Abstract
Fat build-up is determined by the balance between lipogenesis and lipolysis/fatty acid oxidation. In the past few years, our understanding of the nutritional, hormonal and particularly transcriptional regulation of lipogenesis has expanded greatly. Lipogenesis is stimulated by a high carbohydrate diet, whereas it is inhibited by polyunsaturated fatty acids and by fasting. These effects are partly mediated by hormones, which inhibit (growth hormone, leptin) or stimulate (insulin) lipogenesis. Recent research has established that sterol regulatory element binding protein-1 is a critical intermediate in the pro- or anti-lipogenic action of several hormones and nutrients. Another transcription factor implicated in lipogenesis is the peroxisome proliferator activated receptor γ. Both transcription factors are attractive targets for pharmaceutical intervention of disorders such as hypertriglyceridemia and obesity.
Introduction
In the past several decades, obesity has become extremely common, with prevalence rates skyrocketing among certain groups and communities (Kopelman, 2000). Inasmuch as traditional dietary approaches to combat obesity have largely failed, the scientific community has become increasingly interested in the molecular regulation of triglyceride synthesis and in pharmaceutical approaches to reduce fat storage. Accordingly, a heavy research effort is currently directed towards the identification of molecular targets for fat storage, and on the development of drugs that specifically reduce adipose tissue mass.
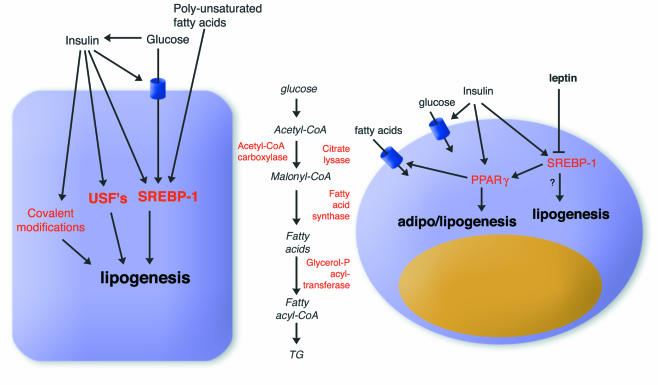
Fat accumulation is determined by the balance between fat synthesis (lipogenesis) and fat breakdown (lipolysis/fatty acid oxidation). Lipogenesis encompasses the processes of fatty acid synthesis and subsequent triglyceride synthesis, and takes place in both liver and adipose tissue (Figure 1). Lipogenesis should not be confused with adipogenesis, which refers to the differentiation of pre-adipocytes into mature fat cells. A comprehensive review on the regulation of adipogenesis has appeared recently (Rosen and Spiegelman, 2000).
Fig. 1. Regulation of lipogenesis in hepatocytes (left) and adipocytes (right). The effects of nutrients and hormones on the expression of lipogenic genes are mostly mediated by SREBP-1 and, in adipose tissue, by PPARγ. Lipogenesis entails a number of discrete steps, shown in the middle, which are controlled via allosteric interactions, by covalent modification and via changes in gene expression.
Nutritional regulation of lipogenesis
Lipogenesis is very responsive to changes in the diet. Polyunsaturated fatty acids decrease lipogenesis by suppressing gene expression in liver, including that of fatty acid synthase, spot14 and stearoyl-CoA desaturase (Jump et al., 1994). Conversely, a diet rich in carbohydrates stimulates lipogenesis in both liver and adipose tissue, leading to elevated postprandial plasma triglyceride levels. Fasting reduces lipogenesis in adipose tissue, which, combined with an increased rate of lipolysis, leads to net loss of triglycerides from fat cells. In contrast, in liver, because of the large amounts of fatty acids arriving from the adipose tissue, triglyceride synthesis is increased, resulting in a mild form of hepatosteatosis (fatty liver) (Kersten et al., 1999). This happens despite a reduced rate of fatty acid synthesis and decreased expression of numerous genes involved in lipogenesis (Shimano et al., 1999).
It can be reasoned that, somehow, the signal of reduced or excess food intake has to be translated into altered expression levels of lipogenic genes. This concept can be illustrated by examining the effects of fasting, which is associated with a decrease in plasma glucose and an increase in plasma-free fatty acids. Plasma glucose levels stimulate lipogenesis via several mechanisms. First, glucose itself is a substrate for lipogenesis. By being glycolytically converted to acetyl-CoA, glucose promotes fatty acid synthesis. Secondly, glucose induces the expression of lipogenic genes, the mechanisms of which are explained below. Finally, glucose increases lipogenesis by stimulating the release of insulin and inhibiting the release of glucagon from the pancreas.
Hormonal regulation of lipogenesis
Fasting is associated with significant changes in plasma hormone concentrations, such as a decrease in plasma insulin and leptin, and an increase in plasma growth hormone and glucagon. Insulin is probably the most important hormonal factor influencing lipogenesis. By increasing the uptake of glucose in the adipose cell via recruitment of glucose transporters to the plasma membrane, as well as activating lipogenic and glycolytic enzymes via covalent modification, insulin potently stimulates lipogenesis (Figure 1). These effects are achieved by the binding of insulin to the insulin receptor at the cell surface, thus activating its tyrosine kinase activity and inducing a plethora of downstream effects via tyrosine phosphorylation (Lane et al., 1990; Nakae and Accili, 1999). Insulin also has long-term effects on the expression of lipogenic genes (Assimacopoulos-Jeannet et al., 1995), probably via the transcription factor sterol regulatory element binding protein-1 (SREBP-1) (Figure 1 and see below). In addition, insulin causes SREBP-1 to induce the expression and activity of glucokinase, thereby increasing the concentration of a glucose metabolite that supposedly mediates the effects of glucose on lipogenic gene expression (Foretz et al., 1999a).
Another hormone that has an important influence on lipogenesis is growth hormone (GH). GH dramatically reduces lipogenesis in adipose tissue, resulting in significant fat loss, with a concomitant gain of muscle mass (Etherton, 2000). These effects appear to be mediated by two pathways. In one case, GH decreases insulin sensitivity, resulting in down-regulation of fatty acid synthase expression in adipose tissue (Yin et al., 1998). The details of this mechanism are still unknown, but GH probably interferes with insulin signaling at the post-receptor level. In the second case, GH may decrease lipogenesis by phosphorylating the transcription factors Stat5a and 5b. The loss of Stat5a and 5b in a knock-out model was recently shown to decrease fat accumulation in adipose tissue (Teglund et al., 1998). The mechanism by which Stat5 proteins enhance fat storage remains to be determined.
Leptin is another hormone that may be involved in lipogenesis. There is a growing consensus that leptin limits fat storage not only by inhibiting food intake, but also by affecting specific metabolic pathways in adipose and other tissues. Leptin stimulates the release of glycerol from adipocytes (Siegrist-Kaiser et al., 1997), by both stimulating fatty acid oxidation and inhibiting lipogenesis (Bai et al., 1996; Wang et al., 1999). The latter effect is achieved by down-regulating the expression of genes involved in fatty acid and triglyceride synthesis, as was nicely demonstrated recently by oligonucleotide micro-array analysis (Soukas et al., 2000). Interestingly, another negative target of leptin is probably SREBP-1, suggesting that this transcription factor may be involved in mediating the inhibitory effect of leptin on lipogenic gene expression (Kakuma et al., 2000; Soukas et al., 2000).
A final endocrine/autocrine factor connected with triglyceride synthesis is acylation stimulating protein (ASP). ASP is a small peptide that is identical to C3adesArg, a product of the complement factor C3 (Sniderman et al., 2000). ASP is produced by adipose tissue and supposedly acts via an autocrine loop. Numerous in vitro studies have shown that ASP stimulates triglyceride accumulation in adipose cells (Sniderman et al., 2000). This is achieved by an increase in triglyceride synthesis, as well as by a simultaneous decrease in adipose tissue lipolysis. Intraperitoneal injection of ASP has been reported to stimulate triglyceride clearance from plasma, indicating that ASP has a similar effect in vivo (Murray et al., 1999a). This is supported by studies with female ASP null mice, which display a pronounced reduction in adipose tissue mass despite an increased energy intake (Murray et al., 2000). However, whereas Murray et al. (1999b) reported a delayed postprandial triglyceride clearance in ASP null mice, another group failed to find any differences between null and wild-type mice (Wetsel et al., 1999). The reason for this discrepancy is unclear. Very little is known about how ASP stimulates triglyceride synthesis. It probably binds to some kind of cell surface receptor, thereby activating a signaling cascade that involves phosphodiesterase 3 (Van Harmelen et al., 1999).
Transcriptional regulation of lipogenesis
Evidence that has been gathered over the past few years indicates that the effects of various nutrients and hormones on the expression of lipogenic genes are mediated by the SREBPs (Hua et al., 1993; Tontonoz et al., 1993; Yokoyama et al., 1993). SREBPs are transcription factors that regulate the expression of genes connected with cholesterol and fatty acid metabolism. They belong to the group of basic helix–loop–helix (bHLH)-leucine zipper transcription factors, and can be separated into three types: SREBP-2, SREBP-1a and SREBP-1c (also called ADD1). SREBP-1a and -1c, of which SREBP-1c is considered the most physiologically relevant, are products from a single gene that differ in their first exon. Since its discovery in 1993, the molecular mode of action of SREBP-2 has been very well characterized. When levels of free cholesterol in the cell are high, SREBP-2 is present as a large immature precursor bound to the endoplasmic reticulum. When the cellular concentration of cholesterol declines, the precursor molecule is proteolytically cleaved to release a mature fragment that translocates to the nucleus. In the nucleus, mature SREBP-2 binds to a so-called sterol response element in the promoter region of target genes and thereby activates their transcription.
Studies in transgenic mice that overexpress SREBP-2 in the liver suggested that SREBP-2 stimulates the expression of genes involved in cholesterol metabolism, such as the LDL receptor, farnesyl pyrophosphate synthase and HMG-CoA reductase genes. Interestingly, in mice that overexpress SREBP-1a or SREBP-1c in liver, a dramatic build-up of hepatic triglycerides and elevated expression levels of lipogenic genes were observed. This led to the conclusion that SREBP-1 activates genes connected with lipogenesis in liver (reviewed in Horton and Shimomura, 1999).
Surprisingly, the phenotype of SREBP-1 null mice revealed that SREBP-1 probably has a somewhat different role in adipose tissue. In these mice, adipose tissue mass was not affected, nor was the adipose tissue expression of fatty acid synthase and acetyl-CoA carboxylase (Shimano et al., 1997). Further evidence suggesting a different role of SREBP-1 in adipose tissue came from studies with transgenic mice that express SREBP-1c under control of the aP2 promoter (for adipose tissue-specific overexpression). In white adipose tissue of these mice, expression of genes implicated in cholesterol metabolism was markedly elevated, whereas the expression of genes implicated in fatty acid and triglyceride synthesis remained unchanged (Shimomura et al., 1998). An even more striking and counterintuitive observation in these mice was that their adipose tissue mass was reduced to less than half that of wild-type mice. The explanation behind the diminished fat mass remains elusive, but could be related to decreased expression of the adipogenic transcription factors peroxisome proliferator activated receptor γ (PPARγ) and CCAAT enhancer binding protein (C/EBPα). Overall, these data suggest that the roles of SREBP-1 in liver and adipose tissue may differ.
It is becoming more and more evident that the induction of lipogenic gene expression in liver by insulin and glucose is mediated by SREBP-1. Indeed, SREBP-1 null mice display impaired up-regulation of lipogenic gene expression on a fasting/refeeding protocol (Shimano et al., 1999). Insulin and glucose affect SREBP-1 transcriptional activity via several mechanisms. First, insulin has been shown to stimulate SREBP-1 mRNA expression in adipocytes (Kim et al., 1998) and hepatocytes (Foretz et al., 1999b), an effect that is probably mediated by the phosphatidylinositol 3-kinase pathway (Azzout-Marniche et al., 2000). In addition, insulin probably increases transcriptional activation by SREBP-1, independently of changes in its mRNA levels, via MAP-kinase-dependent phosphorylation (Roth et al., 2000). Like insulin, glucose stimulates SREBP-1 promoter activity and mRNA expression (Hasty et al., 2000). The relative increase in the nuclear form of SREBP-1 after carbohydrate refeeding (Horton et al., 1998) suggests that insulin and glucose may also stimulate SREBP-1-dependent gene transcription by activating the proteolytic cleavage of membrane-bound SREBP-1. However, a direct effect of insulin or glucose on the proteolytic cleavage of the SREBP-1 precursor could not be demonstrated (Azzout-Marniche et al., 2000; Hasty et al., 2000).
Polyunsaturated fatty acids also regulate expression of lipogenic genes. However, in contrast to glucose and insulin, they down-regulate gene expression. This effect is achieved by inhibiting the mRNA expression of SREBP-1 (Kim et al., 1999; Mater et al., 1999; Xu et al., 1999; Yahagi et al., 1999), as well as by inhibiting the proteolytic processing of the SREBP-1 precursor (Thewke et al., 1998).
SREBP-1 clearly plays a pivotal role in mediating the effects of insulin on gene expression, but it is probably not the only transcription factor involved. In vitro studies have clearly established the importance of the upstream stimulatory factors (USFs) in the regulation of the fatty acid synthase promoter by insulin. USFs are ubiquitous bHLH-leucine zipper transcription factors that are able to interact as homo- and/or heterodimers with E boxes of CANNTG sequence (Wang and Sul, 1997). Such an E box is present in the promoter of fatty acid synthase. Mutations that weaken binding of USF1 and USF2 to this E box abolish the insulin-dependent activation of the fatty acid synthase promoter. Recent studies with mice lacking USF1 and/or USF2 have provided very compelling evidence that USF1 and USF2 are involved in mediating the stimulatory effect of insulin/glucose on fatty acid synthase expression (Casado et al., 1999). The effects of USFs and SREBP-1 seem to be additive and independent (Latasa et al., 2000). Finally, glucose may regulate expression of lipogenic genes via a carbohydrate response transcription factor (ChoRF), which has yet to be cloned. Specific response elements that bind this transcription factor have been identified in the promoter of target genes, such as pyruvate kinase (Koo and Towle, 2000).
An important transcription factor in adipose tissue is the nuclear hormone receptor PPARγ. Despite its name, this protein is not activated by peroxisome proliferators but by fatty acids and their eicosanoid derivatives, as well as by drugs of the thiazolidinedione class (Kersten et al., 2000a). PPARγ is part of the adipocyte differentiation program, inducing the differentiation of pre-adipocytes into mature fat cells. To date, only a limited number of genes are known to be regulated by PPARγ in adipose tissue. These encode the adipocyte fatty acid binding protein, lipoprotein lipase, fatty acid transport protein (FATP), acyl-CoA synthetase, phospho-enol pyruvate carboxykinase and the fasting-induced adipose factor FIAF/PPARγ angiopoietin related PGAR (Kersten et al., 2000b; Yoon et al., 2000). Based on the identities of these genes, coupled with the observation that PPARγ expression is stimulated by insulin (Vidal-Puig et al., 1997) and by SREBP-1 (Fajas et al., 1999), one would expect PPARγ to have not only an adipogenic effect, but also a lipogenic effect. This is supported by clinical data, showing that patients taking synthetic PPARγ activators frequently gain weight (Fuchtenbusch, 2000). Furthermore, heterozygous PPARγ mutant mice exhibit smaller fat stores on a high fat diet (Kubota et al., 1999; Miles et al., 2000). Inducible and tissue-specific PPARγ knock-out models should be highly informative in terms of gaining further insight into the function of PPARγ in mature fat cells. With respect to the liver, although PPARγ is normally only minimally expressed in hepatocytes, hepatic triglyceride accumulation is associated with a dramatic increase in PPARγ expression, suggesting that PPARγ may play a role in stimulating lipogenesis (Chao et al., 2000).
In conclusion, the past few years have brought a deluge of new data about the mechanisms of regulation of lipogenesis by nutrients and hormones. It is now clear that SREBP-1, and to a lesser extent USF1 and USF2, play a pivotal role in mediating the effects of nutrients and hormones on lipogenic gene expression in the liver. In adipose tissue, another transcription factor, PPARγ, is critical for the regulation of both adipogenesis and lipogenesis. The role that SREBP-1 plays in adipose tissue has yet to be clearly defined. Overall, however, SREBP-1 and PPARγ have become attractive targets for pharmaceutical interventions of disorders such as hypertriglyceridemia and obesity.
Acknowledgments
Acknowledgements
The author would like to thank Michael Müller for advice on the manuscript. The support of the Royal Netherlands Academy of Arts and Sciences is gratefully acknowledged.
References
- Assimacopoulos-Jeannet F., Brichard, S., Rencurel, F., Cusin, I. and Jeanrenaud, B. (1995) In vivo effects of hyperinsulinemia on lipogenic enzymes and glucose transporter expression in rat liver and adipose tissues. Metabolism, 44, 228–233. [DOI] [PubMed] [Google Scholar]
- Azzout-Marniche D., Becard, D., Guichard, C., Foretz, M., Ferre, P. and Foufelle, F. (2000) Insulin effects on sterol regulatory-element-binding protein-1c (SREBP-1c) transcriptional activity in rat hepatocytes. Biochem. J., 350, 389–393. [PMC free article] [PubMed] [Google Scholar]
- Bai Y., Zhang, S., Kim, K.S., Lee, J.K. and Kim, K.H. (1996) Obese gene expression alters the ability of 30A5 preadipocytes to respond to lipogenic hormones. J. Biol. Chem., 271, 13939–13942. [DOI] [PubMed] [Google Scholar]
- Casado M., Vallet, V.S., Kahn, A. and Vaulont, S. (1999) Essential role in vivo of upstream stimulatory factors for a normal dietary response of the fatty acid synthase gene in the liver. J. Biol. Chem., 274, 2009–2013. [DOI] [PubMed] [Google Scholar]
- Chao L., Marcus-Samuels, B., Mason, M.M., Moitra, J., Vinson, C., Arioglu, E., Gavrilova, O. and Reitman, M.L. (2000) Adipose tissue is required for the antidiabetic, but not for the hypolipidemic, effect of thiazolidinediones. J. Clin. Invest., 106, 1221–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etherton T.D. (2000) The biology of somatotropin in adipose tissue growth and nutrient partitioning. J. Nutr., 130, 2623–2625. [DOI] [PubMed] [Google Scholar]
- Fajas L. et al. (1999) Regulation of peroxisome proliferator-activated receptor γ expression by adipocyte differentiation and determination factor 1/sterol regulatory element binding protein 1: implications for adipocyte differentiation and metabolism. Mol. Cell. Biol., 19, 5495–5503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foretz M., Guichard, C., Ferre, P. and Foufelle, F. (1999a) Sterol regulatory element binding protein-1c is a major mediator of insulin action on the hepatic expression of glucokinase and lipogenesis-related genes. Proc. Natl Acad. Sci. USA, 96, 12737–12742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foretz M. et al. (1999b) ADD1/SREBP-1c is required in the activation of hepatic lipogenic gene expression by glucose. Mol. Cell. Biol., 19, 3760–3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchtenbusch M., Standl, E. and Schatz, H. (2000) Clinical efficacy of new thiazolidinediones and glinides in the treatment of type 2 diabetes mellitus. Exp. Clin. Endocrinol. Diabetes, 108, 151–163. [DOI] [PubMed] [Google Scholar]
- Hasty A.H. et al. (2000) Sterol regulatory element-binding protein-1 is regulated by glucose at the transcriptional level. J. Biol. Chem., 275, 31069–31077. [DOI] [PubMed] [Google Scholar]
- Horton J.D. and Shimomura, I. (1999) Sterol regulatory element binding proteins: activators of cholesterol and fatty acid biosynthesis. Curr. Opin. Lipidol., 10, 143–150. [DOI] [PubMed] [Google Scholar]
- Horton J.D., Bashmakov, Y., Shimomura, I. and Shimano, H. (1998) Regulation of sterol regulatory element binding proteins in livers of fasted and refed mice. Proc. Natl Acad. Sci. USA, 95, 5987–5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua X., Yokoyama, C., Wu, J., Briggs, M.R., Brown, M.S., Goldstein, J.L. and Wang, X. (1993) SREBP-2, a second basic-helix–loop–helix-leucine zipper protein that stimulates transcription by binding to a sterol regulatory element. Proc. Natl Acad. Sci. USA, 190, 11603–11607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jump D.B., Clarke, S.D., Thelen, A. and Liimatta, M. (1994) Coordinate regulation of glycolytic and lipogenic gene expression by polyunsaturated fatty acids. J. Lipid Res., 35, 1076–1084. [PubMed] [Google Scholar]
- Kakuma T., Lee, Y., Higa, M., Wang, Z.W., Pan, W., Shimomura, I. and Unger, R.H. (2000) Leptin, troglitazone, and the expression of sterol regulatory element binding proteins in liver and pancreatic islets. Proc. Natl Acad. Sci. USA, 97, 8536–8541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersten S., Seydoux, J., Peters, J.M., Gonzalez, F.J., Desvergne, B. and Wahli, W. (1999) Peroxisome proliferator-activated receptor α mediates the adaptive response to fasting. J. Clin. Invest., 103, 1489–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersten S., Desvergne, B. and Wahli, W. (2000a) Roles of PPARs in health and disease. Nature, 405, 421–424. [DOI] [PubMed] [Google Scholar]
- Kersten S., Mandard, S., Tan, N.S., Escher, P., Metzger, D., Chambon, P., Gonzalez, F.J., Desvergne, B. and Wahli, W. (2000b) Characterization of the fasting-induced adipose factor FIAF, a novel peroxisome proliferator-activated receptor target gene. J. Biol. Chem., 275, 28488–28493. [DOI] [PubMed] [Google Scholar]
- Kim H.J., Takahashi, M. and Ezaki, O. (1999) Fish oil feeding decreases mature sterol regulatory element-binding protein 1 (SREBP-1) by down-regulation of SREBP-1c mRNA in mouse liver. A possible mechanism for down-regulation of lipogenic enzyme mRNAs. J. Biol. Chem., 274, 25892–25898. [DOI] [PubMed] [Google Scholar]
- Kim J.B., Sarraf, P., Wright, M., Yao, K.M., Mueller, E., Solanes, G., Lowell, B.B. and Spiegelman, B.M. (1998) Nutritional and insulin regulation of fatty acid synthetase and leptin gene expression through ADD1/SREBP1. J. Clin. Invest., 101, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo S.H. and Towle, H.C. (2000) Glucose regulation of mouse S(14) gene expression in hepatocytes. Involvement of a novel transcription factor complex. J. Biol. Chem., 275, 5200–5207. [DOI] [PubMed] [Google Scholar]
- Kopelman P.G. (2000) Obesity as a medical problem. Nature, 404, 635–643. [DOI] [PubMed] [Google Scholar]
- Kubota N. et al. (1999) PPARγ mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Mol. Cell, 4, 597–609. [DOI] [PubMed] [Google Scholar]
- Lane M.D. et al. (1990) Insulin-receptor tyrosine kinase and glucose transport. Diabetes Care, 13, 565–575. [DOI] [PubMed] [Google Scholar]
- Latasa M.J., Moon, Y.S., Kim, K.H. and Sul, H.S. (2000) Nutritional regulation of the fatty acid synthase promoter in vivo: sterol regulatory element binding protein functions through an upstream region containing a sterol regulatory element. Proc. Natl Acad. Sci. USA, 97, 10619–10624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mater M.K., Thelen, A.P., Pan, D.A. and Jump, D.B. (1999) Sterol response element-binding protein 1c (SREBP1c) is involved in the polyunsaturated fatty acid suppression of hepatic S14 gene transcription. J. Biol. Chem., 274, 32725–32732. [DOI] [PubMed] [Google Scholar]
- Miles P.D., Barak, Y., He, W., Evans, R.M. and Olefsky, J.M. (2000) Improved insulin-sensitivity in mice heterozygous for PPAR-γ deficiency. J. Clin. Invest., 105, 287–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray I., Sniderman, A.D. and Cianflone, K. (1999a) Enhanced triglyceride clearance with intraperitoneal human acylation stimulating protein in C57BL/6 mice. Am. J. Physiol., 277, E474–E480. [DOI] [PubMed] [Google Scholar]
- Murray I., Sniderman, A.D. and Cianflone, .K. (1999b) Mice lacking acylation stimulating protein (ASP) have delayed postprandial triglyceride clearance. J. Lipid Res., 40, 1671–1676. [PubMed] [Google Scholar]
- Murray I., Havel, P.J., Sniderman, A.D. and Cianflone, K. (2000) Reduced body weight, adipose tissue, and leptin levels despite increased energy intake in female mice lacking acylation-stimulating protein. Endocrinology, 141, 1041–1049. [DOI] [PubMed] [Google Scholar]
- Nakae J. and Accili, D. (1999) The mechanism of insulin action. J. Pediatr. Endocrinol. Metab., 12, 721–731. [PubMed] [Google Scholar]
- Rosen E.D. and Spiegelman, B.M. (2000) Molecular regulation of adipogenesis. Annu. Rev. Cell Dev. Biol., 16, 145–171. [DOI] [PubMed] [Google Scholar]
- Roth G., Kotzka, J., Kremer, L., Lehr, S., Lohaus, C., Meyer, H.E., Krone, W. and Muller-Wieland, D. (2000) MAP kinases Erk1/2 phosphorylate sterol regulatory element-binding protein (SREBP)-1a at serine 117 in vitro. J. Biol. Chem., 275, 33302–33307. [DOI] [PubMed] [Google Scholar]
- Shimano H., Shimomura, I., Hammer, R.E., Herz, J., Goldstein, J.L., Brown, M.S. and Horton, J.D. (1997) Elevated levels of SREBP-2 and cholesterol synthesis in livers of mice homozygous for a targeted disruption of the SREBP-1 gene. J. Clin. Invest., 100, 2115–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimano H. et al. (1999) Sterol regulatory element-binding protein-1 as a key transcription factor for nutritional induction of lipogenic enzyme genes. J. Biol. Chem., 274, 35832–35839. [DOI] [PubMed] [Google Scholar]
- Shimomura I., Hammer, R.E., Richardson, J.A., Ikemoto, S., Bashmakov, Y., Goldstein, J.L. and Brown, M.S. (1998) Insulin resistance and diabetes mellitus in transgenic mice expressing nuclear SREBP-1c in adipose tissue: model for congenital generalized lipodystrophy. Genes Dev., 12, 3182–3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegrist-Kaiser C.A. et al. (1997) Direct effects of leptin on brown and white adipose tissue. J. Clin. Invest., 100, 2858–2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sniderman A.D., Maslowska, M. and Cianflone, K. (2000) Of mice and men (and women) and the acylation-stimulating protein pathway. Curr. Opin. Lipidol., 11, 291–296. [DOI] [PubMed] [Google Scholar]
- Soukas A., Cohen, P., Socci, N.D. and Friedman, J.M. (2000) Leptin-specific patterns of gene expression in white adipose tissue. Genes Dev., 14, 963–980. [PMC free article] [PubMed] [Google Scholar]
- Teglund S. et al. (1998) Stat5a and Stat5b proteins have essential and nonessential, or redundant, roles in cytokine responses. Cell, 93, 841–850. [DOI] [PubMed] [Google Scholar]
- Thewke D.P., Panini, S.R. and Sinensky, M. (1998) Oleate potentiates oxysterol inhibition of transcription from sterol regulatory element-1-regulated promoters and maturation of sterol regulatory element binding proteins. J. Biol. Chem., 273, 21402–21407. [DOI] [PubMed] [Google Scholar]
- Tontonoz P., Kim, J.B., Graves, R.A. and Spiegelman, B.M. (1993) ADD1: a novel helix–loop–helix transcription factor associated with adipocyte determination and differentiation. Mol. Cell. Biol., 13, 4753–4759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Harmelen V., Reynisdottir, S., Cianflone, K., Degerman, E., Hoffstedt, J., Nilsell, K., Sniderman, A. and Arner, P. (1999) Mechanisms involved in the regulation of free fatty acid release from isolated human fat cells by acylation-stimulating protein and insulin. J. Biol. Chem., 274, 18243–18251. [DOI] [PubMed] [Google Scholar]
- Vidal-Puig A.J., Considine, R.V., Jimenez-Linan, M., Werman, A., Pories, W.J., Caro, J.F. and Flier, J.S. (1997) Peroxisome proliferator-activated receptor gene expression in human tissues. Effects of obesity, weight loss, and regulation by insulin and glucocorticoids. J. Clin. Invest., 99, 2416–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D. and Sul, H.S. (1997) Upstream stimulatory factor binding to the E-box at –65 is required for insulin regulation of the fatty acid synthase promoter. J. Biol. Chem., 272, 26367–26374. [DOI] [PubMed] [Google Scholar]
- Wang M.-Y., Lee, Y. and Unger, R.H. (1999) Novel form of lipolysis induced by leptin. J. Biol. Chem., 274, 17541–17544. [DOI] [PubMed] [Google Scholar]
- Wetsel R.A., Kildsgaard, J., Zsigmond, E., Liao, W. and Chan, L. (1999) Genetic deficiency of acylation stimulating protein (ASP(C3ades-Arg)) does not cause hyperapobetalipoproteinemia in mice. J. Biol. Chem., 274, 19429–19433. [DOI] [PubMed] [Google Scholar]
- Xu J., Nakamura, M.T., Cho, H.P. and Clarke, S.D. (1999) Sterol regulatory element binding protein-1 expression is suppressed by dietary polyunsaturated fatty acids. A mechanism for the coordinate suppression of lipogenic genes by polyunsaturated fats. J. Biol. Chem., 274, 23577–23583. [DOI] [PubMed] [Google Scholar]
- Yahagi N. et al. (1999) A crucial role of sterol regulatory element-binding protein-1 in the regulation of lipogenic gene expression by polyunsaturated fatty acids. J. Biol. Chem., 274, 35840–35844. [DOI] [PubMed] [Google Scholar]
- Yin D., Clarke, S.D., Peters, J.L. and Etherton, T.D. (1998) Somatotropin-dependent decrease in fatty acid synthase mRNA abundance in 3T3-F442A adipocytes is the result of a decrease in both gene transcription and mRNA stability. Biochem. J., 331, 815–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama C., Wang, X., Briggs, M.R., Admon, A., Wu, J., Hua, X., Goldstein, J.L. and Brown, M.S. (1993) SREBP-1, a basic-helix–loop–helix-leucine zipper protein that controls transcription of the low density lipoprotein receptor gene. Cell, 75, 187–197. [PubMed] [Google Scholar]
- Yoon J.C., Chickering, T.W., Rosen, E.D., Dussault, B., Qin, Y., Soukas, A., Friedman, J.M., Holmes, W.E. and Spiegelman, B.M. (2000) Peroxisome proliferator-activated receptor γ target gene encoding a novel angiopoietin-related protein associated with adipose differentiation. Mol. Cell. Biol., 20, 5343–5349. [DOI] [PMC free article] [PubMed] [Google Scholar]