Abstract
Listeria monocytogenes is an intracellular bacterial pathogen that elicits a strong cellular immune response following infection and therefore has potential use as a vaccine vector. However, while infections by L. monocytogenes are fairly rare and can readily be controlled by a number of antibiotics, the organism can nevertheless cause meningitis and death, particularly in immunocompromised or pregnant patients. We therefore have endeavored to isolate a highly attenuated strain of this organism for use as a vaccine vector. d-Alanine is required for the synthesis of the mucopeptide component of the cell walls of virtually all bacteria and is found almost exclusively in the microbial world. We have found in L. monocytogenes two genes that control the synthesis of this compound, an alanine racemase gene (dal) and a d-amino acid aminotransferase gene (dat). By inactivating both genes, we produced an organism that could be grown in the laboratory when supplemented with d-alanine but was unable to grow outside the laboratory, particularly in the cytoplasm of eukaryotic host cells, the natural habitat of this organism during infection. In mice, the double-mutant strain was completely attenuated. Nevertheless, it showed the ability, particularly under conditions of transient suppression of the mutant phenotype, to induce cytotoxic T-lymphocyte responses and to generate protective immunity against lethal challenge by wild-type L. monocytogenes equivalent to that induced by the wild-type organism.
Listeria monocytogenes is a gram-positive facultative intracellular microorganism which has been used for decades as a model pathogen for the study of cell-mediated immunity (24). Immunization of mice with a sublethal L. monocytogenes infection results in the generation of immunity which is largely major histocompatibility complex (MHC) class I mediated. Such infections generate CD8+ T cells, which can adoptively transfer immunity and specifically recognize and kill Listeria-infected target cells (3, 6, 17, 19).
Recently, the cell biology of L. monocytogenes intracellular growth has been defined (47). Subsequent to internalization, the bacteria escape from a phagocytic vacuole and replicate in the host cell cytosol. Hence, secreted proteins of L. monocytogenes are delivered directly into the cytosol and into the MHC class I pathway of antigen processing and presentation (1, 5). Mutants of L. monocytogenes which are unable to enter the cytosol are absolutely avirulent and fail to immunize mice, and cells infected by such mutants are not recognized by L. monocytogenes-immune CD8+ T cells (6, 28).
The natural properties of L. monocytogenes make it particularly attractive as a potential live vaccine vector for the induction of cell-mediated immunity to foreign antigens. Indeed, recombinant L. monocytogenes expressing such antigens successfully has been used to protect mice against lymphocytic choriomeningitis virus (15, 40) and influenza virus (22) infections and against lethal tumor cell challenge (32, 33). We have suggested the use of L. monocytogenes for the induction of cytolytic T cells directed against human immunodeficiency virus (HIV) antigens and have shown that strong cell-mediated immune responses against HIV-1 Gag protein can be induced in mice infected with recombinant L. monocytogenes carrying a chromosomal copy of the HIV-1 gag gene (13).
Because of the potential broad use of this organism as a vaccine vector in infectious disease and cancer, the safety of L. monocytogenes becomes an important issue. While infections by L. monocytogenes are fairly rare and can readily be controlled by a number of antibiotics, the organism can nevertheless cause meningitis and death, particularly in immunocompromised or pregnant patients. An ideal vaccine strain of L. monocytogenes would be absolutely avirulent but fully immunogenic. We therefore sought to isolate a mutant which could enter the cytosol but have limited growth potential both in vivo and in the environment.
d-Alanine is required for the synthesis of the mucopeptide component of the cell walls of virtually all bacteria, including L. monocytogenes (21, 23, 43), and is also found in the lipoteichoic acids of this and some other gram-positive organisms (11, 37). However, it is present in only trace quantities and fails to accumulate in vertebrates; the likely origin of these trace quantities is the breakdown products of intestinal and food bacteria (16, 20, 25, 29). We hypothesized that a strain of L. monocytogenes that is unable to synthesize this compound could be grown in the laboratory when supplemented with d-alanine but should be unable to grow outside the laboratory, particularly in the cytoplasm of eukaryotic host cells, the natural habitat of this organism during infection. The isolation of such a mutant of L. monocytogenes required the identification and inactivation of two genes, dal and dat. dal encodes alanine racemase, which catalyzes the reaction: l-alanine↔d-alanine. dat encodes d-amino acid aminotransferase, which catalyzes the reaction d-glutamic acid + pyruvate↔α-ketoglutaric acid + d-alanine. The dal dat double-mutant strain had the anticipated phenotype and in addition showed the ability to induce cytotoxic T-lymphocyte (CTL) responses and to generate protective immunity against lethal challenge by wild-type L. monocytogenes in infected mice under restricted conditions.
MATERIALS AND METHODS
Bacteria and plasmids.
L. monocytogenes 10403S (34) was the wild-type organism used in most of these studies. It was grown in brain heart infusion medium (BHI; Difco Laboratories). Escherichia coli DH5α, used for cloning, was grown in Luria broth (38). Plasmid pKSV7, used for allellic exchange reactions in L. monocytogenes, is a shuttle vector capable of replication in E. coli, where it is selected in the presence of 50 μg of ampicillin per ml of medium, and in L. monocytogenes, where its replication is temperature sensitive and selection is in the presence of 10 μg of chloramphenicol per ml of medium (42). Plasmid DNA from E. coli and total DNA (chromosomal and plasmid) from L. monocytogenes were isolated by standard methods (38).
Identification of genes in L. monocytogenes by homology.
Based on sequences of alanine racemase (dal) genes in two gram-positive organisms, Bacillus subtilis (10) and B. stearothermophilis (46), we devised consensus oligonucleotide sequences from highly conserved regions at the 5′ and 3′ ends of the gene and then modified these sequences to reflect preferred codon usage in L. monocytogenes. These 5′ and 3′ consensus oligonucleotides, 5′-GGG-[AAGCTT(HindIII)]-AAAGC(A/T)AA(C/T)GC(A/T)TATGG(A/T)CATGG-3′ and 5′-GGG-[AAGCTT(HindIII)]-GATCCAT(A/G)CAAAT(A/G)CG(A/G)CC-3′, respectively, were used as primers in a PCR using chromosomal DNA from either L. monocytogenes or B. subtilis as the template. A product of about 850 nucleotides was obtained from each. Translation of the sequenced product from the L. monocytogenes template showed that it resembled the alanine racemases of the gram-positive organisms.
A similar strategy was used to infer the presence and to sequence the central portion of a d-amino acid aminotransferase (dat) gene of L. monocytogenes, based on published sequences from B. sphaericus (12), Bacillus sp. strain YM-1 (45), and Staphylococcus haemolyticus (35). The 5′ and 3′ oligonucleotide primers were 5′-GGG-[AAGCTT(HindIII)]-GGTTATGT(A/T)TT(T/C)GGTGATGG-3′ and 5′-GGG-[AAGCTT(HindIII)]-TTTAATATCACA(A/G)CG(T/A)AA/GCC-3′, respectively. In this case, we obtained a PCR product of about 400 nucleotides whose DNA sequence and translation showed significant homology with the aminotransferase genes of the other organisms.
Strategy for sequence determination of the complete genes.
We determined the sequence of the remaining portions of the L. monocytogenes dal gene adjoined to the 5′ and 3′ ends of the original 850-bp PCR product by anchored PCRs (36). Briefly, this strategy used a BglII restriction digest (for the 5′ portion of the gene) or an XbaI digest (for the 3′ portion of the gene) of Listeria chromosomal DNA, onto the ends of which was then ligated a small fragment of DNA containing the T7 promoter. A 5′-portion PCR product and a 3′-portion PCR product were then synthesized and sequenced by using primers from within the central dal gene PCR product and a second primer homologous to the T7 promoter fragment. This procedure permitted determination of the entire sequence of the gene.
The sequence of the remainder of the dat gene was determined by use of an inverse PCR (8, 49). Briefly, a HindIII digest of Listeria chromosomal DNA was permitted to self-ligate under conditions of low DNA concentration so that mainly single circular molecules were produced. Outward-directing primers with homologies at the two ends of the original PCR segment of the gene were then used to make a new PCR product that began at the 5′ end of the original PCR segment and continued to the 5′ end of the gene, through the HindIII self-ligation site, and into the 3′ end of the gene. Using this method, we obtained the sequence of the entire gene.
Production of mutations in the dal and dat genes.
The dal gene was initially inactivated by means of a double-allelic exchange between the chromosomal gene and the temperature-sensitive shuttle plasmid pKSV7 (42) carrying an erythromycin resistance gene (39) between a 450-bp fragment from the 5′ end of the original 850-bp dal gene PCR product and a 450-bp fragment from the 3′ end of the dal gene PCR product, following the protocol of Camilli et al. (7). Subsequently a dal deletion mutant covering 82% of the gene was constructed by a similar exchange reaction with pKSV7 carrying homology regions from the 5′ and 3′ ends of the intact gene (including sequences upstream and downstream of the gene) surrounding the desired deletion. PCR analysis was used to confirm the structure of this chromosomal deletion.
The chromosomal dat gene of L. monocytogenes was inactivated by a similar allelic exchange reaction. pKSV7 was modified to carry 450-bp fragments derived by PCR from both the 5′ and 3′ ends of the intact dat gene (including sequences upstream and downstream of the gene). These two fragments were ligated by appropriate PCR. Exchange of this construct into the chromosome resulted in the deletion of 30% of the central bases of the dat gene, which was confirmed by PCR analysis.
Infection of cells in culture.
To examine the intracellular growth of the attenuated strain of L. monocytogenes in cell culture, monolayers of J774 cells, a murine macrophage-like cell line, primary murine bone marrow-derived macrophages, and the human HeLa line were grown on glass coverslips and infected as described previously (34). To enhance the efficiency of infection of HeLa cells, a naturally nonphagocytic cell line, the added bacteria were centrifuged onto the HeLa cells at 543 × g for 15 min. At various times after infection, samples of the cultures were taken for differential staining, for the determination of viable intracellular bacteria, or for immunohistochemical analysis.
Immunohistochemistry.
Coverslips with infected macrophages or HeLa cells were washed with phosphate-buffered saline, fixed in 3.2% formalin, and permeabilized with 0.05% Tween 20. Bacteria were detected with fluorescein isothiocyanate (FITC)-labeled rabbit anti-Listeria antiserum (Molecular Probes, Eugene, Oreg.) or with rabbit anti-Listeria antiserum (Listeria O Antiserum Poly; Difco Laboratories) followed by lissamine rhodamine sulfonyl chloride (LSRSC)-labeled donkey anti-rabbit antibodies or coumarin-labeled goat anti-rabbit antibodies. Actin was detected with FITC- or tetramethylrhodamine isothiocyanate (TRITC)-labeled phalloidin. To distinguish extracellular (or phagosomal) from intracytosolic bacteria, the former were stained prior to permeabilization.
Induction of LLO-specific CTLs.
Female BALB/c mice, 6 to 8 weeks of age (Charles River Laboratories, Raleigh, N.C.), were immunized by intraperitoneal inoculation with either the wild-type or dal dat strain of L. monocytogenes. After 14 days, some of the mice were boosted with a second inoculation at the same number of microorganisms. Ten or more days after the last inoculation, 6 × 107 splenocytes from a given animal were incubated in Iscove’s modified Dulbecco modified Eagle medium with 3 × 107 splenocytes from that same animal that had been loaded with 10 μM LLO peptide 91-99 during a 60-min incubation at 37°C. After 5 days of in vitro stimulation, the resulting cultures were assayed for the presence of CTL activity capable of recognizing LLO peptide-labeled P815 cells as previously described (13, 53). Every determination of lytic activity was corrected for activity on unlabeled target cells, which showed between 1 and 10% lysis in different experiments.
Animal studies.
Fifty percent lethal dose (LD50) assays were performed by injecting female BALB/c mice (Bantin-Klingman, Freemont, Calif.) at 8 weeks of age with 0.2 ml of three- to fivefold serial dilutions of bacteria as described previously (2). The LD50 of wild-type L. monocytogenes strain 10403 in these animals is approximately 104. The LD50 of the dal dat double-mutant strain of L. monocytogenes was found to be >8 × 108 or, when injected in the presence of 20 mg of d-alanine in the 0.2-ml injection volume, approximately 7 × 107.
To examine the protection produced by immunization with the dal dat mutant, groups of four to five BALB/c mice were injected with viable wild-type or dal dat double-mutant bacteria (in the presence or absence of 20 mg of d-alanine) by tail vein injection. Three to four weeks following immunization, the mice were challenged with approximately 10 LD50 of viable wild-type L. monocytogenes 10403 in 0.2 ml by tail vein injection. Spleens were removed 48 h later and homogenized individually in 4.5 ml of phosphate-buffered saline–1% proteose peptone in a tissue homogenizer (Tekmar). The homogenates were serially diluted and plated onto BHI agar. Log10 protection was determined by subtracting the mean of the log10 CFU/spleen values of the test group from the mean of the log10 CFU/spleen values of the untreated control group.
Nucleotide sequence accession numbers.
The nucleotide sequences of the L. monocytogenes dal and dat genes, shown in Fig. 1 and 3, respectively, have GenBank accession no. AF038438 and AF038439.
FIG. 1.
Nucleotide sequence and translation of the alanine racemase (dal) gene of L. monocytogenes. The gene was inactivated either by insertion of a 1.35-kb fragment of DNA encoding erythromycin resistance at a SpeI site at nucleotide 517 or by deletion of nucleotides 44 to 948.
FIG. 3.
Nucleotide sequence and translation of the d-amino acid aminotransferase (dat) gene of L. monocytogenes. The gene was inactivated by deletion of nucleotides 370 to 636.
RESULTS
Construction of a strain of L. monocytogenes defective in cell wall synthesis.
We determined whether L. monocytogenes harbors genes expected to be required for the synthesis of d-alanine. The alanine racemase (dal) gene, used by many microorganisms for the synthesis of d-alanine, has been sequenced in Salmonella typhimurium (14, 51), B. subtilis (10), and B. stearothermophilis (46) but has not been found in L. monocytogenes. To search for evidence of the gene in this organism, we synthesized primers based on the sequences (adjusted for preferred codon usage in L. monocytogenes) of two highly conserved regions of the gene present in the two gram-positive organisms and used these in a PCR on L. monocytogenes chromosomal DNA. A product that showed significant homology with the published dal gene sequences was obtained. The sequence of the remainder of the L. monocytogenes dal gene was determined (see Materials and Methods) and is shown in Fig. 1. The translated protein sequence showed 44 to 53% identity with the alanine racemase proteins of the gram-positive microorganisms and is shown in comparison with these sequences in Fig. 2.
FIG. 2.
Linear alignment of deduced protein sequences of alanine racemases of L. monocytogenes (LMDAL), B. stearothermophilus (BSTDAL), and B. subtilis (BSUBDAL). Identical amino acids are boxed.
The gene was inactivated by insertion of a 1.35-kb fragment of DNA encoding erythromycin resistance at a SpeI site near the center of the gene. The resulting dal bacteria were found to grow both in rich bacteriological medium (BHI) and in a synthetic medium (48) in the presence or absence of d-alanine (not shown). A mutation of the dal gene constructed by an in-frame deletion covering 82% of the gene (from nucleotides 44 to 949) had the same properties.
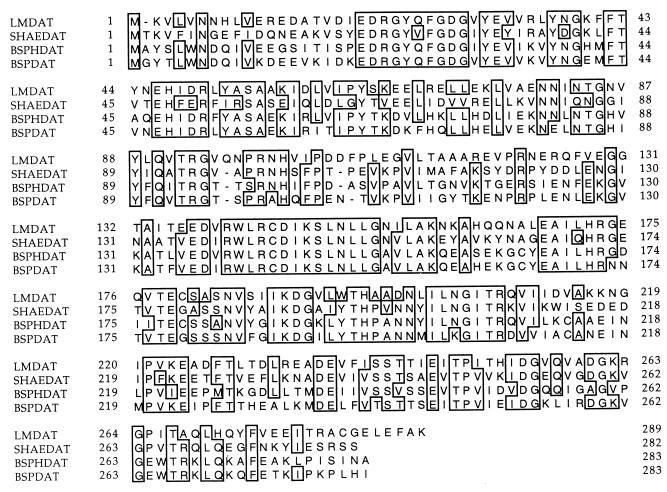
A second enzyme used by some bacteria for synthesis of d-alanine is d-amino acid aminotransferase, encoded by the dat gene (12, 35, 45). Using a strategy similar to that used to detect the dal gene in L. monocytogenes, we obtained a PCR product that showed significant sequence homology with known dat genes and gene products. The sequence of the remainder of the dat gene was determined (see Materials and Methods) and is shown in Fig. 3. Its deduced protein sequence showed 49 to 51% identity with sequences of aminotransferases of other gram-positive organisms. Comparison of these dat gene products is shown in Fig. 4.
FIG. 4.
Linear alignment of deduced protein sequences of d-amino acid aminotransferases of L. monocytogenes (LMDAT), S. haemolyticus (SHAEDAT), B. sphaericus (BSPHDAT), and Bacillus sp. strain YM-1 (BSPDAT). Identical amino acids are boxed.
This gene was inactivated by in-frame deletion of 31% of its central region (from nucleotides 370 to 636). The growth of the resulting dat bacteria in broth and synthetic media was again found to be independent of the presence of d-alanine.
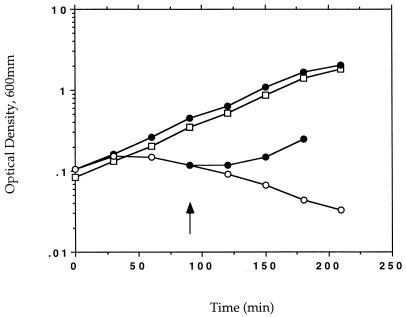
A dal dat double-mutant strain of L. monocytogenes was produced by a double-allelic exchange reaction between the erythromycin-resistant dal organism and the shuttle vector carrying the dat gene deletion. The growth of the resulting double mutant in bacteriological media was found to be completely dependent on the presence of d-alanine (Fig. 5). A double mutant containing deletions in both of the genes had the same phenotype. The growth defect of the double-deletion strain in the absence of d-alanine could be complemented by a plasmid carrying the dal gene of B. subtilis (not shown). All of the experiments reported used the single-deletion strain.
FIG. 5.
Growth requirement for d-alanine of the dal dat double-mutant strain of L. monocytogenes. The dal dat (○, •) and wild-type (□) strains of L. monocytogenes were grown in liquid culture (BHI medium) in the presence (•) or absence (○, □) of exogenous d-alanine (100 μg/ml) at 37°C. An aliquot of the mutant culture was provided d-alanine at 90 min. The starting cultures were in log phase of growth. As d-alanine had no effect on the growth of wild-type L. monocytogenes, those data are not shown.
Defective growth of the dal dat double mutant in eukaryotic cells.
Our original hypothesis was that a defect in the ability of L. monocytogenes to synthesize d-alanine would be expressed as an inability to replicate in the cytoplasm of eukaryotic cells due to the absence of the required substrate at that site. To test this hypothesis, several cell lines and primary cells in culture were examined after infection with the wild-type and mutant strains of the organism.
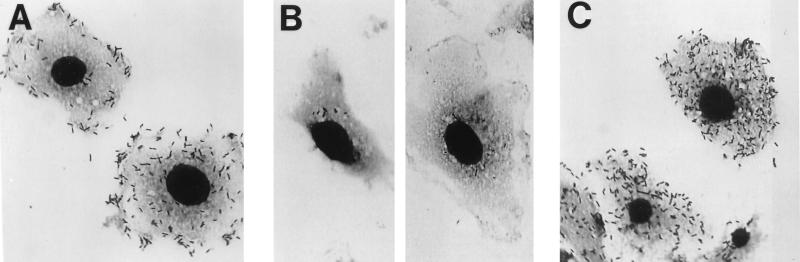
J774 cells are mouse macrophage-like cells that readily take up L. monocytogenes by phagocytosis and permit its cytoplasmic growth following escape of the bacteria from the phagolysosome (47). Figures 6A and B show typical cells seen at 5 h after infection with wild-type L. monocytogenes and with the dal dat double mutant, respectively. Whereas large numbers of bacteria were associated with those mouse cells infected with the wild-type strain, few bacteria could be found in any cells following infection with the double mutant. Infection by double-mutant bacteria in culture medium containing d-alanine permitted bacterial growth indistinguishable from that seen after wild-type infection (Fig. 6C).
FIG. 6.
Light micrographs showing the growth of wild-type (A) and dal dat double-mutant (B) strains of L. monocytogenes in J774 macrophages at 5 h after infection (at approximately 5 bacteria per mouse cell). (C) Infection by double-mutant bacteria in the continuous presence of d-alanine (80 μg/ml).
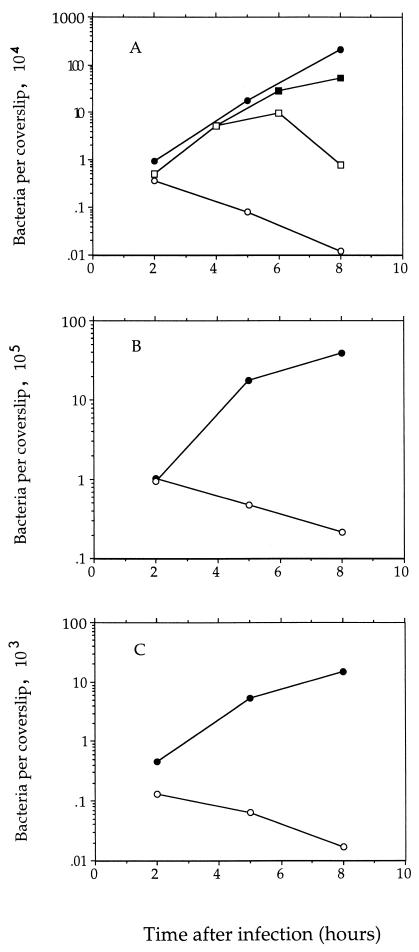
The number of intracellular bacteria (defined by gentamicin resistance) that could form colonies on medium containing d-alanine was determined at several times after infection (at a multiplicity of infection [MOI] of about 0.05 bacteria per mouse cell) (Fig. 7A). The data clearly show that the double mutant was unable to replicate in J774 cells and in fact slowly died during the course of the experiment. Figure 7A also shows that the replication-defective phenotype of the double mutant could be suppressed by the inclusion of d-alanine (at 100 μg/ml) in the tissue culture medium at the time of infection and that the suppression was reversed 2 h after removal of the d-alanine. We also examined the phenotype of the mutant bacteria in primary mouse bone marrow-derived macrophages and in the HeLa line of human epithelial cells and found that the double mutant was unable to replicate in either of those cell types as well (Fig. 7B and C).
FIG. 7.
Infection of mammalian cells with the dal dat double-mutant (○) and wild-type strains of L. monocytogenes (•). (A) J774 murine macrophage-like cells (MOI of about 0.05). Mutant infection in one culture (■) was in the continuous presence of d-alanine (100 μg/ml); cells in an aliquot of that culture (□) were resuspended in d-alanine-free medium at 4 h. (B) Primary murine bone marrow-derived macrophages (MOI of about 5). (C) Human epithelial cells (HeLa) (MOI of about 5). Starting cultures of L. monocytogenes were in stationary phase of growth.
Within a few hours after infection of cells by L. monocytogenes when the bacteria have escaped from the phagosome, host actin filaments form a dense cloud around intracytosolic bacteria and then rearrange to form a polarized tail which propels the bacteria through the cytoplasm (9, 47). The bacterium-associated actin can readily be visualized by fluorescence-tagged phalloidin, while total bacteria can be detected with appropriately labelled anti-Listeria antibodies. To determine the intracytoplasmic status of the double-mutant bacteria following infection, we examined the distribution of cytoplasmic actin in the infected cells.
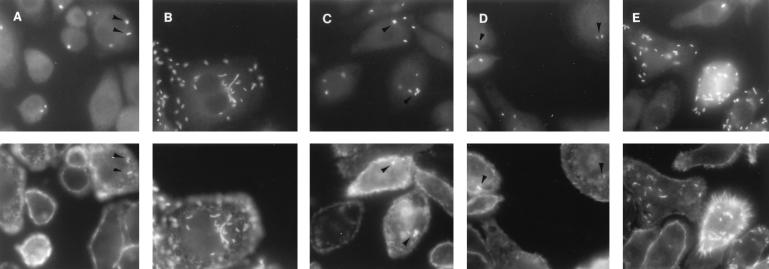
As shown in Fig. 8A and Table 1, at 2 h after infection (at an MOI of about 5 bacteria per cell), we found that 25.2% of wild-type bacteria associated with J774 macrophages were surrounded with a halo of stained actin and therefore were intracytosolic. By 5 h, 100% showed actin staining, some with long actin tails (Fig. 8B). However, the staining of actin in double-mutant-infected macrophages was much rarer (less than 2%). Nevertheless, if d-alanine was present during only the 30-min period of bacterial adsorption, at 2 h after infection 22% of the cell-associated mutant bacteria were surrounded with actin (Fig. 8C). At 5 h (d-alanine absent from 0.5 to 5 h), the number of intracytosolic bacteria was still only 26.7% (Fig. 8D). This indicated that few additional bacteria had entered the cytosol after removal of the d-alanine and that any bacteria already present in the cytosol had not replicated. If d-alanine was present during the entire infection (Fig. 8E), the results at 2 and 5 h were virtually indistinguishable from the wild-type infection.
FIG. 8.
Association of actin with intracytosolic wild-type L. monocytogenes (A, 2 h; B, 5 h) or with the dal dat double mutant (C, 2 h with d-alanine [100 μg/ml] present from 0 to 30 min; D, 5 h with d-alanine present from 0 to 30 min; E, 5 h with d-alanine present continuously) following infection of J774 cells. Photomicrographs in the top row show the binding of FITC-labeled antilisterial antibodies to total bacteria; those below show the binding of TRITC-labeled phalloidin to actin. Arrowheads indicate some actin-associated bacteria in the sparsely infected cells.
TABLE 1.
Intracytoplasmic status of bacteria following infection of J774 cells and bone marrow-derived macrophages
| Cells | % Intracytosolic bacteriaa
|
|
|---|---|---|
| 2 h | 5 h | |
| J774 | ||
| L. monocytogenes wild type | 25.2 (35.6) | 100 (100) |
| dal dat mutant | ||
| 5 h d-alab | 22.7 (44.4) | 95 (95) |
| 30 min d-alac | 22.0 (37.5) | 26.7 (47.6) |
| No d-ala | <2 (<5) | <2 (<5) |
| Bone marrow macrophages | ||
| L. monocytogenes+ wild type | 20.0 (68.0) | 75.8 (77.8) |
| dal dat mutant | ||
| 5 h d-ala | 19.4 (65.4) | 75.0 (80.0) |
| 30 min d-ala | 18.2 (52.9) | 24.8 (46.4) |
| No d-ala | 6.8 (36.4) | 13.6 (40.6) |
Percentage of total cell-associated bacteria that are intracytosolic, as defined by their staining for bacteria-associated actin. Numbers in parentheses represent percentages of infected macrophages containing intracytosolic, actin-associated bacteria.
d-Alanine (100 μg/ml) was present throughout the infection.
d-Alanine (100 μg/ml) was present only during the first 30 min of infection.
Since J774 cells have been culture adapted and reflect few of the normal properties of tissue macrophages, we examined the entry of the mutant bacteria into the cytosol of primary bone marrow-derived macrophages which had been in culture for only 6 days. Because these cells show significant bacterial killing capacity, they were infected at a ratio of about 50 bacteria per cell. In this experiment, at 2 h after infection, 6.8% of the double-mutant bacteria were found to be associated with actin, and this number increased to the level seen after wild-type infection (20%) by inclusion of d-alanine during the first 30 min of the infection (18.2%) or during the entire 2-h infection (19.4%). Therefore, depending on the cell type examined, mutant bacteria in the absence of d-alanine had either a low or a moderate efficiency of entering the host cytosol (or, alternatively, showed reduced binding of actin onto their surface). However, the brief presence of d-alanine during the initial phase of infection allowed a normal fraction of bacteria to enter the cytosol and bind actin.
In vivo attenuation of the dal dat double mutant.
The LD50 of wild-type L. monocytogenes in BALB/c mice is approximately 104 (2). To determine the virulence of the dal dat double-mutant strain relative to wild-type bacteria, groups of mice were injected with graded doses of the mutant organism. The LD50 of the double mutant was found to be greater than 8 × 108, indicating that this strain was highly attenuated. When the inoculum in these experiments contained d-alanine (20 mg), the LD50 was found to be lowered 10-fold to approximately 7 × 107.
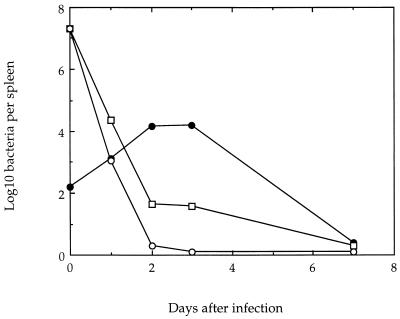
After sublethal infection of mice with wild-type L. monocytogenes, the bacteria survive and replicate in the spleens and livers of infected animals for up to 5 to 7 days, followed by the onset of a sterilizing immunity. The extent of the in vivo persistence of the mutant bacteria in the spleens of infected animals was therefore examined following infection with 2 × 107 mutant bacteria and compared with the result of infection by 4 × 102 wild-type organisms. The results in Fig. 9 show that whereas this low dose of wild-type L. monocytogenes resulted in a peak of replication at 2 to 3 days, increasing the number of bacteria in this organ by several logs, the mutant bacteria fell to almost undetectable levels within 2 days. The presence of d-alanine in the inoculum allowed a small number of organisms to survive somewhat longer.
FIG. 9.
Recovery of bacteria from spleens of BALB/c mice following sublethal infection with wild-type L. monocytogenes (•), the dal dat mutant in the absence of d-alanine (○), and the dal dat mutant in the presence of 20 mg of d-alanine in the inoculation fluid (□). The points at day 0 show the total number of viable organisms injected, not bacteria per spleen.
Induction of an immune response with the dal dat double mutant.
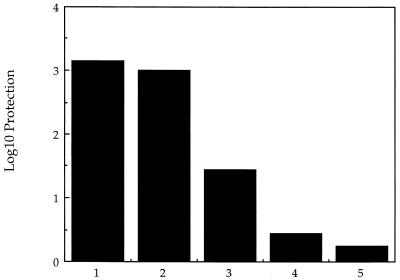
Infection of mice by L. monocytogenes produces a long-lived state of specific immunologic memory that enables the infected host to resist lethal challenge by the same organism for months after the primary infection. We determined whether infection of mice with sublethal doses of the dal dat bacteria could induce this important long-lasting state of protective immunity. Mice were injected intravenously with 2 × 107 double-mutant bacteria and challenged 3 to 4 weeks later with 10 LD50 of wild-type L. monocytogenes. d-Alanine (20 mg) was provided in the initial inoculum of mutant organisms to be certain that the organisms were fully viable at the time of initial infection. The data in Fig. 10 shows that following a single infection with the mutant bacteria, the level of antilisterial protection was approximately 3 log10, similar to the protection generated by immunization with 4 × 102 wild-type organisms. Infection with 2 × 107 mutant bacteria without d-alanine provided little protection. The almost complete protection obtained with mutant bacteria occurred despite the fact that by 2 days postinfection more than 100-fold-fewer bacteria were detected in the spleens of mutant-infected mice than in animals infected with wild-type organisms (Fig. 9).
FIG. 10.
Protection of BALB/c mice against challenge with 10 × LD50 of wild-type L. monocytogenes by immunization with the dal dat double-mutant strain of L. monocytogenes. Column numbers represent groups of five mice immunized with the following organisms: 1, 4 × 102 CFU of wild-type L. monocytogenes; 2, 2 × 107 CFU of dal dat mutant (plus 20 mg of d-ala), 3, 2 × 105 CFU of dal dat mutant (plus d-ala); 4, 2 × 104 CFU of dal dat mutant (plus d-ala), 5, 2 × 107 CFU of dal dat mutant (no d-ala). Mice were challenged 21 to 28 days later. Log10 protection was calculated as described in Materials and Methods. The largest error seen in all mouse groups was 0.17 log10.
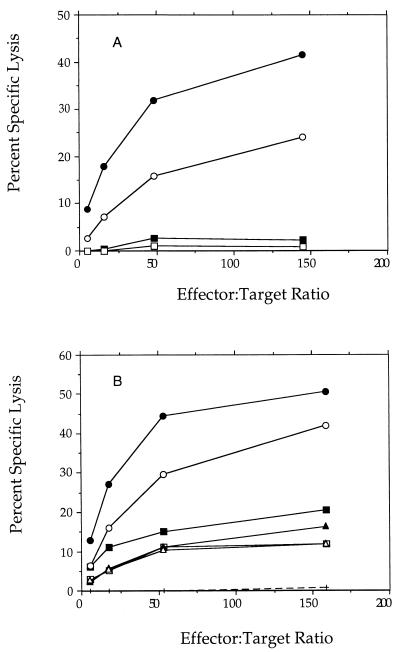
LLO peptide 91-99 is the major epitope of the LLO protein and one of the major epitopes to which mice respond when mounting a cell-mediated immune response against L. monocytogenes infection (4, 17, 31). To determine whether the protective immunity generated by infection with the attenuated dal dat bacteria was associated with the induction of cytolytic T cells, splenocytes from infected animals were assayed for the ability to lyse target cells loaded with this peptide. Figure 11B shows that animals that had been infected intraperitoneally with 3 × 107 double-mutant bacteria and provided d-alanine subcutaneously (40 mg) before and after the infection showed strong CTL responses directed against the LLO peptide. Likewise, mice provided with d-alanine in their drinking water (0.2 or 2 mg/ml) before and after infection mounted a modest CTL response after single infection with 3 × 107 mutant bacteria. In the absence of d-alanine, animals infected and boosted one time with 3 × 107 double-mutant bacteria, or animals singly infected with 3 × 108 bacteria (data not shown), also showed a modest CTL response to LLO peptide 91-99. Single infection with 3 × 107 double-mutant bacteria in the absence of d-alanine produced no significant response (Fig. 11B).
FIG. 11.
Cytolytic activity of splenocytes isolated from mice 10 to 14 days after infection with wild-type L. monocytogenes (•, ○) or naive controls (■, □) (A). Open and closed symbols represent independent experiments; d-alanine was not provided in either experiment. (B) dal dat double mutant: 3 × 107 bacteria (+), 3 × 107 bacteria with boost at 10 days (▴, ▵); 3 × 107 bacteria with animals provided d-alanine subcutaneously (40 mg at −6 h, time of infection, 6 h, and 12 h) (•, ○), 3 × 107 bacteria with d-alanine (2 mg/ml; ■) or d-alanine (0.2 mg/ml; □) in drinking water from 24 h before infection to 36 h post-infection. Open and closed symbols represent independent experiments.
DISCUSSION
L. monocytogenes is a gram-positive facultative intracellular bacterium which has been used for decades as a model for the study of cell-mediated immunity (6, 26, 27). Long-term resistance to infection by this microorganism resides primarily in the MHC class I-restricted CD8+ T cells that are induced following primary infection (3, 17). These cells act by directly lysing antigen-expressing target cells, as well as through the action of the cytokines gamma interferon and tumor necrosis factor alpha (18). We and other investigators have been exploring the use of recombinant forms of L. monocytogenes as a vehicle for the delivery of foreign antigens into the MHC class I pathway of antigen presentation (13, 15, 22, 32, 33, 40).
This strategy for vaccine development suffers, however, from the known pathogenicity of the organism, which is the cause of listeriosis, a food-borne disease characterized by meningitis, septicemia, abortion, and often a high rate of mortality. We therefore attempted to develop a suitably attenuated form of L. monocytogenes that could be used as a safe vaccine and adjuvant. Since virtually all bacterial species contain cell wall components that are unique to these organisms, we deduced that a strain of L. monocytogenes unable to synthesize one of these components, d-alanine (21, 43), would be crippled unless specifically supplied with this substrate.
d-Alanine appears to be synthesized by different pathways in different organisms. E. coli and S. typhimurium possess two weakly homologous alanine racemases, one constitutive and one inducible, which can convert l-alanine to d-alanine. d-Alanine produced by the first enzyme is apparently used for peptidoglycan formation, while the latter enzyme may be utilized to provide substrate to a d-alanine dehydrogenase that converts the compound to pyruvate and ammonia (50, 52). In S. typhimurium, a mutation in either gene alone permits the synthesis of sufficient d-alanine for cell growth, while a double mutant displays the expected phenotype of an exogenous d-alanine requirement (50). In two Bacillus species, alanine racemases have also been identified (10, 46). Mutation of this gene in B. subtilis leads to a d-alanine requirement only when the bacteria are grown in rich broth or in synthetic media that contain l-alanine (10). A second enzyme, d-amino acid aminotransferase, that can convert d-glutamic acid and pyruvate to α-ketoglutarate and d-alanine has also been identified in several gram-positive species and could be a source of d-alanine (12, 35, 45). Indeed, we have found that in L. monocytogenes, both a racemase gene and an aminotransferase gene are present, and both genes must be inactivated in order to produce a requirement for exogenous d-alanine.
The dal dat double mutant was found to be unable to replicate in bacteriological culture media devoid of added d-alanine. It was also unable to replicate following infection of several different lines of eukaryotic cells growing in standard tissue culture media. On infection of BALB/c mice with 107 of these bacteria, few organisms survived for longer than 1 to 2 days. Consequently, the dal dat strain was completely attenuated in BALB/c mice and showed an LD50 in these animals of >8 × 108 (compared with 104 for the wild-type organism). These results support the view that if any d-alanine is present in eukaryotic cells and in mice, the levels are below the threshold required for growth of the d-alanine-requiring strain of Listeria.
Can this attenuated strain of L. monocytogenes induce an immune response in mice? When ∼0.1 LD50 of the double mutant was administered intravenously in the absence of d-alanine, little protection against a lethal challenge by the wild-type organism was obtained. Likewise, this dose of the double mutant (given intraperitoneally) produced no detectable Listeria-specific CTL response. A booster infection of mice produced a modest increase in the Listeria-specific CTL response, as did single immunization with a higher dose of the mutant organisms. These weak responses were not surprising in view of the complete absence of any replication of these bacteria in eukaryotic cells. Indeed, the lack of d-alanine might in addition cause down-regulation of the synthesis of enzymes necessary for entry of the microorganism to the host cytosol, the minimum requirement for induction of a cell-mediated immune response. Our assays for intracytosolic bacteria (by the detection of binding of cytoplasmic actin) suggested that their entry to the cytosol was far less efficient than for wild-type L. monocytogenes.
However, the presence of d-alanine at the time of infection of cells in culture allowed the normal number of the double-mutant bacteria to enter the cytosol of infected cells. Additionally, we showed that eukaryotic cells were able to take up sufficient d-alanine from their growth medium to satisfy the growth requirement of the double-mutant bacteria. This uptake was reversible, so that when d-alanine was subsequently removed from the medium, the attenuation phenotype returned within several hours. It seemed possible that a similar paradigm could function in vivo to produce a novel means of in vivo suppression of the attenuating double mutation, resulting in both entry to the cytosol and transient survival and replication of the organism to generate a moderate to strong immune response. In vertebrates, d-amino acids, which originate almost exclusively from intestinal and food bacteria, are efficiently removed from the system by the action of d-amino acid oxidases (16, 20, 25, 29).
Indeed, intravenous inoculation of these organisms (at ∼0.1 LD50 in the presence of d-alanine) led to virtually full protection against a lethal challenge by wild-type organisms. Likewise, transient suppression of the defective phenotype of the double mutant by providing the host with d-alanine by subcutaneous injection or by inclusion in the drinking water at the time of intraperitoneal infection produced moderate or strong CTL responses against the major L. monocytogenes T-cell epitope, LLO peptide 91-99. Transient suppression of the attenuation thus appears to be an effective mechanism to produce a good immune response to these organisms.
The strong response seen in the protection experiment suggests that protective immunity is adequately stimulated within the first 1 or 2 days of infection, since after this time the bulk of the attenuated bacteria are gone. This result appears to differ from an early observation that showed that ampicillin administered 24 h after L. monocytogenes infection greatly reduced protection against subsequent challenge (30). A significant difference between these two experiments is the larger mass of antigen administered in 0.1 LD50 of our attenuated strain.
Other modifications of L. monocytogenes have been suggested for use as vaccine vectors. Mutations in the hly gene produce a defective hemolysin and prevent the ingested organism from escaping into the host cytosol. Such mutants can be completely avirulent (28), but they fail to present antigens to CD8+ T cells (6) and therefore are a poor choice of vector for potential induction of CTL responses. It has been reported that heat-killed L. monocytogenes, which also should fail to enter the cytosol of infected cells, was able to induce protective CD8+ T lymphocytes under appropriate circumstances. However, protection was short-lived (44). actA mutants are able to grow in the cytoplasm of infected cells but, because they fail to nucleate host actin, are unable to propagate the infection through cell-to-cell spread. These bacteria are capable of inducing effective CTL responses (15). However, such mutants are still virulent and persist for up to 7 days in the livers of infected mice. They also grow at normal rates in standard media; such growth represents a continuing source of bacteria that might through various genetic mechanisms become altered to regain greater virulence or even, unreverted, show virulence under unanticipated circumstances.
The hyperattenuated dal dat strain of L. monocytogenes described in this report, in which the pathways for synthesis of d-alanine have been abolished, appears to provide good immunogenicity while being unable to replicate in infected animals or in usual media and thus may represent a useful tool as a safe vaccine and adjuvant. The use of cell wall auxotrophy, as exploited here, has been explored previously as a mechanism for attenuation of virulence in Shigella (41).
ACKNOWLEDGMENTS
We appreciate the continuous interest and insights of Y. Paterson and M. Moors.
This study was supported by a University of Pennsylvania Research Foundation award (F.R.F.) and National Institutes of Health grants AI-26919 and AI-27655 (D.A.P.).
REFERENCES
- 1.Ada G L. The immunological principles of vaccination. Lancet. 1990;335:523–526. doi: 10.1016/0140-6736(90)90748-t. [DOI] [PubMed] [Google Scholar]
- 2.Barry R A, Bouwer H G A, Portnoy D A, Hinrichs D J. Pathogenicity and immunogenicity of Listeria monocytogenes small-plaque mutants defective for intracellular growth and cell-to-cell spread. Infect Immun. 1992;60:1625–1632. doi: 10.1128/iai.60.4.1625-1632.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bishop D K, Hinrichs D J. Adoptive transfer of immunity to Listeria monocytogenes: the influence of in vitro stimulation on lymphocyte subset requirements. J Immunol. 1987;139:2005–2009. [PubMed] [Google Scholar]
- 4.Bouwer H G A, Hinrichs D J. Cytotoxic-T-lymphocyte responses to epitopes of listeriolysin O and p60 following infection with Listeria monocytogenes. Infect Immun. 1996;64:2515–2522. doi: 10.1128/iai.64.7.2515-2522.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braciale T J, Morrison L A, Sweetser M T, Sambrook J, Gething M J, Braciale V L. Antigen presentation pathways to class I and class II MHC-restricted T lymphocytes. Immunol Rev. 1987;98:95–114. doi: 10.1111/j.1600-065x.1987.tb00521.x. [DOI] [PubMed] [Google Scholar]
- 6.Brunt L M, Portnoy D A, Unanue E R. Presentation of Listeria monocytogenes to CD8+ T cells requires secretion of hemolysin and intracellular bacterial growth. J Immunol. 1990;145:3540–3546. [PubMed] [Google Scholar]
- 7.Camilli A, Tilney L G, Portnoy D A. Dual roles of plcA in Listeria monocytogenes pathogenesis. Mol Microbiol. 1993;8:143–157. doi: 10.1111/j.1365-2958.1993.tb01211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins F S, Weissman S M. Directional cloning of DNA fragments at a large distance from an initial probe: a circularization method. Proc Natl Acad Sci USA. 1984;81:6812–6816. doi: 10.1073/pnas.81.21.6812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dabiri G A, Sanger J M, Portnoy D A, Southwick F S. Listeria monocytogenes moves rapidly through the host-cell cytoplasm by inducing directional actin assembly. Proc Natl Acad Sci USA. 1990;87:6068–6072. doi: 10.1073/pnas.87.16.6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrari E, Henner D J, Yang M Y. Isolation of an alanine racemase gene from Bacillus subtilis and its use for plasmid maintenance in B. subtilis. Bio/Technology. 1985;3:1003–1007. [Google Scholar]
- 11.Fischer W. Bacterial phospholipids and lipoteichoic acids. In: Kates M, editor. Glycolipids, phosphoglycolipids, and sulfoglycolipids. New York, N.Y: Plenum Press; 1990. pp. 123–234. [Google Scholar]
- 12.Fotheringham, I. G., P. P. Taylor, and S. A. Bledig. 1995. Unpublished data.
- 13.Frankel F R, Hedge S, Lieberman J, Paterson Y. Induction of cell-mediated immune responses to human immunodeficiency virus type 1 Gag protein by using Listeria monocytogenes as a live vaccine vector. J Immunol. 1995;155:4775–4782. [PubMed] [Google Scholar]
- 14.Galakatos N G, Daub E, Botstein D, Walsh C T. Biosynthetic alr alanine racemase from Salmonella typhimurium: DNA and protein sequence determination. Biochemistry. 1986;25:3255–3260. doi: 10.1021/bi00359a026. [DOI] [PubMed] [Google Scholar]
- 15.Goossens P L, Milon G, Cossart P, Saron M-F. Attenuated Listeria monocytogenes as a live vector for induction of CD8+ T cells in vivo: a study with the nucleoprotein of the lymphocytic choriomeningitis virus. Int Immunol. 1995;7:797–802. doi: 10.1093/intimm/7.5.797. [DOI] [PubMed] [Google Scholar]
- 16.Hamase K, Homma H, Takigawa Y, Fukushima T, Santa T, Imai K. Regional distribution and postnatal changes of d-amino acids in rat brain. Biochim Biophys Acta. 1997;1334:214–222. doi: 10.1016/s0304-4165(96)00095-5. [DOI] [PubMed] [Google Scholar]
- 17.Harty J T, Bevan M J. CD8+ T cells specific for a single nonamer epitope of Listeria monocytogenes are protective in vivo. J Exp Med. 1992;175:1531–1538. doi: 10.1084/jem.175.6.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harty J T, Lenz L L, Bevan M J. Primary and secondary immune responses to Listeria monocytogenes. Curr Opin Immunol. 1996;8:526–530. doi: 10.1016/s0952-7915(96)80041-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harty J T, Pamer E G. CD8 T lymphocytes specific for the secreted p60 antigen protect against Listeria monocytogenes infection. J Immunol. 1995;154:4642–4650. [PubMed] [Google Scholar]
- 20.Hashimoto A, Nishikawa T, Konno R, Niwa A, Yasumura Y, Oka T, Takahashi K. Free d-serine, d-aspartate and d-alanine in central nervous system and serum in mutant mice lacking d-amino acid oxidase. Neurosci Lett. 1993;152:33–36. doi: 10.1016/0304-3940(93)90476-2. [DOI] [PubMed] [Google Scholar]
- 21.Holden J T, Snell E E. The vitamin B6 group. XVII. The relation of d-alanine and vitamin B6 to growth of lactic acid bacteria. J Biol Chem. 1949;178:799–809. [PubMed] [Google Scholar]
- 22.Ikonomidis G, Portnoy D, Gerhard W, Paterson Y. Influenza-specific immunity induced by recombinant Listeria monocytogenes vaccines. Vaccine. 1997;15:433–440. doi: 10.1016/s0264-410x(96)00188-0. [DOI] [PubMed] [Google Scholar]
- 23.Kamisango K, Saiki I, Tanio Y, Okumura H, Araki Y, Sekikawa I, Azuma I, Yamamura Y. Structure and biological activities of peptidoglycans of Listeria monocytogenes and Propionibacterium acnes. J Biochem. 1982;92:23–33. doi: 10.1093/oxfordjournals.jbchem.a133918. [DOI] [PubMed] [Google Scholar]
- 24.Kaufmann S H E. Immunity to intracellular bacteria. Annu Rev Immunol. 1993;11:129–163. doi: 10.1146/annurev.iy.11.040193.001021. [DOI] [PubMed] [Google Scholar]
- 25.Konno R, Oowada T, Ozaki A, Iida T, Niwa A, Yasumura Y, Mizutani T. Origin of d-alanine present in urine of mutant mice lacking d-amino-acid oxidase activity. Am J Physiol. 1993;265:G699–G703. doi: 10.1152/ajpgi.1993.265.4.G699. [DOI] [PubMed] [Google Scholar]
- 26.Mackaness G B. Cellular resistance to infection. J Exp Med. 1962;116:381–406. [PubMed] [Google Scholar]
- 27.Mackaness G B. The influence of immunologically committed lymphoid cells on macrophage activity in vivo. J Exp Med. 1969;129:973–992. doi: 10.1084/jem.129.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Michel E, Reich K A, Favier R, Berche P, Cossart P. Attenuated mutants of the intracellular bacterium Listeria monocytogenes obtained by single amino acid substitutions in listeriolysin O. Mol Microbiol. 1990;4:2167–2178. doi: 10.1111/j.1365-2958.1990.tb00578.x. [DOI] [PubMed] [Google Scholar]
- 29.Nagata Y, Masui R, Akino T. The presence of free d-serine, d-alanine and d-proline in human plasma. Experientia. 1992;48:986–988. doi: 10.1007/BF01919147. [DOI] [PubMed] [Google Scholar]
- 30.North R J, Berche P A, Newborg M F. Immunologic consequences of antibiotic-induced abridgement of bacterial infection: effect on generation and loss of protective T cells and level of immunologic memory. J Immunol. 1981;127:342–346. [PubMed] [Google Scholar]
- 31.Pamer E G, Harty J T, Bevan M J. Precise prediction of a dominant class I MHC-restricted epitope of Listeria monocytogenes. Nature. 1991;353:852–855. doi: 10.1038/353852a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pan Z-K, Ikonomidis G, Lazenby A, Pardoll D, Paterson Y. A recombinant Listeria monocytogenes vaccine expressing a model tumour antigen protects mice against lethal tumour cell challenge and causes regression of established tumours. Nat Med. 1995;1:471–477. doi: 10.1038/nm0595-471. [DOI] [PubMed] [Google Scholar]
- 33.Paterson Y, Ikonomidis G. Recombinant Listeria monocytogenes cancer vaccines. Curr Opin Immunol. 1996;8:664–669. doi: 10.1016/s0952-7915(96)80083-5. [DOI] [PubMed] [Google Scholar]
- 34.Portnoy D A, Jacks P S, Hinrichs D J. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J Exp Med. 1988;167:1459–1471. doi: 10.1084/jem.167.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pucci M J, Thanassi J A, Ho H-T, Falk P J, Dougherty T J. Staphylococcus haemolyticus contains two d-glutamic acid biosynthetic activities, a glutamate racemase and a d-amino acid transaminase. J Bacteriol. 1995;177:336–342. doi: 10.1128/jb.177.2.336-342.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rubin H, Salem J S, Li L-S, Yang F, Mama S, Wang Z, Fisher A, Hamann C S, Cooperman B S. Cloning, sequence determination, and regulation of the ribonucleotide reductase subunits from Plasmodium falciparum: a target for antimalarial therapy. Proc Natl Acad Sci USA. 1993;90:9280–9284. doi: 10.1073/pnas.90.20.9280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruhland G J, Fiedler F. Occurrence and biochemistry of lipoteichoic acids in the genus Listeria. Syst Appl Microbiol. 1987;9:40–46. [Google Scholar]
- 38.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 39.Shaw J H, Clewell D B. Complete nucleotide sequence of macrolide-lincosamide-streptogramin B-resistance transposon Tn917 in Streptococcus faecalis. J Bacteriol. 1985;164:782–796. doi: 10.1128/jb.164.2.782-796.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shen H, Slifka M K, Matloubian M, Jensen E R, Ahmed R, Miller J F. Recombinant Listeria monocytogenes as a live vaccine vehicle for the induction of protective anti-viral cell-mediated immunity. Proc Natl Acad Sci USA. 1995;92:3987–3991. doi: 10.1073/pnas.92.9.3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sizemore D R, Branstrom A A, Sadoff J C. Attenuated Shigella as a DNA delivery vehicle for DNA-mediated immunization. Science. 1995;270:299–302. doi: 10.1126/science.270.5234.299. [DOI] [PubMed] [Google Scholar]
- 42.Smith K, Youngman P. Use of a new integrational vector to investigate compartment-specific expression of the Bacillus subtilis spoIIM gene. Biochimie. 1992;74:705–711. doi: 10.1016/0300-9084(92)90143-3. [DOI] [PubMed] [Google Scholar]
- 43.Snell E E, Radin N S, Ikawa M. The nature of d-alanine in lactic acid bacteria. J Biol Chem. 1955;217:803–818. [PubMed] [Google Scholar]
- 44.Szalay G, Ladel C H, Kaufmann S H E. Stimulation of protective CD8+ T lymphocytes by vaccination with nonliving bacteria. Proc Natl Acad Sci USA. 1995;92:12389–12392. doi: 10.1073/pnas.92.26.12389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tanizawa K, Asano S, Masu Y, Kuramitsu S, Kagamiyama H, Tanaka H, Soda K. The primary structure of thermostable d-amino acid aminotransferase from a therophilic Bacillus species and its correlation with l-amino acid aminotransferases. J Biol Chem. 1989;264:2450–2454. [PubMed] [Google Scholar]
- 46.Tanizawa K, Oshima A, Scheidegger A, Inagaki K, Tanaka H, Soda K. Thermostable alanine racemase from Bacillus stearothermophilus: DNA and protein sequence determination and secondary structure prediction. Biochemistry. 1988;27:1311–1316. doi: 10.1021/bi00404a033. [DOI] [PubMed] [Google Scholar]
- 47.Tilney L G, Portnoy D A. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J Cell Biol. 1989;109:1597–1608. doi: 10.1083/jcb.109.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tomasz A. A chemically defined medium for Streptococcus pneumoniae. Bacteriol Proc. 1964;64:29. . (Abstract.) [Google Scholar]
- 49.Triglia T, Peterson M G, Kemp D J. A procedure for in vitro amplification of DNA segments that lie outside the boundaries of known sequences. Nucleic Acids Res. 1988;16:8186. doi: 10.1093/nar/16.16.8186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walsh C T. Enzymes in the d-alanine branch of bacterial cell wall peptidoglycan assembly. J Biol Chem. 1989;264:2393–2396. [PubMed] [Google Scholar]
- 51.Wasserman S A, Daub E, Grisafi P, Botstein D, Walsh C T. Catabolic alanine racemase from Salmonella typhimurium: DNA sequence, enzyme purification, and characterization. Biochemistry. 1984;23:5182–5187. doi: 10.1021/bi00317a015. [DOI] [PubMed] [Google Scholar]
- 52.Wasserman S A, Walsh C T, Botstein D. Two alanine racemase genes in Salmonella typhimurium that differ in structure and function. J Bacteriol. 1983;153:1439–1450. doi: 10.1128/jb.153.3.1439-1450.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wipke B T, Jameson S C, Bevan M J, Pamer E G. Variable binding affinities of listeriolysin O peptides for the H-2Kd class I molecule. Eur J Immunol. 1993;23:2005–2010. doi: 10.1002/eji.1830230842. [DOI] [PubMed] [Google Scholar]