Abstract
The sex of an individual is generally determined genetically by genes on one of the two sex chromosomes. In mammals, for instance, the presence of the male-specific Y chromosome confers maleness, whereas in Drosophila melanogaster and Caenorhabditis elegans it is the number of X chromosomes that matters. For birds (males ZZ, females ZW), however, the situation remains unclear. The recent discovery that the Z-linked DMRT1 gene, which is conserved across phyla as a gene involved in sexual differentiation, is expressed early in male development suggests that it might be the number of Z chromosomes that regulate sex in birds. On the other hand, the recent identification of the first protein unique to female birds, encoded by the W-linked PKCIW gene, and the observation that it is expressed early in female gonads, suggests that the W chromosome plays a role in avian sexual differentiation. Clearly defining the roles of the DMRT1 and PKC1W genes in gonadal development, and ultimately determining whether avian sex is dependent on Z or W, will require transgenic experiments.
Introduction
Our knowledge about the molecular process behind sex determination has improved significantly in recent years, notably through the insights gained into the mechanisms that control sexual development in mammals, Drosophila melanogaster and Caenorhabditis elegans (see e.g. Meyer, 2000; Schutt and Nothiger, 2000; Vaiman and Pailhoux, 2000). In contrast to these advances, the molecular determinants behind sexual development in birds have largely remained a mystery (Clinton, 1998; Clinton and Haines, 1999; Ellegren, 2000). We know that the process is different from that in mammals, as it has not been possible to identify an SRY (a gene that confers maleness in mammals) homolog in avian genomes (Griffiths, 1991). Moreover, the failure to identify an avian SRY probably is a reflection of what appears to be a general, but perhaps surprising, phenomenon. Despite the fact that the occurrence of two sexes is a nearly universal feature throughout the animal kingdom, the genes involved in directing the process of sexual development seem virtually unrelated among metazoan phyla. The differences obviously raise obstacles for comparative or candidate gene approaches in studies of sexual development. Nevertheless, the first hints toward the genetic mechanism that underlies sex determination in birds recently have come from studies of the expression pattern and chromosomal localization of two genes potentially associated with avian sexual differentiation. Here, I review these findings in an evolutionary context.
Avian sex chromosomes
In birds, females are the heterogametic sex, carrying one copy each of the Z and W sex chromosomes. Males are homogametic (ZZ). Although there is a small pseudoautosomal region on Z and W with an obligate crossing-over at meiosis, most of the W chromosome does not recombine (this is analogous to the situation in X and Y of mammals). Critical to the question of sex determination in birds is the role of the two types of sex chromosomes. It has not been clear whether it is the presence of the female-specific W chromosome that triggers female development (Figure 1A), or the dose of Z chromosome that confers maleness (Figure 1B). The former situation would at least partly be analogous to that in mammals, where the presence of the Y chromosome triggers male development (strictly speaking, it is the SRY gene on the Y chromosome that triggers this process, although other Y-specific genes are also important for testis development). The latter situation, on the other hand, would be analogous to sex determination in Drosophila, which is dependent on dose or genic balance (that is, the number of Z chromosomes would regulate the sex of the individual).
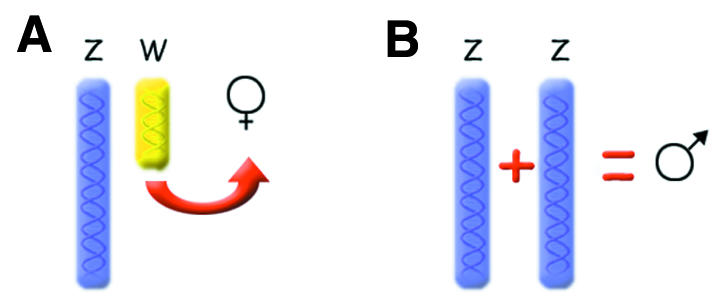
Fig. 1. Two potential mechanisms for chromosomal sex determination among birds. (A) the presence of the W chromosome triggers femaleness or (B) the presence of two Z chromosomes confers maleness.
Mammalian geneticists began to understand the principles underlying sex determination from studies of the phenotypes of sex chromosome aneuploids, individuals with unusual combinations of sex chromosomes (Vogel and Motulsky, 1998). For instance, the observations that XXY (Klinefelter’s syndrome) and XO (Turner’s syndrome) humans develop into phenotypic males and females, respectively, conclusively demonstrate a role of the Y chromosome in triggering maleness in mammals. Despite intensive karyotype analysis in birds, however, equally informative avian germline sex chromosome aneuploids have been difficult to identify (Clinton, 1998). Although the pattern of sexual development in ZO birds would be most revealing (see Figure 2A), unfortunately, no such bird has yet been reported. Similarly, ZZW birds (see Figure 2B) seem to be extremely rare and it has not been possible to analyse this form with modern molecular genetic techniques (there is one old report of such a chicken but the data provided are not conclusive; Crew, 1933; Clinton and Haines, 1999). For some reason, sex chromosome aneuploids appear more or less lethal among birds. Cytology may therefore not provide a successful route towards deciphering avian sex determination.
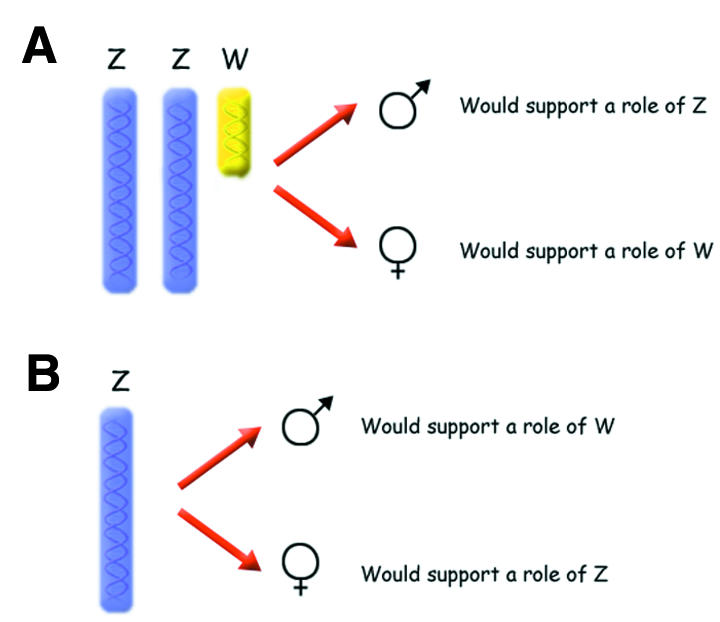
Fig. 2. Transgenic strategies for revealing the mechanism of sex determination among birds. (A) a ZZW bird, (B) a Z0 bird.
Does the dose of DMRT1 trigger maleness in birds?
As stated above, the genes and mechanisms underlying sex determination are poorly conserved between phyla. To this should be added that sex-determining genes seem to evolve particularly rapidly, even between related species (Whitfield et al., 1993; de Bono and Hodgkin, 1996). However, there is one distinct exception. The C. elegans mab-3 (male abnormal; Shen and Hodgkin, 1988) and the D. melanogaster dsx (doublesex; Burtis and Baker, 1989) sexual regulatory genes share a strongly conserved DNA-binding motif, the DM domain (for dsx and mab-3). Both genes encode proteins that control sex-specific neuroblast differentiation and yolk protein gene transcription. Importantly, the expression of D. melanogaster dsx in mab-3 transgenic mutant nematodes restores male development, suggesting that invertebrates share a common evolutionary origin of sexual development (Raymond et al., 1998).
Recent data suggest that proteins with DM domains are also involved in sexual differentiation in vertebrates, including birds (Raymond et al., 1999a, 2000; Smith et al., 1999; Kettlewell et al., 2000; Marchand et al., 2000). The chicken (Gallus gallus) DMRT1 (doublesex- and mab-3-related transcription factor) gene which has been mapped to the Z chromosome (Nanda et al., 1999) contains a highly conserved DM domain. The protein that it encodes is specifically expressed in the gonad during urogenital development, and expression is enhanced in developing male, relative to female, gonads (Raymond et al., 1999a; Smith et al., 1999; Shan et al., 2000). Using whole-mount in situ hybridization, Raymond et al. (1999a) found expression of DMRT1 in the genital ridge and in the Wolffian ducts (progenitors of male-specific internal reproductive structures) of chicken embryos from stage 25. This is prior to sexual differentiation, suggesting that DMRT1 acts upstream in the cascade of proteins involved in male development. After the onset of sexual differentiation, DMRT1 becomes testis-specific and, in adult birds, it is exclusively expressed in testis (Shan et al., 2000).
Data on DMRT1 expression in mammals (human and mouse), reptiles (alligator) and fish (trout and tilapia) are similar to those for birds. DMRT1 is expressed in the genital ridge of human and mouse embryos of both sexes prior to sexual differentiation, but becomes restricted specifically to the seminiferous tubules of the testis as gonadogenesis proceeds (Raymond et al., 1999a). This was an exciting finding in light of the fact that previously, the only regulatory gene that had been found to be expressed exclusively in gonads prior to sexual differentiation was the unconserved SRY protein. Furthermore, mouse XY Dmrt1–/– knock-outs fail to produce differentiated testes and also experience germ cell death (Raymond et al., 2000), suggesting that DMRT1 is required for mammalian testis differentiation. In fish, DMRT1 expression is also specific to testicular differentiation (Marchand et al., 2000) and in alligator, whose sex determination is temperature-dependent, DMRT1 expression is upregulated at male-producing temperatures (Smith et al., 1999). It is interesting to note that the role of DMRT1 might be related even between organisms that have genetic and environmental basis of sex determination, respectively.
In humans, DMRT1 maps to chromosome 9p24.3, and deletions in distal 9p have been implicated in gonadal dysgenesis and sex reversal in 46 XY individuals (Bennet et al., 1993). Hemizygosity of this region thus impairs normal male (testis) development. The minimal critical region of chromosome 9p in which deletions cause sex reversal has been narrowed down to an interval of ∼250 kb. This region contains DMRT1 and several other genes, including at least two novel genes with DM-domains (DMRT2 and DMRT3; Raymond et al., 1999b; Ottolenghi and McElreavey, 2000; Ottolenghil et al., 2000). Available data are consistent with the sex reversal phenotype being a consequence simply of haploinsufficiency, rather than the deletion of the normal allele in a heterozygous individual. A double-dose of DMRT1, or perhaps of one of the other genes in this region, therefore, seems to be required for testis development.
Birds probably do not regulate the expression of genes located on the Z chromosome using dosage compensation (Baverstock et al., 1982). Therefore, in the absence of other regulatory mechanisms, males would be expected to express a double-dose of Z-linked genes. In this context, it is intriguing that avian DMRT1 maps to the Z chromosome. On the basis of the chromosomal location and expression profile, it is tempting to nominate DMRT1 as a candidate for a, or perhaps the only, avian testis-determining factor. In this scenario, the presence of two copies of DMRT1 in ZZ birds would be the factor that is required to ultimately trigger avian maleness. The presence of only a single copy, as in ZW birds, would not be sufficient for development of the male phenotype.
Or is it the presence of PKCIW that triggers femaleness?
If avian sex determination is primarily a matter of Z chromosome dosage, the W chromosome may be required only for the proper segregation of the sex chromosomes upon cell division. Alternatively, W may indeed carry genes required for female fertility, but these genes would only be required after sex determination is complete. Some researchers have found this unlikely, however, and there has been a long hunt for genes on the avian W chromosome. The first two such genes identified (CHD1W, Ellegren, 1996; Griffiths et al., 1996 and ATP5A1W, Fridolfsson et al., 1998) seem not to be involved in sexual differentiation (see below). The recent identification of an altered form of a protein kinase C inhibitor (PKCIW) gene that is widely conserved and localizes to the avian W chromosome is therefore all the more interesting. [Hori et al. (2000) refer to this gene as Wpkci, where W denotes its chromosome location. O’Neill et al. (2000) refer to the same gene as ASW (Avian Sex-specific W-linked). Assuming that the gene represents a true avian ortholog of mammalian PKCI, the appropriate gene symbol in birds should be PKCIW, following the nomenclature for other sex-linked genes in birds and mammals. This designation is further motivated by the fact that the gene also exists in a copy on the avian Z chromosome, and this copy accordingly should be referred to as PKCIZ.]
PKCIW has been independently isolated and characterized by O’Neill et al. (2000), using differential display and representational difference analysis, and by Hori et al. (2000), using subtraction (female-minus-male) cloning. Expression of PKCIW has been found in the female genital ridge from day 4 to day 5 in chicken embryos, i.e. prior to sexual differentiation. Expression is restricted to the urogenital system, with higher transcript levels detectable in the genital ridge than in the mesonephros on day 5. Expression is higher in the medulla of the developing ovary than in the overlying cortex on day 6. Expression in the ovary continues throughout embryonic development, though at decreasing levels. PKCIW is also expressed in various tissues of adult female birds (Hori et al., 2000, O’Neill et al., 2000).
PKCI belongs to a family of HIT proteins, so designated because they contain an invariant HisXHisXHis motif (histidine triad) in which X is a hydrophobic amino acid (Séraphin, 1992). In vitro experiments using both yeast and human PKCI suggest that the histidine triad is essential for enzymatic hydrolyzation of adenosine polyphosphates (Lima et al., 1993; Robinson et al., 1993). Whereas avian PKCIW shows significant amino acid homology outside the HIT region, HIT itself is absent. Moreover, PKCIW also differs from other PKCI proteins in that it contains a unique Leu- and Arg-rich region. The significance of these differences remains to be elucidated.
Relevant to the function of PKCIW may be the recent discovery of another PKCI-encoding gene in avian genomes (Hori et al., 2000). This gene, which is actually more similar to mammalian PKCI genes than to PKCIW itself (e.g. it has a HIT region and lacks the Leu- and Arg-rich region of PKCIW), is located on the Z chromosome; I will refer to it as PKCIZ. Finding related genes on the Z chromosome and the non-recombining region of the W chromosome is not surprising. Like sex chromosomes in general, avian sex chromosomes are thought to have evolved from an ancestral pair of autosomes (Fridolfsson et al., 1998; Ellegren and Carmichael, 2001). The observation of related genes on Z and W thus likely reflects a remnant of this ancestral chromosome homology. As mentioned above, PKCIW is not the first gene identified on the avian W chromosome. Like PKCIW, the two previously characterized genes, CHD1W and ATP5A1W, have related counterparts on the Z chromosome (CHD1Z and ATP5A1Z; Ellegren, 2000). However, while PKCIW and PKCIZ show significant differences at the amino acid level, CHD1W and ATP5A1W are almost identical to their Z chromosome counterparts (Carmichael et al., 2000; Fridolfsson and Ellegren, 2000). PKCIW thus represents the first identified protein that might be functionally unique to female birds. This definitely adds to its merit as a candidate for governing sexual differentiation in avian female embryos.
PKCI proteins dimerize and an α-helix region has been suggested to be the site of intermolecular contact in the formation of a PKCI homodimer (Lima et al., 1993). This region is conserved in the avian PKCIW protein. Hori et al. (2000) have suggested various models for avian sex determination that postulate a role for PKCI dimers composed of PKCIW, and possibly also PKCIZ. Perhaps the most attractive idea is that the PKCIW:PKCIZ heterodimer might block or activate a still unidentified maleness factor, thereby triggering female gonadal differentiation in bird embryos.
Perspectives
Until a year ago we had few clues about the mechanisms whereby sex is determined in birds—even the basic question of whether it is the presence of the W chromosome or the dose of the Z chromosome that matters remained largely unresolved. Now, evidence for the involvement of two unrelated sex-linked genes in the process of avian sex determination is accumulating. Paradoxically, however, data for these two genes point in opposite directions regarding the roles of the W and Z chromosomes in regulating the process; the DMRT1 studies support the dominance of the Z chromosome, whereas the analyses of PKCIW favour W as the sex-determining chromosome. How robust are the data? It should be noted that neither of the avian studies has demonstrated a causal relationship between expression of DMRT1 or PKCIW and sexual differentiation. The evidence presented so far is only indirect. The critical experiment will be to construct transgenic birds in which either an extra copy of DMRT1 is expressed in a ZW background or a PKCIW transgene is expressed in a ZZ background (analogous to the scenarios illustrated in Figure 2). Male gonadal development in the former case, or female development in the latter, would provide strong support for the respective gene triggering sexual development. If no effect is obtained in any of these experiments, the quest for the deciding factors will have to continue.
Finally, an intriguing possibility regarding the answer to the question of whether it is Z or W that matters may be—both! That there is not always a single sex chromosome mediating all aspects of sex differentiation is known from marsupials. In the kangaroo and its relatives, Y acts as a dominant testis determining chromosome, while X chromosome dosage determines the choice between pouch and scrotum (Renfree et al., 1995). An XXY kangaroo is intersexual, with testes and a pouch (Watson et al., 1997). Maybe a system where the two sex chromosomes mediate different aspects of sex differentiation is also used in birds. If so, however, the outcome of the transgenic experiments suggested above may not help reveal the complete pattern.

Hans Ellegren
Acknowledgments
Acknowledgements
An anonymous reviewer is acknowledged for helpful comments on the manuscript, and members of my laboratory, especially Lori Lawson, for discussions. Financial support was obtained from the Swedish Natural Sciences Research Council. H.E. is a Royal Swedish Academy of Sciences Research Fellow supported by a grant from the Knut and Alice Wallenberg Foundation.
REFERENCES
- Baverstock P.R., Adams, M., Polkinghorne, R.W. and Gelder, M. (1982) A sex-linked enzyme in birds—Z-chromosome conservation but no dosage compensation. Nature, 296, 763–766. [DOI] [PubMed] [Google Scholar]
- Bennet C.P., Docherty, Z., Robb, S.A., Ramani, P., Hawkins, J.R. and Grant, D. (1993) Deletion 9p and sex reversal. J. Med. Genet., 46, 597–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burtis K.C. and Baker, B.S. (1989) Drosophila doublesex gene controls somatic sexual differentiation by producing alternatively spliced mRNAs encoding related sex-specific polypeptides. Cell, 56, 997–1010. [DOI] [PubMed] [Google Scholar]
- Carmichael A., Fridolfsson, A.-K. and Ellegren, H. (2000) Male-biased mutation rates revealed from Z- and W-chromosome linked ATP synthase a-subunit (ATP5A1) sequences in birds. J. Mol. Evol., 50, 443–447. [DOI] [PubMed] [Google Scholar]
- Clinton M. (1998) Sex determination and gonadal development: a bird’s eye view. J. Exp. Zool., 281, 457–465. [DOI] [PubMed] [Google Scholar]
- Clinton M. and Haines, L.C. (1999) An overview of factors influencing sex deter-mination and gonadal development in birds. Cell. Mol. Life Sci., 55, 876–886. [Google Scholar]
- Crew F.A.E. (1933) A case of non-disjunction in the fowl. Proc. R. Soc. Edin., 53, 89–105. [Google Scholar]
- de Bono M. and Hodgkin, J. (1996) Evolution of sex determination in Caenorhabditis: unusually high divergence of tra-1 and its functional consequences. Genetics, 144, 587–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellegren H. (1996) First gene on the avian W chromosome (CHD) provides a tag for universal sexing of non-ratite birds. Proc. R. Soc. Lond. B, 263, 1635–1641. [DOI] [PubMed] [Google Scholar]
- Ellegren H. (2000) Evolution of the avian sex chromosomes and their role in sex determination. Trends Ecol. Evol., 15, 188–192. [DOI] [PubMed] [Google Scholar]
- Ellegren H. and Carmichael, A. (2001) Multiple and independent cessation of recombination between avian sex chromosomes. Genetics, 149, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridolfsson A.-K. and Ellegren, H. (2000) Molecular evolution of the avian CHD1 genes on the Z and W sex chromosomes. Genetics, 155, 1903–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridolfsson A.-K. et al. (1998) Evolution of the avian sex chromosomes from an ancestral pair of autosomes. Proc. Natl Acad. Sci. USA, 95, 8147–8152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths R. (1991) The isolation of conserved DNA sequences related to the human sex-determining region Y gene from the lesser-backed gull (Larus fuscus). Proc. R. Soc. Lond. B, 224, 123–128. [DOI] [PubMed] [Google Scholar]
- Griffiths R., Daan, S. and Dijkstra, C. (1996) Sex identification in birds using two CHD genes. Proc. R. Soc. Lond. B, 263, 1251–1256. [DOI] [PubMed] [Google Scholar]
- Hori T., Asakawa, S., Itoh, Y., Shimizu, N. and Mizuno, S. (2000) Wpkci, encoding an altered form of PKCI, is conserved widely on the avian W chromosome and expressed in early female embryos: implication of its role in female sex determination. Mol. Biol. Cell, 11, 3545–3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettlewell J.R., Raymond, C.S. and Zarkower, D. (2000) Temperature-dependent expression of turtle Dmrt1 prior to sexual differentiation. Genesis, 26, 174–178. [PubMed] [Google Scholar]
- Lima C.D., Klein, M.G., Weinstein, I.B. and Hendrickson, W.A. (1993) Three-dimensional structure of human protein kinase C interacting protein 1. Proc. Natl Acad. Sci. USA, 93, 5357–5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand O., Govoroun, M., D’Cotta, H., McMeel, O., Lareyre, J.-J., Bernot, A., Laudet, V. and Guiguen, Y. (2000) DMRT1 expression during gonadal differentiation and spermatogenesis in the rainbow trout, Oncorhynchus mykiss. Biochim. Biophys. Acta, 1493, 180–187. [DOI] [PubMed] [Google Scholar]
- Meyer B.J. (2000) Sex in the worm—counting and compensating X-chromosome dose. Trends Genet., 16, 247–253. [DOI] [PubMed] [Google Scholar]
- Nanda I. et al. (1999) 300 million years of conserved synteny between chicken Z and human chromosome 9. Nature Genet., 21, 258–259. [DOI] [PubMed] [Google Scholar]
- O’Neill M., Binder, M., Smith, C., Andrews, J., Reed, K., Smith, M., Millar, C., Lambert, D. and Sinclair, A. (2000) ASW: a gene with conserved avian W-linkage and female specific expression in chick embryonic gonad. Dev. Genes Evol., 210, 243–249. [DOI] [PubMed] [Google Scholar]
- Ottolenghi C. and McElreavey, K. (2000) Deletions of 9p and the quest for a conserved mechanism of sex determination. Mol. Genet. Metabol., 71, 397–404. [DOI] [PubMed] [Google Scholar]
- Ottolenghil C., Veitia, R., Quintana-Murci, L., Torchard, D., Scapoli, L., Souleyreau-Therville, N., Beckmann, J., Fellous, M. and McElreavey, K. (2000) The region on 9p associated with 46,XY sex reversal contains several transcripts expressed in the urogenital system and a novel doublesex-related domain. Genomics, 64, 170–178. [DOI] [PubMed] [Google Scholar]
- Raymond C.S., Shamu, C.E., Shen, M.M., Seifert, K.J., Hirsch, B., Hodgkin, J. and Zarkower, D. (1998) Evidence for evolutionary conservation of sex-determining genes. Nature, 391, 691–695. [DOI] [PubMed] [Google Scholar]
- Raymond C.S., Kettlewell, J.R., Hirsch, B., Bardwell, V.J. and Zarkower, D. (1999a) Expression of Dmrt1 in the genital ridge of mouse and chicken embryos suggests a role in vertebrate sexual development. Dev. Biol., 215, 208–220. [DOI] [PubMed] [Google Scholar]
- Raymond C.S. et al. (1999b) A region of human chromosome 9p required for testis development contains two genes related to known sexual regulators. Hum. Mol. Genet., 8, 989–996. [DOI] [PubMed] [Google Scholar]
- Raymond C.S., Murphy, M.W., O’Sullivan, M.G., Bardwell, V.J. and Zarkower, D. (2000) Dmrt1, a gene related to worm and fly sexual regulators, is required for mammalian testis differentiation. Genes Dev., 14, 2587–2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renfree M.B., Harry, J.L. and Shaw, G. (1995) The marsupial male: a role model for sexual development. Philos. Trans. R. Soc. Lond. B, 350, 243–251. [DOI] [PubMed] [Google Scholar]
- Robinson A.K., de la Pena, C.E. and Barnes, L.D. (1993) Isolation and characterization of diadenosine tetraphosphate (Ap4A) hydrolase from Schizosaccharomyces pombe. Biochim. Biophys. Acta, 1161, 139–148. [DOI] [PubMed] [Google Scholar]
- Schutt C. and Nothiger, R. (2000) Structure, function and evolution of sex-determining systems in Dipteran insects. Development, 127, 667–677. [DOI] [PubMed] [Google Scholar]
- Séraphin B. (1992) The HIT protein family: A new family of proteins present in prokaryotes, yeast and mammals. Sequences, 3, 177–179. [DOI] [PubMed] [Google Scholar]
- Shan Z., Nanda, I., Wang, Y., Schmid, M., Vortkamp, A. and Haaf, T. (2000) Sex-specific expression of an evolutionarily conserved male regulatory gene, DMRT1, in birds. Cytogenet. Cell Genet., 89, 252–257. [DOI] [PubMed] [Google Scholar]
- Shen M.M. and Hodgkin, J. (1988) mab-3, a gene required for sex-specific yolk protein expression and a male-specific lineage in C. elegans. Cell, 54, 1019–1031. [DOI] [PubMed] [Google Scholar]
- Smith C.A., McClive, P.J., Western, P.S., Reed, K.J. and Sinclair, A.H. (1999) Conservation of a sex-determining gene. Nature, 402, 601–602. [DOI] [PubMed] [Google Scholar]
- Vaiman D. and Pailhoux, E. (2000) Mammalian sex reversal and intersexuality: deciphering the sex-determination cascade. Trends Genet., 16, 488–494. [DOI] [PubMed] [Google Scholar]
- Vogel F. and Motulsky, A.G. (1998) Human Genetics: Problems and Approaches. 3rd edn. Springer Verlag, New York.
- Watson C.M., Johnston, P.G., Rodger, K.A., McKenzie, L.M., O’Neill, R.J. and Cooper, D.W. (1997) SRY and karyotypic status of one abnormal and two intersexual marsupials. Reprod. Fertil. Dev., 9, 233–241. [DOI] [PubMed] [Google Scholar]
- Whitfield L.S., Lovell-Badge, R. and Goodfellow, P.N. (1993) Rapid sequence evolution of the mammalian sex-determining gene SRY. Nature, 364, 713–715. [DOI] [PubMed] [Google Scholar]


