Synopsis
Background
Aztreonam/avibactam is a combination agent that shows promise in treating infections caused by highly antibiotic-resistant MBL-producing Enterobacterales. This combination can be achieved by combining two FDA-approved drugs: ceftazidime/avibactam and aztreonam. It is unknown whether ceftazidime in the combination ceftazidime/aztreonam/avibactam has a synergistic or antagonistic effect on the in vitro activity of aztreonam/avibactam by significantly increasing or decreasing the MIC.
Objective
To determine whether increasing ceftazidime concentrations affect the MICs of aztreonam/avibactam alone.
Methods
A custom 8×8 chequerboard broth microdilution (BMD) panel was made using a digital dispenser (Hewlett-Packard, Corvallis, OR). The panel included orthogonal two-fold dilution series of aztreonam and ceftazidime ranging from 0.5 mg/L to 64 mg/L. Avibactam was kept constant at 4 mg/L throughout the chequerboard. Thirty-seven Enterobacterales isolates from the CDC & FDA AR Isolate Bank or CDC’s internal collection with intermediate or resistant interpretations to aztreonam and ceftazidime/avibactam were included for testing. All isolates harbored at least one of the following MBL genes: blaIMP, blaNDM, or blaVIM.
Results
Regardless of the concentration of ceftazidime, aztreonam/avibactam with ceftazidime MICs for all 37 isolates were within ±1 two-fold doubling dilution of the aztreonam/avibactam MIC.
Conclusions
Ceftazidime, in the combination ceftazidime/avibactam/aztreonam, did not affect the in vitro activity of aztreonam/avibactam in this sample of isolates. These findings can help assure clinical and public health laboratories that testing of aztreonam/avibactam by BMD can act as a reliable surrogate test when the combination of ceftazidime/avibactam and aztreonam is being considered for treatment of highly antibiotic-resistant MBL-producing Enterobacterales.
Introduction
CDC’s 2019 Antibiotic Resistance Threats in the United States report once again recognized carbapenem-resistant Enterobacterales (CRE) as an urgent public health threat.1 Infections caused by CRE are associated with higher morbidity and mortality rates and often present clinicians with limited treatment options.2 A therapeutic approach for the treatment of CRE is to combine an existing β-lactam (BL) with a β-lactamase inhibitor (BLI), such as ceftazidime with avibactam or meropenem with vaborbactam. While these BLIs often inhibit Ambler class A (e.g., KPC, ESBLs), C (e.g., AmpCs), and some D (e.g., OXA-48-like) β-lactamases, they do not inhibit class B MBLs (e.g. IMP, NDM or VIM).3 This leaves a dearth of antimicrobial agents for treating infections caused by MBL-producing organisms. MBLs alone cannot hydrolyze aztreonam; however, organisms with MBLs often harbor other class A, C or D enzymes that hydrolyze aztreonam, rendering monotherapy of aztreonam ineffective. A clever strategy for combatting these MBL-producing organisms is to exploit the MBL’s inability to hydrolyze aztreonam, by the addition of a BLI. Avibactam, a non-β-lactam BLI, is especially potent against organisms carrying class A, class C and some class D β-lactamases.4 In combination with aztreonam, avibactam provides protection from many concomitant β-lactamases, restoring the in vitro activity of aztreonam against some organisms that produce an MBL.
Aztreonam/avibactam is pending phase 3 clinical trials, but the combination can currently be achieved by administering two FDA-approved drugs, ceftazidime/avibactam and aztreonam. This practice is suggested in the Sanford Guide as a last resort recommendation for infections caused by highly antibiotic-resistant MBL-producing Enterobacterales.5 Support for this strategy includes a few case reports and series 6–8 and large in vitro studies 9–12 demonstrating the activity of this combination against MBL-producing Enterobacterales.
A limitation when testing novel drug combinations is the absence of established quality control (QC) parameters to ensure the test can be validated by a laboratory and performs accurately when testing patient samples. CLSI established QC ranges for aztreonam/avibactam, but none exist for the combination currently used in practice, ceftazidime/avibactam and aztreonam. To date, there is no literature describing the effect of ceftazidime with aztreonam/avibactam in the setting of in vitro susceptibility testing. Here we used custom chequerboard BMD panels to determine whether the presence of ceftazidime at increasing concentrations alters the MIC of aztreonam/avibactam for a study set of MBL-producing Enterobacterales isolates.
Methods
Bacterial Strains
A total of 37 MBL-producing Enterobacterales isolates were selected from either the CDC & FDA Antibiotic Resistance Isolate Bank (n=36) or from CDC’s internal collection of clinical isolates (n=1) (Table 1). MBL genes (blaIMP, blaNDM, or blaVIM) were confirmed by WGS or real-time PCR. All isolates had interpretations of intermediate or resistant for aztreonam (≥8 mg/L) and ceftazidime/avibactam (≥16/4 mg/L) according to CLSI’s M100 30th edition interpretive criteria.13 The following QC strains were used for susceptibility testing: Escherichia coli ATCC 35218, E. coli ATCC 25922, Klebsiella pneumoniae ATCC 700603, Pseudomonas aeruginosa ATCC 27853, and Staphylococcus aureus ATCC 29213 (American Type Culture Collection, Manassas, VA).
Table 1.
Modal minimum inhibitory concentrations of aztreonam/avibactam with added concentrations of ceftazidime for 37 highly antibiotic-resistant MBL-producing Enterobacterales isolates
| AR Bank ID | Organism | CP gene(s)a | Control MICsb | MIC (mg/L) of aztreonam/avibactam with increasing concentrations (mg/L) of ceftazidime | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||
| CAZ | CZA | ATM | AZA | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | |||
| 0038 | Enterobacter cloacae | NDM-1 | >64 | >64/4 | >64 | ≤0.5/4 | 1/4c | ≤0.5/4 | ≤0.5/4 | 1/4c | 1/4c | 1/4c | ≤0.5/4 | ≤0.5/4 |
| 0040 | Klebsiella pneumoniae | VIM-27 | >64 | >64/4 | >64 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 |
| 0046 | Klebsiella pneumoniae | VIM-27 | >64 | >64/4 | >64 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 |
| 0048 | Escherichia coli | NDM-1 | >64 | >64/4 | >64 | 4/4 | 8/4 | 4/4 | 4/4 | 4/4 | 4/4 | 4/4 | 8/4c | 4/4 |
| 0049 | Klebsiella pneumoniae | NDM-1 | >64 | >64/4 | >64 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | 1/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 |
| 0055 | Escherichia coli | NDM-1 | >64 | >64/4 | 32 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 |
| 0057 | Morganella morganii | NDM-1 | >64 | >64/4 | >64 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 |
| 0068 | Klebsiella pneumoniae | OXA-232, NDM-1 |
>64 | >64/4 | >64 | 1/4 | 1/4 | 1/4 | 1/4 | 1/4 | 1/4 | 1/4 | 1/4 | 1/4 |
| 0069 | Escherichia coli | NDM-1 | >64 | >64/4 | 8 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 |
| 0080 | Klebsiella pneumoniae | IMP-4 | >64 | >64/4 | >64 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 |
| 0106 | Klebsiella pneumoniae | NDM-1 | >64 | >64/4 | >64 | 1/4 | 1/4 | 1/4 | 1/4 | 1/4 | 1/4 | 1/4 | 1/4 | 1/4 |
| 0118 | Escherichia coli | NDM-1 | >64 | >64/4 | 64 | 4/4 | 8/4 | 4/4 | 4/4 | 4/4 | 4/4 | 4/4 | 4/4 | 4/4 |
| 0119 | Escherichia coli | NDM-1 | >64 | >64/4 | >64 | 8/4 | 8/4 | 8/4 | 16/4 | 16/4 | 8/4 | 8/4 | 8/4 | 8/4 |
| 0127 | Salmonella enterica serotype Senftenberg | NDM-1 | >64 | >64/4 | 64 | ≤0.5/4 | ≤0.5/4 | 1/4 | 1/4 | ≤0.5/4 | 1/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 |
| 0135 | Klebsiella pneumoniae | VIM-1 | >64 | >64/4 | >64 | 1/4 | 1/4 | 1/4 | 1/4 | 1/4 | 1/4 | 1/4 | 1/4 | 1/4 |
| 0137 | Escherichia coli | NDM-6 | >64 | >64/4 | >64 | 8/4 | 8/4 | 8/4 | 16/4 | 16/4 | 16/4 | 8/4 | 16/4 | 8/4 |
| 0138 | Klebsiella pneumoniae | NDM-7 | >64 | >64/4 | >64 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 |
| 0139 | Klebsiella pneumoniae | NDM-1 | >64 | >64/4 | >64 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 |
| 0143 | Klebsiella pneumoniae | NDM-1 | >64 | >64/4 | >64 | 2/4 | 2/4 | 2/4 | 2/4 | 4/4c | 2/4 | 2/4 | 2/4 | 2/4 |
| 0150 | Escherichia coli | NDM-5 | >64 | >64/4 | 32 | 8/4 | 8/4 | 8/4 | 8/4 | 8/4 | 8/4 | 8/4 | 8/4 | 8/4 |
| 0153 | Klebsiella pneumoniae | OXA-232, NDM-1 |
>64 | >64/4 | >64 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | 1/4 | 1/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 |
| 0154 | Enterobacter cloacae | VIM-1 | >64 | >64/4 | >64 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 |
| 0157 | Citrobacter freundii | NDM-1 | >64 | >64/4 | >64 | ≤0.5/4 | 1/4 | ≤0.5/4 | 1/4 | 1/4 | 1/4 | 1/4 | ≤0.5/4 | ≤0.5/4 |
| 0161 | Klebsiella aerogenes | IMP-4 | 64 | >64/4 | 64c | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 |
| 0435 | Escherichia coli | NDM-1 | >64 | >64/4 | >64 | 8/4 | 8/4 | 8/4 | 8/4 | 8/4 | 8/4 | 8/4 | 8/4 | 8/4 |
| 0448 | Enterobacter cloacae | NDM-1 | >64 | >64/4 | >64 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 |
| 0502 | Enterobacter cloacae | IMP-8 | >64 | >64/4 | >64 | 1/4 | 1/4 | 1/4 | 1/4 | 2/4 | 1/4 | 1/4 | 1/4 | 1/4 |
| 0503 | Escherichia coli | NDM-1, OXA-181 |
>64 | >64/4 | >64 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 |
| 0505 | Klebsiella pneumoniae | NDM-1 | >64 | >64/4 | 32 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 |
| 0506 | Klebsiella pneumoniae | NDM-1 | >64 | >64/4 | >64 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 |
| 0507 | Klebsiella pneumoniae | OXA-232, NDM-1 |
>64 | >64/4 | >64 | 1/4 | 1/4 | 1/4 | 1/4 | 1/4 | 1/4 | 1/4 | 1/4 | 1/4 |
| 0555 | Klebsiella pneumoniae | OXA-232, NDM-5 |
>64 | >64/4 | >64 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 |
| 0557 | Klebsiella pneumoniae | NDM, OXA-232 |
>64 | >64/4 | >64 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 |
| 0559 | Escherichia coli | NDM-1 | >64 | >64/4 | 16 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 |
| 0598 | Escherichia coli | NDM-1 | >64 | >64/4 | >64 | 8/4 | 8/4 | 8/4 | 8/4 | 8/4 | 8/4 | 8/4 | 8/4 | 8/4 |
| 0636 | Klebsiella pneumoniae | NDM-1 | >64 | >64/4 | >64 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 |
| CDC-1 | Proteus mirabilis | NDM | >64 | >64/4 | 64 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 | ≤0.5/4 |
AR Bank isolates were characterized by whole genome sequencing. CDC-1 was characterized by real-time PCR. CP, Carbapenemase.
CAZ, ceftazidime; CZA, ceftazidime/avibactam; ATM, aztreonam; AZA, aztreonam/avibactam.
MICs where mode was not available, and a median was used.
Media and Reagents
Growth media used were BBL™ blood agar plates with 5% sheep blood (BD, Franklin Lakes, NJ) and CAMHB. CAMHB was made in-house using Difco Mueller Hinton broth base powder (BD, Franklin Lakes, NJ), and the cation concentrations were adjusted to CLSI requirements.14
Drug stocks were made using drug powders of aztreonam (United States Pharmacopeia; Rockville, MD), ceftazidime pentahydrate (TOKU-E; Bellingham, WA) and avibactam sodium (Sigma Aldrich; St. Louis, MO) and diluted to previously validated stock concentrations of 3000 mg/L, 3000 mg/L, and 1400 mg/L, respectively.15 For drug stock preparation, solvents and diluents were used in accordance with CLSI recommendations.13 Drug stocks were assessed for quality by using a digital dispenser (Hewlett-Packard; Corvallis, OR) to prepare BMD panels with expanded dilution ranges of 0.03 mg/L – 64 mg/L for ceftazidime, aztreonam, ceftazidime/avibactam, and aztreonam/avibactam. The drug stocks were aliquoted for single-use and stored at −70⁰C for up to one week.
Chequerboard AST and Analysis
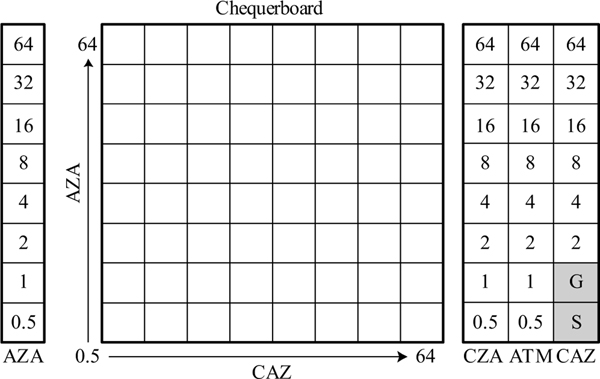
All isolates were tested on a custom BMD panel made using a digital dispenser (Hewlett-Packard; Corvallis, OR) and the Sensititre Automated Inoculation Delivery System (AIM) (ThermoFisher; Waltham, MA) as previously described.15 The custom BMD panel consisted of an 8×8 chequerboard with orthogonal two-fold dilution series of aztreonam and ceftazidime with avibactam at a constant of 4 mg/L. Also included on this custom BMD panel were additional control dilutions for aztreonam, aztreonam/avibactam, ceftazidime, and ceftazidime/avibactam (Figure 1). The use of the digital dispenser requires that drug stock solutions contain a surfactant, such as 0.1% of Triton X-100. Despite lack of guidance for the use of small amounts of surfactant in BMD tests, we had previously shown BMD panels made by the digital dispenser are equivalent to those prepared by reference methods.15 Inoculated BMD panels were incubated at 35⁰C ± 2⁰C and read at 18 ± 2 hours.
Figure 1.
Custom broth microdilution panel layout consisting of a chequerboard area and series of control dilutions.
AZA, aztreonam/avibactam; CAZ, ceftazidime; CZA, ceftazidime/avibactam; ATM, aztreonam; G, growth control well; S, sterility control well. Avibactam is kept constant at 4 mg/L when in combination and throughout the chequerboard.
All isolates were tested in triplicate using separate inocula and modal MICs were reported. If a modal MIC was not available, a median was reported. To evaluate the interaction of ceftazidime with aztreonam and avibactam, we compared the MICs of aztreonam/avibactam with increasing concentrations of ceftazidime from the chequerboard to the control MIC of aztreonam/avibactam alone. Isolates where a concentration of ceftazidime decreased or increased the aztreonam/avibactam MIC by ≥2 doubling dilutions, but not within ±1 doubling dilution of the ceftazidime/avibactam control MIC, were defined as displaying additional synergy or antagonism, respectively.
Ethics
This study was performed under the General Protocol for ethical approval by the Internal Review Board of CDC.
Results
Of 37 MBL-producing Enterobacterales isolates tested, the presence of ceftazidime at varying concentrations (0.5 mg/L – 64 mg/L) did not affect any of the MICs of aztreonam/avibactam by more than the ±1 doubling dilution allowable margin due to the inherent variation of BMD (Table 1). Aztreonam/avibactam MICs ranged from ≤0.5/4 mg/L to 8/4 mg/L. All MICs from the QC strains were acceptable across all testing dates.
Discussion
Several studies have suggested that aztreonam/avibactam may be a promising combination for treating severe infections caused by MBL-producing Enterobacterales.6–12 Although current use of this combination is achievable by using ceftazidime/avibactam and aztreonam, no CLSI QC ranges are established for this triple combination, but they do exist for aztreonam/avibactam.13 For this reason, we believe a laboratory validation and reporting of aztreonam/avibactam MICs is appropriate. Our study findings support that the MIC of aztreonam/avibactam can act as a reliable surrogate for susceptibility of the combination being administered currently to patients.
One limitation of our study was the small sample size. Isolates exhibiting the phenotypic and genotypic characteristics targeted in our study (i.e., MBL-producing Enterobacterales organisms that were not susceptible to both ceftazidime/avibactam and aztreonam) occur infrequently and their detection requires enhanced laboratory testing capacities. A second study limitation was that not all isolates had combination MIC results on-scale, thus we were unable to determine if ceftazidime influences aztreonam/avibactam MICs below 0.5/4 mg/L. We chose the aforementioned two-fold dilution series to assess a concentration range of aztreonam/avibactam where variability could impact forthcoming interpretive criteria. We believe any synergy occurring below the range of the panel are unlikely to affect treatment decisions. A final limitation is that we only evaluated the in vitro effects of ceftazidime/avibactam/aztreonam. Further in vivo studies are necessary to demonstrate the optimal pharmacokinetics and pharmacodynamic properties of this combination and to investigate for potential toxicity of this drug combination.
With the global threat of antibiotic resistance, novel antibiotics and antibiotic combinations are needed now more than ever. The treatment of infections caused by MBL-producing Enterobacterales remains a challenge but in vitro data show support for the clinical development of a novel BL-BLI combination, aztreonam/avibactam, by demonstrating potent activity against organisms with MBL carbapenemases.9–12 Even though administering ceftazidime/avibactam and aztreonam together can be used to attain the same effect of aztreonam plus avibactam, most large in vitro studies assess MICs of aztreonam/avibactam,9–12 not ceftazidime/avibactam and aztreonam. Among the highly antibiotic-resistant MBL-producing Enterobacterales isolates we evaluated, our data support the hypothesis that ceftazidime, when used in the triple combination, does not impact the in vitro activity of aztreonam/avibactam. This finding was critical to the successful establishment of aztreonam/avibactam AST capacity in CDC’s Antibiotic Resistance Laboratory Network. This data can be used to help inform clinical decision-making when therapy with ceftazidime/avibactam and aztreonam is being considered for treatment of infections caused by highly antibiotic-resistant organisms.
Acknowledgements
The authors wish to thank the CDC & FDA AR Isolate Bank staff members for their contributions to this study.
Funding
This work was supported by the Centers for Disease Control and Prevention’s internal funding.
The findings and conclusions in this report are those of the author(s) and do not represent the official position of the Centers for Disease Control and Prevention or the Association of Public Health Laboratories.
Footnotes
Transparency Declarations
All authors reported no conflict of interest.
References
- 1.CDC. Antibiotic resistance threats in the United States, 2019. 2019. [Google Scholar]
- 2.Falagas ME, Tansarli GS, Karageorgopoulos DE et al. Deaths attributable to carbapenem-resistant Enterobacteriaceae infections. Emerging Infect Dis 2014; 20: 1170–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pogue JM, Bonomo RA, Kaye KS. Ceftazidime/Avibactam, Meropenem/Vaborbactam, or Both? Clinical and Formulary Considerations. Clin Infect Dis 2019; 68: 519–24. [DOI] [PubMed] [Google Scholar]
- 4.Ehmann DE, Jahic H, Ross PL et al. Avibactam is a covalent, reversible, non-beta-lactam beta-lactamase inhibitor. Proc Natl Acad Sci U S A 2012; 109: 11663–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The Sanford guide to antimicrobial therapy. In: Gilbert DN, Eliopoulos GM, Chambers HF et al. , eds. Sperryville, VA, USA: Antimicrobial Therapy, Inc., 2019. [Google Scholar]
- 6.Marshall S, Hujer AM, Rojas LJ et al. Can Ceftazidime-Avibactam and Aztreonam Overcome beta-Lactam Resistance Conferred by Metallo-beta-Lactamases in Enterobacteriaceae? Antimicrob Agents Chemother 2017; 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Falcone M, Tiseo G, Antonelli A et al. Clinical Features and Outcomes of Bloodstream Infections Caused by New Delhi Metallo-beta-Lactamase-Producing Enterobacterales During a Regional Outbreak. Open Forum Infect Dis 2020; 7: ofaa011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaw E, Rombauts A, Tubau F et al. Clinical outcomes after combination treatment with ceftazidime/avibactam and aztreonam for NDM-1/OXA-48/CTX-M-15-producing Klebsiella pneumoniae infection. J Antimicrob Chemother 2017; 73: 1104–6. [DOI] [PubMed] [Google Scholar]
- 9.Biedenbach DJ, Kazmierczak K, Bouchillon SK et al. In vitro activity of aztreonam-avibactam against a global collection of Gram-negative pathogens from 2012 and 2013. Antimicrob Agents Chemother 2015; 59: 4239–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vasoo S, Cunningham SA, Cole NC et al. In vitro Activities of Ceftazidime-Avibactam, Aztreonam-Avibactam, and a Panel of Older and Contemporary Antimicrobial Agents against Carbapenemase-Producing Gram-Negative Bacilli. Antimicrob Agents Chemother 2015; 59: 7842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karlowsky JA, Kazmierczak KM, de Jonge BLM et al. In Vitro Activity of Aztreonam-Avibactam against Enterobacteriaceae and Pseudomonas aeruginosa Isolated by Clinical Laboratories in 40 Countries from 2012 to 2015. Antimicrob Agents Chemother 2017; 61(9):e00472-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sader HS, Mendes RE, Pfaller MA et al. Antimicrobial Activities of Aztreonam-Avibactam and Comparator Agents against Contemporary (2016) Clinical Enterobacteriaceae Isolates. Antimicrobial Agents and Chemotherapy 2018; 62: e01856–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.CLSI. M100 Performance Standards for Antimicrobial Susceptibility Testing- 30th Edition. Clinical and Laboratory Standards Institute, Wayne, PA, 2020. [Google Scholar]
- 14.CLSI. Methods for Dilution Antimicrobial Susceptbility Tests for Bacteria that Grow Aerobically; Approved Standard- 10th Edition. CLSI Document M07-A10. Clinical and Laboratory Standards Institute, Wayne, PA, 2015. [Google Scholar]
- 15.Ransom E, Bhatnagar A, Patel JB et al. Validation of Aztreonam-Avibactam Susceptibility Testing Using Digitally Dispensed Custom Panels. J Clin Microbiol 2020; 58(4):e01944–19. [DOI] [PMC free article] [PubMed] [Google Scholar]