Abstract
High cost of chemical fertilizers and poor nutrient content in conventional organic sources (manure, compost, charcoal etc.) can be addressed through development of enriched organic amendments. However, there is a need to evaluate enriched organic amendments as a potential alternative of chemical fertilizers. Therefore, an effort was made to prepare enriched organic amendments through blending distillation waste of aromatic plant biomass (DWB) with naturally available low-grade rock phosphate (RP) and waste mica (WM). Enrich compost (ENC) was produced from DWB in a natural composting process, blended with mineral powder, whereas biochar fortified mineral (BFM) was prepared by blending biochar, derived from DWB through hydrothermal reaction, with mineral powder. The main aims of the present study were to investigate the impacts of ENC and BFM applications on soil properties, and herbage yield and quality of a medicinal herb Senna (Cassia angustifolia Vahl.). The performances of ENC and BFM at two different rates (2.5 and 5 t ha−1) were compared with the application of conventional farmyard manure (FYM, 5 t ha−1) and chemical fertilizers (CF, NPK 60-40-20 kg ha−1) in two different soils in a pot experiment. Both, ENC and EBC improved soil quality and fertility by increasing soil organic carbon, available nutrients, microbial biomass and enzyme activity. The ENC and BFM increased total herbage yields by 21 and 16.3 % compared to FYM. In both soils, the CF treatment produced the maximum dry herbage yields (32.7–37.4 g pot−1), which however were comparable to ENC (31.9–33.7 g pot−1) and BFM (30.7–35.1 g pot−1) treatments. Bioactive compound (sennoside) production in senna was significantly improved by ENC and BFM compared to CF. The present study indicates that ENC and BFM could not only help to overcome the limitation of conventional FYM, but also have the potentials to substitute costly chemical fertilizers, particularly in medicinal plant cultivation.
Keywords: Distillation waste biomass, Biochar, Compost, Mineral enrichment, Medicinal herb
1. Introduction
The commercial fertilizers, especially P and K, are expensive in developing countries due to lack of suitable raw materials for commercial production. The cost of chemical P and K fertilizers has also been steadily rising [1]. The global demand of P and K fertilizers has increased from 44.48 to 35.43 million tonnes in 2016 to 49.10 and 40.23 million tonnes in 2020, respectively [2]. Small and marginal farmers find it difficult to afford fertilizers due to the increased costs. Additionally, environmental pollution caused by injudicious application of chemical fertilizer has become rather complicated, with negative impacts [3]. Therefore, more efficient fertilizer management strategies are needed to be explored by using cost effective local available nutrient sources, like waste minerals and plant biomass [4,5]. Production of local compost and biochar from plant biomass and waste minerals, and their application could reduce the need of costly chemical fertilizers [6].
Organic amendments including manure, compost and biochar have been found as potential sources of nutrients in organic and ecological farming [7,8]. Recently, biochar and compost have been recognized as important organic fertilizers for enhancing crop yield, and soil quality and fertility by retaining carbon and increasing nutrient availability [[9], [10], [11]]. However, the plant nutrient content of these organic sources (FYM, compost and biochar) may not always meet crop requirements, particularly in terms of major nutrients [5]. One potential method of enhancing the nutrient content of traditional organic amendments is to blend low-grade minerals with waste biomass during co-composting or co-pyrolysis [11,12].
India has 200 million tonnes of low-grade rock phosphates (RP), however they are unsuitable for production of commercial phosphate fertilizers due to their low P contents [13]. On the contrary, low-grade silicate mineral powder (SMP), produced as a byproduct of mining, is rich in mica and a viable source of potassium [14]. However, poor solubility of RP and SMP, particularly in neutral and alkaline soils, restricts their direct application in agriculture [15]. Co-composting and co-pyrolysis with waste biomass could boost the bioavailability of phosphorus (P) and potassium (K) from low-grade RP and SMP [[15], [16], [17]]. The bioavailability of P from low-grade RP was found to be improved through composting processes [15]. Co-composting of straw biomass and low-grade RP significantly improves water-soluble and Olsen P content in final product [6]. Co-pyrolysis of biomass and mineral powder and subsequent hydrothermal reaction lead to formation of biochar-mineral complexes [17,18]. The complex formation results in improvement of surface area, surface functional groups and nutrient content of pristine biochar [5]. The high surface area and surface functional groups impart slow release property, which makes the biochar-mineral complex more efficient soil amendment [5,19].
The medicinal plants require less but a steady supply of nutrients for optimal growth and bioactive principal. Organic amendments enriched with mineral powder could potentially meet this need in neutral and saline soils [20,21]. The essential oil distillation of aromatic plants produces a large amount of solid waste biomass as a byproduct [22]. Thus, the huge amount of distillation waste biomass (DWB) can be effectively recycled and utilized to produce enriched compost (ENC) and biochar fortified mineral (BFM) [14,23]. Senna (Cassia angustifolia Vahl.) is a widely known medicinal plant, used in almost all systems of medicine for its laxative properties. Sennosides, which are found in economic parts (leaves and pods) of senna, are responsible for laxative properties [21]. Senna has the largest share in the export of medicinal herb from India [24]. Senna grows well in marginal soils in semi-arid regions [25]. It can tolerate a significant level of soil salinity and grow well in soil with pH of 7.0–8.5 [26]. Senna is a legume that responds well to nutrient management practices. However, the beneficial effects of enriched amendments in Senna cultivation could vary with soil types. The organic amendments are believed to be more beneficial in coarsely textured soil than fine texture soil [27]. Furthermore, it has become a popular practice to add organic amendments (FYM and compost) to saline soils in order to improve soil fertility [28] and plant productivity [20]. However, growing commercially important medicinal herbs, such as senna, by using cost-effective enriched amendments in various soil conditions have not been studied in detail.
The scope of enriched amendments in medicinal plant cultivation and soil fertility improvement has not been investigated thoroughly yet. Very few studies have been carried out till date to evaluate the effect of enriched amendments on yield and quality of medicinal plants. There is still lack of evidence about the changes in soil properties and nutrient dynamics as influenced by enriched amendments. Hence, it is hypothesized that the ENC and BFM (Fig. 1) could be more efficient than traditional organic amendments (FYM) and a potential substitution of chemical fertilizers in improving soil quality, plant growth, yield and bioactive principle in Senna. The main aims of this study were to (1) investigate the changes in soil properties due to applications of ENC and BFM under cultivation of a medicinal plant (Senna) in comparison to conventional FYM and CF in two contrasting soil types, and (2) evaluate the potentials of ENC and BFM in improving yield and bioactive principle of Senna.
Fig. 1.
Photograph sowing (a) enriched compost and (b) biochar fortified mineral produced from distillation waste biomass and mineral powder.
2. Materials and methods
2.1. Preparation and characterization of enriched compost and biochar
For preparation of compost and biochar, distillation waste biomass of Palmarosa (Cymbopogon martini (Roxb.) Wats.) was obtained from the hydro-distillation unit of the ICAR-Directorate of Medicinal and Aromatic Plants Research, Anand. The waste biomass was dried and cut into small pieces (≤5 cm). The low-grade rock phosphate (RP) and silicate mineral powder (SMP) were collected from rock phosphate mine, Udaipur, Rajasthan, India, and mica mine of Nellore district of Andhra Pradesh, India, respectively. The collected mineral samples were ground in a Wiley mill to make powder (150 μm size) for further use. The RP sample contained 0.02, 17, and 94.1 g kg−1 water-soluble, citrate-soluble, and total phosphorus (P), respectively [13]. SMP sample had 0.11, 0.21, 1.56, 80.7 g kg−1 waster-soluble, exchangeable, non-exchangeable, and total potassium (K), respectively [14]. According to the procedure given by Biswas et al. [15], the enriched compost was made from the waste biomass fortified with RP and SMP in equal proportions (2 % of the total composting biomass) and mixed with cow dung slurry which served as natural inoculum for composting. Cow dung slurry was made by mixing fresh cow dung and water at 1:1 ratio and allowed it 24 h for fermentation. For every 100 kg of biomass, 10 kg of cow dung slurry was added and mixed with RP and SMP. So, proportional quantity of biomass, cow dung slurry and mineral powder was applied at the ratio of 100:10:4. Following that, all compostable materials were placed in a composting bin, which was turned and watered frequently to ensure adequate aeration and moisture during the composting process. The mature compost (Fig. 1a) was obtained after 120 days of composting, when C:N ratio reached below 20:1.
The dried chopped biomass was ground into small particle (≤2 mm). The ground biomass sample was pyrolyzed at 350 °C in muffle furnace under limited oxygen environment for preparation of biochar [23]. The biochar fortified mineral (BFM) was produced by mixing biochar, clay, minerals and organics in a hydrothermal reaction, with certain modifications to previously reported methods [18,19,29]. In this case, FYM was used as replacement of chicken manure, while RP and SMP were used in place of commercial calcium carbonate, magnesium sulphate and ilmenite. To improve the porosity and functional groups in the surface, the biochar sample was pre-treated with aqueous solution of phosphoric acid (10 % v/v). In a batch hydrothermal reactor, the BFM was made with proportional mixture of biochar (35 g), minerals (i.e., 30 g kaolinite clay, 7.5 g RP, and 7.5 g WM), and 20 g FYM. The finished product (BFM) was kept in a sealed container (Fig. 1b) for further use. FYM, ENC and BFM were finely powdered (≤2 mm) for further analysis. pH was measured in digital pH meter by suspending samples in deionized water ratio of 1:5 (w/v). Cation exchange capacity (CEC) was measured by the method as described by Sumner and Miller [30]. Total carbon and nitrogen content were estimated by using a CNH analyzer. For the determination of total P and K, samples were digested in a di-acid mixture HNO3/HClO4 at a ratio of 9:4 (v/v) [31]. The P content in the acid digest was analyzed by developing yellow-colored complex measured with a Spectrophotometer [32]. The K content in the acid extract was determined using a flame photometer. Available and water-soluble P was extracted with 0.5 M NaHCO3 solution [33] and ultra-pure water, respectively. The P content in the solution was estimated in a Spectrophotometer by measuring blue-colored complex [32]. Exchangeable and water-soluble K was extracted with neutral 1 N NH4OAc solution [34] and ultra-pure water, respectively. The K content in solution was determined using a flame photometer. Physicochemical properties of ENC and BFM in comparison to FYM were provided in Table 1. BFM had the lowest total C of all amendments, followed by ENC. BFM was found to be somewhat acidic (pH = 6.8), in contrast to the alkaline nature of biochar (pH = 8.13) [23]. The highest CEC (63.8 cmol p+ kg−1) was found in BFM, which was much greater than ENC and FYM. The BFM had a lower total C content (8.9 %), but a higher nutrient content as compared to pristine biochar [23] and FYM (Table 1). However, ENC found to have the highest total N and P content, while BFM had the highest K content. Overall, nutrient content and availability were higher in ENC and BFM than in FYM.
Tables 1.
Physicochemical characteristic of farmyard manure (FYM), enriched compost (ENC) and biochar fortified mineral (BFM).
| Properties | FYM | ENC | BFM | |
|---|---|---|---|---|
| pH | 6.51 ± 0.09a | 7.40 ± 0.11 | 6.82 ± 0.07 | |
| Cation Exchange capacity (cmol (p+) kg−1) | 43.2 ± 1.2 | 47.9 ± 2.2 | 63.8 ± 2.3 | |
| Total C (g kg−1) | 316.2 ± 3.1 | 246.7 ± 3.4 | 89.0 ± 7.9 | |
| Total nitrogen (mg kg−1) | 5143 ± 73 | 13127 ± 486 | 4557 ± 694 | |
| Phosphorus | Total P (mg kg−1) | 2312 ± 43 | 24128 ± 907 | 13709 ± 721 |
| Olsen P (mg kg−1) | – | 609 ± 8.4 | 911 ± 11.7 | |
| Water soluble P (mg kg−1) | 89.7 ± 2.1 | 206.8 ± 2.8 | 187.5 ± 2.6 | |
| Potassium | Total K (mg kg−1) | 4719 ± 109 | 19223 ± 878 | 27243 ± 628 |
| Exchangeable K (mg kg−1) | – | 9329 ± 351 | 12706 ± 447 | |
| Water soluble K (mg kg−1) | 91.3 ± 1.7 | 209.3 ± 2.7 | 141.7 ± 2.1 | |
Mean ± standard error (n = 3).
2.2. Experimental site and soils
Senna was grown as test crop in the pot at the net house of ICAR-DMAPR, Boriavi, Anand. It is located at a latitude of 22° 35′ 57″– 22° 36′ 06″ N and longitude of 73° 27′ 57″– 73° 27′ 16″ E, with 45.11 m altitude. The experimental site is located in a semi-arid and subtropical climate, with hot summers (maximum temperature of 41.5 °C) and cool winters (minimum temperature of 9 °C). The average annual rainfall is 860 mm, largely occurring between the months of August and September. For the pot culture experiments, the bulk soil samples were collected from a fallow site of ICAR-DMAPR, Anand (Fluvic Cambisol) and Bharuch, Gujarat (Haplic Vertisol) [35]. The soil from ICAR-DMAPR, Anand has a sandy loam texture, but the soil from Bharuch has a clay texture. The experimental soils were taken at a depth of 0–15 cm and analyzed for its physicochemical parameters. pH and electrical conductivity (EC) were determined by digital pH-EC meter in 1: 2.5 (w/v) soil: deionized water suspension [36]. Soil texture was analyzed by hydrometer method [37]. Organic carbon in soil was estimated by oxidation-titration method [38]. 2 M KCl solution was used to extract the mineral N (NH4++NO3−) from the soil [39]. This was followed by estimation in micro-Kjeldahl distillation and titration. Alkaline 0.5 M NaHCO3 solution (pH 8.5) was used to extract available P from the soil [33] followed by colorimetric determination of blue color by spectrophotometer [32]. A neutral ammonium acetate (1 N NH4OAc) solution was used to extract available K from soil [34], followed by estimation of K in the extract using flame photometer. The detail physicochemical characteristics of the experimental soil are presented in Table 2.
Table 2.
Physicochemical property of experimental soils.
| Soil Parameters | Soil 1 (Slightly Saline soil) | Soil 2 (Non-saline soil) |
|---|---|---|
| Particle size distribution | ||
| Sand (%) | 15.5 ± 0.23a | 69.1 ± 0.87 |
| Silt (%) | 28.2 ± 0.57 | 14.2 ± .033 |
| Clay (%) | 56.3 ± 1.28 | 16.7 ± 0.72 |
| Texture | Clayey soil | Sandy loam |
| pH | 8.1 ± 0.11 | 7.8 ± 0.09 |
| EC (dSm−1) | 2.62 ± 0.07 | 0.28 ± 0.03 |
| Organic C (g kg−1) | 4.45 ± 0.17 | 2.92 ± 0.13 |
| Mineral N (mg kg−1) | 51.7 ± 1.78 | 39.6 ± 1.29 |
| Available phosphorus (mg kg−1) | 10.5 ± 0.92 | 16.3 ± 1.23 |
| Available potassium (mg kg−1) | 201.7 ± 4.28 | 89.7 ± 2.52 |
Mean ± standard error (n = 3).
2.3. Plant growth experiment
The senna plant (ALFT 2 cultivar) was grown in the pot at the net house of ICAR-DMAPR during the rainy season (June to September). The experiment was a factorial study with a completely randomized design (CRD). Two levels (2.5 and 5 t ha−1) of BFM and ENC were compared to conventional organic manure (FYM) and recommended dose of chemical fertilizer. All together there were seven treatments comprised of: T1: Control; T2: Farmyard manure (5 t ha−1); T3: Enriched compost (2.5 t ha−1); T4: Enriched compost (5 t ha−1); T5: Biochar fortified mineral (2.5 t ha−1); T6: Biochar fortified mineral (5 t ha−1), and T7: Chemical fertilizer (CF). All of the treatments in the pot culture experiment were tested in two different soils: slightly saline soil (S1) and non-saline soil (S2). All the treatments were repeated three times, with a total of 42 pots were used in the experiment. The collected soil samples for pot experiment (<5-mm size) were placed on a clean polythene sheet. A calculated amount of FYM, ENC, and BFM as per the treatments T2, T3, T4, T5, and T6, respectively, was added to soil and mixed thoroughly (Table 3). The chemical fertilizers were applied as per recommended dose (60-40-20 NPK kg ha−1) [26]. The calculated amount of N (26.67 mg kg−1 soil), P (17.77 mg kg−1 soil), and K (8.89 mg kg−1 soil) were supplied through urea, diammonium phosphate (DAP) and muriate of potash (MOP), respectively in T7 and mixed thoroughly with the soil. The soils treated with FYM, ENC BFM and CF were finally placed in earthen pots, each of which contained 20 kg of soil. Senna seeds were soaked in a solution of Trichoderma harizanum (0.4 %) for 2 h, followed by an hour of drying in the shade. Five senna seeds were sown in each pot, and after germination, thinning was done to maintain single plant in each pot. Throughout the experimental period (120 days), irrigation was done at regular intervals to maintain soil moisture level at field capacity. Weeding was done once at 30 days after sowing of senna.
Table 3.
Nutrient application rate calculated from various treatment applied in pot experiment.
| Nutrients added (mg kg soil−1) | Treatments |
||||||
|---|---|---|---|---|---|---|---|
| T1: Control | T2: FYM (5 t ha−1) | T3: ENC (2.5 t ha−1) | T4: ENC (5 t ha−1) | T5: BFM (2.5 t ha−1) | T6: BFM (5 t ha−1) | T7: CF | |
| Nitrogen | 0 | 11.42 | 14.58 | 29.16 | 5.07 | 10.13 | 26.67 |
| Phosphorus | 0 | 5.15 | 26.80 | 53.60 | 15.24 | 30.44 | 17.78 |
| Potassium | 0 | 10.49 | 21.33 | 42.71 | 30.27 | 60.53 | 8.89 |
FYM: Farmyard manure; ENC: Enriched compost; BFM: Biochar fortified mineral; CF: Chemical fertilizer.
2.4. Plant biometric parameters
The plant height (cm) was recorded at flowering stage from ground level to the base of fully opened last leaf. Number of primary branches (plant−1) was recorded at the time of harvest (120 days after sowing). For fresh leaf and pod weights (g plant−1), the plants were uprooted and weighed immediately with an electronic balance. The fresh samples collected from each plant were dried at 40 °C in an oven and leaf dry weights (g plant−1) and pod dry weights (g plant−1) were recorded. Total fresh and dry herbage yields (g plant−1) were derived from fresh and dry yields (g plant−1) of leaf and pod, respectively. The total dry herbage yield per plant was calculated by weighing dry leaf and pod samples.
2.5. Bioactive compound analysis
The matured leaves (up to the third leaf from the top) and green pods from each plant were collected at 120 days after sowing. The leaf and pod samples were dried in the shade and then placed in an oven set at 60 ± 0.5 °C for 48 h. The dried sample was ground by a Willey mill into a fine powder for sennoside analysis. Total sennoside content was quantitatively determined using High Performance Liquid Chromatography (HPLC) method [40]. Using a sonication bath, dried fine powder sample (100 mg) was extracted for 10 min in 20 mL of 70 % aqueous methanol. Standard solutions and samples were centrifuge and filtered through membrane filter (0.45 μm) and analyzed in a HPLC instrument (LC-20A series, Shimadzu Corporation, Kyoto, Japan). Sennoside-A and sennoside-B were used as reference standards (Sigma-Aldrich, Bangalore, India) for quantification of sennosides in the samples. The total sennoside yield was calculated and presented following the procedure given by Srivastava et al. [41].
2.6. Soil sampling and analysis
After harvesting of senna plants, representative soil samples were collected from each pot. For the analysis of biological parameters, a portion of each soil sample was immediately stored in a refrigerator (4 °C). The remaining portion of the soil samples was dried, pulverized and processed for the study of physicochemical parameters. Analysis of physicochemical properties of the soil viz., pH, EC, organic carbon, mineral N (NH4+ + NO3−), available P and K were done as per the standard procedure already described in the preceding sections. The samples from the refrigerator were brought to normal room temperature before being analyzed for microbial biomass carbon (MBC) and dehydrogenase enzyme activity. MBC in soil was measured using a fumigation-extraction method [42]. Dehydrogenase activity was measured by colorimetric method through estimation of tri-phenyl formazan (TPF) formed and expressed in the terms of TPF per hour per gram of soil (TPF g−1 soil h−1) [43].
2.7. Statistical analysis
Data obtained from both laboratory and pot culture studies were presented as the mean of three replicates, and a completely randomized design (CRD) was used for analysis of variance (ANOVA). The means of the individual treatment were compared by using multiple comparison Duncan test (at 5 % significance level). Duncan test and correlation matrix were performed in SPSS software version 24 (SPSS Inc. Chicago, USA). Microsoft Excel (Microsoft Corporation, USA) software package was used for data processing, tabulation and graphical presentations.
3. Results and discussion
3.1. Soil quality
3.1.1. Soil pH, EC and SOC
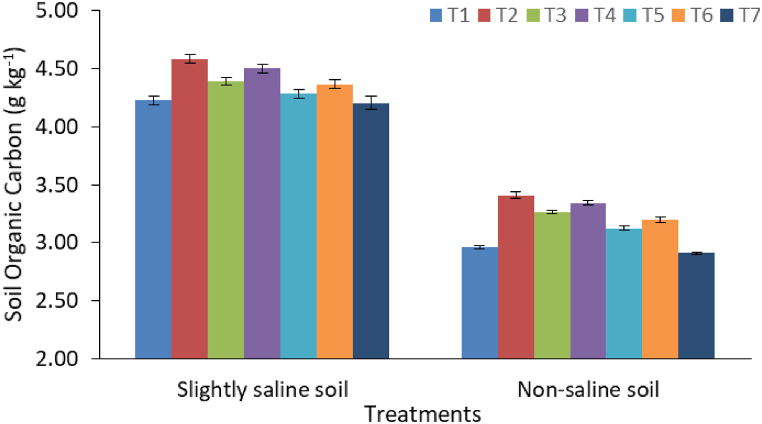
The results of soil physicochemical properties as influenced by various treatments were presented in Table 4. Following the harvest of senna, significant changes in soil pH, EC (dSm−1) and SOC (g kg−1) were found as results of the application of ENC and BFM. Higher soil pH (7.85) and EC (1.23 dSm−1) were reported in slightly saline soil than non-saline soil (7.67 and 0.36 dSm−1, respectively). Application of BFM (5 t ha−1) and ENC (5 t ha−1) resulted in relatively higher soil pH than FYM and CF. On the other hand, application of chemical fertilizer (CF) recorded the lowest pH values regardless of the soil, which may be due to their acidic in nature. The BFM treatment recorded the highest EC values, followed by ENC application in both soils. On the other hand, CF treatment (T7) resulted in EC values that were comparable to ENC application. Application of BFM increased EC of non-saline soil over the initial values due to higher EC value of BFM itself (Table 1). Due to its inherent salinity, the slightly saline soil always recorded higher pH and EC than the non-saline (Anand) soil. On the other hand, regardless of soil types, the treatment receiving FYM had the highest SOC (3.41–4.58 g kg−1) content (Fig. 2). The CF treatment resulted in the lowest SOC in both soils. When compared to control and CF, ENC and BFM significantly (p ≤ 0.05) improved SOC in both soils. The SOC contents were significantly improved by the application of higher doses of ENC (5 t ha−1) and BFM (5 t ha−1) than the lower doses of ENC (2.5 t ha−1) and BFM (2.5 t ha−1) in both the soils.
Table 4.
Soil physicochemical property and nutrient availability as influenced by application of enriched amendments and chemical fertilizer.
| Soil physicochemical properties | Treatments |
ANOVA |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| T1: Control | T2: FYM (5 t ha−1) | T3: ENC (2.5 t ha−1) | T4: ENC (5 t ha−1) | T5: BFM (2.5 t ha−1) | T6: BFM (5 t ha−1) | T7: CF | Treatment | Soil | Interaction | ||
| pH | S1 | 7.96 ± 0.04ab | 7.70 ± 0.04efg | 7.91 ± 0.03b | 8.01 ± 0.05a | 7.79 ± 0.02cd | 7.82 ± 0.04c | 7.77 ± 0.03cde | *** | *** | *** |
| S2 | 7.63 ± 0.01gh | 7.57 ± 0.01hi | 7.67 ± 0.01fg | 7.75 ± 0.01cdef | 7.71 ± 0.03defg | 7.81 ± 0.01c | 7.53 ± 0.01i | ||||
| EC (dSm−1) | S1 | 1.17 ± 0.02de | 1.13 ± 0.02e | 1.22 ± 0.02cd | 1.24 ± 0.02bc | 1.28 ± 0.02b | 1.37 ± 0.02a | 1.23 ± 0.03bc | *** | *** | ** |
| S2 | 0.30 ± 0.01h | 0.34 ± 0.01gh | 0.36 ± 0.01fgh | 0.37 ± 0.01fg | 0.39 ± 0.01fg | 0.41 ± 0.01f | 0.35 ± 0.01gh | ||||
| SOC (g kg−1) | S1 | 4.23 ± 0.03e | 4.58 ± 0.02a | 4.39 ± 0.02c | 4.50 ± 0.02b | 4.28 ± 0.02d | 4.37 ± 0.02c | 4.20 ± 0.02e | *** | *** | *** |
| S2 | 2.96 ± 0.01j | 3.41 ± 0.01e | 3.26 ± 0.01g | 3.34 ± 0.02f | 3.12 ± 0.01i | 3.20 ± 0.01h | 2.91 ± 0.01j | ||||
| Mineral Na(mg kg−1) | S1 | 48.47 ± 1.64cd | 51.47 ± 1.82bc | 51.53 ± 1.17bc | 54.60 ± 1.62ab | 48.17 ± 2.20cd | 54.87 ± 1.28ab | 57.17 ± 1.56a | *** | *** | NS |
| S2 | 37.37 ± 1.13f | 41.37 ± 0.98ef | 43.03 ± 0.83e | 45.10 ± 0.79de | 41.23 ± 0.84ef | 43.73 ± 0.75e | 48.50 ± 0.51cd | ||||
| Available P (mg kg−1) | S1 | 11.23 ± 0.55g | 13.53 ± 0.69g | 16.00 ± 0.93f | 17.77 ± 0.97def | 11.53 ± 0.69g | 13.57 ± 1.18g | 18.43 ± 1.04de | *** | *** | NS |
| S2 | 16.57 ± 0.55ef | 19.87 ± 0.78cd | 21.57 ± 0.63bc | 23.70 ± 0.52ab | 16.87 ± 0.38ef | 18.80 ± 0.85de | 24.77 ± 0.29a | ||||
| Available K (mg kg−1) | S1 | 188.57 ± 2.60c | 202.93 ± 2.88b | 208.33 ± 2.58b | 216.27 ± 3.78a | 218.13 ± 2.98a | 222.50 ± 2.82a | 223.30 ± 3.32a | *** | *** | NS |
| S2 | 91.57 ± 1.10h | 105.27 ± 1.15g | 110.67 ± 0.90fg | 115.93 ± 1.47ef | 115.80 ± 1.16ef | 121.50 ± 1.69de | 124.97 ± 1.14d | ||||
FYM: Farmyard manure; ENC: Enriched compost; BFM: Biochar fortified mineral; CF: Chemical fertilizer; S1: Slightly Saline soil and S2: Non-saline soil; ANOVA: Analysis of variance; NS: Non-significant.
All the data are presented as mean values ± standard error of three independent experiments (n = 3).
*, ** and ***Significant at P < 0.05, P < 0.01 and P < 0.001, respectively. Different letters within a same row indicate significant differences among treatments at p < 0.05 as per as per the Duncan multiple mean comparison test at 5 % significance.
Mineral N (NH4+-N + NO3− –N).
Fig. 2.
Soil organic carbon content as application of enriched amendments and chemical fertilizer. Bars are standard errors (n = 3).
Treatment details: T1: Control; T2: Farmyard manure (5 t ha−1); T3: Enriched compost (2.5 t ha−1); T4: Enriched compost (5 t ha−1); T5: Biochar fortified mineral (2.5 t ha−1); T6: Biochar fortified mineral (5 t ha−1), and T7: Chemical fertilizer (CF).
Application of FYM and enriched amendments (ENC and BFM) resulted in a decrease in soil pH and an increase in EC and SOC. The organic amendments apparently functioned to buffer the pH of the soils. Adsorption of Na, chelation of Ca and Mg by organic anions, and production of organic acids due to decomposition of organic matter (FYM and ENC) perhaps decreased pH and EC of the soils [44]. However, presence of soluble cations (Ca, Mg and K) and recalcitrant carbon in BFM might have increased the soil EC. Similar trend was observed in previous studies [23,45], where application of biochar and biochar based product increased soil pH and EC. Application of FYM and enriched amendments led to a significant buildup of SOC when compared to chemical fertilizers. The results indicate that the enriched amendments contributed significantly to native SOC pools because the enriched amendments themselves had higher total C contents (Table 1) than that of the experimental soils. The organic manures and composts play a synergistic role in building up of SOC pool in cultivated soils over a period of time. However, duration of present investigation was not enough to claim the stability of the SOC pool. On the other hand, rapid mineralization of native soil C in the CF treatment led to decrease in SOC content [46]. The positive impacts of FYM and enriched amendments on soil pH, EC and SOC in different soil types have been reported in different studies [17,20,47,48]. In previous investigations, application of FYM and enriched compost recorded 17.3 % and 10.2 % higher SOC contents, respectively, compared to application of CF [6].
3.1.2. Nutrient availability
Application of both conventional (FYM) and enriched amendments (ENC and BFM) had a positive influence on the mineral N, available P, and K (Table 4). Available nutrient status was improved significantly (p ≤ 0.05) in soil by the addition of enriched amendments (ENC and BFM) as well as CF treatments. Available K was significantly higher in ENC and BFM treatments in both soil types as compared to FYM. In both soils, the highest mineral N (48.5–57.2 mg kg−1), available P (18.4–24.8 mg kg−1) and available K (125–223 mg kg−1) contents were observed under CF treatments. Higher dose (5 t ha−1) of ENC and BFM application did not show any significant improvement in available nutrient status over lower dose (2.5 t ha−1) of ENC and BFM applications. However, available P (17.8–23.7 mg kg−1) measured in ENC (5 t ha−1) treatment and available K (121.5–222.5 mg kg−1) measured in BFM (5 t ha−1) treatment were comparable to CF. In slightly saline soil, the mineral N (NH4+ + NO3−) and available K were higher than non-saline soil, while the opposite was true for available P. Overall, there was no evidence of a significant (p ≤ 0.05) relationship between the different treatments and soil types. Overall, enriched amendments (ENC and BFM) were found comparable or almost equally as effective as CF in maintaining available nutrient status in both the soils. The application of ENC and BFM (Table 1), which were made from P and K bearing minerals, might have improved nutrient retention and availability in the soils. The BFM is a nutrient rich amendment with improved physicochemical properties, which might have derived from organo-mineral complex formation [29]. The scanning electron microscopy (SEM) and fourier transform infrared spectroscopy (FTIR) data proved the evidence of such complex formation. Thus, enriched amendments (ENC and BFM) might have enriched the available N pool by improving mineral N in soil as well as higher retention in soil [45]. These results follow a similar trend to that in other medicinal crops, where significantly higher mineral N was built up by vermicompost [49] and enriched compost [6] applications than FYM. Organic matter present in enriched amendments prevents soluble P from fixation [21] in the soil by occupying the adsorption sites in the soil matrix [6]. Similarly, rapid conversion of available P into microbial biomass may also prevent soluble P from fixation in the soil matrix [50]. Thus, both the mechanism perhaps indirectly contributed to greater available P in soils. Significant higher amounts of K were added to soil through both ENC and BFM (Table 3). Furthermore, mobilization of K from mineral through bio-activation by organic matter present in enriched amendments might have contributed higher available K in soil. Similar observation was found in previous study [14], where application of silicate mineral enriched compost improved available K content in soil. Thus, enriched amendments (ENC and BFM) made from natural minerals and waste biomass can effectively improve nutrient use efficiency as well as available nutrient status of the soil [6,17,47,51].
3.1.3. Soil biological properties
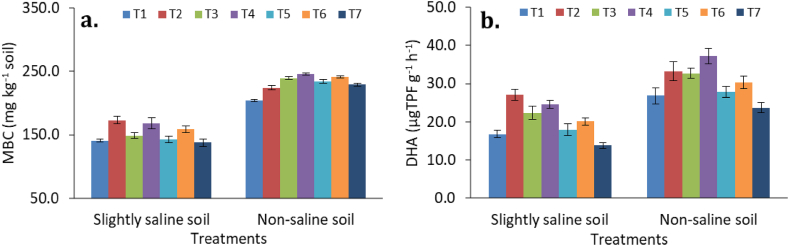
In comparison to CF, enriched amendments (ENC and BFM) significantly (p ≤ 0.05) increased the microbial biomass carbon (MBC) and dehydrogenase activity (DHA) in both slightly saline and non-saline soils (Fig. 3). The highest soil MBC was measured (245.4 mg kg−1) in ENC treatment (5 t ha−1), which was comparable to BFM (5 t ha−1) treatment (240.6 mg kg−1). However, in slightly saline soil, FYM (173.1 mg kg−1) performed even better than ENC (5 t ha−1) in improving MBC (Figure: 3a). Similar trend was also observed for DHA, where ENC (5 t ha−1) (37.23 μTPF g−1 h−1) and FYM (27.07 μTPF g−1 h−1) treatments performed better than CF in both slightly saline soil and non-saline soil, respectively (Figure: 3b). High dose (5 t ha−1) of ENC and BFM applications significantly improved MBC and DHA in soils over low dose (2.5 t ha−1) of ENC and BFM applications. Application of FYM was also found quite effective in improving DHA in slightly saline soil, while the use of chemical fertilizers (CF) reduced the enzyme activity in both soil types. Application of ENC recorded significantly higher DHA than BFM in both the soils. Organic fertilizers improve soil physicochemical environment, available nutrients and the metabolic substrates, which create ideal environment for soil microorganisms [52]. Thus, organic fertilizer improves microbial growth and soil enzyme activity instead of chemical fertilizer [46,53]. MBC is the most active component of SOC, which responds quickly to the input supply [54]. Both, ENC and BFM performed better than CF in improving MBC. The ENC and BFM act as excellent substrates for the rapid microbial growth due to the balanced supply of both carbon and nutrients in the soil [6,51]. The DHA is a metabolic enzyme that represents the microbial population in soil, more specifically the metabolically active microorganisms [55]. Application of bio-organic fertilizer was found to improve soil environment by increasing beneficial microbes in soil [56]. In the present study, the enriched amendments (ENC and BFM) might serve as a balanced source of organic carbon and essential elements, which boosted DHA in soil. In comparison to slightly saline soil, non-saline soil was found to have better biological properties in terms of MBC and DHA. This might be due to more soil congenial environment was prevailing in non-saline soil than slightly alkaline soil. There was a significant interaction between the treatments and soil types in the case of soil MBC, but not for DHA. The findings of the present investigation are in agreement with other studies, where soil MBC and DHA were improved by the application of enriched composts [6,20] and biochar [11,51,54]. Therefore, enriched amendments, such as ENC and BFM, could serve as alternative nutrient sources in improving soil biological properties.
Fig. 3.
Soil microbial biomass carbon (a) and dehydrogenase activity (b) as influenced by application of enriched amendments and chemical fertilizer. Bars are standard errors (n = 3).
Treatment details: T1: Control; T2: Farmyard manure (5 t ha−1); T3: Enriched compost (2.5 t ha−1); T4: Enriched compost (5 t ha−1); T5: Biochar fortified mineral (2.5 t ha−1); T6: Biochar fortified mineral (5 t ha−1), and T7: Chemical fertilizer (CF).
4. Plant growth and yield
The growth and yield parameters of senna as influenced by different organic amendments were presented in Table 5. Enriched amendments (ENC and BFM) and CF significantly increased plant growth in both the soils compared to control (unfertilized). The highest plant growth (plant height and number of branches) was recorded under CF treatment in both soils, followed by ENC (5 t ha−1) and BFM (5 t ha−1) in slightly saline and non-saline soil, respectively. However, there was not statistically significant (p ≤ 0.05) difference among CF, ENC and BFM treatments. Application of ENC (5 t ha−1) and BFM (5 t ha−1) increased plant height by 30.0 % and 25.4 % (slightly saline soil) and 18.8 % and 22.7 % (non-saline soil), respectively over FYM. Although CF produced the highest number of branches, was almost par with ENC (5 t ha−1) and BFM (5 t ha−1) treatments. In both the soils, application of enriched amendments (ENC and BFM) resulted in considerably higher plant height and number of branches than FYM, regardless of doses (2.5 and 5 t ha−1). Higher level of BFM (5 t ha−1) produced significantly (p ≤ 0.05) higher plant height than lower level of EBC (2.5 t ha−1) treatment. However, non-saline soil (Anand) demonstrated better plant growth than slightly saline soil irrespective of treatments and there was no significant (p ≤ 0.05) interaction between nutrient sources and soil types.
Table 5.
Plant biometric parameters as influenced by application of enriched amendments and chemical fertilizer.
| Plant biometric parameters | Treatments |
ANOVA |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| T1: Control | T2: FYM (5 t ha−1) | T3: ENC (2.5 t ha−1) | T4: ENC (5 t ha−1) | T5: BFM (2.5 t ha−1) | T6: BFM (5 t ha−1) | T7: CF | Treatment | Soil | Interaction | ||
| Plant height (cm) | S1 | 31.33 ± 0.74i | 34.67 ± 0.99hi | 42.43 ± 0.38ef | 45.07 ± 0.63cde | 39.03 ± 1.05fg | 43.47 ± 0.41de | 47.37 ± 0.98cd | *** | *** | NS |
| S2 | 36.43 ± 0.75gh | 43.67 ± 1.60de | 48.37 ± 3.43bc | 51.90 ± 0.71ab | 47.03 ± 1.91cd | 53.60 ± 0.46a | 54.63 ± 0.44a | ||||
| Number of branches (plant−1) | S1 | 9.67 ± 0.88e | 11.00 ± 0.58de | 13.00 ± 0.58abcd | 13.33 ± 0.88abcd | 12.33 ± 0.33bcde | 12.67 ± 0.88abcd | 14.67 ± 0.88ab | *** | ** | NS |
| S2 | 11.00 ± 1.15de | 11.67 ± 0.88cde | 14.33 ± 0.88abc | 14.67 ± 0.88ab | 13.67 ± 0.88abcd | 15.33 ± 0.88a | 15.33 ± 0.88a | ||||
| Dry leaf yield (g plant−1) | S1 | 20.07 ± 0.92i | 23.07 ± 0.75gh | 25.30 ± 1.21efg | 27.80 ± 0.59bcd | 23.80 ± 0.95fgh | 26.70 ± 1.21cde | 28.40 ± 0.36bcd | *** | *** | NS |
| S2 | 22.83 ± 0.85h | 26.07 ± 0.73def | 27.80 ± 0.59bcd | 28.43 ± 0.41bcd | 29.10 ± 0.47bc | 29.80 ± 0.38ab | 31.80 ± 0.55a | ||||
| Dry pod yield (g plant−1) | S1 | 2.38 ± 0.13h | 3.33 ± 0.05g | 3.82 ± 0.06ef | 4.15 ± 0.14d | 3.71 ± 0.05f | 4.01 ± 0.11de | 4.28 ± 0.08d | *** | *** | * |
| S2 | 3.54 ± 0.17fg | 4.09 ± 0.11de | 4.83 ± 0.06c | 5.27 ± 0.06b | 5.15 ± 0.04b | 5.28 ± 0.04b | 5.63 ± 0.10a | ||||
| Total dry herbage yield (g plant−1) | S1 | 22.44 ± 0.86i | 26.40 ± 0.79h | 29.12 ± 1.19fg | 31.95 ± 0.61cde | 27.51 ± 0.98gh | 30.71 ± 1.24def | 32.68 ± 0.33bcd | *** | *** | NS |
| S2 | 26.38 ± 0.70h | 30.16 ± 0.84ef | 32.63 ± 0.62bcd | 33.71 ± 0.45bc | 34.25 ± 0.44bc | 35.08 ± 0.41b | 37.43 ± 0.64a | ||||
FYM: Farmyard manure; ENC: Enriched compost; BFM: Biochar fortified mineral; CF: Chemical fertilizer; S1: Slightly Saline soil and S2: Non-saline soil; ANOVA: Analysis of variance; NS: Non-significant.
All the data are presented as mean values ± standard error of three independent experiments (n = 3).
*, ** and ***Significant at P < 0.05, P < 0.01 and P < 0.001, respectively. Different letters within a same row indicate significant differences among treatments at p < 0.05 as per as per the Duncan multiple mean comparison test at 5 % significance.
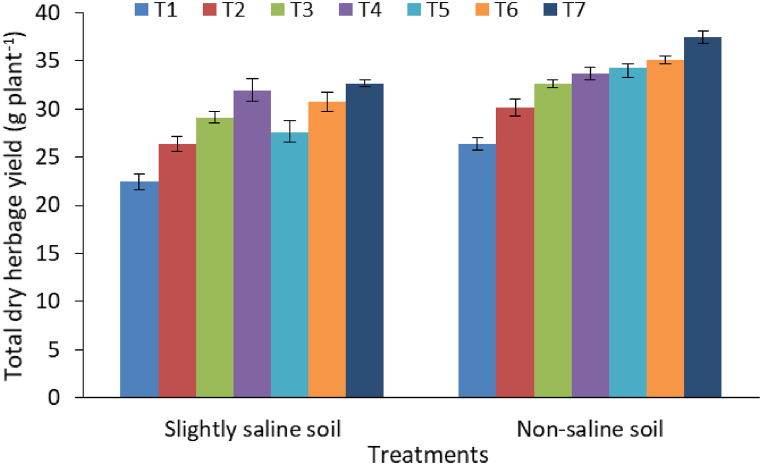
Positive effect of different enriched amendments (ENC and BFM) and CF was also reflected in the improvement of total dry herbage (leaf + pod) yield of senna (Fig. 4). Averaged over two soils, significantly (p ≤ 0.05) higher total dry herbage yield was obtained under CF than other treatments. However, total dry herbage yield under ENC (5 t ha−1) was almost equal to CF treatment in slightly saline soil.
Fig. 4.
Total dry herbage yield of senna as influenced by application of enriched amendments and chemical fertilizer. Bars are standard errors (n = 3).
Treatment details: T1: Control; T2: Farmyard manure (5 t ha−1); T3: Enriched compost (2.5 t ha−1); T4: Enriched compost (5 t ha−1); T5: Biochar fortified mineral (2.5 t ha−1); T6: Biochar fortified mineral (5 t ha−1), and T7: Chemical fertilizer (CF).
The highest dry leaf yield (28.4–31.8 g plant−1) was produced by CF treatment in both soil types, followed by ENC (5 t ha−1) and BFM (5 t ha−1). However, ENC and BFM produced higher dry leaf yield than FYM in both the soils (Table 5). Unlike leaf yield, dry pod yield in the CF treatment was found equivalent to treatment receiving ENC (5 t ha−1) and BFM (5 t ha−1) in slightly saline soils (Table 5). Higher doses of ENC (5 t ha−1) and BFM (5 t ha−1) recorded considerably higher fresh and dry herbage yields than lower dose of ENC (2.5 t ha−1) and BFM (2.5 t ha−1) in both the soils. Application of both ENC and BFM produced significantly higher leaf, pod and total herbage yield (dry weight basis) than FYM. Significant interaction between treatment and soil type was observed in case of dry pod yield, but dry leaf yield did not show such interaction effect. Overall, non-saline soil recorded higher herbage yield than slightly saline soil irrespective of treatments.
The plant growth and yield are important components responsible for the economic yield of senna, which were influenced by CF and enriched amendments in our study. Both ENC and BFM outperformed conventional organic manure like FYM. The ENC and BFM probably supplied balance nutrients and organic matter ideal for plant growth (Table 3). Furthermore, adding of low-grade RP and SMP during hydrothermal reaction and composting process improved nutrient content in the final product (ENC & BFM) than FYM (Table 1) The higher nutrient supplying potential of the enriched amendments might have contributed higher plant growth and yield as compared to conventional organic manure (FYM). The enriched amendments attributed to enhanced plant growth and herbage yield of senna due to their better nutrient retention and higher biological activities (Table 3, Table 4). The application of enriched amendments (ENC & BFM) created a better soil environment by adding organic matter and essential plant nutrients together [47]. Moreover, higher nutrient retention capacity of enriched amendment contributed nutrient supply for longer period of time [12,45]. This phenomenon might have contributed higher cellular metabolisms and biomass production. The findings of this study are in consistent with earlier reports, where enriched biochar formulation boosted plant growth and yield of lettuce [57], wheat [51] and ginger [17], including medicinal plants, where application of enriched amendments increased economic yields of isabgol (Plantogo ovata Forsk.) [6], senna (Cassia angustifolia Vahl.) [21], Centella asiatica (L.) [58] and ashwagandha (Withania somnifera (L.) Dunal [20] (see Fig. 4).
4.1. Bioactive compound content and yield
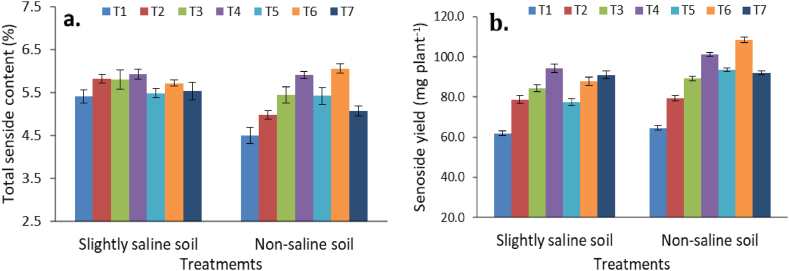
Sennoside content and yield as influenced by various amendments and chemical fertilization in two different soil types were presented in Fig. 5. The treatments receiving FYM and enriched amendments (ENC and BFM) produced higher leaf sennoside content over the control. However, sennoside content in pods was unaffected by various fertilizer treatments in both soil types. In slightly saline soil, different treatments did not influence total sennoside content in senna. However, application of ENC and BFM resulted in significantly (p ≤ 0.05) higher total sennoside contents than CF and FYM treatments in non-saline soil (Fig. 5a). The BFM treatment (5 t ha−1) under non-saline soil had the highest sennoside content (3.13 %), which are 21.5 % and 29.2 % higher than FYM and CF, respectively. Unlike sennoside content, total sennoside yield in both soils varied significantly by different treatments (Fig. 5b). The treatment that received BFM (5 t ha−1) had the highest total sennoside yield (108.46 mg plant−1) in non-saline soil, followed by ENC (5 t ha−1) (101.23 mg plant−1). High doses of ENC (5 t ha−1) and BFM (5 t ha−1) recorded significantly higher sennoside yields than low doses of ENC (2.5 t ha−1) and BFM (2.5 t ha−1) in both the soils. However, in slightly saline soil, the highest total sennoside yield was obtained in ENC treatment followed by CF treatment. Overall, higher sennoside yield was found in non-saline soil than slightly saline soil.
Fig. 5.
Bio active compound content (a) and yield (b) of senna as influenced by application of enriched amendments and chemical fertilizer. Bars are standard errors (n = 3).
Treatment details: T1: Control; T2: Farmyard manure (5 t ha−1); T3: Enriched compost (2.5 t ha−1); T4: Enriched compost (5 t ha−1); T5: Biochar fortified mineral (2.5 t ha−1); T6: Biochar fortified mineral (5 t ha−1), and T7: Chemical fertilizer (CF).
The production of secondary metabolites, including alkaloids, is very much related to nutrient sources [59] including organic fertilizers that can significantly influence secondary metabolites in medicinal plant [60]. Organic manures and fertilizers supply all essential nutrients throughout the growth period with the improvement in soil properties that influence secondary metabolisms of plants [45]. In the present study, sennoside content and yield responses to various fertilization treatments followed quite different patterns. This could be due to the fact that both herbage yield and sennoside content influenced the sennoside yield. The enriched soil amendments contain more essential nutrients as well as organic carbon, which boost the nutrient availability that promote the production of secondary metabolites in the plant [20]. In the present study, the consistent supply of essential plant nutrients from the enriched amendments (ENC and BFM) improved nutrient transformation, availability and assimilation, resulting in a boost in synthesis of primary and secondary metabolites in senna [61]. Traditional organic manures (FYM and farm compost) are good sources of organic carbon, but poor in essential plant nutrient contents [6,47]. Due to a balance combination of organic and mineral sources, ENC and BFM served better than FYM in our study [12,17] Application of enriched amendment might have facilitated a congenial plant growth environment for the synthesis of bioactive compound in Senna (sennoside) [21]. A better congenial environment in terms of pH, electrical conductivity and biological parameters prevailed in non-saline soil during plant growth period resulted in higher sennoside yield as compared to slightly saline soil. The similar results were reported by previous studies in which application of organic amendments recorded higher sennoside yields in non-saline soil as compared saline soil [46]. One of the reasons for better performance of ENC in slightly saline soil could be higher organic carbon content in ENC than BFM. ENC was found more effective than BFM in improving the soil properties particularly SOC and available nutrients in slightly saline soil as evident in our study. On the other hand, BFM improved nutrient use efficiency in non-saline due to better nutrient retention capacity than other organic as evident in previous study [45]. The findings of our study are also in line with previous work where enriched compost were found to perform better than biochar in saline soil [62] and enriched biochar performed better than compost in non-saline soil [45].
Based on the data on soil and plant parameters, correlation matrix was developed for each soil type (Table 6, Table 7). There were significant positive correlations of total herbage yield with plant height (r = 0.80, p ≤ 0.01), soil EC (0.70, p ≤ 0.01), mineral N (0.83, p ≤ 0.01), available P (0.53, p ≤ 0.01), available K (0.94, p ≤ 0.01) and MBC (0.71, p ≤ 0.01) in non-saline soil (Table 7). Sennoside content was significantly correlated with soil pH (0.68) and organic carbon (0.49). Almost similar trend was observed in slightly saline soil (Table 6). However, sennoside content did not show any significant correlation with soil parameters. On the other hand, the soil microbial biomass carbon and dehydrogenase activity were significantly correlated with organic carbon in both the soils. The result indicated a positive influence of available nutrients and MBC on herbage yield of senna. The available nutrients were correlated well with soil biological properties. Thus, enriched organic amendment improved available nutrients, which might have contributed to plant growth and yield. The outcome of the present investigation is in agreement with previous studies [6,21].
Table 6.
Correlations among plant and soil parameters of senna pot experiment in slightly saline soil.
| Plant height | Leaf dry weight | Pod dry weight | Total herbage yield | Total sennoside | Soil pH | EC | Organic carbon | Mineral N | Available P | Available K | MBC | DHA | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plant height | 1 | ||||||||||||
| Leaf dry weight | .821** | 1 | |||||||||||
| Pod dry weight | .922** | .848** | 1 | ||||||||||
| Total herbage yield | .857** | .996** | .894** | 1 | |||||||||
| Total sennoside | .146 | .056 | .039 | .054 | 1 | ||||||||
| Soil pH | .009 | −.064 | −.163 | −.083 | .259 | 1 | |||||||
| EC | .485* | .480* | .471* | .489* | .012 | .056 | 1 | ||||||
| Organic carbon | .131 | .152 | .284 | .178 | .055 | .091 | .129 | 1 | |||||
| Mineral N | .569** | .686** | .587** | .684** | .048 | .011 | .316 | .075 | 1 | ||||
| Available P | .740** | .658** | .704** | .680** | .057 | .146 | .043 | .128 | .604** | 1 | |||
| Available K | .751** | .798** | .814** | .818** | −.140 | −.231 | .623** | .176 | .570** | .391 | 1 | ||
| MBC | −.027 | .088 | .112 | .094 | −.027 | −.076 | −.096 | .877** | .077 | .051 | .023 | 1 | |
| DHA | −.182 | .023 | .039 | .027 | −.046 | −.012 | −.259 | .798** | −.034 | .014 | −.117 | .848** | 1 |
Table 7.
Correlations among plant and soil parameters of senna pot experiment in non-saline soil.
| Plant height | Plant height | Leaf dry weight | Pod dry weight | Total herbage yield | Total sennoside | Soil pH | EC | Organic carbon | Mineral N | Available P | Available K | MBC | DHA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plant height | 1 | ||||||||||||
| Leaf dry weight | .773** | 1 | |||||||||||
| Pod dry weight | .857** | .910** | 1 | ||||||||||
| Total herbage yield | .801** | .996** | .941** | 1 | |||||||||
| Total sennoside | .239 | .101 | .353 | .153 | 1 | ||||||||
| Soil pH | .259 | .126 | .307 | .163 | .677** | 1 | |||||||
| EC | .599** | .688** | .704** | .700** | .280 | .633** | 1 | ||||||
| Organic carbon | .369 | .186 | .362 | .223 | .468* | .821** | .650** | 1 | |||||
| Mineral N | .799** | .819** | .813** | .830** | .041 | −.029 | .411 | .114 | 1 | ||||
| Available P | .620** | .517* | .567** | .535* | −.099 | −.209 | .032 | .059 | .828** | 1 | |||
| Available K | .907** | .925** | .934** | .940** | .209 | .233 | .733** | .303 | .829** | .526* | 1 | ||
| MBC | .776** | .679** | .797** | .711** | .309 | .555** | .771** | .781** | .588** | .464* | .753** | 1 | |
| DHA | .074 | −.168 | −.032 | −.144 | .043 | .423 | .184 | .741** | −.055 | .176 | −.036 | .460* | 1 |
*p ≤ 0.05, **p ≤ 0.01 significant correlation co-efficient n = 21.
EC: Electrical conductivity; MBC: Microbial biomass carbon; DHA: Dehydrogenase activity.
In the current study, we investigated the potential of enriched amendments application (ENC and BFM) in senna cultivation. The ENC and BFM were found superior to conventional organic amendments, such as FYM, as an alternative of CF. However, the results of the present study was based on the one season pot experiment. The effect of ENC and BFM may also vary between pot and field conditions. Therefore, further studies, including field experiments, would be helpful to determine the full potential of these enriched amendments in sustainable production of high value medicinal herbs. In this study, the economic feasibility was not investigated for ENC and BFM in comparison to FYM and CF applications. This aspect might be important for farmer adaptation of enriched amendments in cultivation of high value medicinal herbs.
5. Concluding remarks
This study demonstrated that blending of waste biomass and mineral powder in hydrothermal and composting process significantly increased the essential nutrients content in the biochar fortified mineral (BFM) and enriched compost (ENC). Application of both ENC and BFM boosted the soil quality and fertility via improving available nutrients status, microbial biomass, and enzyme activity. Thus, present findings support our hypothesis of soil properties improvement through enriched amendment application. As a result, the enriched amendments (ENC and BFM) were significantly more effective than traditional organic manures (FYM) in plant growth and herbage yield of the medicinal herb Senna. The ENC performed better in slightly saline soil while BFM in non-saline soil. Higher doses of ENC (5 t ha−1) and BFM (5 t ha−1) performed better than the lower dose of ENC (2.5 t ha−1) and BFM (2.5 t ha−1) in both the soils. Although application of chemical fertilizer produced overall the highest total herbage yield of Senna, the enriched amendments (ENC and BFM) were found more effective than chemical fertilizer in slightly saline soil with respect to bioactive compound (sennoside) content and its yield. Thus, findings of the study support our hypothesis of enriched amendments as potential substitute of chemical fertilizers. We conclude that application of enriched amendments (ENC and BFM) is better than conventional FYM in Senna cultivation in term of total herbage and sennoside yields. Furthermore, these enriched amendments have the potentials to serve as an alternative of costly chemical fertilizers, particularly regarding sennoside yields in Senna cultivation.
Data availability statement
-
•
Data included in article/supplementary material/referenced in article
-
•
Data will be made available on request
CRediT authorship contribution statement
Prem Kumar B: Writing – original draft, Data curation. B.B. Basak: Supervision, Conceptualization. V.J. Patel: Software, Visualization. Nimai Senapati: Writing – review & editing. V.P. Ramani: Visualization. N.A. Gajbhiye: Methodology.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Authors gratefully acknowledge ICAR-Directorate of Medicinal and Aromatic Plants Research, Anand for providing the required facilities to undertake this work. The senior author gratefully acknowledges National Talent Scholarship (NTS) scheme of Indian Council of Agricultural Research for financial support during his research work.
References
- 1.World Bank . 2018. Commodity Prices.www.worldbank.org/en/research/commodity-markets [Google Scholar]
- 2.FAO . Food and Agriculture Organization of the United Nations (FAO); Rome, Italy: 2019. World Fertilizer Trends and Outlook to 2022. [Google Scholar]
- 3.Chen Y., Hu S., Guo Z., Cui T., Zhang L., Lu C., Yu Y., Luo Z., Jin Y. Effect of balanced nutrient fertilizer: a case study in Pinggu District, Beijing, China. Sci. Total Environ. 2021;754 doi: 10.1016/j.scitotenv.2020.142069. [DOI] [PubMed] [Google Scholar]
- 4.Abou-Sreea A.I., Rady M.M., Roby M.H., Ahmed S.M., Majrashi A., Ali E.F. Cattle manure and bio-nourishing royal jelly as alternatives to chemical fertilizers: potential for sustainable production of organic Hibiscus sabdariffa L. J. Appl. Res. Med. Aromat. Plants. 2021;25 [Google Scholar]
- 5.Buss W., Wurzer C., Manning D.A., Rohling E.J., Borevitz J., Masek O. Mineral-enriched biochar delivers enhanced nutrient recovery and carbon dioxide removal. Commun. Earth Environ. 2022;3:1–11. [Google Scholar]
- 6.Basak B.B., Saha A. Recycling of isabgol (Plantago ovata Forsk.) straw biomass and mineral powder with bio-inoculants as an effective soil amendment for isabgol cultivation. Pedosphere. 2022;32:686–697. [Google Scholar]
- 7.Ayaz M., Stulpinaite U., Feiziene D., Tilvikiene V., Akthar K., Baltrėnaitė‐Gedienė E., Striugas N., Rehmani U., Alam S., Iqbal R., Toleikiene M. Pig manure digestate‐derived biochar for soil management and crop cultivation in heavy metals contaminated soil. Soil Use Manag. 2022;38:1307–1321. [Google Scholar]
- 8.Saha A., Basak B.B., Banerjee A. In-vitro antioxidant evaluation and production of biochar from distillation waste biomass of Mentha arvensis. J. Appl. Res. Med. Aromat. Plants. 2022;31 [Google Scholar]
- 9.Zhongqi H.E., Pagliari P.H., Waldrip H.M. Applied and environmental chemistry of animal manure: a review. Pedosphere. 2016;26:779–816. [Google Scholar]
- 10.Ilyas M., Arif M., Akhtar K., Riaz M., Wang H. Diverse feedstock's biochars as supplementary K fertilizer improves maize productivity, soil organic C and KUE under semiarid climate. Soil Tillage Res. 2021;211 [Google Scholar]
- 11.Basak B.B., Sarkar B., Saha A., Sarkar A., Mandal S., Biswas J.K., Wang H., Bolan N.S. Revamping highly weathered soils in the tropics with biochar application: what we know and what is needed. Sci. Total Environ. 2022;822 doi: 10.1016/j.scitotenv.2022.153461. [DOI] [PubMed] [Google Scholar]
- 12.Kizito S., Luo H., Lu J., Bah H., Dong R., Wu S. Role of nutrient-enriched biochar as a soil amendment during maize growth: exploring practical alternatives to recycle agricultural residuals and to reduce chemical fertilizer demand. Sustainability. 2019;11:3211. [Google Scholar]
- 13.Basak B.B. Evaluation of Indian rock phosphates for predicting agronomic potential through chemical and biological methods. Arch. Agron Soil Sci. 2019;65:1599–1609. [Google Scholar]
- 14.Basak B.B. Recycling of waste biomass and mineral powder for preparation of potassium-enriched compost. J. Mater. Cycles Waste Manag. 2018;20:1409–1415. [Google Scholar]
- 15.Biswas D.R., Narayanasamy G., Datta S.C., Singh G., Begum M., Maiti D., Mishra A., Basak B.B. Changes in nutrient status during preparation of enriched organomineral fertilizers using rice straw, low-grade rock phosphate, waste mica, and phosphate solubilizing microorganism. Commun. Soil Sci. Plant Anal. 2009;40:2285–2307. [Google Scholar]
- 16.Kim J.A., Vijayaraghavan K., Reddy D.H.A., Yun Y.S. A phosphorus-enriched biochar fertilizer from bio-fermentation waste: a potential alternative source for phosphorus fertilizers. J. Clean. Prod. 2018;196:163–171. [Google Scholar]
- 17.Farrar M.B., Wallace H.M., Xu C.Y., Nguyen T.T.N., Tavakkoli E., Joseph S., Bai S.H. Short-term effects of organo-mineral enriched biochar fertiliser on ginger yield and nutrient cycling. J. Soils Sediments. 2019;19:668–682. [Google Scholar]
- 18.Chia C.H., Singh B.P., Joseph S., Graber E.R., Munroe P. Characterization of an enriched biochar. J. Anal. Appl. Pyrolysis. 2014;108:26–34. [Google Scholar]
- 19.Blackwell P., Joseph S., Munroe P., Anawar H.M., Storer P., Gilkes R.J., Solaiman Z.M. Influences of biochar and biochar-mineral complex on mycorrhizal colonisation and nutrition of wheat and sorghum. Pedosphere. 2015;25:686–695. [Google Scholar]
- 20.Gupta M., Srivastava P.K., Niranjan A., Tewari S.K. Use of a bioaugmented organic soil amendment in combination with gypsum for Withania somnifera growth on sodic soil. Pedosphere. 2016;26:299–309. [Google Scholar]
- 21.Basak B.B., Gajbhiye N.A. Phosphorus enriched organic fertilizer, an effective P source for improving yield and bioactive principle of Senna (Cassia angustifolia Vahl.) Ind. Crop. Prod. 2018;115:208–213. [Google Scholar]
- 22.Saha A., Basak B.B. Scope of value addition and utilization of residual biomass from medicinal and aromatic plants. Ind. Crop. Prod. 2020;145 [Google Scholar]
- 23.Saha A., Basak B.B., Gajbhiye N.A., Kalariya K.A., Manivel P. Sustainable fertilization through co-application of biochar and chemical fertilizers improves yield, quality of Andrographis paniculata and soil health. Ind. Crop. Prod. 2019;140 [Google Scholar]
- 24.Sastry K.P., Rao B.R.R., Rajput D.K., Singh C.P., Singh K. first ed. Hyderabad.; 2015. Cultivation and Processing of Cassia Angustifolia Vahl. Tamil Nadu, Advances in Medicinal Plants; pp. 123–129. [Google Scholar]
- 25.Tripathi Y.C. Cassia angustifolia, a versatile medicinal crop. Int. Tree Crops J. 1999;10:121–129. [Google Scholar]
- 26.Jat R.S., Reddy N.R., Bansal R., Manivel P. ICAR-Directorate of Medicinal and Aromatic Plants Research; Anand, Gujarat: 2015. Good Agricultural Practices for Senna. [Google Scholar]
- 27.Butnan S., Deenik J.L., Toomsan B., Antal M.J., Vityakon P. Biochar characteristics and application rates affecting corn growth and properties of soils contrasting in texture and mineralogy. Geoderma. 2015;237:105–116. [Google Scholar]
- 28.Lashari M.S., Liu Y., Li L., Pan W., Fu J., Pan G., Yu X. Effects of amendment of biochar-manure compost in conjunction with pyroligneous solution on soil quality and wheat yield of a salt-stressed cropland from Central China Great Plain. Field Crops Res. 2013;144:113–118. [Google Scholar]
- 29.Lin Y., Munroe P., Joseph S., Ziolkowski A., Van Zwieten L., Kimber S., Rust J. Chemical and structural analysis of enhanced biochars: thermally treated mixtures of biochar, chicken litter, clay and minerals. Chemosphere. 2013;91:35–40. doi: 10.1016/j.chemosphere.2012.11.063. [DOI] [PubMed] [Google Scholar]
- 30.Sumner M.E., Miller W.P. Cation exchange capacity and exchange coefficients. Methods of Soil Analysis: Part 3 Chemical methods. 1996;5:1201–1229. [Google Scholar]
- 31.Jackson M.L. Prentice Hall of India Pvt. Ltd.; New Delhi: 1973. Soil Chemical Analysis. [Google Scholar]
- 32.Watanabe F.S., Olsen S.R. Test of ascorbic acid method for determining phosphorus in water and sodium bicarbonate extracts of soils. Soil Sci. Soc. Am. Proc. 1965;29:677–678. [Google Scholar]
- 33.Olsen S.R., Cole C.W., Watanabe F.S., Dean L.A. US Department of Agriculture; Circular: 1954. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; p. 939. [Google Scholar]
- 34.Hanway J.J., Heidel H. Soil analysis methods as used in Iowa state college soil testing laboratory. Iowa Agric. 1952;57:1–13. [Google Scholar]
- 35.IUSS Working Group WRB . fourth ed. International Union of Soil Sciences (IUSS); Vienna, Austria: 2022. World Reference Base for Soil Resources. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; p. 234. [Google Scholar]
- 36.Richards L.A. 1954. Diagnosis and Improvement of Saline and Alkali Soils USDA Handbook; p. 60. [Google Scholar]
- 37.Bouyoucos G.J. Hydrometer method improved for making particle size analyses of soils. Agron. J. 1962;54:464–465. [Google Scholar]
- 38.Nelson D.W., Sommers L.E. In: Method of Soil Analysis, Part 3: Chemical Methods. Sparks D., Page A.L., Helmke P.A., Leoppert R.A., Soltanpour P.N., Tabatabai M.A., Johnston C.T., Sumner M.E., editors. Soil Science Society of America; Madison, USA: 1996. Total carbon, organic carbon, and organic matter; pp. 995–996. [Google Scholar]
- 39.Keeney D.R., Nelson D.W. In: Methods of Soil Analysis. Part 2: Chemical and Microbiological Properties. second ed. Page A.L., Miller R.H., Keeney D.R., editors. American Society of Agronomy and Soil Science Society; Madison Wisconsin: 1982. Nitrogen-inorganic forms; pp. 643–698. [Google Scholar]
- 40.Reddy N.R.R., Mehta R.H., Soni P.H., Makasana J., Gajbhiye N.A., Ponnuchamy M., Kumar J. Next generation sequencing and transcriptome analysis predicts biosynthetic pathway of sennosides from Senna (Cassia angustifolia Vahl.), a non model plant with potent laxative properties. PLoS One. 2015;10 doi: 10.1371/journal.pone.0129422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Srivastava V.K., Maheshwari M.L., Mandal S. Investigation of chemical assay of sennoside in Senna (Cassia angustifolia Vahl.) Int. J. Trop. Agric. 1983;1:231–238. [Google Scholar]
- 42.Jenkinson D.S., Powlson D.S. The effects of biocidal treatment on metabolism in soil. I. Fumigation with chloroform. Soil Biol. Biochem. 1976;8:167–177. [Google Scholar]
- 43.Klein D.A., Loh T.C., Goulding R.L. A rapid procedure to evaluate the dehydrogenase activity of soils low in organic matter. Soil Biol. Biochem. 1971;3:385–387. [Google Scholar]
- 44.Tejada M., Garcia C., Gonzalez J.L., Hernandez M.T. Use of organic amendment as a strategy for saline soil remediation: influence on the physical, chemical and biological properties of soil. Soil Biol. Biochem. 2006;38:1413–1421. [Google Scholar]
- 45.Basak B.B., Saha A., Sarkar B., Kumar B.P., Gajbhiye N.A., Banerjee A. Repurposing distillation waste biomass and low-value mineral resources through biochar-mineral-complex for sustainable production of high-value medicinal plants and soil quality improvement. Sci. Total Environ. 2021;760 doi: 10.1016/j.scitotenv.2020.143319. [DOI] [PubMed] [Google Scholar]
- 46.Basak B.B., Chinchmalatpure A.R., Saha A., Lodaya D.H., Patel P., Gajbhiye N.A. Integrated nutrient management fostered economic yield and bioactive principle of medicinal herb (Cassia angustifolia Vahl.) in saline soil of semi-arid region of Western India. Commun. Soil Sci. Plant Anal. 2023;54:611–626. [Google Scholar]
- 47.Ye J., Zhang R., Nielsen S., Joseph S.D., Huang D., Thomas T. A combination of biochar–mineral complexes and compost improves soil bacterial processes, soil quality, and plant properties. Front. Microbiol. 2016;7:372. doi: 10.3389/fmicb.2016.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meena M.D., Narjary B., Sheoran P., Jat H.S., Joshi P.K., Chinchmalatpure A.R. Changes in physical and chemical properties of saline soil amended with municipal solid waste compost and chemical fertilizers in a mustard–pearl millet cropping system. Land Degrad. Dev. 2022;33:1177–1688. [Google Scholar]
- 49.Basak B.B., Saha A., Gajbhiye N.A., Manivel P. Potential of organic nutrient sources for improving yield and bioactive principle of ashwagandha (Withania somnifera) through enhanced soil fertility and biological functions. Commun. Soil Sci. Plant Anal. 2020;51:779–793. [Google Scholar]
- 50.Basak B.B. Soil phosphorus dynamics and P uptake by medicinal crops as influenced by locally available organic amendments in light-textured soil of semi-arid western India. J. Soil Sci. Plant Nutr. 2023;23:2190–2201. [Google Scholar]
- 51.Joseph S., Anawar H.M., Storer P., Blackwell P., Chia C., Lin Y., Munroe P., Donne S., Horvat J., Wang J., Solaiman Z.M. Effects of enriched biochars containing magnetic iron nanoparticles on mycorrhizal colonisation, plant growth, nutrient uptake and soil quality improvement. Pedosphere. 2015;25:749–760. [Google Scholar]
- 52.Zang Z., Yang Q., Liang J., Yang Y., Li N., Wang H., Guo J., Yang L. Alternate micro-sprinkler irrigation and organic fertilization decreases root rot and promotes root growth of Panax notoginseng by improving soil environment and microbial structure in rhizosphere soil. Ind. Crop. Prod. 2023;202 [Google Scholar]
- 53.Yue X., Yang Q., Liang J., Tang J., Yang Y. Alternate micro-sprinkler irrigation synergized with organic fertilizer: a sustainable water-fertilizer management technology of improving quality and increasing efficiency in Panax notoginseng production. Ind. Crop. Prod. 2023;194 [Google Scholar]
- 54.Nielsen S., Minchin T., Kimber S., van Zwieten L., Gilbert J., Munroe P., Joseph S., Thomas T. Comparative analysis of the microbial communities in agricultural soil amended with enhanced biochars or traditional fertilisers. Agric. Ecosyst. Environ. 2014;191:73–82. [Google Scholar]
- 55.Nannipieri P., Trasar-Cepeda C., Dick R.P. Soil enzyme activity: a brief history and biochemistry as a basis for appropriate interpretations and meta-analysis. Biol. Fertil. Soils. 2018;54:11–19. [Google Scholar]
- 56.Liu X., Zhang Y., Jiang Z., Yue X., Liang J., Yang Q., Li J., Li N. Micro-moistening irrigation combined with bio-organic fertilizer: an adaptive irrigation and fertilization strategy to improve soil environment, edible Rose yield, and nutritional quality. Ind. Crop. Prod. 2023;196 [Google Scholar]
- 57.Gunes A., Inal A., Taskin M.B., Sahin O., Kaya E.C., A Atakol A.R.D. Effect of phosphorus enriched biochar and poultry manure on growth and mineral composition of lettuce (Lactuca sativa L. cv.) grown in alkaline soil. Soil Use Manag. 2014;30:182–188. [Google Scholar]
- 58.Trisilawati O., Hartoyo B., Bermawie N., Pribadi E.R. IOP Conference Series. Earth Environ. Sci.; 2019. Application of AMF (Arbuscular Mycorrhizal Fungi) and organic fertilizer to increase the growth, biomass and bioactive content of centella. 292. [Google Scholar]
- 59.Mohammadi S.M., Sefidkon F., Asadi‐Sanam S., Kalatejari S. The changes of carvacrol content and essential oil yield of Satureja khuzestanica Jamzad in response to different fertilizer sources. Flavour Fragrance J. 2023;38:37–48. [Google Scholar]
- 60.Díaz-Gutiérrez C., Hurtado A., Ortíz A., Poschenrieder C., Arroyave C., Peláez C. Increase in steviol glycosides production from Stevia rebaudiana Bertoni under organo-mineral fertilization. Ind. Crop. Prod. 2020;147 [Google Scholar]
- 61.Zhu Z., Liang Z., Han R., Wang X. Impact of fertilization on drought response in the medicinal herb Bupleurum chinense DC.: growth and saikosaponin production. Ind. Crop. Prod. 2009;29:629–633. [Google Scholar]
- 62.Dashti M., Kafi M., Mirza M. Chemical variation in the essential oil of salvia leriifolia benth. In response to organic and biological fertilizers. J. Med. plants By-prod. 2022;11:93–101. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
-
•
Data included in article/supplementary material/referenced in article
-
•
Data will be made available on request