Abstract
Background.
Respiratory viral infections are a major cause of morbidity and hospitalization in young children. Nevertheless, the population burden of respiratory viral infections, especially asymptomatic cases, is not known due to the lack of prospective community-based cohort studies with intensive monitoring.
Methods.
To address this gap, we enacted the PREVAIL cohort, a Centers for Disease Control and Prevention–sponsored birth cohort in Cincinnati, Ohio, where children were followed from 0 to 2 years of age. Weekly text surveys were administered to record acute respiratory illnesses (ARIs), which were defined as the presence of cough or fever (≥38°C). Weekly midturbinate nasal swabs were collected and tested using the Luminex Respiratory Pathogen Panel, which detected 16 viral pathogens. Viral infection was defined as ≥1 positive tests from the same virus or viral subtype ≤30 days of a previous positive test. Maternal report and medical chart abstractions identified healthcare utilization.
Results.
From 4/2017 to 7/2020, 245 mother–infant pairs were recruited and followed. From the 13 781 nasal swabs tested, a total of 2211 viral infections were detected, of which 821 (37%) were symptomatic. Children experienced 9.4 respiratory viral infections/child-year; half were rhinovirus/enterovirus. Viral ARI incidence was 3.3 episodes/child-year. Emergency department visits or hospitalization occurred with only 15% of respiratory syncytial virus infections, 10% of influenza infections, and only 4% of all viral infections. Regardless of pathogen, most infections were asymptomatic or mild.
Conclusions.
Respiratory viral infections are common in children 0–2 years. Most viral infections are asymptomatic or non–medically attended, underscoring the importance of community-based cohort studies.
Keywords: birth cohort, influenza virus, respiratory viruses, RSV, viral infections
Respiratory viral infections contribute a significant burden of disease in infants and young children [1, 2]. Respiratory viruses are a leading cause of acute respiratory illnesses (ARIs) globally and cause a wide spectrum of respiratory syndromes from asymptomatic infection to bronchiolitis and death [3–6]. Acquisition of viral infections in early life can also be associated with long-term morbidity, including wheezing, asthma, and recurrent respiratory infections [7, 8].
In hospitalized children, the burden of respiratory viruses such as respiratory syncytial virus (RSV), influenza virus, parainfluenza virus, and rhinovirus, is well documented [9–12]. However, the natural history of these pathogens and their community-level burden in US children is inadequately characterized. Most studies have relied on hospital-based surveillance to identify infections, leading to an overrepresentation of children with symptomatic disease requiring medical care or hospital admission [13, 14]. Furthermore, most existing studies focus on recruiting children with specific respiratory symptoms, limiting our understanding of the full spectrum of asymptomatic and symptomatic illnesses. Community-based cohort studies, including birth cohorts, attempt to overcome these limitations through prospective, longitudinal data collection [15]. Cohort studies provide a comprehensive picture of the burden, spectrum, and natural history of respiratory viral infections, which can inform our prevention and control strategies [14, 16–18].
The Pediatric Respiratory and Enteric Virus Acquisition and Immunogenesis Longitudinal (PREVAIL) cohort is a Centers for Disease Control and Prevention (CDC)–funded investigation that conducted prospective, longitudinal, community-based surveillance for endemic respiratory and enteric pathogens through weekly nasal swab and stool testing of a birth cohort. The objective of this analysis is to describe the overall burden, symptomatology, and patterns of medical attendance among children less than 2 years of age with respiratory viral infections.
METHODS
Healthy mother–infant pairs in the greater Cincinnati region were enrolled into the PREVAIL cohort from April 2017 to July 2018. As enrollment occurred on a rolling basis, participants were followed from birth until 2 years of age across multiple respiratory seasons between April 2017 and July 2020. Pregnant mothers aged 18 years and older, who were at least at 34 weeks of gestation with a singleton pregnancy, were eligible and invited to participate in the study. Mothers who elected to participate provided written consent and were provisionally enrolled, but they were excluded if they did not complete their first postnatal study visit at week 2. Details on the study design, enrollment strategy, inclusion criteria, data collection, sample collection, and cohort methodology have been extensively described elsewhere [19]. Methods relevant to this work are described here. This study was reviewed and approved by the institutional review boards at the CDC, Cincinnati Children’s Hospital Medical Center, and the enrolling birth hospitals: The Christ Hospital and University of Cincinnati Medical Center.
Sample Collection and Testing
Mothers collected midturbinate nasal swabs from infants on a weekly basis. Nasal samples were delivered to the study laboratory via courier within 48 hours of sample collection. Multiplex polymerase chain reaction (PCR) testing was performed on all samples using NxTAG Respiratory Pathogen Panel (Luminex Molecular Diagnostics, Toronto, Canada), which contains 18 viral targets, including adenovirus, human bocavirus, endemic human coronaviruses (types 229E, HKU1, NL63, and OC43), influenza viruses (type A and B), human metapneumovirus, parainfluenza viruses (types 1, 2, 3, and 4), rhinovirus/enterovirus, and RSV (subtype A and B). Viral controls along with an internal control, bacteriophage MS2 (Emesvirus zingeri), were run to ensure adequate sample collection and assay validity.
Data Collection
Automated weekly text-messaging surveys were sent to enrolled mothers via mobile phone to ascertain the presence of fever and/or cough. Reports of fever or cough triggered additional questions, including start date of symptoms, if symptoms were ongoing or resolved, and the presence of additional respiratory and gastrointestinal symptoms such as earache, runny nose, wheezing, vomiting, and diarrhea. Once resolved, end-of-illness surveys were sent to capture summary information on the episode, including duration of symptoms, all medically attended visits, and antimicrobial medication use. Abstraction of medical and immunization records further identified unreported medical office attendance, emergency department visits, and hospital admissions.
Outcome Definitions
An acute respiratory infection/ARI was defined as the presence of maternally reported fever (temperature ≥ 38°C rectally or ≥37°C axillary) or cough at any time in the past week. A viral infection was defined as a positive viral detection from a nasal swab. The detection was considered part of the same viral infection if the same virus or viral subtype (when applicable) was detected in 2 respiratory samples 30 days or less apart, regardless of any interval negative swabs. A viral ARI was defined as a viral infection associated with ARI symptoms reported within ±7 days of the first day of the viral infection.
Statistical Analysis
For analyses involving symptomatology and healthcare-seeking behavior, viral infections with detection of multiple respiratory viruses were excluded because symptoms and severity could not be attributed to a single virus [15]. The full cohort was used for most analyses except for calculating the proportion of the cohort who experienced at least 1 viral infection by swab collection. In this calculation, a subcohort of 101 highly adherent participants who submitted at least 70% of eligible weekly samples and were followed to at least 18 months of age were used, as consistency of follow-up would significantly impact these calculations.
Descriptive analysis of age at first viral infection and median duration of viral ARI was performed using medians and interquartile ranges (IQRs). Nonparametric, pairwise comparisons were made using the Kruskal–Wallis rank-sum test with Holm–Sidak corrections. The denominators (weeks at risk) used for pathogen-specific viral infection incidence rates were calculated using the number of unique epidemiological weeks in which a swab was submitted per participant, minus all weeks in which that specific pathogen was detected. Weeks at risk for pathogen-specific viral ARIs were similarly calculated, but by subtracting the weeks during which the specific viral ARI occurred. Weeks at risk for incidence of any viral infection was calculated using the total weeks at risk without subtractions as it is assumed that a child may be at risk for any respiratory virus for each swab. Weeks at risk for total viral ARI incidence was calculated by subtracting the number of weeks with any viral ARI from the total number of unique weeks of submitted swabs.
The odds ratio (OR) with the 95% confidence interval (CI) of a viral infection being symptomatic and being medically attended were calculated for each respiratory virus using logistic regression with generalized estimating equation (GEE) modeling and an exchangeable correlation matrix to account for clustering among participants, comparing each respiratory virus with all other respiratory viral pathogens combined as a composite reference group. The respiratory virus–specific attributable fraction (AF) was then calculated from the OR of viral infections being asymptomatic versus symptomatic using the following formula: AF = (OR − 1)/OR. An AF of 0 indicates that the odds of detecting a specific virus during an asymptomatic viral infection was equal to the odds of detecting that virus during a symptomatic viral infection (OR = 1). All statistical analysis was performed using STATA (StataCorp, College Station, TX) and the R Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna, Austria) [20].
RESULTS
Demographics and Cohort Characteristics
A total of 265 mothers were enrolled in the third trimester of pregnancy, of whom 245 met the final postpartum eligibility criteria. Most mothers were 25–34 years of age (63%), married or partnered (67%), and publicly insured (57%) (Table 1). A plurality of households reported an annual income of $50 000 or higher (43%), while nearly one-third reported incomes below $25 000 (32%). Most children were born at greater than 38 gestational weeks (78%), initiated breastfeeding (87%), and received at least 1 dose of influenza vaccine (70%).
Table 1.
Demographic Characteristics of Mother–Infant Pairs Enrolled in the PREVAIL Cohort (N = 245): Cincinnati, Ohio, April 2017–July 2020
| Characteristics | n (%) |
|---|---|
| Maternal age | |
| 18–24y | 50 (20.4%) |
| 25–34 y | 155 (63.3%) |
| ≥35 y | 40 (16.3%) |
| Maternal race | |
| White | 127 (51.8%) |
| Black | 106 (43.3%) |
| Other | 12 (4.9%) |
| Insurance | |
| Public | 139 (56.7%) |
| Private | 106 (43.3%) |
| Marital status | |
| Married/partnered | 163 (66.5%) |
| Single | 82 (33.5%) |
| Maternal education | |
| Less than high school | 22 (9.0%) |
| High school graduate | 93 (38.0%) |
| Associate/trade | 35 (14.3%) |
| College graduate | 95 (38.8%) |
| Annual household income | |
| <US $25 000 | 78 (31.8%) |
| US $25 000–$49 999 | 49 (20.0%) |
| ≥US $50 000 | 106 (43.3%) |
| Unknown | 12 (4.9%) |
| Infant sex | |
| Female | 127 (51.8%) |
| Male | 118 (48.2%) |
| Infant gestational age | |
| 35–36 wk | 11 (4.5%) |
| 37 wk | 44 (18.0%) |
| 38–42 wk | 190 (77.6%) |
| Breastfeeding | |
| Initiated | 212 (86.5%) |
| Never breastfed | 33 (13.5%) |
| Infant influenza vaccine status | |
| Never received influenza vaccine | 74 (30.2%) |
| Received at least 1 dose of influenza vaccine | 171 (69.8%) |
Abbreviation: PREVAIL, Pediatric Respiratory and Enteric Virus Acquisition and Immunogenesis Longitudinal.
Of 13 781 nasal swabs tested, 4238 (31%) were positive for at least 1 respiratory virus. Based on our outcome definitions, these positive detections were further categorized into 2211 viral infections and 821 viral ARIs, of which 1470 (66%) viral infections and 508 (62%) viral ARIs did not involve co-detections of multiple respiratory viruses.
Viral Infection Incidence and Odds of Symptomatic Infection
Enrolled children contributed a total of 12 242 unique child-weeks (or 235.4 child-years) of follow-up based on weeks of nasal swab submission. The overall incidence of any respiratory viral infection was 9.4 infections per child-year and the overall incidence of viral ARI was 3.3 infections per child-year (Table 2). The incidence of any respiratory viral infection and viral ARIs was similar between the highly adherent cohort (n = 101) and the non–highly adherent cohort (n = 144), with 8.5 versus 10.4 infections per child-year for any viral infection and 3.4 versus 3.8 symptomatic infections per child-year, respectively.
Table 2.
Swab Positivity and Incidence of Viral Infections and Viral ARIs by Respiratory Virus in the PREVAIL Cohort (N = 245), by Respiratory Virus
| Viral Infections | Viral ARIs | ||||
|---|---|---|---|---|---|
| Pathogen | Number of Positive Weeks, n (%) | n | Incidence per Child-yeara (95% CI) | n | Incidence per Child-yeara (95% CI) |
| Adenovirus | 302 (7.8%) | 196 | .86 (.74, .99) | 81 | .35 (.28, .44) |
| Bocavirus | 764 (19.7%) | 257 | 1.19 (1.05, 1.34) | 70 | .32 (.25, .41) |
| Coronavirus 229E | 19 (0.5%) | 13 | .06 (.03, .09) | 1 | .00 (.00, .02) |
| Coronavirus HKU1 | 78 (2.0%) | 62 | .27 (.20, .34) | 20 | .09 (.05, .13) |
| Coronavirus NL63 | 138 (3.6%) | 74 | .32 (.25, .40) | 26 | .11 (.07, .16) |
| Coronavirus OC43 | 134 (3.5%) | 88 | .38 (.30, .47) | 37 | .16 (.11, .22) |
| Influenza A | 50 (1.3%) | 37 | .16 (.11, .22) | 25 | .11 (.07, .16) |
| Influenza B | 10 (0.3%) | 8 | .03 (.01, .07) | 5 | .02 (.01, .05) |
| Human metapneumovirus | 108 (2.8%) | 75 | .32 (.25, .40) | 38 | .16 (.12, .22) |
| Parainfluenza 1 | 30 (0.8%) | 25 | .11 (.07, .16) | 15 | .06 (.04, .11) |
| Parainfluenza 2 | 25 (0.6%) | 23 | .10 (.06, .15) | 16 | .07 (.04, .11) |
| Parainfluenza 3 | 129 (3.3%) | 100 | .43 (.35, .52) | 55 | .24 (.18, .31) |
| Parainfluenza 4 | 62 (1.6%) | 48 | .21 (.15, .27) | 26 | .11 (.07, .16) |
| Rhinovirus/enterovirus | 2462 (63.4%) | 1076 | 6.42 (6.04, 6.81) | 317 | 1.89 (1.69, 2.11) |
| RSV A | 112 (2.9%) | 69 | .30 (.23, .38) | 45 | .19 (.14, .26) |
| RSV B | 79 (2.0%) | 60 | .26 (.20, .33) | 44 | .19 (.14, .25) |
| Any virus detected | … | 2211 | 9.39 (9.00, 9.78)b | 691c | 3.30 (3.01, 3.50)d |
Abbreviations: ARI, acute respiratory illness; CI, confidence interval; PREVAIL, Pediatric Respiratory and Enteric Virus Acquisition and Immunogenesis Longitudinal; RSV, respiratory syncytial virus.
Incidence rate and 95% CI provided in child-years.
Total duration of follow-up (235.40 y) used as denominator as it is assumed a child may be at risk for any virus for each swab.
Excluded viral infections associated with the same ARI episode.
Years-at-risk calculated as total duration of follow-up (235.40 y) minus years of viral ARI (23.22 y) = 212.17 years as it is assumed a child with a current viral ARI is not at risk for a new viral ARI.
Rhinovirus/enterovirus accounted for nearly half of all viral infections detected in the cohort (49%; n = 1076/2211 viral infections) and had the highest incidence of both viral infection and viral ARIs among all respiratory viruses (6.42 viral infections per child-year and 1.89 viral ARIs per child-year). The lowest incidence was seen with influenza B virus, with only 8 viral infections detected, accounting for an incidence of 0.03 viral infections per child-year and 0.02 viral ARIs per child-year. The seasonality of respiratory viruses across study years is shown in Supplementary Figure 1.
Among the 2211 viral infections identified in our cohort, 63% (n = 1390) were reported as asymptomatic. The odds of a detected viral infection being symptomatic varied widely across different respiratory viruses and viral subtypes (Table 3). RSV B (OR = 8.9; 95% CI: 3.94, 20.05; P < .001), parainfluenza 2 (OR = 6.36; 95% CI: 1.96, 20.65; P = .002), and RSV A (OR = 4.97; 95% CI: 2.66, 9.28; P < .001) viral infections had the highest odds of being symptomatic compared with all other respiratory viruses detected by PCR combined as a reference group. Endemic coronaviruses (any type), bocavirus, and rhinovirus/enterovirus viral infections, when detected, were less likely to be symptomatic, although this was only statistically significant for bocavirus and rhinovirus/enterovirus.
Table 3.
Asymptomatic and Symptomatic Viral Infections, Odds Ratio of Symptomatic Infection, and Attributable Fraction in the PREVAIL Cohort (N = 245), by Respiratory Virus
| Pathogena | Viral Infectionsb | Asymptomatic Infections | Symptomaticc Infections | Odds of Symptomatic Infection, Compared With All Other Pathogens (95% CI) | P | Attributable Fraction in Exposed (95% CI) |
|---|---|---|---|---|---|---|
| RSV B | 37 | 6 | 31 | 8.89 (3.94, 20.05) | <001 | 89% (75%, 95%) |
| Parainfluenza 2 | 16 | 4 | 12 | 6.36 (1.96, 20.65) | .002 | 84% (49%, 95%) |
| RSV A | 47 | 14 | 33 | 4.97 (2.66, 9.28) | <001 | 80% (62%, 89%) |
| Influenza A | 26 | 9 | 17 | 3.85 (1.60, 9.22) | .003 | 74% (38%, 89%) |
| Influenza B | 5 | 2 | 3 | 3.69 (.71, 19.11) | .119 | 73% (−41%, 95%) |
| Parainfluenza 1 | 19 | 8 | 11 | 2.91 (1.15, 7.40) | .025 | 66% (13%, 86%) |
| Parainfluenza 4 | 37 | 17 | 20 | 2.52 (1.29, 4.93) | .007 | 60% (23%, 80%) |
| Parainfluenza 3 | 67 | 33 | 34 | 2.09 (1.32, 3.30) | .002 | 52% (24%, 70%) |
| Human metapneumovirus | 56 | 28 | 28 | 2.05 (1.20, 3.49) | .009 | 52% (17%, 71%) |
| Adenovirus | 68 | 34 | 34 | 1.81 (1.04, 3.15) | .036 | 45% (4%, 68%) |
| Coronavirus OC43 | 61 | 41 | 20 | .91 (.52, 1.59) | .736 | −10% (−92%, 37%) |
| Coronavirus NL63 | 45 | 31 | 14 | .86 (.49, 1.51) | .596 | −16% (−104%, 34%) |
| Coronavirus HKU1 | 40 | 28 | 12 | .75 (.38, 1.50) | .417 | −33% (−163%, 33%) |
| Rhinovirus/enterovirus | 822 | 602 | 220 | .45 (.36, 0.56) | <.001 | −122% (−178%, −79%) |
| Bocavirus | 115 | 97 | 18 | .36 (.22, 0.60) | <001 | −178% (−355%, −67%) |
| Coronavirus 229E | 9 | 8 | 1 | .22 (.02, 3.02) | .258 | −355% (−4900%, 67%) |
P values ≤ 0.05 bolded.
Abbreviations: CI, confidence interval; PREVAIL, Pediatric Respiratory and Enteric Virus Acquisition and Immunogenesis Longitudinal; RSV, respiratory syncytial virus.
Ordered by odds ratio of symptomatic infection, highest to lowest.
Viral infections without co-detection of multiple respiratory viruses (n = 1470).
Symptomatic is defined as the presence of fever and/or cough with or without the presence of other respiratory or gastrointestinal symptoms.
Viral Infection Acquisition by Age
The median age and range of ages at which a participant acquired their first viral infection detected by PCR were variable based on the type of respiratory virus detected (Table 4). Children acquired their first rhinovirus/enterovirus infection significantly earlier (median age: 2 months; IQR: 1–3 months; P < .0001) when compared with all other respiratory viruses. For all other respiratory viruses, the median age at first viral infection occurred after 6 months of age.
Table 4.
Age at First Viral Infection and Proportion of Cohort Who Experienced at Least 1 Respiratory Viral Infection Detected by PCR of a Nasal Swab in the Highly Adherent PREVAIL Cohort (n = 101), by Respiratory Virus
| Proportion of Cohort With at Least 1 Viral Infection Detected by Nasal Swab | |||
|---|---|---|---|
| Pathogen | Age at First Viral Infection,a mo | By 12 Months | By 24 Months |
| Adenovirus | 8 (6–11)b | 48% | 59% |
| Bocavirus | 10 (6–15)c | 50% | 77% |
| Endemic coronavirus | 11 (6–17) | 55% | 79% |
| 229E | 18 (11–19) | 3% | 9% |
| HKU1 | 13 (7–18) | 20% | 45% |
| NL63 | 11 (5–15.5) | 23% | 44% |
| OC43 | 11 (6–13) | 31% | 50% |
| Influenza virus | 17 (10–20) | 11% | 27% |
| Influenza A | 17 (10–20) | 9% | 25% |
| Influenza B | 11.5 (6–20) | 2% | 4% |
| Human metapneumovirus | 11 (8–18) | 24% | 47% |
| Parainfluenza virus | 12 (7–17) | 50% | 82% |
| PIV1 | 18.5 (15–21.5) | 3% | 20% |
| PIV2 | 9 (7–13) | 9% | 17% |
| PIV3 | 10 (6–14) | 38% | 57% |
| PIV4 | 15 (9–19) | 10% | 31% |
| Rhino/enterovirus | 2 (1–3)d | 97% | 100% |
| Respiratory syncytial virus | 12 (7–18) | 40% | 66% |
| RSV A | 15.5 (9–19) | 16% | 42% |
| RSV B | 9 (6–17) | 25% | 41% |
Abbreviations: PCR, polymerase chain reaction; PREVAIL, Pediatric Respiratory and Enteric Virus Acquisition and Immunogenesis Longitudinal; RSV, respiratory syncytial virus.
Presented as median (interquartile range).
Significantly differs from median age at first viral infection of influenza virus (P = .005), parainfluenza virus (P = .013), and RSV (P = .018).
Significantly differs from median age at first viral infection of influenza virus (P = .005).
Significantly differs from median age at first viral infection of all other respiratory virus using Kruskal–Wallis, P ≤ .0001.
By 12 months of age, 97% of the highly adherent cohort had at least 1 rhinovirus/enterovirus infection detected, but a minority of participants at 12 months of age had experienced an infection from adenovirus (48%), RSV (40%), human metapneumovirus (24%), and influenza virus (11%). By 2 years of age, all participants had experienced at least 1 rhinovirus/enterovirus infection and most participants had experienced at least 1 parainfluenza virus (82%), endemic coronavirus (79%), bocavirus (77%), RSV (66%), and adenovirus (59%). A minority of participants had human metapneumovirus (47%) and influenza virus (27%) detected by 2 years of age.
Symptomatology of Viral Infections
The proportion of maternally reported fever, earache, cough, vomiting, and diarrhea as well as the overlap between these symptoms varied widely by respiratory virus type (Figure 1 and Supplementary Figure 2). A combination of fever and cough were reported in 70% (n = 14/20) of influenza ARIs and 54% (n = 33/64) of RSV ARIs, while isolated fever or cough was uncommon (5% and 15% for influenza and 3% and 41% for RSV for fever and cough, respectively) for both viruses (Supplementary Table 1). Isolated cough was seen in 61% (n = 17/28) of human metapneumovirus, 54% (n = 118/220) of rhinovirus/enterovirus, and 50% (n = 9/18) of bocavirus ARIs, while isolated fever was seen in 35% (n = 12/34) of adenovirus ARIs.
Figure 1.
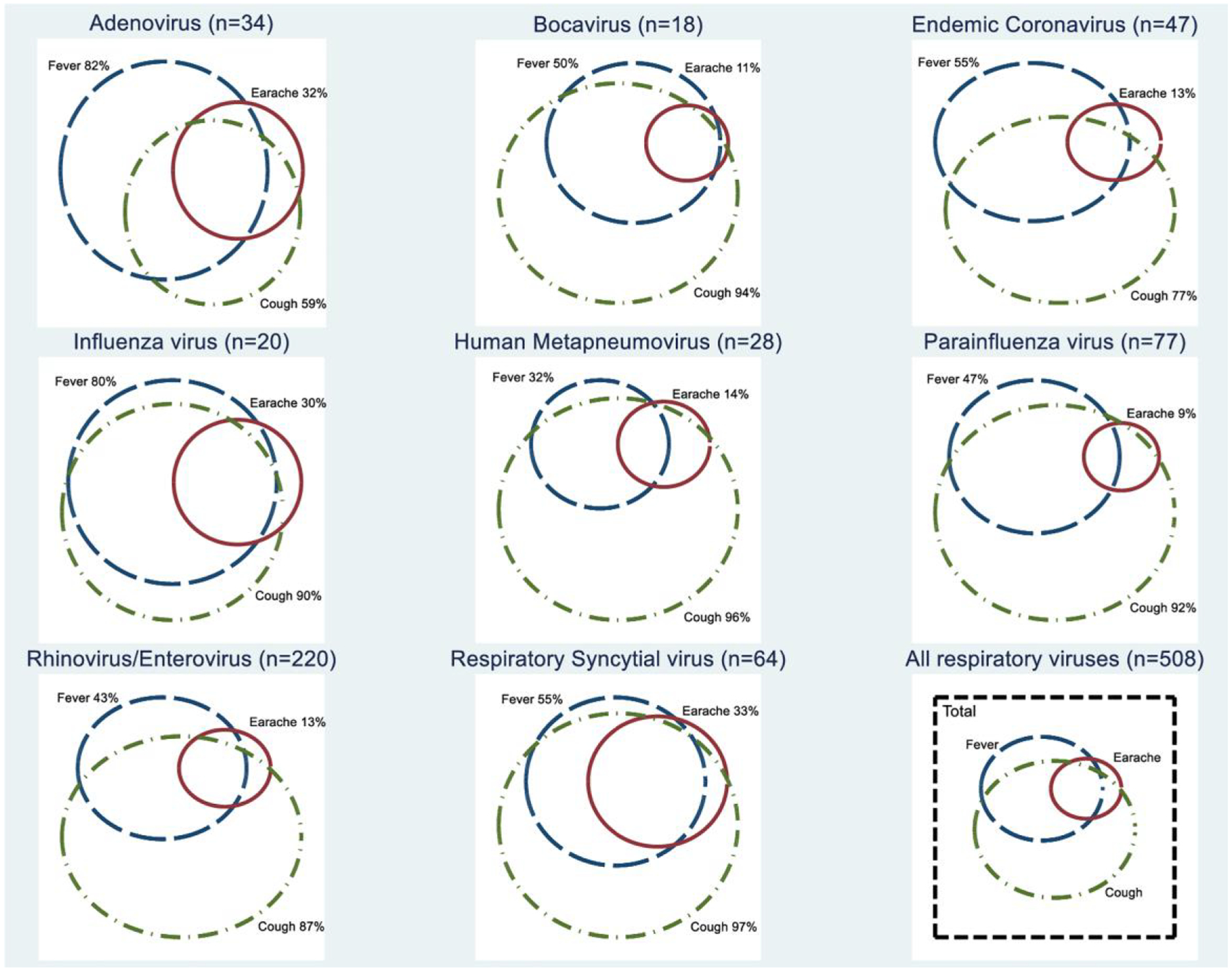
Proportional Venn diagram of symptom overlap seen during viral ARIs is without co-detection (n = 508) in the PREVAIL cohort (n = 245), by respiratory viruses (subtypes combined). Abbreviations: ARI, acute respiratory illness; PREVAIL, Pediatric Respiratory and Enteric Virus Acquisition and Immunogenesis Longitudinal.
The median duration of symptoms during an ARI episode for all respiratory viruses was 5 days (IQR: 3–9 days) (Supplementary Table 1). There was no significant difference in median duration of ARI between most respiratory viruses, except that ARIs associated with RSV (7 days) were significantly longer than ARIs associated with adenovirus or endemic coronavirus (5 days each; P < .05).
Healthcare Utilization
Among all 1470 viral infections without co-detections, 17% (n = 255) were medically attended. Among the patients with medically attended infections, 13% (n = 192) were evaluated by a primary care physician or at an urgent care clinic, 4% (n = 55) were evaluated at an emergency department, and 0.5% (n = 8) were hospitalized (Figure 2). The proportions of viral infections that were medically attended varied, with RSV infections having the highest proportion of medical attendance (56%; n = 47/84) while endemic coronavirus (12%; n = 19/155), rhinovirus/enterovirus (12%; n = 102/822), and bocavirus (6%; n = 7/115) infections had the smallest proportion of infections that were medical attended. The odds of a viral infection being medically attended was highest for RSV infections (OR: 7.03; 95% CI: 4.48, 11.02; P < .001) compared with all other respiratory viruses; bocavirus (OR: .32; 95% CI: .15, 0.67; P = .002) and rhinovirus/enterovirus (OR: .45; 95% CI: .34, .59; P < .001) infections had the lowest odds of medical attendance. Adenovirus (OR: 2.49; 95% CI: 1.48, 4.19; P = .001) and influenza virus (OR: 2.43; 95% CI: 1.15, 5.15; P = .02) infections had similar odds of medical attendance.
Figure 2.
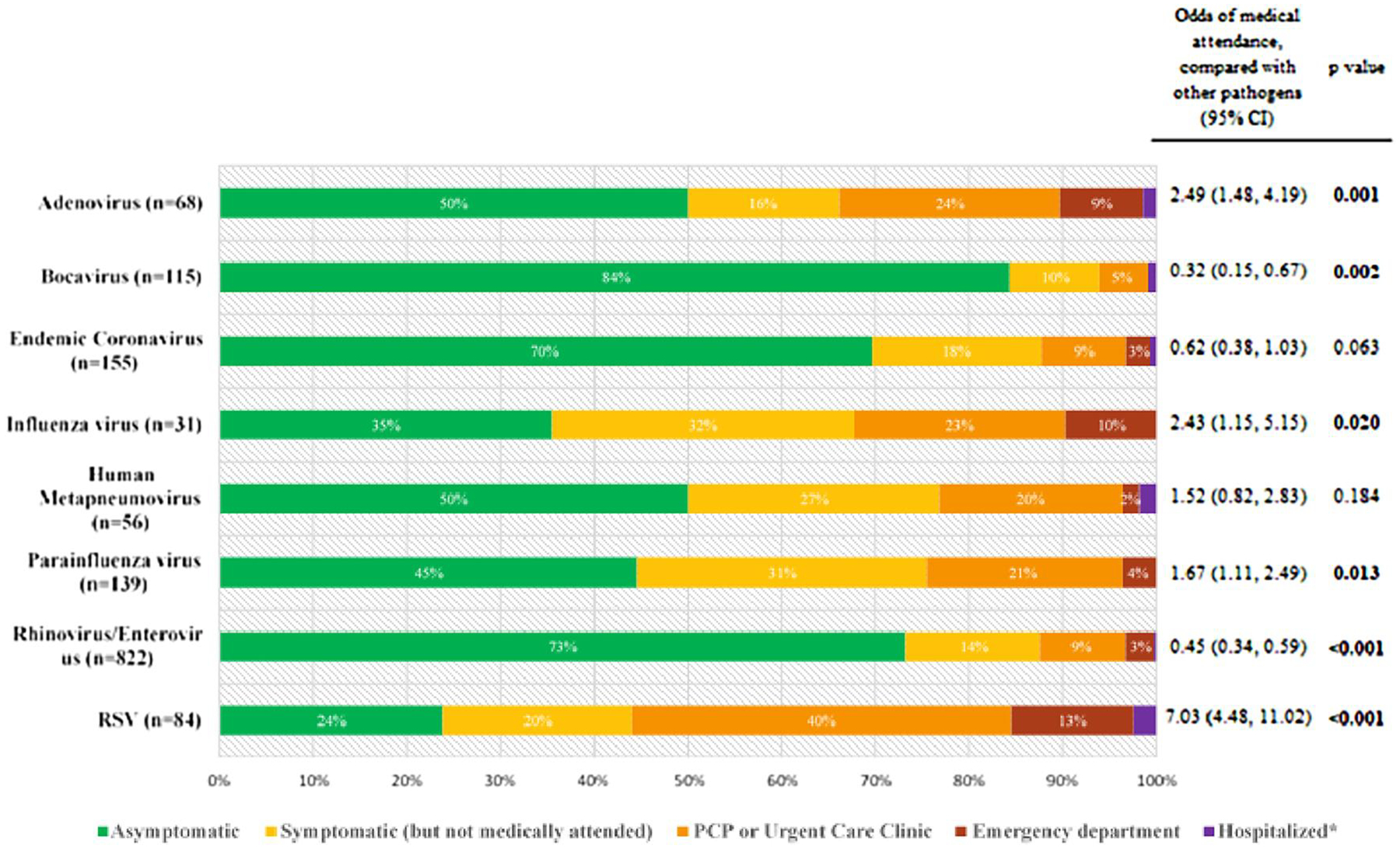
Medical utilization patterns of viral infections without co-detection of other viruses (n = 1470) and odds ratio of a viral infection being medically attended in the PREVAIL cohort (n = 245), by respiratory virus (subtype combined). *Hospitalized: adenovirus (1%), bocavirus (1%), endemic coronavirus (1%), influenza virus (0%), human metapneumovirus (2%), parainfluenza virus (0%), rhinovirus/enterovirus (0.2%), RSV (2%). Abbreviations: CI, confidence interval; PCP, primary care provider; PREVAIL, Pediatric Respiratory and Enteric Virus Acquisition and Immunogenesis Longitudinal; RSV, respiratory syncytial virus.
Hospitalizations were rare across all respiratory viruses. Two RSV infections, 2 rhinovirus/enterovirus infections, as well as 1 human metapneumovirus, 1 coronavirus NL63, 1 bocavirus, and 1 adenovirus infection each resulted in hospitalization. There were no hospitalizations for viral infections due to influenza viruses or parainfluenza viruses in our cohort.
DISCUSSION
In our community-based, prospective US birth cohort, respiratory viral infections were common in children during the first 2 years of life. We observed an average of 9 respiratory viral infections per child annually, with detection of rhinovirus/enterovirus accounting for almost half (49%) of all infections. While the timing and frequency of viral acquisition were diverse across the cohort, most respiratory viral infections were asymptomatic and not medically attended. However, the odds of symptoms and medical attendance varied widely by respiratory virus. Viruses that are typically associated with severe disease, such as influenza and RSV, were also more likely to be symptomatic and medically attended in our cohort. However, the vast majority of influenza (90%) and RSV (85%) infections never required evaluation at an emergency department or hospital admission. Among non–influenza and RSV infections, parainfluenza viral infections, especially type 2 infections, had higher odds of being symptomatic than other viruses, while adenovirus infections had higher odds of medical attendance than other viruses. Bocavirus, endemic coronavirus, and rhinovirus/enterovirus infections had lower odds of both developing symptoms and medical attendance compared with all other viruses.
Our overall incidence rate estimate of 9.42 viral infections/child-year is comparable to those reported in the Observational Research in Childhood Infectious Disease (ORChID) birth cohort in Australia [15]. Similar to our study, ORChID also utilized routine weekly nasal sampling regardless of symptoms, which better captures both symptomatic and asymptomatic childhood viral infections. Our findings supplement those of the ORChID cohort, which was geographically distinct and performed during different respiratory seasons. In contrast, most birth cohorts rely on the ascertainment of specific respiratory symptoms in order to determine the timing of sample collection and report a lower incidence of viral infections [21–29]. The disparity in incidence rates likely reflects the underdetection of asymptomatic and subclinical infections, leading to an underestimation of the true incidence of respiratory viral infections.
We found that few (4%) of all viral infections in our cohort were evaluated in the emergency department or resulted in hospitalization, suggesting that reliance on hospital-based surveillance also may miss a substantial number of asymptomatic and symptomatic viral infections among infants and young children. Also, studies that rely on hospital or emergency department surveillance may overrepresent viruses associated with symptomatic and severe infections, such as influenza virus and RSV. Additional community-based cohort studies that focus on routine longitudinal multiviral testing are needed to better characterize the experiences of respiratory viral infections in different settings and patient populations. The few published birth cohort studies that performed weekly or longitudinal viral sampling typically focused on the epidemiology of a single viral pathogen [30–34].
There are several limitations in the interpretation of results related to our cohort methodology. The definition of a viral infection and parameters used to attribute symptoms to a viral infection were similar to comparable cohort studies [1, 15, 16], but no standardized or universally agreed definition exists. Given the intermittency of viral detection, the cutoff utilized for defining a viral infection may imprecisely capture true episodes. This may be especially problematic for viruses such as human rhinovirus and bocavirus that are known to intermittently shed over a prolonged period [32, 33, 35]. Another important limitation is our inability to differentiate between rhinovirus and enterovirus, which is a common challenge found with many commercial multiplex PCR platforms. Utilizing the full cohort of 245 mother–infant pairs allows us to better describe the full diversity of respiratory viral infections, but adherence to weekly nasal sample submissions varied; 41% (n = 101) of mother–infant pairs submitted at least 70% of eligible swabs and are demographically different from our full cohort (Supplementary Table 2) [19]. However, in our sensitivity analysis, we found that the incidence of viral infections and viral ARIs as well as the proportion of medically attended infections were similar between the highly adherent and non–highly adherent cohort, a finding that is likely due to using weeks of viral sample testing as the person-time denominator. The adjunctive use of serology and molecular tests has been shown to better capture the full extent of viral infections experienced by children [36]. While the use of weekly longitudinal nasal specimen collection allows us to better capture the spectrum of asymptomatic and symptomatic viral infections, the addition of respiratory viral serological assays would supplement future studies from our cohort. Finally, our ARI symptom ascertainment focused on fever and/or cough, which may miss symptomatic patients with ARIs who presented with other isolated respiratory symptoms. However, given our study design and reliance on self-reported symptom surveys, fever and/or cough were targeted as these are specific and verifiable, while indicating a degree of clinical concern for children less than 2 years.
Overall, there was high adherence to our study protocol, including high rates of median weekly survey responses and nasal sample submission [19]. The collection of respiratory samples on a weekly basis across 4 respiratory seasons provided a more complete overview of the respiratory viral experience among young children in the community. In addition, Cincinnati Children’s Hospital Medical Center is the major in-patient and outpatient pediatric provider for the region and serves more than 97% of children living in Hamilton County. The combination of data from medical chart abstraction and self-reported medical visits allowed us to confidentially capture most medically attended visits for participating children. As nearly all samples (98%) were collected prior to the first reported case of coronavirus disease 2019 (COVID-19) in the United States, our study also provides a unique opportunity to characterize childhood respiratory viral infections in the pre-pandemic era [37].
In conclusion, our community-based, prospective birth cohort provides a comprehensive overview on the incidence, symptomatology, and severity of viral infections in children 0–2 years of age and how these varied by respiratory virus. Viral infections were commonly detected during the first 2 years of life, but the majority of infections, irrespective of virus or viral subtype, were asymptomatic and not medically attended. The high proportions of asymptomatic and non–medically attended infections have implications on our understanding of the dynamics of respiratory viral transmission in the community, and future research studies and public health policies must account for these infections.
Supplementary Material
Acknowledgments.
The authors gratefully acknowledge the participation of the PREVAIL birth cohort families. The authors also thank their researchers at the University of Cincinnati, Cincinnati Children’s Hospital, and The Christ Hospital—Cincinnati. They also gratefully acknowledge the hard work of the dedicated PREVAIL staff. This publication was made possible, in part, using the Cincinnati Children’s Shubert Research Center (SRC).
Financial support.
This work was supported by a cooperative agreement from the US Centers for Disease Control and Prevention (CDC), the Molecular Epidemiology in Children’s Environmental Health Training program, and the Center for Clinical and Translational Science and Training at the University of Cincinnati and Cincinnati Children’s Hospital Medical Center. The US CDC was involved in the study design, data collection, data analysis, data interpretation, and writing of the report. R. M. B. reports support for this work from the CDC as a government employee (paid) and then an unpaid guest researcher (work on this manuscript was performed as part of regular job and research duties, with no additional payment or compensation).
Potential conflicts of interest.
M. A. S. reports research funding from National Institutes of Health (NIH), Cepheid, Open Philanthropy/Good Ventures, and Pfizer; payment or honoraria from Up-To-Date for Articles on Care of Internationally Adopted Children. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Footnotes
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention (CDC).
References
- 1.Sarna M, Ware RS, Sloots TP, Nissen MD, Grimwood K, Lambert SB. The burden of community-managed acute respiratory infections in the first 2-years of life. Pediatr Pulmonol 2016; 51:1336–46. [DOI] [PubMed] [Google Scholar]
- 2.Iwane MK, Edwards KM, Szilagyi PG, et al. Population-based surveillance for hospitalizations associated with respiratory syncytial virus, influenza virus, and parainfluenza viruses among young children. Pediatrics 2004; 113:1758–64. [DOI] [PubMed] [Google Scholar]
- 3.Troeger C, Blacker B, Khalil IA, et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis 2018; 18:1191–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nair H, Nokes DJ, Gessner BD, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 2010; 375:1545–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jansen RR, Wieringa J, Koekkoek SM, et al. Frequent detection of respiratory viruses without symptoms: toward defining clinically relevant cutoff values. J Clin Microbiol 2011; 49:2631–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galanti M, Birger R, Ud-Dean M, et al. Rates of asymptomatic respiratory virus infection across age groups. Epidemiol Infect 2019; 147:e176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Steenhuijsen Piters WAA, Watson RL, de Koff EM, et al. Early-life viral infections are associated with disadvantageous immune and microbiota profiles and recurrent respiratory infections. Nat Microbiol 2022; 7:224–37. [DOI] [PubMed] [Google Scholar]
- 8.Fauroux B, Simoes EAF, Checchia PA, et al. The burden and long-term respiratory morbidity associated with respiratory syncytial virus infection in early childhood. Infect Dis Ther 2017; 6:173–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weinberg GA, Hall CB, Iwane MK, et al. Parainfluenza virus infection of young children: estimates of the population-based burden of hospitalization. J Pediatr 2009; 154:694–9. [DOI] [PubMed] [Google Scholar]
- 10.Poehling KA, Edwards KM, Griffin MR, et al. The burden of influenza in young children, 2004–2009. Pediatrics 2013; 131:207–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall CB, Weinberg GA, Blumkin AK, et al. Respiratory syncytial virus-associated hospitalizations among children less than 24 months of age. Pediatrics 2013; 132: e341–8. [DOI] [PubMed] [Google Scholar]
- 12.Iwane MK, Prill MM, Lu X, et al. Human rhinovirus species associated with hospitalizations for acute respiratory illness in young US children. J Infect Dis 2011; 204:1702–10. [DOI] [PubMed] [Google Scholar]
- 13.Hall CB, Weinberg GA, Iwane MK, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med 2009; 360:588–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor S, Lopez P, Weckx L, et al. Respiratory viruses and influenza-like illness: epidemiology and outcomes in children aged 6 months to 10 years in a multi-country population sample. J Infect 2017; 74:29–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarna M, Lambert SB, Sloots TP, et al. Viruses causing lower respiratory symptoms in young children: findings from the ORChID birth cohort. Thorax 2018; 73:969–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarna M, Ware RS, Lambert SB, Sloots TP, Nissen MD, Grimwood K. Timing of first respiratory virus detections in infants: a community-based birth cohort study. J Infect Dis 2018; 217:418–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turner S Childhood respiratory cohort studies: do they generate useful outcomes? Breathe 2012; 8:194. [Google Scholar]
- 18.Byington CL, Ampofo K, Stockmann C, et al. Community surveillance of respiratory viruses among families in the Utah Better Identification of Germs–Longitudinal Viral Epidemiology (BIG-LoVE) study. Clin Infect Dis 2015; 61: 1217–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morrow AL, Staat MA, DeFranco EA, et al. Pediatric respiratory and enteric virus acquisition and immunogenesis in US mothers and children aged 0–2: PREVAIL cohort study. JMIR Res Protoc 2021; 10:e22222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2020. Available at: http://www.r-project.org/index.html. [Google Scholar]
- 21.van Benten I, Koopman L, Niesters B, et al. Predominance of rhinovirus in the nose of symptomatic and asymptomatic infants. Pediatr Allergy Immunol 2003; 14:363–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kusel MM, de Klerk NH, Holt PG, Kebadze T, Johnston SL, Sly PD. Role of respiratory viruses in acute upper and lower respiratory tract illness in the first year of life: a birth cohort study. Pediatr Infect Dis J 2006; 25:680–6. [DOI] [PubMed] [Google Scholar]
- 23.von Linstow ML, Holst KK, Larsen K, Koch A, Andersen PK, Hogh B. Acute respiratory symptoms and general illness during the first year of life: a population-based birth cohort study. Pediatr Pulmonol 2008; 43:584–93. [DOI] [PubMed] [Google Scholar]
- 24.van der Zalm MM, Uiterwaal CS, Wilbrink B, et al. Respiratory pathogens in respiratory tract illnesses during the first year of life: a birth cohort study. Pediatr Infect Dis J 2009; 28:472–6. [DOI] [PubMed] [Google Scholar]
- 25.Toivonen L, Karppinen S, Schuez-Havupalo L, et al. Burden of recurrent respiratory tract infections in children: a prospective cohort study. Pediatr Infect Dis J 2016; 35:e362–9. [DOI] [PubMed] [Google Scholar]
- 26.Regamey N, Kaiser L, Roiha HL, et al. Viral etiology of acute respiratory infections with cough in infancy: a community-based birth cohort study. Pediatr Infect Dis J 2008; 27:100–5. [DOI] [PubMed] [Google Scholar]
- 27.Kumar P, Mukherjee A, Randev S, et al. Effect of acute respiratory infections in infancy on pulmonary function test at 3 years of age: a prospective birth cohort study. BMJ Open Respir Res 2020; 7:e000436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Emanuels A, Hawes SE, Newman KL, et al. Respiratory viral coinfection in a birth cohort of infants in rural Nepal. Influenza Other Respir Viruses 2020; 14:739–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anders KL, Nguyen HL, Nguyen NM, et al. Epidemiology and virology of acute respiratory infections during the first year of life: a birth cohort study in Vietnam. Pediatr Infect Dis J 2015; 34:361–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galanti M, Shaman J. Direct observation of repeated infections with endemic coronaviruses. J Infect Dis 2021; 223:409–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frank AL, Taber LH, Wells CR, Wells JM, Glezen WP, Paredes A. Patterns of shedding of myxoviruses and paramyxoviruses in children. J Infect Dis 1981; 144:433–41. [DOI] [PubMed] [Google Scholar]
- 32.Martin ET, Kuypers J, McRoberts JP, Englund JA, Zerr DM. Human bocavirus 1 primary infection and shedding in infants. J Infect Dis 2015; 212:516–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loeffelholz MJ, Trujillo R, Pyles RB, et al. Duration of rhinovirus shedding in the upper respiratory tract in the first year of life. Pediatrics 2014; 134:1144–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Munywoki PK, Koech DC, Agoti CN, et al. The source of respiratory syncytial virus infection in infants: a household cohort study in rural Kenya. J Infect Dis 2014; 209:1685–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Howard LM, Johnson M, Gil AI, et al. Molecular epidemiology of rhinovirus detections in young children. Open Forum Infect Dis 2016; 3:ofw001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y, Sakthivel SK, Bramley A, et al. Serology enhances molecular diagnosis of respiratory virus infections other than influenza in children and adults hospitalized with community-acquired pneumonia. J Clin Microbiol 2017; 55:79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med 2020; 382:929–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


