Abstract
Autophagy is an intracellular bulk protein degradation system. Beclin is known to be involved in this process; however, its role is unclear. In this study, we showed that Beclin was co-immunoprecipitated with phosphatidylinositol (PtdIns) 3-kinase, which is also required for autophagy, suggesting that Beclin is a component of the PtdIns 3-kinase complex. Quantitative analyses using a cross-linker showed that all Beclin forms a complex with PtdIns 3-kinase, whereas ∼50% of PtdIns 3-kinase remains free from Beclin. Indirect immunofluorescence microscopy demonstrated that the majority of Beclin and PtdIns 3-kinase localize to the trans-Golgi network (TGN). Some PtdIns 3-kinase is also distributed in the late endosome. These results suggest that Beclin and PtdIns 3-kinase control autophagy as a complex at the TGN.
INTRODUCTION
Autophagy is an intracellular degradation system in which the cytoplasmic components are transported to the lysosome/vacuole to be degraded (Seglen and Bohley, 1992). A portion of the cytoplasm is first enclosed by a double-membrane structure, the autophagosome, which then fuses with the lysosome to become an autophagolysosome, where the contents are degraded by lysosomal hydrolases. Previously, we identified a large number of yeast mutants (apg) defective in autophagy (Tsukada and Ohsumi, 1993). Cloning of APG genes revealed that they are novel genes except for Apg6p, which is identical to Vps30p, a protein required for targeting of carboxypeptidase Y (CPY) to the vacuole (Kametaka et al., 1998). Most Apg proteins have related proteins in mammalian cells, suggesting that the molecular mechanism of autophagy is conserved in eukaryotes. However, there is only limited knowledge about autophagy in mammalian cells. Recently, Beclin, a human homolog of Vps30p/Apg6p, and one class (class III) of phosphoinositide (PI) 3-kinase were shown to be required for autophagy (Liang et al., 1999; Petiot et al., 2000).
PI 3-kinase is involved in several signal transduction pathways which regulate many diverse physiological functions, including adhesion, actin rearrangement, cell growth and cell survival by producing the signaling molecules phosphatidylinositol (PtdIns) 3,4,5-triphosphate [PtdIns(3,4,5)P3] and PtdIns(3,4)P2 (Toker and Cantley, 1997). However, the role of PtdIns(3)P, which is constitutively present, has long been unclear. The discovery that one of the yeast VPS (vacuolar protein sorting) genes, VPS34, encodes a PI 3-kinase (Schu et al., 1993) revealed an essential role of PtdIns(3)P in vesicle-mediated transport. Although Vps34p is the sole PI 3-kinase in yeast (Schu et al., 1993), several PI 3-kinases exist in mammalian cells, and can be divided into three classes (Domin and Waterfield, 1997). The class III PI 3-kinases are made up of Vps34p and its mammalian homologs. A PI 3-kinase of this class specifically phosphorylates PtdIns, but not PtdIns(4)P or PtdIns(4,5)P2, and hence is a PtdIns 3-kinase (Stack and Emr, 1994; Volinia et al., 1995). We refer to the mammalian homolog of Vps34p as PtdIns 3-kinase hereafter. In mammalian cells, soluble lysosomal hydrolases are sorted away from secretory proteins at the trans-Golgi network (TGN) (Hille-Rehfeld, 1995). Treatment of cultured cells with wortmannin or LY294002, two chemically distinct PI 3-kinase inhibitors, leads to missorting of the lysosomal enzymes to the cell surface (Brown et al., 1995; Davidson, 1995), suggesting that PtdIns 3-kinase is involved in lysosomal protein transport.
We have recently found that, in yeast, Vps34 PtdIns 3-kinase forms at least two multi-subunit complexes: one, containing Vps15p, Vps30p/Apg6p and Apg14p, functions in autophagy, and the other, containing Vps15p, Vps30p/Apg6p, and Vps38p, functions in CPY sorting (Kihara et al., 2001). In the present study, we showed that Beclin interacts with PtdIns 3-kinase by co-immunoprecipitation and cross-linking experiments. Our results suggest that, in mammals, Beclin also plays an important role in PtdIns(3)P production as a subunit of the PtdIns 3-kinase complex, which may be essential for autophagy as well as for lysosomal enzyme transport. Indirect immunofluorescence microscopy demonstrated that membrane-associated forms of Beclin and PtdIns 3-kinase localize to the trans-Golgi network (TGN). Some PtdIns 3-kinase was found in the late endosome. These results suggest that Beclin–PtdIns 3-kinase complex produces PtdIns(3)P at the TGN, which then functions in sorting of autophagosomal components and of lysosomal proteins.
RESULTS
Physical interaction between Beclin and PtdIns 3-kinase
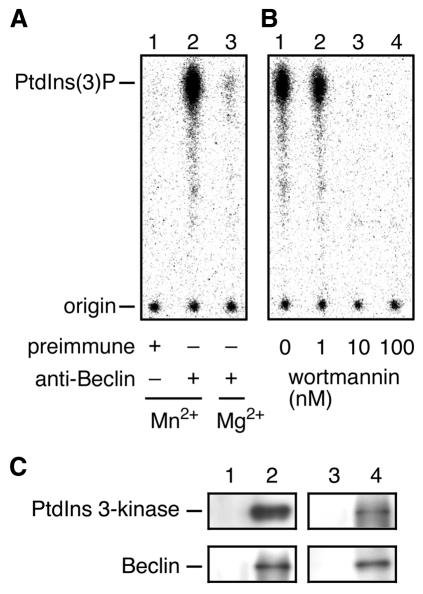
Both Beclin and PtdIns 3-kinase were shown to be required for autophagy (Liang et al., 1999; Petiot et al., 2000). However, the role of Beclin in autophagy remains unclear. Recently, we found that Vps30p/Apg6p, a yeast homolog of Beclin, is a regulatory subunit of Vps34 PtdIns 3-kinase. To test the possibility that Beclin can also function as a subunit of PtdIns 3-kinase, we first investigated the association of PI 3-kinase activity to Beclin. Cell homogenates prepared from HeLa cells were solubilized with Triton X-100 and immunoprecipitated with anti-Beclin antibodies. The immunoprecipitates were then assayed for PI 3-kinase activity. As shown in Figure 1A, PI 3-kinase activity was detected in the immunoprecipitates (lane 2). Control immunoprecipitates using preimmune sera did not show any PI 3-kinase activity (lane 1). The PI 3-kinase activity associated with Beclin was reduced when Mg2+ was used instead of Mn2+ in the reaction mixture (lane 3). The activity was completely inhibited by 10 nM wortmannin, a well-known PI 3-kinase inhibitor (Figure 1B, lane 3). The cation dependence and high sensitivity to wortmannin are strongly characteristic of PtdIns 3-kinase (Volinia et al., 1995).
Fig. 1. Beclin and PtdIns 3-kinase form a complex. (A) Cell lysates prepared from HeLa cells were solubilized with Triton X-100 and subjected to immunoprecipitation using preimmune sera (lane 1) or anti-Beclin antibodies (lanes 2 and 3). The immunoprecipitates were incubated with phosphatidylinositol, [γ-32P]ATP and 60 µM cold ATP in the presence of Mn2+ (lanes 1 and 2) or Mg2+ (lane 3) for 5 min at 30°C. (B) Immunoprecipitates of anti-Beclin antibodies prepared as described in (A) were assayed for PI 3-kinase activity in the presence of wortmannin at concentrations of 0 nM (lane 1), 1 nM (lane 2), 10 nM (lane 3), 100 nM (lane 4). The labeled lipids were extracted and separated by thin layer chromatography, followed by detection by autoradiography using a bioimaging analyzer BAS2000 (Fuji Film). (C) Cell lysates prepared from HeLa cells were solubilized with Triton X-100 and incubated with protein A-immobilized anti-Beclin (lane 2) or anti-PtdIns 3-kinase antibodies (lane 4). As controls, preimmune sera of the respective antibodies (lanes 1 and 3) were used. Retained proteins were separated by SDS–PAGE and detected by immunoblotting with anti-PtdIns 3-kinase (upper panels) and anti-Beclin (lower panels) antibodies.
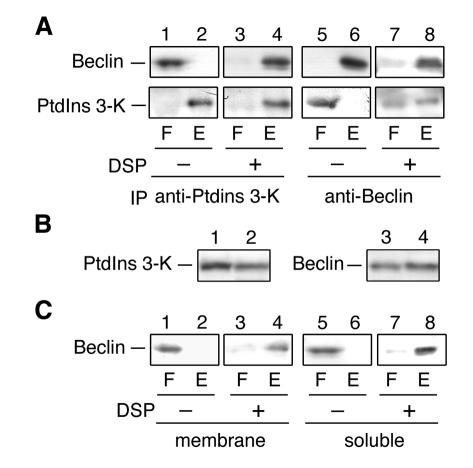
To further confirm that Beclin binds to PtdIns 3-kinase, we performed co-immunoprecipitation experiments. Immunoprecipitates of either Beclin or PtdIns 3-kinase prepared from Triton X-100-solubilized HeLa cells were separated by SDS–PAGE and detected by immunoblotting with anti-Beclin and anti-PtdIns 3-kinase antibodies. In immunoprecipitates using anti-Beclin antibodies, PtdIns 3-kinase was detected in addition to Beclin (Figure 1C, lane 2). Control immunoprecipitates using the preimmune sera contained neither Beclin nor PtdIns 3-kinase (lane 1). Complex formation of PtdIns 3-kinase and Beclin was also confirmed by immunoprecipitation using anti-PtdIns 3-kinase antibodies (lane 4). Next, we performed cross-linking experiments. Cell lysates were prepared from HeLa cells and treated with 3,3′-dithiobis-(succinimidyl propionate) (DSP), a homo-bifunctional, amino-reactive and thiol-cleavable cross-linker. After cross-linking, proteins were solubilized in SDS, immunoprecipitated with anti-Beclin or anti-PtdIns 3-kinase antibodies, and separated by SDS–PAGE under reducing conditions where cross-linked partners were cleaved. When immunoprecipitated with anti-PtdIns 3-kinase antibodies, almost all Beclin and PtdIns 3-kinase were detected in the eluant (Figure 2A, lane 4). Without cross-linking, Beclin was detected in the flow-through fraction (lane 1). In contrast, only ∼50% of PtdIns 3-kinase was immunoprecipitated with anti-Beclin antibodies when cross-linked (Figure 2A, lane 8). These results indicated that all Beclin forms a complex with PtdIns 3-kinase, whereas only ∼50% of PtdIns 3-kinase forms a complex with Beclin.
Fig. 2. Cross-linking of Beclin and PtdIns 3-kinase. (A) Total cell lysates were treated with DSP (lanes 3, 4, 7 and 8) or its solvent, dimethylsulfoxide (lanes 1, 2, 5 and 6), at 4°C for 2 h. Proteins were solubilized with SDS and subjected to immunoprecipitation using protein A-immobilized anti-PtdIns 3-kinase (PtdIns 3-K) (lanes 1–4) or Beclin (lanes 5–8) antibodies. Immunoprecipitates were eluted with 1× SDS sample buffer (62.5 mM Tris–HCl pH 6.8, 2% SDS, 10% glycerol, a trace amount of Bromophenol blue). After treatment with 5% 2-mercaptoethanol, proteins were separated by SDS–PAGE, followed by detection by immunoblotting with anti-Beclin (upper panels) and anti-PtdIns 3-kinase (lower panels) antibodies. (B) Cell lysates prepared from HeLa cells were subjected to subcellular fractionation by centrifugation at 100 000 g for 1 h. The membrane (lanes 1 and 3) and supernatant (lanes 2 and 4) fractions were subjected to SDS–PAGE, followed by immunoblotting with anti-PtdIns 3-kinase (lanes 1 and 2) and anti-Beclin (lanes 3 and 4) antibodies. (C) Membrane (lanes 1–4; 100 000 g, 30 min, pellet) and soluble fractions (lanes 5–8; 100 000 g, 30 min, supernatant) prepared from HeLa cells were treated with DSP (lanes 3, 4, 7 and 8) or dimethylsulfoxide (lanes 1, 2, 5 and 6). Proteins were solubilized with SDS and subjected to immunoprecipitation using protein A-immobilized anti-PtdIns 3-kinase antibodies. Immunoprecipitates were separated by SDS–PAGE under a reducing condition, followed by detection by immunoblotting with Beclin antibodies. F and E indicate flow-through and elute fracions, respectively.
Localization of Beclin and PtdIns 3-kinase
To reveal the localization of Beclin and PtdIns 3-kinase, we examined their subcellular distribution by centrifugation. Cell homogenates prepared from HeLa cells were centrifuged at 100 000 g, and the resulting supernatant and pellet fractions were analyzed by immunoblotting. Approximately 40% of PtdIns 3-kinase was found in the supernatant fraction (Figure 2B, lane 2), and 60% was found in the pellet fraction (lane 1). Beclin was also divided between both fractions, but Beclin was slightly more abundant in the supernatant fraction (54%) (Figure 2B, lane 4) than in the pellet fraction (46%) (lane 3). These results suggest that about half of Beclin and PtdIns 3-kinase are distributed in some membrane compartments.
To investigate whether the soluble or membrane-associated form of Beclin interacts with PtdIns 3-kinase, we performed cross-linking experiments using total membrane and soluble fractions. As shown in Figure 2C, all Beclin, regardless of its localization, was cross-linked with PtdIns 3-kinase. Thus, Beclin is stably associated with PtdIns 3-kinase. These results suggest that the Beclin–PtdIns 3-kinase complex cycles between the cytosol and the membrane.
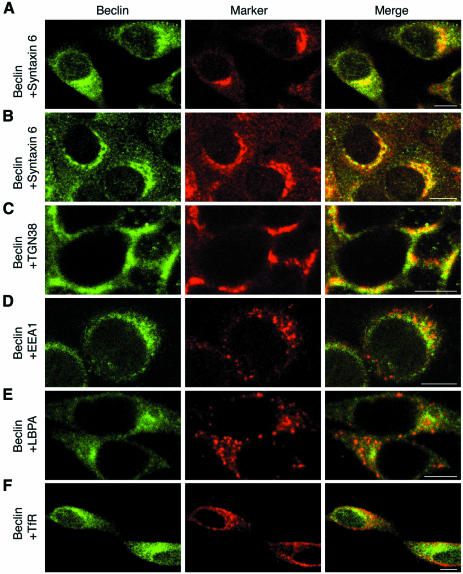
To determine the precise localization of the Beclin–PtdIns 3-kinase complex in HeLa cells, indirect immunofluorescence microscopy was performed. HeLa cells were fixed and then subjected to immunostaining by anti-Beclin antibodies. By confocal imaging, Beclin was detected throughout the cytosol and was also concentrated at the perinuclear region (Figure 3A, left panel). Removal of cytosol by permeabilization with digitonin prior to fixation improved the staining of membrane-associated Beclin (Figure 3D and 3E, left panels). Next, we double-stained HeLa cells with antibodies against syntaxin 6, a TGN marker. We observed apparent colocalization of Beclin with syntaxin 6 (Figure 3A, right panel). These results indicate that Beclin localizes to the TGN in HeLa cells. The TGN localization of Beclin was not specific to the cell line used. In BNL CL.2 cells, Beclin was also stained in a perinuclear pattern overlapping with syntaxin 6 (Figure 3B). Moreover, another well-known TGN marker, TGN38, colocalized with Beclin in H-4-II-E cells (Figure 3C). Treatment with the microtubule polymerization inhibitor, nocodazole, is known to disperse the TGN (Reaves and Banting, 1992). Under these conditions, Beclin was still associated with scattered syntaxin 6-positive structures (data not shown). In yeast, Vps30p/Apg6p, a Beclin homolog, has been proposed to act at a step essential for recycling of the sorting receptor for CPY, Vps10p, from the endosome to the TGN/late Golgi. Therefore, it is important to determine whether Beclin also localizes to endosomes in addition to the TGN. To test this, we double-stained HeLa cells with Beclin and either EEA1, an early endosome marker, lysobisphosphatidic acid (LBPA), a late endosome marker (Kobayashi et al., 1998), or transferrin receptor (TfR), a recycling endosome marker. Co-localization of Beclin with these endosome markers was hardly observed (Figure 3D–F). From these results, we concluded that Beclin localizes exclusively to the TGN, but not to endosomes.
Fig. 3. Beclin localizes to the TGN. HeLa (A, D, E and F), BNL CL.2 (B) and H-4-II-E cells (C) were grown on cover slips. Cells were permeabilized with digitonin before (C, D and E) or after (A, B and F) fixation with 3% paraformaldehyde. Cells were double-labeled with anti-Beclin (A–F, left panels) and either anti-syntaxin 6 (A and B, middle panels), anti-TGN38 (C, middle panel), anti-EEA1 (D, middle panel), anti-LBPA (E, middle panel) or anti-TfR (F, middle panel) antibodies. In the merged images (right panels), yellow indicates co-localization. Bar, 10 µm.
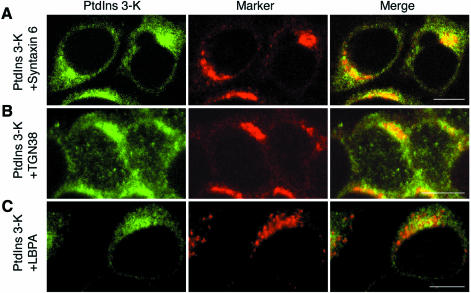
Next, we examined the subcellular localization of PtdIns 3-kinase by immunofluorescence microscopy. HeLa cells were first permeabilized to remove the cytosol, and then were subjected to fixation and immunofluorescence staining. Like Beclin, PtdIns 3-kinase showed perinuclear staining patterns in both HeLa (Figure 4A and C, left panels) and H-4-II-E cells (Figure 4B, left panel). PtdIns 3-kinase was also detected in the cytosol of cells that had not been pre-permeabilized (data not shown). We then double-stained the pre-permeabilized cells with anti-PtdIns 3-kinase antibodies and several other organelle markers. The staining pattern of PtdIns 3-kinase overlapped significantly with those of syntaxin 6 (Figure 4A) and TGN38 (Figure 4B). On the other hand, EEA1 did not co-localize with PtdIns 3-kinase (data not shown). Although most of LBPA-positive structures were not PtdIns 3-kinase-positive, partial co-localization of PtdIns 3-kinase with LBPA was detected (Figure 4C). These results indicate that PtdIns 3-kinase localizes to the TGN, although a small fraction exists in the late endosome.
Fig. 4. Immunofluorescence localization of PtdIns 3-kinase. HeLa (A and C) and H-4-II-E cells (B) were permeabilized, fixed, and then subjected to immunostaining. Cells were double-labeled with anti-PtdIns 3-kinase (A–C, left panels) and either anti-syntaxin 6 (A, middle panel), anti-TGN38 (B, middle panel) or anti-LBPA (C, middle panel) antibodies. Bar, 10 µm.
DISCUSSION
Although autophagy was discovered by electron microscopy in the 1960s in mammalian cells (Seglen and Bohley, 1992), its molecular basis is poorly understood. Recently, it was shown that Beclin and PtdIns 3-kinase are involved in mammalian autophagy (Liang et al., 1999; Petiot et al., 2000), but their precise roles are still unclear. Here, we show that Beclin forms a complex with PtdIns 3-kinase. Indirect immunofluorescence microscopy revealed that Beclin–PtdIns 3-kinase complex localizes to the cytosol and the TGN. Since the substrate of PtdIns 3-kinase, PtdIns, is a lipid molecule, the PtdIns 3-kinase complex must function on the membrane but not in the cytosol. Therefore, we propose that Beclin–PtdIns 3-kinase complex produces PtdIns(3)P on the TGN membrane.
We have found recently that, in yeast, Vps30p/Apg6p interacts with Vps34p, which is mediated by an adaptor protein, Apg14p or Vps38p (Kihara et al., 2001). These observations indicate that function of the Beclin/Vps30p–PtdIns 3-kinase/Vps34p complex is conserved. We speculate that the interaction of Beclin and PtdIns 3-kinase may also be mediated by a putative adaptor protein. It is conceivable that the production of PtdIns(3)P at the TGN is a fundamental step in the autophagy process in both yeast and mammals, although localization of the complex in yeast is unclear at this moment.
Our discovery of a Beclin–PtdIns 3-kinase complex on the TGN gives rise to a possibility that the complex sorts putative autophagosomal components at the TGN. Some proteins that must have been glycosylated at the Golgi apparatus were detected in the autophagosome (Yamamoto et al., 1990); on the other hand, Dunn (1990) reported that the compartments contain some ER membrane proteins. Therefore, it is likely that some autophagosomal proteins are supplied from the TGN, while others originate in the ER. In addition, PtdIns(3)P itself may be an essential lipid for autophagosome formation. Further work is needed to explore this model.
Roles of Beclin in lysosomal protein transport have not been examined. However, since PtdIns 3-kinase is also required for lysosomal enzyme transport (Brown et al., 1995; Davidson, 1995), it is likely that Beclin–PtdIns 3-kinase complex functions in such transport as well as autophagy, in a manner analogous to the yeast complex (Herman and Emr, 1990; Kametaka et al., 1998; Kihara et al., 2001). Thus, it remains unclear whether recently reported Beclin-dependent suppression of tumorigenesis (Liang et al., 1999) is only due to autophagy. It is possible that tumorigenesis is induced by impairment of lysosomal protein transport, or of both it and autophagy.
In this study, we showed that almost all Beclin was complexed with PtdIns 3-kinase, whereas only ∼50% of PtdIns 3-kinase was associated with Beclin. Moreover, we observed partial overlap of PtdIns 3-kinase with LBPA, a late endosome marker, in contrast to Beclin which was exclusively localized to the TGN. These findings mean that PtdIns 3-kinases may also function at endosomes for other membrane trafficking events independently of Beclin. Wortmannin treatment inhibits delivery of internalized Semliki Forest virus to the lysosome (Martys et al., 1996). Electron microscopy revealed that PtdIns(3)P is concentrated on the internal membranes of the late endosome (Gillooly et al., 2000). Therefore, it is likely that another PtdIns 3-kinase complex functions in endocytosis and multivesicular body formation. Beclin may anchor PtdIns 3-kinase to the TGN or be a regulatory component of the complex for autophagy. In conclusion, we reveal here that Beclin forms a complex with PtdIns 3-kinase and localizes to the TGN. These findings are quite important to understand the involvement of a specific lipid in formation of autophagosome and origin of the organelle.
METHODS
Cell culture. HeLa cells, H-4-II-E cells derived from rat hepatoma, and BNL CL.2 cells derived from mouse embryonic normal liver were grown in DMEM containing 10% fetal calf serum supplemented with 5 U/ml penicillin and 50 µg/ml streptomycin in a 5% CO2 incubator at 37°C.
Antibodies. Anti-Beclin and anti-PtdIns 3-kinase antisera were raised against recombinant full-length Beclin and PtdIns 3-kinase proteins expressed as His6-Myc or His6-fusion proteins, respectively. Other antibodies used were anti-syntaxin 6 (Transduction Laboratories), anti-EEA1 (Transduction Laboratories), anti-TGN38 (Affinity Bioreagents Inc.), anti-LBPA (6C4; Kobayashi et al., 1998), anti-TfR (N-2; Yoshimori et al., 1988), Cy2-labeled goat anti-rabbit IgG antibodies (Amersham Pharmacia Biotech) and Cy5-labeled goat anti-mouse IgG antibodies (Amersham Pharmacia Biotech).
Immunoprecipitation of PI 3-kinase activity. HeLa cells suspended in buffer A (50 mM HEPES–NaOH pH 7.5, 100 mM NaCl, 3 mM EGTA, 10% glycerol, 1 mM PMSF, 10 mM 2-mercaptoethanol) were lysed by sonication and solubilized with 1% Triton X-100, followed by centrifugation at 100 000 g for 30 min. The supernatant was divided into 300 µl aliquots, each of which was incubated with antisera bound to protein A–SepharoseTM CL-4B (Amersham Pharmacia Biotech) for 2 h at 4°C. The immunoprecipitates were then subjected to a PI 3-kinase assay described previously (Walsh et al., 1991).
Cross-linking. HeLa cells suspended in buffer B [50 mM HEPES–NaOH pH 7.5, 20% glycerol, 150 mM NaCl, 1 mM PMSF, 0.1 mM dithiothreitol (DTT)] were lysed by sonication and subjected to cross-linking using DSP as described previously (Kihara et al., 1996).
Subcellular fractionation. HeLa cells were washed, harvested, and lysed in buffer C [20 mM Tricine-NaOH pH 7.8, 8% sucrose, 1 mM EDTA, 1 mM PMSF, 1 mM DTT, 1× protease inhibitor mixture (Complete, EDTA-free; Roche)] by extrusion through a polycarbonate filter with 8 µm pores according to the method of Vida and Gerhardt (1999). Observation by light microscopy showed that most of the cells were disrupted with organelles apparently intact. The filter effluent was centrifuged at 300 g for 5 min to remove cell debris. The supernatant was centrifuged at 100 000 g for 1 h to generate the pellet and supernatant fractions.
Other methods. Immunofluoresence microscopic analysis was carried out as described previously (Yoshimori et al., 2000). Cells were permeabilized with 50 µg/ml digitonin for 5 min before or after fixation as indicated in figure legends. Immunoblotting was carried out as described previously (Yoshimori et al., 2000). Co-immunoprecipitaion was carried out as described (Kihara et al., 2001).
Acknowledgments
ACKNOWLEDGEMENTS
We thank Toshihide Kobayashi (RIKEN, Wako, Japan) for providing antibodies. A.K. was supported by a Japan Society for the Promotion of Science (JSPS) Research Fellowship for Young Scientists. This work was supported by grants-in-aid for Scientific Research from the Ministry of Education, Science, Culture and Sports of Japan.
REFERENCES
- Brown W.J., DeWald, D.B., Emr, S.D., Plutner, H. and Balch, W.E. (1995) Role for phosphatidylinositol 3-kinase in the sorting and transport of newly synthesized lysosomal enzymes in mammalian cells. J. Cell Biol., 130, 781–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson H.W. (1995) Wortmannin causes mistargeting of procathepsin D. Evidence for the involvement of a phosphatidylinositol 3-kinase in vesicular transport to lysosomes. J. Cell Biol., 130, 797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domin J. and Waterfield, M.D. (1997) Using structure to define the function of phosphoinositide 3 kinases family members. FEBS Lett., 410, 91–95. [DOI] [PubMed] [Google Scholar]
- Dunn W.A. Jr, (1990) Studies on the mechanisms of autophagy: formation of the autophagic vacuole. J. Cell Biol., 110, 1923–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillooly D.J., Morrow, I.C., Lindsay, M., Gould, R., Bryant, N.J., Gaullier, J.-M., Parton, R.G. and Stenmark, H. (2000) Localization of phosphatidylinositol 3-phosphate in yeast and mammalian cells. EMBO J., 19, 4577–4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman P.K. and Emr, S.D. (1990) Characterization of VPS34, a gene required for vacuolar protein sorting and vacuole segregation in Saccharomyces cerevisiae.Mol. Cell. Biol., 10, 6742–6754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille-Rehfeld A. (1995) Mannose 6-phosphate receptors in sorting and transport of lysosomal enzymes. Biochim. Biophys. Acta, 1241, 177–194. [DOI] [PubMed] [Google Scholar]
- Kametaka S., Okano, T., Ohsumi, M. and Ohsumi, Y. (1998) Apg14p and Apg6p/Vps30p form a protein complex essential for autophagy in the yeast, Saccharomyces cerevisiae. J. Biol. Chem., 273, 22284–22291. [DOI] [PubMed] [Google Scholar]
- Kihara A., Akiyama, Y. and Ito, K. (1996) A protease complex in the Escherichia coli plasma membrane: HflKC (HflA) forms a complex with FtsH (HflB), regulating its proteolytic activity against SecY. EMBO J., 15, 6122–6131. [PMC free article] [PubMed] [Google Scholar]
- Kihara A., Noda, T., Ishihara, N. and Ohsumi, Y. (2001) Two distinct Vps34 phosphatidylinositol 3-kinase complexes function in autophagy and CPY sorting in Saccharomyces cerevisiae. J. Cell Biol., 152, 519–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T., Stang, E., Fang, K.S., de Moerloose, P., Parton, R.G. and Gruenberg, J. (1998) A lipid associated with the antiphospholipid syndrome regulates endosome structure and function. Nature, 392, 193–197. [DOI] [PubMed] [Google Scholar]
- Liang X.H., Jackson, S., Seaman, M., Brown, K., Kempkes, B., Hibshoosh, H. and Levine, B. (1999) Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature, 402, 672–676. [DOI] [PubMed] [Google Scholar]
- Martys J.L., Wjasow, C., Gangi, D.M., Kielian, M.C., McGraw, T.E. and Backer, J.M. (1996) Wortmannin-sensitive trafficking pathway in chinese hamster ovary cells. J. Biol. Chem., 271, 10953–10962. [DOI] [PubMed] [Google Scholar]
- Petiot A., Ogier-Denis, E., Blommaart, E.F., Meijer, A.J. and Codogno, P. (2000) Distinct classes of phosphatidylinositol 3′-kinases are involved in signaling pathways that control macroautophagy in HT-29 cells. J. Biol. Chem., 275, 992–998. [DOI] [PubMed] [Google Scholar]
- Reaves B. and Banting, G. (1992) Perturbation of the morphology of the trans-Golgi network following Brefeldin A treatment: redistribution of a TGN-specific integral membrane protein, TGN38. J. Cell Biol., 116, 85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schu P.V., Takegawa, K., Fry, M.J., Stack, J.H., Waterfield, M.D. and Emr, S.D. (1993) Phosphatidylinositol 3-kinase encoded by yeast VPS34 gene essential for protein sorting. Science, 260, 88–91. [DOI] [PubMed] [Google Scholar]
- Seglen P.O. and Bohley, P. (1992) Autophagy and other vacuolar protein degradation mechanisms. Experientia, 48, 158–172. [DOI] [PubMed] [Google Scholar]
- Stack J.H. and Emr, S.D. (1994) Vps34p required for yeast vacuolar protein sorting is a multiple specificity kinase that exhibits both protein kinase and phosphatidylinositol-specific PI 3-kinase activities. J. Biol. Chem., 269, 31552–31562. [PubMed] [Google Scholar]
- Toker A. and Cantley, L.C. (1997) Signaling through the lipid products of phosphoinositide-3-OH kinase. Nature, 387, 673–676.9192891 [Google Scholar]
- Tsukada M. and Ohsumi, Y. (1993) Isolation and characterization of autophagy-defective mutants of Saccharomyces cerevisiae. FEBS Lett., 333, 169–174. [DOI] [PubMed] [Google Scholar]
- Vida T., and B. Gerhardt. (1999) A cell-free assay allows reconstitution of Vps33p-dependent transport to the yeast vacuole/lysosome. J. Cell Biol., 146, 85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volinia S., Dhand, R., Vanhaesebroeck, B., MacDougall, L.K., Stein, R., Zvelebil, M.J., Domin, J., Panaretou, C. and Waterfield, M.D. (1995) A human phosphatidylinositol 3-kinase complex related to the yeast Vps34p-Vps15p protein sorting system. EMBO J., 14, 3339–3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh J.P., Caldwell, K.K. and Majerus, P.W. (1991) Formation of phosphatidylinositol 3-phosphate by isomerization from phosphatidylinositol 4-phosphate. Proc. Natl Acad. Sci. USA, 88, 9184–9187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto A., Masaki, R. and Tashiro, Y. (1990) Characterization of the isolation membranes and the limiting membranes of autophagosomes in rat hepatocytes by lectin cytochemistry. J. Histochem. Cytochem., 38, 573–580. [DOI] [PubMed] [Google Scholar]
- Yoshimori T., Shimonishi, Y. and Uchida, T. (1988) Binding properties of monoclonal antibody to the cytoplasmic domain of transferrin receptor. Cell Struct. Funct., 13, 311–324. [DOI] [PubMed] [Google Scholar]
- Yoshimori T. et al. (2000) The mouse SKD1, a homologue of yeast Vps4p, is required for normal endosomal trafficking and morphology in mammalian cells. Mol. Biol. Cell, 11, 747–763. [DOI] [PMC free article] [PubMed] [Google Scholar]