Abstract
During cell migration, coordination between membrane traffic, cell substrate adhesion and actin reorganization is required for protrusive activity to occur at the leading edge. Actin organization is regulated by Rho family GTPases and, with a contribution from the endocytic cycle, serves to extend the cell front. The details of the molecular mechanisms that direct membrane traffic at sites of adhesion and rearrange actin at the cell edge are still unknown. However, recent findings show that a number of multi-domain proteins characterized by an ArfGAP domain interact with both actin-regulating and integrin-binding proteins, as well as affecting Rac-mediated protrusive activity and cell migration. Some of these proteins have been shown to localize to endocytic compartments and to have a role in regulating endocytosis. Given the participation of Arf proteins in regulating membrane traffic, one appealing hypothesis is that the ArfGAPs act as molecular devices that coordinate membrane traffic and cytoskeletal reorganization during cell motility.
Membrane traffic and actin dynamics at the leading edge
Cell migration is driven by the protrusive activity at the leading edge of the cell, where continuous remodelling of actin and adhesive contacts is required. It has been hypothesized that membrane internalized from the cell surface is recycled to the front of migrating cells to contribute to the extension of the cell border (Bretscher, 1996). Given the rapid rate of membrane internalization (Hao and Maxfield, 2000), large amounts of recycling membrane would be made available for polarized delivery by such a mechanism. Consistent with this model, recycling transferrin receptors and low density lipoprotein receptors are distributed to the cell front of migrating fibroblasts and to Rac-induced ruffles (Hopkins et al., 1994; Bretscher and Aguado-Velasco, 1998a). Thus, the random reinsertion of internalized membranes at the surface of a resting cell may be redirected to the sites of protrusion when migration is induced by motogenic stimuli (Bretscher and Aguado-Velasco, 1998b).
While our knowledge of the molecular machinery underlying the propulsive mechanism driven by actin and mediated by Rho family GTPases has increased dramatically (Hall, 1998; Borisy and Svitkina, 2000), it is still unclear how, and to what extent, vesicle recycling is incorporated into the extension process. Progress in this direction, however, comes from studies of Arf6, a member of the ADP-ribosylation factor (Arf) family of GTPases. This protein has been implicated in the regulation of membrane traffic between the recycling endosomal compartment and the plasma membrane because of the specific localization of Arf6 in these compartments, and the effects of its overexpression on transferrin uptake and recycling to the cell surface (D’Souza-Schorey et al., 1995; Peters et al., 1995). Moreover, the Arf6-positive intracellular compartment overlaps with the transferrin receptor-positive recycling compartment (D’Souza-Schorey et al., 1998). Based on the fact that Arf1 regulates specifically the formation of vesicles within the Golgi compartment (Roth, 1999), one could speculate that Arf6 would also regulate vesicle formation, in this case during recycling between endosomes and the plasma membrane. Arf6 appears to be functionally linked to Rac1, a Rho family GTPase involved in the formation of actin-rich ruffles and lamellipodia (Ridley et al., 1992). Rac1 and Arf6 colocalize at the plasma membrane and on recycling endosomes, and Rac1-stimulated ruffling is blocked by the GTP binding-defective N27-Arf6 mutant (Radhakrishna et al., 1999). Together, the data regarding Arf6 involvement in vesicle trafficking and the association with Rac1 have led to the suggestion that the ability of Arf6 to influence Rac1-mediated lamellipodial formation depends in part on Arf6-mediated regulation of Rac1 trafficking to the plasma membrane.
The question left largely unsolved relates to what the mechanisms underlying the postulated recycling of membrane to polarized sites of actin organization are. The focus here is on recent findings on a group of proteins that share an Arf-specific GAP (GTPase-activating protein) domain and are thus implicated in the coordination between membrane trafficking and actin reorganization during cell locomotion.
More than just GAPs: a connection
Arfs cycle between the GTP- and GDP-bound forms with the help of specific GAPs and GEFs (guanine nucleotide exchange factors) (Donaldson and Jackson, 2000). Recently, a number of multi-domain proteins with an ArfGAP domain have been identified (Figure 1) and these are capable of interacting with proteins involved in both cell adhesion and actin organization. Given the proposed role of Arf6 in membrane recycling, one attractive hypothesis is that the ArfGAP activity of some of these proteins is required for the regulation of Arf-mediated membrane recycling to sites of protrusion during cell locomotion.
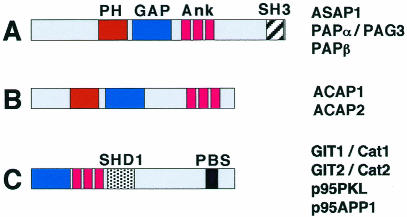
Fig. 1. Schematic representation of three recently identified groups of multi-domain proteins characterized by the presence of an ArfGAP domain (GAP) and by ankyrin repeats (Ank). (A) ASAP1 and two PAP proteins are characterized by the presence of a pleckstrin homology domain (PH) and a C-terminal Src homology type 3 (SH3) domain. (B) ACAP proteins, include a PH domain. (C) Four members of the GIT family are characterized by a Spa2 homology 1 domain (SHD1) (Sheu et al.,1998), and a C-terminal paxillin binding subdomain (PBS). Proteins in (A) and (B) are members of the centaurin family, which is characterized by ArfGAP domain, ankyrin repeats and PH domain.
Support for this hypothesis comes from recent data on ArfGAPs of the centaurin family. Two of these, ASAP1 and PAPα (Figure 1A), show in vitro GAP activity toward Arf1, Arf5 and, to a lesser extent, Arf6. They interact with Src and Pyk2, respectively (Brown et al., 1998; Andreev et al., 1999), two tyrosine kinases that have been implicated in the regulation of integrin-mediated adhesion (Schlaepfer and Hunter, 1998). ASAP1 localizes to peripheral focal complexes, and requires a functional ArfGAP domain for actin remodelling in motile cells (Randazzo et al., 2000). PAPα-paxillin complexes are recruited to the cell periphery. Paxillin is a focal adhesion scaffolding protein (Turner et al., 1990), which has been proposed to play a role in focal adhesion dynamics. Overexpression of wild-type PAPα, but not of a GAP-inactive mutant form, inhibits paxillin recruitment to focal contacts and sites of cell migration, resulting in decrease of the cell migratory activity on extracellular matrix (Kondo et al., 2000). The data support the hypothesis that the recruitment of both structural (paxillin) and signalling (Src and Pyk2) molecules to the leading edges of migrating cells, a step that favours the formation of new adhesive contacts at these sites, is not mediated by simple cytoplasmic diffusion, but rather through these ArfGAPs. Two other centaurins, ACAP1 and ACAP2 (Figure 1B), show strongest GAP activity toward Arf6 (Jackson et al., 2000). Overexpression of ACAPs prevents the formation of Arf6-dependent protrusions and leads to redistribution of ACAPs to endosomal structures, together with activated Arf6. Again, a functional GAP domain is required for these effects, implicating the GAP activity of ACAPs in the regulation of Arf6-mediated membrane recycling from the endosomal compartment (where Arf6 is activated to Arf6-GTP) (Radhakrishna and Donaldson, 1997) back to the plasma membrane.
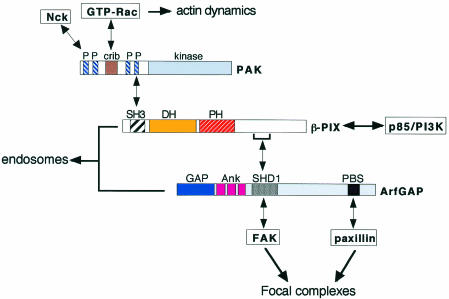
The GIT family of AfrGAPs (Figure 1C) includes multi-domain proteins with an N-terminal ArfGAP domain (Premont et al., 1998). These exhibit in vitro GAP activity toward several Arfs, including Arf6 (Vitale et al., 2000). GIT proteins are components of complexes (Figure 2) which may also include the GEF PIX (Oh et al., 1997; Bagrodia et al., 1998; Manser et al., 1998), the Rac effector PAK (Daniels and Bokoch, 1999), and the adaptor protein Nck (McCarty, 1998; Turner et al., 1999). Existing data support the hypothesis that GIT proteins play a role in Arf6-mediated membrane recycling. It is known that GIT proteins can affect endocytosis (Claing et al., 2000), and dissection of these multi-domain proteins has been useful in identifying possible distinct functions for individual domains. For example, the N-terminal region of p95APP1 colocalizes with the GTP-binding defective mutant N27-Arf6 at endocytic vesicles (Di Cesare et al., 2000), where this inactive GTPase accumulates. The first ankyrin repeat of this protein is necessary for membrane targeting of the ArfGAP domain, which by itself is cytosolic (Di Cesare et al., 2000). Another possible link of GIT proteins to membrane recycling is the partial localization of p95APP1 to large, transferrin receptor-positive endocytic vesicles (Di Cesare et al., 2000), which may represent a functionally altered recycling compartment. This localization is strongly enhanced by co-expression of PIX, and also for a truncated form of p95APP1 that includes the SHD1 PIX-binding domain but lacks the N-terminal region in which the ArfGAP domain resides (Di Cesare et al., 2000). These data point to a role for the SHD1 domain in PIX-mediated recruitment to the recycling compartment. It can be speculated that the resulting lack of the GAP activity at vesicles would interfere with Arf6-mediated membrane recycling, leading to the accumulation of internalized membrane in an abnormal perinuclear recycling compartment.
Fig. 2. Model for the intermolecular interactions and functional connections proposed for members of the GIT family (Figure 1C). Double-ended arrows point to known direct intermolecular interactions; single-ended arrows indicate functional connections. P, Proline-rich region; crib, Cdc42/Rac interactive binding motif; SH3, Src homology type 3; DH, Dbl homology; PH, pleckstrin homology; GAP, ArfGAP domain; Ank, ankyrin repeat; SHD1, Spa2 homology domain type 1; PBS, paxillin binding subdomain; p85/PI3K, 85 kD regulatory subunit of the phosphatidylinositol-3 kinase; ArfGAP = multi-domain ArfGAP protein. Members of the GIT family of ArfGAP proteins (indicated as ArfGAP) can stably interact with PIX and PAK (Bagrodia et al., 1999; Turner et al., 1999; Di Cesare et al., 2000), which mediate the interaction of the complex with active Rac and Cdc42 at the membrane. The complex may regulate actin remodelling at the cell surface by controlling Rac/Cdc42 activity via PAK and PIX. The interaction of ArfGAP proteins with paxillin (Turner et al., 1990, 1999) and FAK (Zhao et al., 2000b) functionally links the complex to integrin-mediated adhesion. Finally, the ArfGAPs are localized to the endosomal compartment by both PIX-dependent and PIX-independent mechanisms.
Most GIT proteins interact with paxillin by their C-terminal region (Turner et al., 1999). A p95APP1 C-terminal paxillin-binding construct strongly enhances the protrusive activity at the cell edge in a Rac- and Arf6-dependent manner, with relocation of paxillin to the sites of protrusion (Di Cesare et al., 2000). Overexpression of GIT1 causes loss of paxillin from existing focal complexes and stimulates cell motility (Zhao et al., 2000b), while inhibition of the interaction between paxillin and p95PKL prevents lamellipodial formation (Turner et al., 1999). Interestingly, PIX-mediated recruitment of p95APP1 to the large endocytic vacuoles is accompanied by recruitment of paxillin from older focal complexes to the same vesicles (Di Cesare et al., 2000). Consistent with this finding, the severe paxillin reduction observed at focal adhesions in response to GIT1 overexpression is accompanied by the localization of paxillin at large perinuclear vesicles (Zhao et al., 2000b). Altogether, these data indicate a possible connection between paxillin and membrane recycling, and implicate the GIT–paxillin complexes in the protrusive activity.
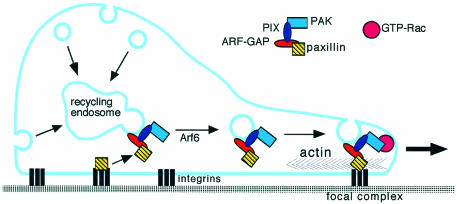
According to the model proposed here (Figure 3), GIT proteins would form stable complexes linking Rho and Arf family GTPases by cycling between the endocytic recycling compartment and the plasma membrane. This mechanism would provide a way to deliver to the cell edge both membranes and the molecules required to stimulate actin polymerization and the formation of new adhesive sites. On one hand, GIT–paxillin complexes would affect adhesion and actin organization by recruiting paxillin to new Rac-induced focal complexes at the cell edge (Nobes and Hall, 1995). On the other, GIT complexes would position PIX and PAK at the cell border to regulate Rac function (Daniels and Bokoch, 1999). In this picture, the ArfGAP activity would be part of the engine required to efficiently re-utilize the endocytosed membrane for cell motility.
Fig. 3. Proposed model for the role of multi-domain ArfGAPs of the GIT family during cell migration. The ArfGAP protein is able to colocalize with Arf6 in the recycling endosomal compartment via its ankyrin repeats and the interaction with PIX. There the internalized membranes converge before recycling. In the proposed model it is speculated that wild-type Arf proteins (including Arf6) and the GAP activity of the ArfGAP are required for the formation of recycling vesicles. Once formed, vesicles may be recruited to Rac-enriched sites of the leading edge of the migrating cell. By recruiting paxillin to the complex, ArfGAP proteins would induce the redistribution of paxillin away from established focal adhesions to the leading edge. Here, paxillin would contribute to the formation of membrane protrusions, by participating in the formation of focal complexes in which paxillin is required for the anchorage of the Rac-induced actin filaments to the sites of substrate adhesion. Regulatory mechanisms must exist to direct distinct pools of the complex to endosomal membranes or to the cell surface.
How are vesicles budding from the recycling compartment targeted to the migrating cell edge? Recent results have indicated that PIX may be recruited to the membrane either via the formation of a complex with PAK and Nck, or by direct association with the p85 regulatory subunit of PI3-kinase (Yoshii et al., 1999). During cell motility, the localized activation of adhesive or guiding receptors by extracellular cues would drive anchoring of the PIX–ArfGAP complex at new sites by one of these mechanisms. As a consequence, in addition to the stimulation of the PAK kinase activity by PIX, PAK would stimulate the exchange factor activity of PIX (Daniels et al., 1999). Alternatively, the localization of the ArfGAP–PIX–PAK complex could be driven by PAK to areas of the plasma membrane that are enriched in GTP-Rac (Figure 3), which, in turn, might be recruited in response to extracellular adhesive and motogenic stimuli (del Pozo et al., 2000).
Perspectives
At this stage, the proposed function of the multi-domain ArfGAP proteins represents mostly a working model, with several open questions to be answered. Several aspects of cell biology will need to be merged to address the issue in a complete way. Among the requirements are a better characterization of the intracellular recycling compartments involved, and of the molecular machinery linking these complexes to the endosomal membranes. It will also be necessary to identify the Arf proteins targeted by all these ArfGAPs in vivo, as well as to demonstrate the role of the ArfGAP activity in membrane recycling in cell-free systems.
Also worthy of attention is the apparent incongruence between the localization of some of these ArfGAPs to the endosomal compartment, and the reported accumulation of active Arf6 mutants at the plasma membrane where known Arf6 GEFs, such as ARNO (Frank et al., 1998) and EFA6 (Franco et al., 1999), have also been shown to reside. One possibility is that the substrate-independent subcellular localization of these proteins represents a way to limit their catalytic activity to sites where endogenous Arf6 needs to be regulated to perform its specific functions, and these sites may not be reflected by the distribution of Arf6 mutants as revealed by morphological analysis. Further work will be required to unravel this issue.
In view of the dynamic nature of the mechanisms in which the ArfGAP complexes are implicated, it is not surprising that their regulation within the cell appears to be extremely complicated. Examples of functional regulation include the PIX-dependent interaction of GIT1 with paxillin (Zhao et al., 2000b), the PIX-mediated localization of PAK to focal complexes (Manser et al., 1998) and the disruption of the PIX–PAK complex as a consequence of PAK activation (Zhao et al., 2000a). Further analysis of these regulatory mechanisms will be fundamental to understanding how these complex interactions may be dynamically and spatially coordinated during cell migration. Although at its primordial stages, an integrated analysis of the mechanisms that lead to polarized delivery of membranes during cell migration should finally lead us to understand, in a more comprehensive way, the fundamental process of cell motility.

Ivan de Curtis
Acknowledgments
Acknowledgements
I thank Jacopo Meldolesi for critical reading of the manuscript. The financial support of Telethon-Italy (Grant n.1171 to I.de C.) is gratefully acknowledged.
REFERENCES
- Andreev J., Simon, J.P., Sabatini, D.D., Kam, J., Plowman, G., Randazzo, P.A. and Schlessinger, J. (1999) Identification of a new Pyk2 target protein with Arf-GAP activity. Mol. Cell. Biol., 19, 2338–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagrodia S., Taylor, S.J., Jordan, K.A., Van Aelst, L. and Cerione, R.A. (1998) A novel regulator of p21-activated kinases. J. Biol. Chem., 273, 23633–23636. [DOI] [PubMed] [Google Scholar]
- Bagrodia S., Bailey, D., Lenard, Z., Hart, M., Guan, J.L., Premont, R.T., Taylor, S.J. and Cerione, R.A. (1999) A tyrosine-phosphorylated protein that binds to an important regulatory region on the cool family of p21-activated kinase-binding proteins. J. Biol. Chem., 274, 22393–22400. [DOI] [PubMed] [Google Scholar]
- Borisy G.G. and Svitkina, T.M. (2000) Actin machinery: pushing the envelope. Curr. Opin. Cell Biol., 12, 104–112. [DOI] [PubMed] [Google Scholar]
- Bretscher M.S. (1996) Getting membrane flow and the cytoskeleton to cooperate in moving cells. Cell, 87, 601–606. [DOI] [PubMed] [Google Scholar]
- Bretscher M.S. and Aguado-Velasco, C. (1998a) EGF induces recycling membrane to form ruffles. Curr. Biol., 8, 721–724. [DOI] [PubMed] [Google Scholar]
- Bretscher M.S. and Aguado-Velasco, C. (1998b) Membrane traffic during cell locomotion. Curr. Opin. Cell Biol., 10, 537–541. [DOI] [PubMed] [Google Scholar]
- Brown M.T., Andrade, J., Radhakrishna, H., Donaldson, J.G., Cooper, J.A. and Randazzo, P.A. (1998) ASAP1, a phospholipid-dependent Arf GTPase-activating protein that associates with and is phosphorylated by Src. Mol. Cell. Biol., 18, 7038–7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claing A., Perry, S.J., Achiriloaie, M., Walker, J.K., Albanesi, J.P., Lefkowitz, R.J. and Premont, R.T. (2000) Multiple endocytic pathways of G protein-coupled receptors delineated by GIT1 sensitivity. Proc. Natl Acad. Sci. USA, 97, 1119–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels R.H. and Bokoch, G.M. (1999) p21-activated protein kinase: a crucial component of morphological signaling? Trends Bioch. Sci., 24, 350–355. [DOI] [PubMed] [Google Scholar]
- Daniels R.H., Zenke, F.T., and Bokoch, G.M. (1999) αPix stimulates p21-activated kinase activity through exchange factor-dependent and -independent mechanisms. J. Biol. Chem., 274, 6047–6050. [DOI] [PubMed] [Google Scholar]
- del Pozo M.A., Price, L.S., Alderson, N.B., Ren, X.D. and Schwartz, M.A. (2000) Adhesion to the extracellular matrix regulates the coupling of the small GTPase Rac to its effector PAK. EMBO J., 19, 2008–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cesare A., Paris, S., Albertinazzi, C., Dariozzi, S., Andersen, J., Mann, M., Longhi, R. and de Curtis, I. (2000) p95-APP1 links membrane transport to Rac-mediated reorganization of actin. Nature Cell Biol., 2, 521–530. [DOI] [PubMed] [Google Scholar]
- Donaldson J.G. and Jackson, C.L. (2000) Regulators and effectors of the ARF GTPases. Curr. Opin. Cell Biol., 12, 475–482. [DOI] [PubMed] [Google Scholar]
- D’Souza-Schorey C., Li, G., Colombo, M.I. and Stahl, P.D. (1995) A regulatory role for ARF6 in receptor-mediated endocytosis. Science, 267, 1175–1178. [DOI] [PubMed] [Google Scholar]
- D’Souza-Schorey C., van Donselaar, E., Hsu, V.W., Yang, C., Stahl, P.D. and Peters, P.J. (1998) ARF6 targets recycling vesicles to the plasma membrane: insights from an ultrastructural investigation. J. Cell Biol., 140, 603–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco M., Peters, P.J., Boretto, J., van Donselaar, E., Neri, A., D’Souza-Schorey, C. and Chavrier, P. (1999) EFA6, a sec7 domain-containing exchange factor for ARF6, coordinates membrane recycling and actin cytoskeleton organization. EMBO J., 181480–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank S., Upender, S., Hansen, S.H. and Casanova, J.E. (1998) ARNO is a guanine nucleotide exchange factor for ADP-ribosylation factor 6. J. Biol. Chem., 273, 23–27. [DOI] [PubMed] [Google Scholar]
- Hall A. (1998) Rho GTPases and the actin cytoskeleton. Science, 279, 509–514. [DOI] [PubMed] [Google Scholar]
- Hao M. and Maxfield, F.R. (2000) Characterization of rapid membrane internalization and recycling. J. Biol. Chem., 275, 15279–15286. [DOI] [PubMed] [Google Scholar]
- Hopkins C.R., Gibson, A., Shipman, M., Strickland, D.K. and Trowbridge, I.S. (1994) In migrating fibroblasts, recycling receptors are concentrated in narrow tubules in the pericentriolar area, and then routed to the plasma membrane of the leading lamella. J. Cell Biol., 125, 1265–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson T.R., Brown, F.D., Nie, Z., Miura, K., Foroni, L., Sun, J., Hsu, V.W., Donaldson, J.G. and Randazzo, P.A. (2000) ACAPs are arf6 GTPase-activating proteins that function in the cell periphery. J. Cell Biol., 151, 627–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo A., Hashimoto, S., Yano, H., Nagayama, K., Mazaki, Y. and Sabe, H. (2000) A new paxillin-binding protein, PAG3/PAPα/KIAA0400, bearing an ADP-ribosylation factor GTPase-activating protein activity, is involved in paxillin recruitment to focal adhesions and cell migration. Mol. Biol. Cell, 11, 1315–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manser E., Loo, T.H., Koh, C.G., Zhao, Z.S., Chen, X.Q., Tan, L., Tan, I., Leung, T. and Lim, L. (1998) PAK kinases are directly coupled to the PIX family of nucleotide exchange factors. Mol. Cell, 1, 183–192. [DOI] [PubMed] [Google Scholar]
- McCarty J.H. (1998) The Nck SH2/SH3 adaptor protein: a regulator of multiple intracellular signal transduction events. BioEssays, 20, 913–921. [DOI] [PubMed] [Google Scholar]
- Nobes C.D. and Hall, A. (1995) Rho, Rac, and Cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell, 81, 53–62. [DOI] [PubMed] [Google Scholar]
- Oh W.K., Yoo, J.C., Jo, D., Song, Y.H., Kim, M.G. and Park, D. (1997) Cloning of a SH3 domain-containing proline-rich protein, p85SPR, and its localization in focal adhesion. Biochem. Biophys. Res. Commun., 235, 794–798. [DOI] [PubMed] [Google Scholar]
- Peters P.J., Hsu V.W., Ooi C.E., Finazzi D., Teal S.B., Oorschot V., Donaldson J.G. and Klausner R.D. (1995) Overexpression of wild-type and mutant ARF1 and ARF6: distinct perturbations of nonoverlapping membrane compartments. J. Cell Biol., 128, 1003–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premont R.T., Claing, A., Vitale, N., Freeman, J.L., Pitcher, J.A., Patton, W.A., Moss, J., Vaughan, M. and Lefkowitz, R.J. (1998) β2-Adrenergic receptor regulation by GIT1, a G protein-coupled receptor kinase-associated ADP ribosylation factor GTPase-activating protein. Proc. Natl Acad. Sci. USA, 95, 14082–14087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishna H. and Donaldson, J.G. (1997) ADP-ribosylation factor 6 regulates a novel plasma membrane recycling pathway. J. Cell Biol., 139, 49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishna H., Al-Awar, O., Khachikian, Z. and Donaldson, J.G. (1999) ARF6 requirement for Rac ruffling suggests a role for membrane trafficking in cortical actin rearrangements. J. Cell Sci., 112, 855–866. [DOI] [PubMed] [Google Scholar]
- Randazzo P.A., Andrade, J., Miura, K., Long, Y.-Q., Stauffer, S., Roller, P. and Cooper, J.A. (2000) The Arf GTPase-activating protein ASAP1 regulates the actin cytoskeleton. Proc. Natl Acad. Sci. USA, 97, 4011–4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley A.J., Paterson, H.F., Johnston, C.L., Diekmann, D. and Hall, A. (1992) The small GTP-binding protein Rac regulates growth factor-induced membrane ruffling. Cell, 70, 401–410. [DOI] [PubMed] [Google Scholar]
- Roth M.G. (1999) Snapshots of ARF1: Implications for mechanisms of activation and inactivation. Cell, 97, 149–152. [DOI] [PubMed] [Google Scholar]
- Schlaepfer D.D. and Hunter, T. (1998) Integrin signalling and tyrosine phosphorylation: just the FAKs? Trends Cell Biol., 8, 151–157. [DOI] [PubMed] [Google Scholar]
- Sheu Y.J., Santos, B., Fortin, N., Costigan, C. and Snyder, M. (1998) Spa2p interacts with cell polarity proteins and signaling components involved in yeast cell morphogenesis. Mol. Cell. Biol., 18, 4053–4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner C.E., Glenney, J.R. and Burridge, K. (1990) Paxillin: a new vinculin-binding protein present in focal adhesions. J. Cell Biol., 111, 1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner C.E., Brown, M.C., Perrotta, J.A., Riedy, M.C., Nikolopoulos, S.N., McDonald, A.R., Bagrodia, S., Thomas, S. and Leventhal, P.S. (1999) Paxillin LD4 motif binds PAK and PIX through a novel 95-kD ankyrin repeat, ARF-GAP protein: A role in cytoskeletal remodeling. J. Cell Biol., 145, 851–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale N., Patton, W.A., Moss, J., Vaughan, M., Lefkowitz, R.J. and Premont, R.T. (2000) GIT proteins, a novel family of phosphatidylinositol 3, 4, 5-trisphosphate-stimulated GTPase-activating proteins for ARF6. J. Biol. Chem., 275, 13901–13906. [DOI] [PubMed] [Google Scholar]
- Yoshii S. et al. (1999) αPIX nucleotide exchange factor is activated by interaction with phosphatidylinositol 3-kinase. Oncogene, 18, 5680–5690. [DOI] [PubMed] [Google Scholar]
- Zhao Z.S., Manser, E. and Lim, L. (2000a) Interaction between PAK and Nck: a template for Nck targets and role of PAK autophosphorylation. Mol. Cell. Biol., 20, 3906–3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z.S., Manser, E., Loo, T.H. and Lim, L. (2000b) Coupling of PAK-interacting exchange factor PIX to GIT1 promotes focal complex disassembly. Mol. Cell. Biol., 20, 6354–6363. [DOI] [PMC free article] [PubMed] [Google Scholar]