Introduction
It was just over a century ago that Santiago Ramon y Cajal first marvelled at the intricate patterns of neuronal connectivity in the nervous system. Finding out how these connections are established has intrigued and challenged generations of developmental neuroscientists. It is, however, only within the last decade that advances in molecular biology, genetics, imaging and electrophysiology have combined to allow the first insights into the molecular and cellular mechanisms that control the formation and refinement of neuronal connections. To provide a forum for the exchange of information and ideas in this rapidly expanding field, Cold Spring Harbor Laboratory hosts a meeting on ‘Axon guidance and neural plasticity’ every two years. The most recent meeting attracted some 400 participants, 265 of whom presented the results of their latest research. Here we review just a small selection of these presentations, highlighting some of the major new themes that emerged.
New guidance mechanisms
New mechanisms of axon guidance continue to be discovered at a rapid pace (Table I). This trend shows no signs of abating. Two highlights of the Cold Spring Harbor meeting were talks reporting the identification of new guidance mechanisms. One of these is implicated in retinal axon targeting in the vertebrate visual system; the other in the inhibition of axon regeneration in the mature mammalian CNS.
Table I. Some of the molecules that guide axons.
| Ligands | Receptors | Functions |
|---|---|---|
| Netrins | DCC (Deleted in colorectal cander) family: DCC, Neogenin, UNC-40, Frazzled | chemoattraction, promote outgrowth |
| Netrins | UNC5 and DCC families | chemorepulsion |
| Ephrins | Eph receptor tyrosine kinases | short-range repulsion or attraction (‘forward’ signalling) |
| Ephs | Ephrins | short-range repulsion or attraction (‘reverse’ signalling) |
| Semaphorins | Plexins, Neuropilins | generally repulsion, but some may also mediate attraction |
| Slits | Robos (Roundabouts) | short- and long-range repulsion |
| Slits | ? | branching and elongation |
| RGM | ? | short-range repulsion |
| Neurotrophins | Trk receptor tyrosine kinases, p75NTR | chemoattraction, promote outgrowth |
| HGF/SF | Met receptor tyrosine kinases | chemoattraction |
| BMPs | heterodimeric serine/threonine kinase receptors | chemorepulsion |
| Cell adhesion molecules | homophilic and heterophilic interactions | short-range attraction, fasciculation |
| Extracellular matrix (laminin, fibronectin, nidogen, etc) | integrins, proteoglycans, etc | short-range attraction and repulsion, and modifying responses to other guidance cues |
| Beats | ? | defasciculation |
| ? | receptor tyrosine phosphatases | defasciculation? |
| Nogo | NgR, ? | inhibitory component of adult myelin |
| MAG | ? | inhibitory component of adult myelin |
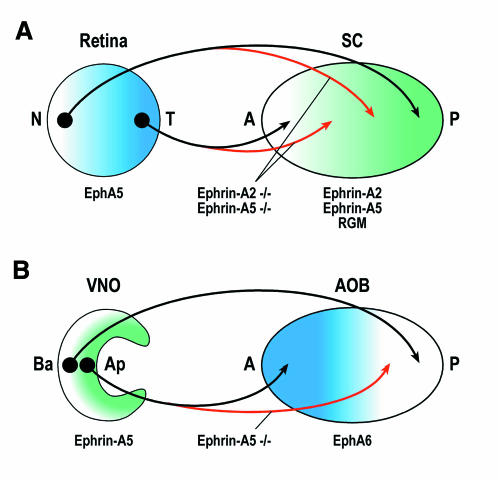
Ever since the pioneering work of Roger Sperry in the 1950s and ’60s, the retinotectal system has been an informative model system for both theoretical and experimental studies of axon pathfinding. Retinal axons project topographically to the tectum (or its mammalian equivalent, the superior colliculus): nasal retinal axons map to posterior and temporal axons to anterior tectum (Figure 1A). In the ’70s and ’80s, Friedrich Bonhoeffer and colleagues showed that temporal axons are repelled by three GPI-linked proteins expressed at high levels in the posterior but not anterior tectum. Two of these have since been identified as Ephrin-A2 and Ephrin-A5. The third, a 33 kDa protein known simply as ‘repulsive guidance molecule’, or RGM, has long remained elusive. At the Cold Spring Harbor meeting, Bernhard Mueller (Tübingen, Germany) reported the successful identification of RGM. It turns out to be a cysteine-rich protein of 434 amino acids, with no familiar structural motifs other than a predicted N-terminal signal sequence and a C-terminal GPI anchor. As anticipated, RGM, like Ephrin-A2 and Ephrin-A5, is expressed in a posterior>anterior gradient in the tectum. In vitro studies indicate that RGM is a potent repellent for temporal retinal axons, and that interfering with its function abolishes the preference of temporal axons for growth on anterior rather than posterior tectal membranes. The results of in vivo studies of RGM function are anxiously awaited. Studies of retinotopic mapping in mice lacking both Ephrin-A2 and Ephrin-A5 had hinted that there must be at least one other source of anterior–posterior positional information (Figure 1A), and RGM now emerges as a strong candidate for this additional guidance cue.
Fig. 1. Topographic mapping by Ephrin-A ligands and their EphA receptors in the mammalian visual system (A) and vomeronasal system (B). SC, superior colliculus; N, nasal; T, temporal; A, anterior; P, posterior; VNO, vomeronasal organ; AOB, accessory olfactory bulb; Ba, basal; Ap, apical. Black shows the wild type projection patterns, red shows typical projection errors in Ephrin-A2, Ephrin-A5 double mutant mice (A) or Ephrin-A5 single mutants (B).
Another major discovery reported at the meeting was the identification of a receptor for Nogo, an inhibitory component of CNS myelin that appears to be at least partly responsible for the limited potential of CNS axons to regenerate after injury or damage. Alyson Fournier and colleagues (with Stephen Strittmatter, New Haven, CT) mapped the active domain of Nogo to a 66 amino acid extracellular loop, and then fused this domain to alkaline phosphatase in order to hunt for Nogo-binding proteins encoded in an expression library prepared from adult rat brains. The protein they identified, NgR, is a 473 amino acid protein with a predicted signal sequence, a series of leucine-rich repeats, and a GPI anchor. It is highly expressed throughout the adult CNS. To test whether NgR is a functional Nogo receptor, Fournier and colleagues first asked whether cleavage of GPI-anchored proteins would block Nogo-induced collapse of dorsal root ganglion axons in vitro. It did. They then asked whether expression of NgR would be sufficient to confer Nogo responsiveness to chick E7 retinal ganglion axons—axons that normally do not respond to Nogo. This too proved to be the case. These data make a compelling case that NgR is indeed a functional Nogo receptor. But NgR is predicted to be GPI anchored, so it most likely acts together with at least one other coreceptor that transduces the Nogo signal across the membrane. Continuing the search for Nogo-binding proteins, and now also NgR-binding proteins, will no doubt soon lead to the identification of this coreceptor. In the meantime, NgR will be an essential tool for studying Nogo function in the adult, and probably also the developing nervous system. Perhaps most importantly, it should now be possible to search for small molecules that inhibit the interaction between Nogo and NgR. Such molecules could prove to be useful therapeutic agents in the treatment of brain and spinal cord injuries.
Slit proteins control axon traffic at the midline
Another powerful model system for studying axon guidance has been the midline of the developing CNS. Biochemical studies in vertebrates and genetic studies in invertebrates have led to the identification of two key families of guidance cues provided by midline cells. These are the Netrins, which act primarily as attractants, and the Slits, which act as repellents. Several presentations provided important new insights into the mechanisms of axon guidance at the midline, in particular revealing some surprising new functions for Slit and its Robo receptors.
The Drosophila Slit protein is expressed by midline glia, and its receptor Robo is expressed on axons in the two longitudinal tracts that run on each side of the midline. Axons that cross the midline transiently downregulate their Robo receptors and thus lose sensitivity to the midline repellent Slit. Once axons have crossed the midline, Robo levels are upregulated to prevent them from crossing again. This model fits nicely with the phenotypes of robo loss-of-function mutations, in which too many axons cross and recross the midline, and of robo gain-of-function mutations, in which too few axons cross.
Julie Simpson (with Corey Goodman, Berkeley, CA) and Srikanth Rajagopalan (with Barry Dickson, Vienna, Austria) presented evidence that Slit does more that just prevent longitudinal axons from crossing the midline. It also organises them into a series of parallel fascicles, each at a specific distance from the midline. Both groups identified two new Slit receptors, Robo2 and Robo3. Unlike the original Robo, which is expressed on all longitudinal axons, Robo2 is only expressed on those in the lateral third of the longitudinal tract, and Robo3 only on axons in the lateral two-thirds. Together the three Robos divide the longitudinal tract into three zones: a medial zone in which only Robo is expressed, an intermediate zone of Robo+Robo3 expression, and a lateral Robo+Robo2+Robo3 zone. Axons in each zone thus have a unique ‘Robo code’, and loss- and gain-of-function experiments show that changing this code results in predictable shifts in the lateral position of axons within the longitudinal tract.
The roles of Slit and Robo in midline crossing decisions have thus far been demonstrated only in Drosophila. At the Cold Spring Harbor meeting, Chi-Bin Chien (Salt Lake City, UT) provided genetic evidence for Robo function in regulating midline guidance events in vertebrates. Chien’s work involved the analysis of a zebrafish mutant called astray. In astray mutants, retinal axons make various guidance errors near the optic chiasm at the midline of the CNS. These errors include aberrant midline crossings, as well as wandering growth cones and axon defasciculation. Using transplantation experiments, Chien showed that astray mutations act autonomously in the retina. Mapping experiments localized the astray mutation to the Robo2 locus. Robo2 is expressed in retinal ganglion cells, consistent with the idea that it might regulate some of their guidance decisions at the optic chiasm.
The current model of Slit function is that it acts as a repellent through its Robo receptors. Elke Stein (with Marc Tessier-Lavigne, San Francisco, CA) expanded this view with the surprising demonstration that Slit can also act as a silencer of chemoattractive responses. Stein’s work took advantage of the in vitro growth cone turning assay, in which Xenopus spinal cord axons turn in response to a chemical gradient. Stein showed that while Slit itself did not induce a turning response in early stage neurons, it could nevertheless abolish their turning response towards a Netrin source. This silencing appears to be mediated by binding of Robo to the Netrin receptor DCC. Such a mechanism may function in the spinal cord to prevent commissural axons that have crossed the midline from being attracted by Netrin expressed at the floorplate. It will now be interesting to re-examine some of the known in vivo functions of Slit, asking if they might not involve repulsion, as previously thought, but rather the silencing of attraction. For example, in Drosophila robo mutants and zebrafish astray mutants, axons might cross and recross the midline not because they are insensitive to the repulsive Slit signal, but rather because they cannot shut off their attraction by Netrins.
Bidirectional and bimodal signalling by Ephrins
Even with these newly ascribed functions, the Slits are no match for the Ephrins when it comes to functional versatility. Ephrins are the membrane-anchored ligands for the Eph receptors, the largest subclass of receptor tyrosine kinases. They have been shown to mediate contact-dependent repulsion in many different processes, including rhombomere patterning, vascularization and axon guidance. As axon guidance molecules, their most celebrated role is in the establishment of topographic projections in the retinotectal (in mammals, the retinocollicular) system. Ephrins come in two flavours: GPI-anchored Ephrin-A ligands and transmembrane Ephrin-B ligands. Ephrin-A ligands bind preferentially to EphA receptors, and Ephrin-B ligands to EphB receptors. The one notable exception to this rule is the EphA4 receptor, which binds not only to Ephrin-A ligands but also Ephrin-B ligands. The Ephrin-B–EphB interaction also has the unusual property that the ligand–receptor relationship is sometimes reversed, with EphB ‘receptors’ sending a signal back through their transmembrane Ephrin-B ‘ligands’.
Klas Kullander (with Rüdiger Klein, Heidelberg, Germany) reported on a series of experiments designed to tease apart the roles of ‘forward’ and ‘reverse’ signalling in the interactions between EphA4 and its Ephrin-B ligands. EphA4 knockout mice exhibit prominent defects in the formation of both the anterior commissure (AC) and the corticospinal tract (CST). The CST delivers motor instructions to the hindlimbs, and it is thought that the defects in this system are responsible for the unusual hopping, or ‘kangaroo’, gait of these animals. To find out whether these defects are due to the loss of forward or reverse signalling by EphA4, Kullander and colleagues generated mice carrying two new EphA4 alleles. In one of these, the tyrosine kinase was rendered inactive (EphA4-KD), and, in the other, critical sites of autophosphorylation were mutated (EphA4-2F). Both the EphA4-KD and EphA4-2F alleles are predicted to disrupt forward but not reverse signalling. These mice showed the same CST defects and kangaroo gait as the null mice, but their AC formed normally. This suggested that EphA4 signals in the forward direction in the CST, and in the reverse direction in the AC. Consistent with this idea, Kullander showed that CST axons express EphA4, whereas AC axons express Ephrin-B ‘ligands’. Strikingly, CST axons avoid midline cells that express Ephrin-B3, while AC axons seem to be attracted by cells expressing EphA4. So not only the direction of signalling, but also the mode (i.e. repulsion or attraction) may be reversed in the two cases.
This theme of attraction or adhesion mediated by Ephrin–Eph interactions was taken up in several other presentations. These studies focussed on the Ephrin-A ligands that have been so well characterized as repellents in the formation of the retinotectal projection. Bernd Knöll (with Uwe Drescher, Tübingen, Germany) examined the distributions of EphA receptors and Ephrin-A ligands in the vomeronasal system, where projections are also organized topographically. Both Ephrin-A5 and EphA6 have graded distributions in the vomeronasal system, just as Ephrin-A ligands and EphA receptors do in the visual system (Figure 1). But there are two striking differences. First, the configuration is reversed, with Ephrin-A5 expressed on the vomeronasal afferents and EphA6 in the target. And secondly, the gradients are parallel. In other words, axons expressing high levels of Ephrin-A5 map to regions of high EphA6 expression, and axons expressing low Ephrin-A5 levels maps to regions of low EphA6 expression. These patterns suggest that, in the vomeronasal system, Ephrin-A5/EphA6 interactions mediate attraction or adhesion rather than repulsion. In support of such a model, Knöll reported that in mice lacking Ephrin-A5, axons that would normally project to regions of high EphA6 expression often wander into regions of lower EphA6 levels (Figure 1B).
Johan Holmberg (with Jonas Frisen, Stockholm, Sweden) noticed another defect in the Ephrin-A5 knockout mice: a failure of the neural tube to close. Cells at the edges of the dorsal neural fold express Ephrin-A5, as well as three different splice variants of the EphA7 receptor—one full-length receptor and two truncated forms that lack the kinase domain. Using a cellular assay, Holmberg could show that cells expressing the full-length EphA7 receptor avoid cells expressing Ephrin-A5, but that coexpression of both a full-length and a truncated receptor switches this response from repulsion to attraction. These data suggest that proper folding of the neural tube may depend on adhesion mediated by Ephrin-A5 and the truncated forms of EphA7.
The picture that emerges from these studies is that of an extraordinarily flexible set of bidirectional and bimodal interactions between the Ephrin ligands and their Eph receptors. The directionality and modality, it seems, are properties of the cellular context rather than of the ligands and receptors themselves.
Rac and Rho function in the growth cone
Another emerging theme at the Cold Spring Harbor meeting was the role of the small GTPases Rac and Rho in growth cone guidance. Evidence has been accumulating over recent years that these GTPases regulate actin dynamics in the growth cone. A popular idea is that attractive guidance cues stimulate Rac to promote directed outgrowth, whereas repulsive or inhibitory cues inhibit Rac and stimulate Rho to induce retraction. This is a nice idea, but there has, until now, been little direct evidence to support it.
Several presentations provided some of the first strong evidence in support of this model, and even made important steps towards defining the molecular pathways involved. For example, Steven Shamah and Michael Lin (with Michael Greenberg, Boston, MA) reported the identification of Ephexin, a Rho family guanine nucleotide exchange factor that interacts with EphA4. Biochemical studies suggest that Ephexin is constitutively associated with EphA4 receptors, and that ligand binding modulates its activity or substrate specificity. This is an attractive model, since it had previously been reported that Ephrin-A5 signalling involves the inhibition of Rac and activation of Rho. Shamah then presented evidence that Ephrin-A stimulation of EphA receptors does indeed reduce the ability of Ephexin to activate Rac. Whether this is accompanied by an increase in its activity towards Rho is still an open question.
While Ephrins inhibit Rac and activate Rho, repulsion by Semaphorins has previously been shown to require Rac activity. Why would Rac activity be required for repulsion? Data presented by Mariëtte Driessens (with Alan Hall, London, UK) offered a potential resolution to this paradox. These authors presented evidence for a direct interaction between activated Rac and the cytoplasmic domain of the Semaphorin receptor Plexin-B1. Surprisingly, however, stimulation of Plexin-B1 receptors in fibroblasts does not produce the lamellipodia one would expect if Rac were activated. Instead, it results in the formation of stress fibres, a hallmark of Rho activation. Nonetheless, the formation of these stress-fibres depended not only on Rho activity, but also on Rac. To reconcile these data, Driessens suggested that, upon ligand binding, Plexin-B receptors might sequester activated Rac and thus prevent it from associating with effectors required for lamellipodia formation. In addition, such Plexin-B–Rac complexes might also trigger activation of Rho via an unknown exchange factor.
These two studies provided a first glimpse of the signalling pathways by which Ephrin and Semaphorin repellents might antagonize Rac and stimulate Rho. On the other side of the coin, two groups presented data suggesting that attraction by the Netrin receptor DCC might involve activation of Rac. Xiaodong Li (with Nathalie Lamarche-Vane, Montreal, Canada) showed that stimulation of DCC receptors with Netrin-1 results in a 3-fold increase in Rac activation, while Zemer Gitai (with Cori Bargmann and Marc Tessier-Lavigne, San Fransisco, CA) reported that some of the defects caused by an activated form of the Caenorhabditis elegans DCC homologue, UNC-40, could be partly suppressed by mutations in one of the worm Rac genes, ced-10.
Riding the waves
Guiding axons to their targets is only the important first step in wiring up the nervous system. Activity-dependent processes then play an important role in refining this initial pattern of connections. Ever since the pioneering discovery of spontaneous waves of action potential sweeping across the retinae of newborn mammals, considerable effort has been devoted to finding out whether such waves can provide the patterns of correlated activity needed for this refinement process, and if so, how they would do it.
The role of spontaneous waves of activity in the retina was addressed at a theoretical level by Daniel Butts (with Dan Rokhsar, Berkeley, CA and Carla Shatz, Boston, MA). Using data from electrophysiological and calcium imaging studies, Butts argued that bursts of action potentials rather than single spikes are required for topographic refinement, and that the timing between bursts is essential to distinguish between retinal ganglion cells that are close together and those that are far apart. Butts proposed that relatively long periods, in the order of seconds, are required to resolve spatial differences between ganglion cells. These findings make sense because neighbouring ganglion cells on a wave front have the same pattern of activity. Therefore, for any given wave, they carry identical information. Because waves travel across the retina in different directions and at different speeds, information relevant to the topographic organization can be extracted over fairly long periods, encompassing many waves. Bursts of action potential activity that are repeatedly correlated between neighbouring ganglion cells may lead to stabilization of inputs from neighbouring ganglion cells, as required for the development of topographic projections.
Continuing on the wave theme, Julius Zhu (with Roberto Malinow, Cold Spring Harbor, NY) presented evidence that spontaneous waves of activity are required for the initial potentiation of synaptic responses in the early postnatal hippocampus. As is the case in the retina, spontaneous waves of activity are postulated to play an essential role in circuit formation in the hippocampus and cortex. At early stages of hippocampal development AMPA type glutamate receptors are composed of GluR4 subunits. Zhu and colleagues used 2-photon microscopy and electrophysiological assays to show that GluR4 tagged with GFP is delivered to synaptic sites. They then showed that synaptic delivery of these receptors depends on spontaneous waves of activity and requires the cytoplasmic tail of the GluR4 subunit. Finally, the group demonstrated that GluR4 plays only a transient role in synaptic transmission during this early window of synaptic development. Later, as the expression of GluR2 and GluR3 subunits increases, AMPA receptors composed of these subunits replace synaptic GluR4-containing receptors. These receptors then maintain synaptic strength over prolonged periods. This receptor exchange mechanism itself is activity-independent and may serve to maintain synaptic strength despite protein turnover.
Conclusion
One of the most enjoyable features of the Cold Spring Harbor meetings is that all the presentations are made by the students and postdocs who do the work. At this year’s meeting, the organizers made a welcome exception to this rule by inviting two keynote speakers. Tim Mitchison (Boston, MA) gave a talk on actin-based motility, describing some of the molecular machinery that is likely to underlie both growth cone guidance and synaptic plasticity. At the other end of the spectrum, Patricia Kuhl (Seattle, WA) discussed the ways in which infants acquire their first language, and the reasons why adults struggle to acquire a second. Neural plasticity, alas, gives way to rigidity. It may seem overly optimistic and naïve to think that the gap between these two talks might some day be bridged. Might we some day even understand the molecular basis of language acquisition? Like the subjects of Kuhl’s studies, the molecular analysis of axon guidance and neural plasticity is still in its infancy. Who knows where this exciting journey might lead?
Meeting on ‘Axon guidance and neural plasticity’ held at Cold Spring Harbor Laboratory, NY, September 20–24, 2000 (organized by Tobias Bonhoeffer, Marc Tessier-Lavigne and Larry Zipursky).
Acknowledgments
Acknowledgements
We wish to thank the organizers, Tobias Bonhoeffer, Marc Tessier-Lavigne and Larry Zipursky, and the hosts, Cold Spring Harbor Laboratories, for putting together such a great meeting. We thank those whose work we have described for their permission to do so, and the helpful suggestions they provided. Our apologies and also our thanks go to the vast majority of presenters whose work we could not include due to space and thematic restrictions—they made this an exceptionally stimulating meeting.
References
- Feller M.B. (1999) Spontaneous correlated activity in developing neural circuits. Neuron, 22, 653–656. [DOI] [PubMed] [Google Scholar]
- Flanagan J.G. and Vanderhaeghen, P. (1998) The ephrins and Eph receptors in neural development. Annu. Rev. Neurosci., 21, 309–245. [DOI] [PubMed] [Google Scholar]
- Mueller B. (1999) Growth cone guidance: first steps towards a deeper understanding. Annu. Rev. Neurosci., 22, 351–388. [DOI] [PubMed] [Google Scholar]
- O’Leary D.D., Yates, P.A. and McLaughlin, T. (1999). Molecular development of sensory maps: representing sights and smells in the brain. Cell, 96, 255–269. [DOI] [PubMed] [Google Scholar]
- Tessier-Lavigne M. and Goodman, C.S. (1996) The molecular biology of axon guidance. Science, 274, 1123–1133. [DOI] [PubMed] [Google Scholar]