three larval instars.
Fig. 1. Injection of LPS induces a systemic immune response in the fat body of first and second instar larvae. (A–D) Distribution of β-gal staining in opened A10 CecA1–lacZ transgenic larvae of first (A, B) and second (C, D) larval instar. Larvae were injected with LPS into the haemocoel (A, C), or were not injected (B, D), kept at 25°C for 3–4 h, opened up with forceps, fixed and stained. Anterior is to the left.
The CecA1 gene is induced in the fat body of larvae which have been infected as embryos
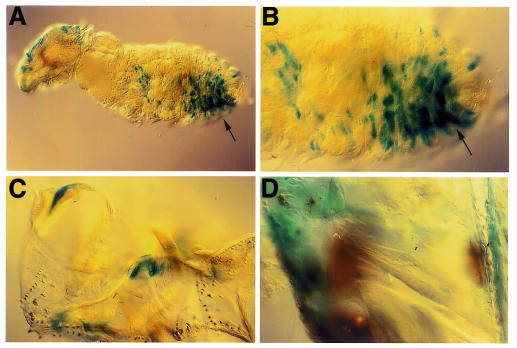
We decided to infect late stage embryos with bacteria and study the effects on CecA1 expression in the hatched larvae. Embryos, which were injected with Gram-negative bacteria and aged to 2–6 h after hatching, displayed similarly patched staining patterns in the larval epidermis, as was observed in late stage embryos (Figure 2A) (Önfelt Tingvall et al., 2001). Most interestingly, however, when the larvae were aged to a later stage of development (10–14 h after hatching), activation of CecA1 expression occurred in the fat body (Figure 2B), while the expression in the epidermis had declined. These results suggest that the CecA1 gene can be induced in the larval fat body by the persistent presence of bacteria, or that cells that were activated in the embryo have a ‘memory’ and provide a secondary signal in the larval stage, which activates the response in the fat body. To differentiate between these two models we repeated the experiment by injecting LPS instead of live bacteria, since LPS is a short-lived molecule in vivo, and not likely to be transferred from the embryo into the hatched larva. LPS-injection of late stage embryos, followed by aging into larvae, mounted a similar pattern of staining in the larval epidermis as seen in late stage embryos (Figure 2C), but could not elicit any response in the larval fat body even after longer aging (data not shown). LPS-induction leads to a peak of CecA1 expression that reaches a maximum after 1–3 h (Samakovlis et al., 1990). Therefore, the epidermal expression of CecA1 in LPS-injected embryos is probably transient and the staining observed in the larval epidermis (Figure 2C), mainly the result of high stability of the β-gal protein. Our conclusion is therefore that live bacteria, but not LPS, transmitted the inducing signal from embryos to larvae, which promoted activation of the CecA1 reporter gene in the larval fat body, but not in the larval epidermis. Thus, a maturation in the immune-competence of the fat body had occurred during a time window ranging from 6 to 14 h of larval development.
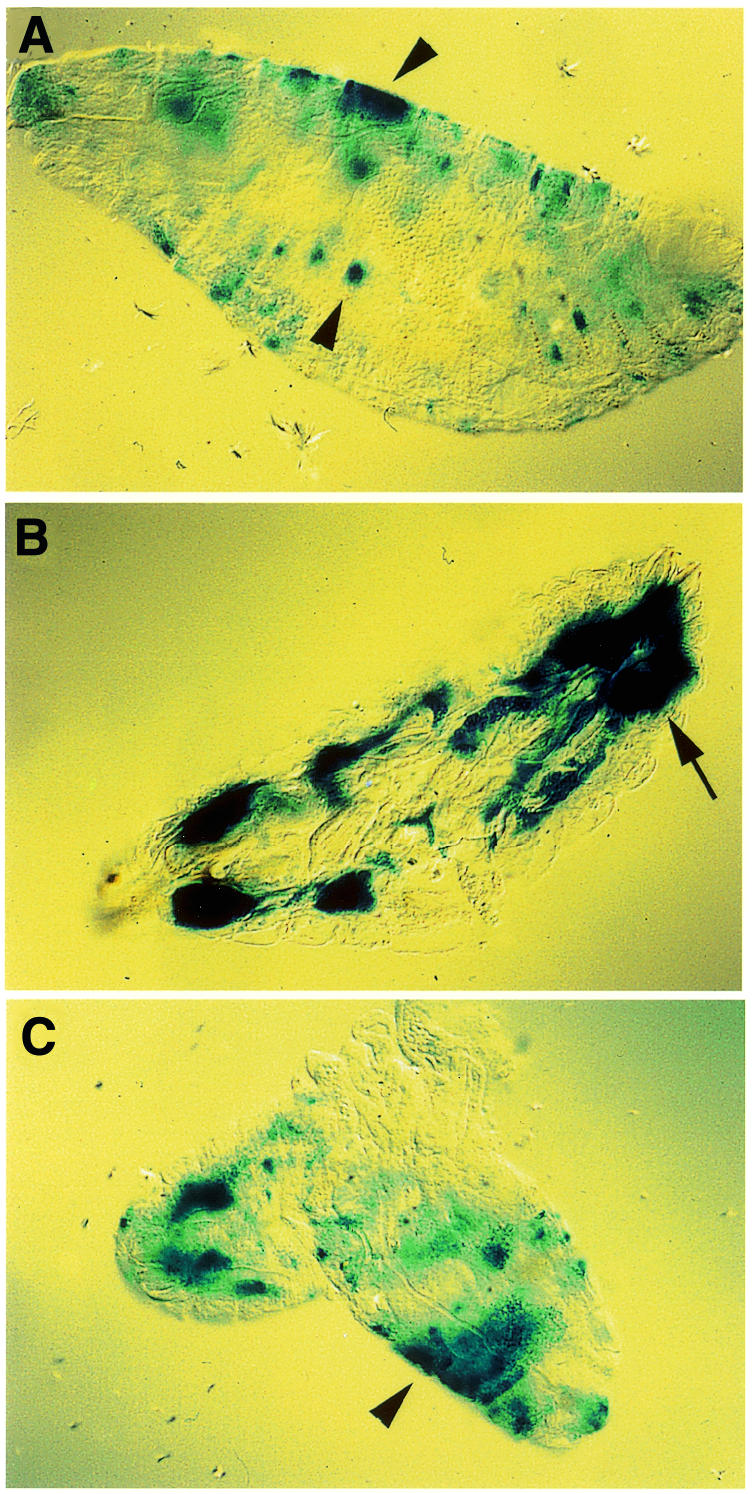
Fig. 2. The fate of embryonally induced CecA1 expression was analysed in hatched first instar larvae after injections of bacteria or LPS in late stage embryos. (A–B) Injection with E. cloacae. (A) 8 h post-injection (2–6 h after hatching) the pattern of induced cells in the larval epidermis (arrowheads) remained the same as in embryos (Önfelt Tingvall et al., 2001), (B) while staining of the fat body (arrow) was predominant in larvae aged to 16 h post-injection (10–14 h after hatching). (C) Injection with LPS promoted expression in the epidermis (arrowheads) but not in the fat body.
Wounding induces local CecA1 expression in the larval epidermis
How do Drosophila larvae deal with skin infections, and what are the effects of non-sterile abrasion on epidermal and fat body CecA1 expression? We tested this by direct challenge of the larval epidermis using sandpaper or the ragged edge of a sharp preparation needle, and inoculation with a solution of Enterobacter cloacae. All three larval instars reacted by turning on the CecA1 promoter in cells in the epidermis while in the fat body the CecA1 promoter remained inactive (Figure 3). The response was local and on the same side as the wound had been inflicted (Figure 3A and B). The expression pattern closely resembled the one found previously in embryos, with a network of stained cells elongated in the dorsovetral axis (Önfelt Tingvall et al., 2001). However, a major difference is that the expression in the larval epidermis was restricted to the wounded region, while in the embryo, it occurred throughout the embryo. Occasionally, a more general β-gal staining occurred, which included the underlying fat body. This was probably due to wounding through the cuticle, allowing bacteria to enter the haemocoel, giving rise to a systemic induction of the immune response. The β-gal expression in the epidermis was in most cases accompanied by a local melanization reaction. To study this in detail, the larval cuticle was dissected and the rest of the larval tissue, including the fat body, was removed carefully. In Figure 3C and D, dissected cuticle from second and third stage larvae show melanized tissues adjacent to the β-gal positive cells.
Fig. 3. Cuticular wounding and exposure to bacteria induces a local immune response in the epidermis of larvae. (A–D) The epidermis layer of larvae carrying the A10 CecA1–lacZ construct was abraded and incubated with E. cloacae, and analysed for reporter gene expression using X-gal. (A) First instar larva, and (B) a close up of the same larva demonstrating the local distribution of β-gal staining in the same areas as had been scratched (arrows). Dissected cuticle of abraded second (C) and third (D) instar larvae showing melanization of the wound (brown) and β-gal staining mimicking CecA1 expression (blue) in adjacent and partly overlapping regions of the cuticle.
Wounding and incubation with LPS instead of live bacteria promoted a weaker response, suggesting that bacteria may trigger several signal transduction pathways, leading to stronger upregulation of CecA1 expression. Abrasion during aseptic conditions did not promote any CecA1 expression in the epidermis, indicating that the presence of bacterial substances is required. In conclusion, we have demonstrated that the larval epidermis of Drosophila is not only a passive barrier against foreign invaders, but has the capacity to induce the synthesis of antimicrobial peptides in response to infected wounds.
The imd signalling pathway is involved in local CecA1 expression in the epidermis of embryos and larvae, but not in the melanization of the wounded cuticle
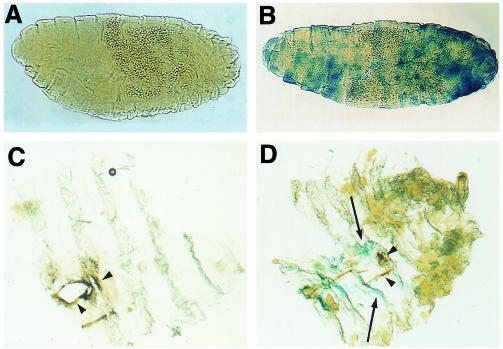
Genetic analysis of the regulation of Drosophila antimicrobial peptide genes implies that several signalling pathways for activation exist (reviewed in Engström, 1999). In the fly mutant imd, systemic expression of the genes for the antimicrobial peptides diptericin, drosocin and cecropin was severely impaired upon bacterial challenge, while inducible expression of the gene for the antifungal peptide drosomycin was unaffected (Lemaitre et al., 1995; Corbo and Levine, 1996). We tested whether the imd pathway was involved in the activation of CecA1 in epidermis by analysing the expression of A10 CecA1–lacZ in homozygous imd embryos and larvae. The β-gal expression in the epidermis of immuno-challenged imd embryos was reduced strikingly. To reveal the differences in β-gal staining between imd and wild-type embryos the incubation time with X-gal was reduced to 1 h. Under these conditions, no β-gal staining was observed in the epidermis or in any other tissues in the imd– mutant background (Figure 4A), while 80% of the A10 embryos revealed substantial staining (Figure 4B). Longer incubation time shows a low level of β-gal staining in 44% of the imd– embryos. This is in correspondence with previous data showing that CecA gene expression is reduced but not abolished in imd mutant larvae and adults (Lemaitre et al., 1995; Corbo and Levine, 1996; Lemaitre et al., 1996).
Fig. 4. The imd gene is required for epidermal CecA1 expression, but not for melanization. (A–B) β-gal staining of A10 CecA1–lacZ in (A) homozygous imd and in (B) wild-type embryos shown in lateral views, anterior to the left, dorsal up. (C–D) Local expression of A10 CecA1–lacZ in the cuticle of wild-type and imd mutant larvae. (C) Dissected cuticle from imd larvae did not reveal any reporter gene staining but clear melanization (brown, arrowheads) in the wounded area. Cuticle from control A10 larvae (D) displayed both reporter gene staining (blue, arrows) and melanization (arrowheads).
We investigated further the role of the imd pathway for the local expression of CecA1 in the larval cuticle. Wounding of the larval cuticle and incubation with E. cloacae did not promote any β-gal staining in the epidermis of the imd– mutant larvae (Figure 4C), in contrast to wild-type larvae (Figure 4D), demonstrating that the imd– gene is crucial for the activation of a local immune response in the epidermis of larvae. Most interestingly, the formation of melanin in the wounded area of the abraded cuticle occurs in both the imd mutant and in the wild type (Figure 4C and D). This indicates that the signalling pathway(s) leading to activation of the pro-phenoloxidase cascade, resulting in melanin formation, is independent of the imd pathway. Taken together, our data show that the imd gene product is an important component of signal-induced, local CecA1 expression in the epidermis of both embryos and larvae.
DISCUSSION
We propose the following model to explain the transition of CecA1 expression in epidermal tissues of the embryo to the larval fat body. During embryogenesis, CecA1 is induced in the epidermis as a result of direct contact between microbial substances in the privitelline fluid and epidermal cells. (Önfelt Tingvall et al., 2001). When the cuticle is formed during late embryogenesis, this direct contact is broken, and the presence of bacteria or LPS in the larval haemocoel does not promote CecA1 expression in the larval epidermis. During the larval stages, CecA1 expression is restricted to wounded areas of the epidermis, suggesting that either the cuticle needs to be removed to allow direct contact between microbial substances and the epithelial cell layer, or that the wounding is an important signal per se. Our conclusion is therefore, that the epidermal cells are immuno-competent from the embryonic stage onwards. The fat body, on the other hand, seems to undergo maturation during the first larval instar. Expression of CecA1 could be induced in the fat body of all three larval instars, but not in embryos. Injecting live bacteria into embryos and aging them into larvae showed that a maturation in the immuno-competence of the fat body occurs during first larval instar.
We were able to restore CecA1-driven reporter gene expression in the larval epidermis by inflicting infected wounds in all three larval instars. In addition to the cuticle being a physical barrier it is possible that the larval epidermis at the stage of hatching normally contains cecropins, since a low level of constitutive CecA1 expression is observed in the epidermis of late stage embryos (Önfelt Tingvall et al., 2001). It was shown recently that some of the antimicrobial peptides have a half-life of 2–3 weeks in Drosophila adults (Uttenweiler-Joseph et al., 1998), suggesting that a low level of expression may also suffice to load the epidermis layer with substantial amounts of antimicrobial peptides. In addition, it was demonstrated recently that Drosophila cecropins are not only active against Gram-negative and Gram-positive bacteria (Samakovlis et al., 1990), but also possess strong antifungal activity (Ekengren and Hultmark, 1999) making them powerful weapons against most classes of microorganisms.
Genetic evidence has provided a model in which at least two different pathways are involved in the activation of antimicrobial peptides, the Toll pathway and the imd pathway, both suggested to activate members of the Drosophila Rel family (Lemaitre et al., 1997; Engström, 1999). Although the systemic expression of the peptide drosomycin was dependent on the Toll pathway and the Rel protein Dif, the local expression of drosomycin in tracheal epithelium was independent of the same pathway (Ferrandon et al., 1998). The present study demonstrates that the imd gene seems to play an important role in the local expression of cecropin. In imd mutants, the expression of CecA1 was impaired dramatically in the epidermis of embryos, and was undetectable in the epidermis underlying the cuticle of wounded larvae. Interestingly, the pro-phenoloxidase cascade was activated by injury in both wild-type and imd larvae, showing that the processes of antimicrobial defence and melanization can be uncoupled. In addition, the inducible, local expression of CecA1 in infected wounds indicates that the insect epidermis is not only a passive barrier against infection, but plays an active role in the innate defence against microorganisms.
In this study, we demonstrate that antimicrobial cecropins are expressed locally in infected wounds in the epidermis of Drosophila larvae, and that this response is amenable to genetic dissection in mutants of the fly’s immune response. This has a strong potential to become valuable for the understanding of innate and epithelial immunity in higher organisms, including mammals.
METHODS
Flies. Transgenic fly strains, with A10 (A10–1, A10–3 and A10–4) reporter constructs have been described previously (Roos et al., 1998). Flies carrying a recombination of A10 to the X-chromosome, the b pr imd stock and the A10; imd stock were generously provided by Bruno Lemaitre. Flies were reared on standard cornmeal agar medium in vials and flasks in humid culture rooms at 25 or 18°C.
Injections and staining of embryos. Embryos were collected on apple juice agar plates for 3 h at 25°C and aged to the desired stage at 18°C. Dechorionated embryos were mounted on a coverslip with double-stick tape and covered with 10 S voltalef oil (Elf Autochem). Injections were with LPS (10 µg/ml) or with a 1:10 dilution of log phase E. cloacae, β12 or with sterile PBS. After LPS injection the embryos were kept at 25°C for 3–4 h, fixed in glutaraldehyde-saturated heptane, devitellinized by hand, and stained using 5-bromo-4-chloro-3-indoyl-β-d-galactopyranoside (X-gal) for 15–18 h or as indicated.
Injection and wounding of larvae. Larvae were injected with LPS (10 µg/ml) on the dorsal side using fine glass capillaries. Injected larvae were kept on humid filter paper at 25°C for 3–4 h before fixation and staining. Abrasion of larvae was done by holding a larva with forceps and scratching the cuticle against sandpaper or a ragged, sharp preparation needle. Larvae with such wounds in the epidermis were inoculated with E. cloacae, 1:10 dilution of an overnight culture in Ringer solution. The abraded larvae were incubated for 18 or 24 h at 18°C, fixed in 1% glutaraldehyde in PBS pH 7.3 for 20 min and stained as described (Roos et al., 1998).
Acknowledgments
ACKNOWLEDGEMENTS
We thank Bruno Lemaitre for fly strains. This work was supported by grants to Y.E. from the Swedish Cancer Society and the Swedish Natural Science Research Council.
REFERENCES
- Ashida M. and Brey, P.T. (1995) Role of the integument in insect defense: pro-phenol oxidase cascade in the cuticular matrix. Proc. Natl Acad. Sci. USA, 92, 10698–10702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boman H.G., Nilsson, I. and Rasmuson, B. (1972) Inducible antibacterial defence system in Drosophila. Nature, 237, 232–235. [DOI] [PubMed] [Google Scholar]
- Brey P.T., Lee, W.J., Yamakawa, M., Koizumi, Y., Perrot, S., Franqis, M. and Ashida, M. (1993) Role of the integument in insect immunity: epicuticular abrasion and induction of cecropin synthesis in cuticular epithelial cells. Proc. Natl Acad. Sci. USA, 90, 6275–6279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulet P., Hetru, C., Dimarcq, J.-L. and Hoffmann, D. (1999) Antimicrobial peptides in insects; structure and function. Dev. Comp. Immunol., 23, 329–344. [DOI] [PubMed] [Google Scholar]
- Corbo J.C. and Levine, M. (1996) Characterization of an immunodeficiency mutant in Drosophila. Mech. Dev., 55, 211–220. [DOI] [PubMed] [Google Scholar]
- Ekengren S. and Hultmark, D. (1999) Drosophila cecropin as an antifungal agent. Insect Biochem. Mol. Biol., 29, 965–972. [DOI] [PubMed] [Google Scholar]
- Engström Y. (1998) Insect immune gene regulation. In Brey, P. and Hultmark, D. (eds), Molecular Mechanisms of Immune Responses in Insects. Chapman and Hall, London, UK, pp. 211–244.
- Engström Y. (1999) Induction and regulation of antimicrobial peptides in Drosophila. Dev. Comp. Immunol., 23, 345–358. [DOI] [PubMed] [Google Scholar]
- Engström Y., Kadalayil, L., Sun, S.-C., Samakovlis, C., Hultmark, D. and Faye, I. (1993) κB-like motifs regulate the induction of immune genes in Drosophila. J. Mol. Biol., 232, 327–333. [DOI] [PubMed] [Google Scholar]
- Ferrandon D., Jung, A.C., Criqui, M.-C., Lemaitre, B., Uttenweiler-Joseph, S., Michaut, L., Reichhart, J.-M. and Hoffmann, J.A. (1998) A drosomycin–GFP reporter transgene reveals a local immune response in Drosophila that is not dependent on the Toll pathway. EMBO J., 17, 1217–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann J.A., Kafatos, F.C., Janeway, C.A. and Ezekowitz, R.A. (1999) Phylogenetic perspectives in innate immunity. Science, 284, 1313–1318. [DOI] [PubMed] [Google Scholar]
- Hultmark D. (1993) Immune reactions in Drosophila and other insects—A model for innate immunity. Trends Genet., 9, 178–183. [DOI] [PubMed] [Google Scholar]
- Lemaitre B., Kromer-Metzger, E., Michaut, L., Nicolas, E., Meister, M., Georgel, P., Reichhart, J.-M. and Hoffmann, J.A. (1995) A recessive mutation, immune deficiency (imd), defines two distinct control pathways in the Drosophila host defense. Proc. Natl Acad. Sci. USA, 92, 9465–9469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaitre B., Nicolas, E., Michaut, L., Reichhart, J.M. and Hoffmann, J.A. (1996) The dorsoventral regulatory gene cassette spätzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell, 86, 973–983. [DOI] [PubMed] [Google Scholar]
- Lemaitre B., Reichhart, J.M. and Hoffmann, J.A. (1997) Drosophila host defense: differential induction of antimicrobial peptide genes after infection by various classes of microorganisms. Proc. Natl Acad. Sci. USA, 94, 14614–14619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmaras V.J., Bournazos, S.N., Katsoris, P.G. and Lambropoulou, M. (1993) Defense mechanisms in insects: certain integumental proteins and tyrosinases are reponsible for non-self recognition and immobilization of Escherichia coli in the cuticle of developing Ceratitis capitata. Arch. Insect Biochem. Physiol., 23, 169–180. [DOI] [PubMed] [Google Scholar]
- Medzhitov R. and Janeway, C.A. (1998) An ancient system of host defense. Curr. Opin. Immunol., 10, 12–15. [DOI] [PubMed] [Google Scholar]
- Önfelt Tingvall T., Roos, E. and Engström, Y. (2001) The GATA factor Serpent is required for the onset of the humoral immune response in Drosophila embryos. Proc. Natl Acad. Sci. USA, 98, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen U.-M., Kadalayil, L., Rehorn, K.-P., Hoshizaki, D.K., Reuter, R. and Engström, Y. (1999) Serpent regulates Drosophila immunity genes in the larval fat body through an essential GATA motif. EMBO J., 18, 4013–4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos E., Björklund, G. and Engström, Y. (1998) In vivo regulation of tissue-specific and LPS-inducible expression of the Drosophila Cecropin genes. Insect Mol. Biol., 7, 51–62. [DOI] [PubMed] [Google Scholar]
- Samakovlis C., Kimbrell, D.A., Kylsten, P., Engström, Å. and Hultmark, D. (1990) The immune response in Drosophila: pattern of cecropin expression and biological activity. EMBO J., 9, 2969–2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder J.-M. (1999) Epithelial peptide antibiotics. Biochem. Pharmacol., 57, 121–134. [DOI] [PubMed] [Google Scholar]
- Uttenweiler-Joseph S., Moniatte, M., Lagueux, M., Van Dorsselaer, A., Hoffmann, J.A. and Bulet, P. (1998) Differential display of peptides induced during the immune response of Drosophila: a matrix-assisted laser desorption ionization time-of-flight mass spectrometry study. Proc. Natl Acad. Sci. USA, 95, 11342–11347. [DOI] [PMC free article] [PubMed] [Google Scholar]