Abstract
The purpose of this inquiry is to provide a conprehensive summary and analysis of the literature concerning the pharmacological properties of components that can be extracted from Desmodium styracifolium, a preparation in Chinese medicine. This study also aims to explore their potential application in elaborating medicinal products for the effective prevention and treatment of such conditions as urolithiasis, cholelithiasis, type 2 diabetes mellitus, metabolic syndrome, pro-oxidant and inflammatory processes, etc.
Several experimental studies confirmed the potential of D. styracifolium to influence mineral metabolism, to decrease the concentration of constituents involved in the formation of urinary calculi, and to reduce mineral encrustation in the urinary tract, as well as to alleviate the damage caused by crystal structures. This beneficial impact is achieved through a combination of antioxidant and anti-inflammatory actions, along with urine alkalinization. The cholelitholytic, choleretic, and hepatoprotective effects of D. styracifolium plants have been confirmed, primarily ascribed to the activation of the hepatic Xα receptor and the bile acid receptor, farnesoid X receptor, by the flavonoid shaftoside.
Special attention is focused on the potential therapeutic applications of flavonoids derived from D. styracifolium for diseases associated with the development of chronic inflammation and systemic response, emphasizing the ability of flavonoids to exert antioxidant and anti-inflammatory effects by acting directly and through the modulation of transcription factors.
It is concluded that new strategies for the prevention and treatment of urolithiasis, cholelithiasis, type 2 diabetes mellitus, metabolic syndrome, acute and chronic inflammatory processes may rely on the promising development of dosage forms of D. styracifolium with their subsequent preclinical and clinical trials.
Keywords: Desmodium styracifolium, Ethnopharmacology, Antiurolithic, Cholelytic, Cholagogic, Hepatoprotective, Nephroprotective, Antioxidant, Anti-inflammatory and antidiabetic effects
1. Introduction
Kidney stone disease (urolithiasis) despite being a non-infectious disease affects large portion of human population regardless of gender and racial features [1]. Some studies provide evidence that urolithiasis can impact nearly 10 % of the global population, with the disease recurring in approximately 2 % of cases even after operative treatment [2]. The prevalence of urolithiasis in Europe ranges between 5 % and 9 %. The management guidelines for urolithiasis primarily focus on the prevention, diagnosis, and medical or surgical management of stone disease [3]. The management of urolithiasis typically encompasses either the disintegration of stones or their dissolution using medicinal preparations that can induce pain, discomfort, and lead to additional costs for patients. Accordingly, the prevention of kidney stone formation presents a more practical and economically feasible option.
Cholelithiasis, another non-infectious disease characterized by the stone formation, also results in substantial management costs. This disease demonstrates a high prevalence rate in Western countries and may lead to comorbidities such as cholecystitis, gall bladder cancer, and pancreatitis. Risk factors related to cholesterol gallstones include dietary habits, lifestyle features, comorbid conditions, and certain medications. These factors have the potential to disrupt the homeostasis of bile, cholesterol, and phospholipids, thus contributing to the formation of cholesterol gallstones. Treatment approaches, whether surgical or non-surgical, carry a risk of complications and can result in high treatment costs [4].
The treatment of urolithiasis and cholelithiasis highly complex and often involves substantial expenses. A prospective approach to manage these conditions effectively may focus on the prevention of stone formation. Desmodium styracifolium, a medication in Chinese medicine, shows promise as a potential mean for the preventive and treatment of urolithiasis and cholelithiasis [5].
The purpose of this inquiry is to provide a conprehensive summary and analysis of the literature concerning the pharmacological properties of components that can be extracted from Desmodium styracifolium. Furthermore, this study aims to explore their potential application in elaborating medicinal products for the effective prevention and treatment of such conditions as urolithiasis, cholelithiasis, type 2 diabetes mellitus, metabolic syndrome, pro-oxidant and inflammatory processes, etc.
A comprehensive search was carried out through electronic databases such as PubMed, Scopus, Web of Science, and the Cochrane Library to identify the relevant literature sources. Traditional manual searching was applied to determine the relevant reference lists using the key terms and phrases associated with our research question: “Desmodium styracifolium”; “apigenin”; “genistein”; “kaemferol”; “luteolin”; “quercetin”; “shaftoside”; “styracifoline”; “polyphenols” OR “phenolic acids” OR “flavonoids” AND “transcription factors”; “polyphenols” OR “phenolic acids” OR “flavonoids” AND “urolithiasis” OR “cholelithiasis” OR “inflammatory response” OR “metabolic disorder” OR “diabetes mellitus”, etc. All experimental and clinical studies, alongside review articles, editorials, patent documentation, and case reports, were included without any restrictions on publication dates; our search yielded 364 sources (315 in English, 49 in other languages). Additionally, textbooks and various reference materials in the domains of pharmacology, pharmacy, ethnopharmacology, traditional oriental medicine, as well as clinical and molecular medicine were scrutinized. The figures were created using the vector graphics editor CorelDRAW 11.
2. Botanical and ethnopharmacological characteristics of the genus Desmodium
Appreciating the place of Desmodium styracifolium in the plant kingdom can contribute to revealing its potential as a source of medicinal substances or as a stand-alone remedy. Investigating the history of Desmodium styracifolium usage in traditional medicine across different countries can offer valuable insights for its applications in medical science and clinical practice nowadays.
The genus Desmodium, a large representative of the legume family (Fabaceae), comprises approximately 350 species that are typically found in tropical and subtropical regions worldwide, with 28 species recorded in China [6]. These plants are mostly herbaceous perennials or deciduous shrubs and subshrubs, with digitate or pinnate leaves and loose clusters of small, pea-like pink or white flowers that appear in late summer and fall. In particular, Desmodium styracifolium (Osbeck) Merr. is a semi-shrub with a cylindrical stem up to 1 m tall, densely covered with yellow ample pubescence. The leaves are alternate, compound, uni- or trifoiliate, ellipticl or elongated, 2–4 cm in diameter; there is a notch at the top and a heart-shaped or bluntly rounded base, and solid edges. The upper surface of the leaves is yellowish-green or greyish-green and glabrous, while the lower surface is densely covered with greyish-white hair. The lateral veins are pinnate, the petiole is 1–2 cm long, and the stipules are lanceolate and approximately 8 mm-long. This plant has a slightly aromatic smell and a slightly sweet taste [7,8].
Desmodium styracifolium (Osbeck) Merr. can be found growing in natural conditions on mountain slopes, meadows, and thickets up to an altitude of 1 km in various regions in China (the provinces of Fujian, Guangdong, Guangxi, Hainan, Hubei, and Yunnan); it also grows in the mountainous regions of Vietnam, India (in the states of Assam, Karnataka, Kerala, Meghalaya, and Sikkim), Bangladesh, Sri Lanka, Thailand, Laos, Cambodia, Myanmar, and Malaysia [7,8].
More that 20 species of the Desmodium genus have been applied over 3000-year history of traditional Eastern medicine, particularly in Chinese and Vietnamese cultures, and according to its theory, they can "alleviate internal heat or fever, neutralize toxins and promote blood circulation" [8]. In China, aqueous decoction of Desmodium plants has been widely used to treat a wide range of illnesses including rheumatism, fever, asthma, typhoid fever, dysentery, wound process, malaria, hepatitis, hemoptysis, etc. [5]. D. styracifolium (广东金钱草, guǎng dōng jīn qián cǎo in Chinese) is a traditional Chinese herbal remedy (Table 1) included in the Chinese Pharmacopoeia. This plant is also used to treat various conditions, including urolithiasis, bladder diseases, stranguria (painful and slow urination caused by bladder and urethra spasms), bile stasis, and inflammatory diseases [5,7,[9], [10], [11], [12]].
Table 1.
The use of the Desmodium styracifolii plants according to traditional Chinese medicine (based on [5,7,[9], [10], [11], [12]]).
| Taste | Slightly sweet |
|---|---|
| External pathogenic factor | Cold |
| Channels entered | Orbis hepaticus, orbis renalis et vesicalis |
| Effects | To ease stranguria, disinhibit urine, and drain moisture in order to reduce jaundice. |
| Symptoms and indications | Diseases of the urinary tract (urolithiasis, haematuria, slow and painful urination, oedema, oliguria), hepatitis, jaundice, bile stasis, rheumatism, fever, dysentery, wound process, cough, malaria, haemoptysis, stomatitis, laryngitis, urticaria. |
| Method of administration and dosage: | The whole plant is used per os in the form of a decoction to treat urinary tract infections (24 g), urolithiasis (24–60 g), cholecystitis (30 g), stomatitis and laryngitis (15–30 g), and urticaria (60 g). For the treatment of infantile hypotrophy, it is used together with pork food. For the management of mastitis, topical application of a poultice from the whole plant is recommended. |
The China's National Medical Products Administration (NMPA) has recently granted marketing approval for D. styracifolium flavonoids capsule, recognizing it as an innovative product based on Chinese traditional medicine. However, it is worth noting that the outcomes of the randomized, double-blind, and placebo-controlled multicenter clinical trials, which aimed to assess the safety and effectiveness of this medicine, have solely been posted on the NMPA newsletter and have not been independently validated within the scientific literature [13]. The findings revealed significant statistical distinctions between the group receiving a placebo and the group receiving treatment, indicating that the medication could be appropriate for managing ureteral calculi in patients with symptoms characterized by the presence of accumulated "damp-heat".
In traditional Vietnamese medicine, D. styracifolium is a medicinal herb used to treat urinary tract infections, fever, kidney stones, cardiovascular diseases, and hepatitis [14].
Experimental studies have demonstrated that extracts of Desmodium plants have a wide range of pharmacological properties, including anti-inflammatory, anti-parasitic, anti-diabetic, litholytic, antibacterial, nootropic effects; they also improve cardiovascular and cerebrovascular functions and regulate the immune system [5,[15], [16], [17], [18], [19]].
Thus, D. styracifolium is used in traditional oriental medicine mainly for the treatment of urinary tract diseases (urolithiasis, haematuria, slow and painful urination, and oliguria), as well as for biliary stasis, hepatitis, and inflammatory diseases, but the lack of clinical trials studying the medicines derived from this plant and being performed in conformity with the requirements of evidence-based medicine leaves some issues unclear.
3. Phytochemical studies
Prior to considering Desmodium styracifolium as a stand-along therapeutic agent or as a potential source of medicinal compounds, it is essential to conduct a thorough analysis of the chemical compounds found in D. styracifolium. This analysis should focus on their respective concentrations and an assessment of their therapeutic potential based on up-to-date medical knowledge.
The use of Desmodium plants in traditional medicine has encouraged further phytochemical research. Scientists have isolated more than 200 compounds from representatives of this genus, including flavonoids, alkaloids, steroids, terpenoids, phenylpropanoids, and other components. However, only a portion of these compounds have been evaluated for biological activity. Polyphenols and alkaloids are considered the primary components responsible for the majority of the pharmacological effects of plants in this genus [5,15,[20], [21], [22]]. Table 2 demonstrates a list of compounds isolated from plants of the D. styracifolium species, according to literature data [5,14,[23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40]]. The greatest importance in the phamacological action of D. styracifolium have following polyphenols: apigenin (IUPAC: 5,7-dihydroxy-2-(4-hydroxyphenyl)chromen-4-one), genistein (IUPAC: 5,7-dihydroxy-3-(4-hydroxyphenyl)chromen-4-one), kaemferol (IUPAC: 3,5,7-trihydroxy-2-(4-hydroxyphenyl)chromen-4-one), luteolin (IUPAC: 2-(3,4-dihydroxyphenyl)-5,7-dihydroxychromen-4-one), quercetin (IUPAC: 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxychromen-4-one), and shaftoside (IUPAC: 5,7-dihydroxy-2-(4-hydroxyphenyl)-6-[(2R,3R,4R,6S, 7R)-3,4,6-trihydroxy-7-(hydroxymethyl)oxan-2-yl]-8-[(2S,3R,4S, 6S)-3,4,6-trihydroxyoxan-2-yl]chromen-4-one).
Table 2.
Chemical components isolated from Desmodium styracifolium plants.
| No | Chemical component (MeSH entry term or IUPAC name) | Part of plant | References |
|---|---|---|---|
| Flavonoids | |||
| 1. | 2,3-Trans-3,5,7,2′,4′-pentahydroxy-flavanone | Aerial parts | [7,27] |
| 2. | 3-O-acetyl (−)-epicatechin | Seeds | [25] |
| 3. | 3′-O-methyl epicatechin | Seeds | [25] |
| 4. | 6-C-glycopyranosyl-8-C-arabinosyl apigenin | Whole plant | [7,26] |
| 5. | 6-C-glycopyranosyl luteolin | Whole plant | [7,26] |
| 6. | 6-C-glycopyranosyl-8-C-glycopyranosyl apigenin | Whole plant | [26] |
| 7. | 6-C-glyeopyranosyl-8-C-xyvloeyl apigenin | Whole plant | [26] |
| 8. | Acacetin | Aerial parts | [40] |
| 9. | Afzelin | Seeds | [25] |
| 10. | Ambonin | Aerial parts | [7,37] |
| 11. | Apigenin | Whole plant/Aerial parts | [7,26,28,29] |
| 12. | Astragalin | Aerial parts | [7,37] |
| 13. | Catechin | Aerial parts/Seeds | [25,29] |
| 14. | Chrysoeriol | Aerial parts | [7,27,29] |
| 15. | Diosmetin | Aerial parts | [40] |
| 16. | Diosmin | Aerial parts | [40] |
| 17. | Hesperidin | Aerial parts | [40] |
| 18. | Homoadonivernite | Whole plant | [7,30] |
| 19. | Hydnocarpin D | Aerial parts | [7,27] |
| 20. | Isoorientin | Aerial parts | [7,31,33,37] |
| 21. | Isoorientin 3′-O-methyl ether | Aerial parts | [7,37] |
| 22. | Isorhamnetin | Aerial parts | [29] |
| 23. | Isoschaftoside | Aerial parts | [7,32,33,37] |
| 24. | Isovitexin | Aerial parts | [7,[30], [31], [32], [33],37] |
| 25. | Kaemferol | Aerial parts | [7,27,29] |
| 26. | Kaempferol-7-O-βD-glucopyranoside | Seeds | [25] |
| 27. | Katuranin | Aerial parts | [7,27] |
| 28. | Luteolin | Aerial parts | [[26], [27], [28], [29]] |
| 29. | Luteolinidin | Aerial parts | [29] |
| 30. | Myricetin | Aerial parts | [29] |
| 31. | Orientin | Aerial parts | [7,37] |
| 32. | Quercetin | Aerial parts | [7,27,29] |
| 33. | Quercetin 3–O-β-d-glucopyranoside (isoquercitrin) | Aerial parts/Seeds | [7,25,37] |
| 34. | Rutin | Aerial parts | [29] |
| 35. | Schaftoside | Whole plant/Aerial parts | [7,8,[29], [30], [31], [32], [33],37] |
| 36. | Vicenin 1, 2, 3 | Whole plant/Aerial parts | [7,[29], [30], [31], [32]] |
| 37. | Vitexin | Whole plant/Aerial parts | [7,29] |
| Isoflavonoids and Coumaronochromones | |||
| 38. | 2′-Hydroxygenistein | Aerial parts | [7,27] |
| 39. | 3,9-Dihydroxypterocarpan | Whole plant | [7] |
| 40. | 3,5,7,4′-Tetrahydroxycoumaronochromone | Aerial parts | [7,27] |
| 41. | 5,7,4′-Trihydroxycoumaronochromone | Aerial parts | [7,27] |
| 42. | 5,7-Dihydroxy-2′,3′,4′-trimethoxy-isoflavanone | Aerial parts | [7,27,37] |
| 43. | 5,7-Dihydroxy-2′-methoxy-3′,4′-methylenedioxyisoflavanone | Aerial parts | [7,27] |
| 44. | 5,7-Dihydroxy-2′,3′,4′-trimethoxy-isoflavanone7-O-β-glucopyranoside | Aerial parts | [7,27] |
| 45. | 5,7-Dihydroxy-2-methoxy-3′,4′-methylenedioxyisoflavanone7-O-β-glucopyranoside | Aerial parts | [7,27] |
| 46. | 5,7-Dihydroxy-2′,4′-dimethoxy-isoflavanone 7-O-β-glucopyranoside |
Aerial parts | [7,27] |
| 47. | 5,7,4′-Trihydroxy-2′,3′-dimethoxy-isoflavanone 7-O-β-glucopyranoside |
Aerial parts | [7,27] |
| 48. | 7,4′-Dihydroxy-3′-methoxy-isoflavone | Aerial parts | [7,27] |
| 49. | Daidzein | Aerial parts | [29] |
| 50. | Dalbergiodin | Aerial parts | [7,27] |
| 51. | Desmoxyphyllin A | Aerial parts | [7,27] |
| 52. | Formononetin | Aerial parts | [7,27] |
| 53. | Genistein | Aerial parts | [7,[27], [28], [29],37,40] |
| 54. | Genistein-7-O-β-d-apiofuranosyl-(1 → 6)-O-β-d-glucopyranoside (ambocin) | Aerial parts | [37] |
| 55. | Genistin | Aerial parts | [37,40] |
| 56. | Homoferreirin | Aerial parts | [7,27,37] |
| 57. | Isoferreirin | Aerial parts | [7,27] |
| 58. | Orobol | Aerial parts | [7,27] |
| 59. | Panchovillin | Aerial parts | [7,37] |
| 60. | Secundiflorol H | Aerial parts | [7,27] |
| Alkaloids | |||
| 61. | (3α,4β,5γ)-4,5-Dihydro-4,5-dimethyl-3 (1-pyrrolyl)-furan-2(3H)-one | Whole plant/Aerial parts | [7,34] |
| 62. | Desmodimine | Whole plant/Aerial parts | [7,23] |
| 63. | Desmodilactone | Aerial parts | [7,14,23,37] |
| 64. | Styracifoline | Aerial parts | [14] |
| Terpenoids | |||
| 65. | (3α,4β,5γ)-4,5-dihydro-4,5-dimethyl-3 (1-pyrrol)-furan-2(3H)-one | Whole plant | [7] |
| 66. | (23Z)-9,19-cycloart-23-ene-3β,25-diol | Aerial parts | [35] |
| 67. | Lupeol | Aerial parts | [7,27] |
| 68. | Lupenone | Aerial parts | [7,24,27] |
| 69. | Sophoradiol | Aerial parts | [7,27] |
| 70. | Soyasapogenol B | Whole plant/Aerial parts | [7,36] |
| 71. | Soyasaponin I | Whole plant | [7,37,38] |
| 72. | Soyasapogenol E | Whole plant | [7,37,38] |
| Steroides | |||
| 73. | β-Dauosterol | Whole plant | [7,26] |
| 74. | β- Sitosterol | Whole plant | [7,26] |
| 75. | τ-Sitosterol | Whole plant/Aerial parts | [7,24] |
| 76. | Stigmasterol | Aerial parts/Seeds | [24,25] |
| 77. | Stigmasterol-3-O-β-d-glucopyranoside | Whole plant | [7,26] |
| Phenolic Acids and Phenylpropanoids | |||
| 78. | 3,4-Dimethoxyphenol | Leave/stem | [34] |
| 79. | Caffeic acid | Aerial parts | [29,40] |
| 80. | Chlorogenic acid | Whole plant/Aerial parts | [7,23,29] |
| 81. | Cimicifugic acid | Aerial parts | [7,27] |
| 82. | Ferulic acid | Aerial parts | [7,29,34] |
| 83. | Gallic acid | Aerial parts | [29] |
| 84. | Protocatechuic acid | Aerial parts | [29] |
| 85. | Salicylic acid | Leave/stem | [7,34] |
| 86. | Vanillaic acid | Leave/Stem | [7,34] |
| Volatile Oils | |||
| 87. | 3,7,11,15-Tetramethyl-2-hexadecon-1-ol | Whole plant/Aerial parts | [7,24] |
| 88. | 4,8,12,16-Tetramethylheptadecan-4-olide | Whole plant/Aerial parts | [7,24] |
| 89. | 6,10,14-Trimethyl-2-pentadecanone | Whole plant/Aerial parts | [7,24] |
| 90. | 9,12-Octadecadienoic acid | Whole plant/Aerial parts | [7,24] |
| 91. | Eicosanoic acid | Whole plant/Aerial parts | [7,23,24] |
| 92. | Eicosanoic acid ethylester | Whole plant/Aerial parts | [7,24] |
| 93. | Eicosyl ester | Whole plant/Aerial parts | [7,23] |
| 94. | Heptadecanoic acid | Whole plant/Aerial parts | [7,24] |
| 95. | n-Hexadecanoic acid | Whole plant/Aerial parts | [7,24] |
| 96. | Hexadecanoic acid ethylester | Whole plant/Aerial parts | [7,24] |
| 97. | Hexadecanoic acid methylester | Whole plant/Aerial parts | [7,24] |
| 98. | Isophytol | Whole plant/Aerial parts | [7,24] |
| 99. | Octadecanoic acid | Whole plant/Aerial parts | [7,24] |
| 100. | Octadecanoic acid ethylester | Whole plant/Aerial parts | [7,24] |
| 101. | Octadecanoic acid methylester | Whole plant/Aerial parts | [7,24] |
| 102. | Oxalic acid | Leave/Stem | [7,34] |
| 103. | Pentadecanoic acid | Whole plant/Aerial parts | [7,24] |
| 104. | Phytol | Whole plant/Aerial parts | [7,24] |
| 105. | Tetradecanoic acid | Whole plant/Aerial parts | [7,24] |
| 106. | Tritriacontane | Whole plant/Aerial parts | [7,23] |
| Others | |||
| 107. | 3-Hydroxy-2-methyl-4-pyrone | Seeds | [25] |
| 108. | Benzoic acid | Seeds | [25] |
| 109. | Dibutyl phthalate | Seeds | [25] |
Phytochemical analysis of D. Styracifolium has revealed a wide array of chemical compounds of high medicinal relevance, including polyphenols, alkaloids, terpenoids, as well as other substances. This analysis suggests that polyphenols can be of particular interest due to their potential as therapeutic agents, given their capacity to influence diverse signalling pathways.
4. Antiurolytic action
The presence of polyphenols with established antiurolytic properties in D. styracifolium, supported with the results of in vitro studies, indicates the potential effectiveness of pharmaceuticals derived from this plant in the therapy for urolithiasis.
This disease affects approximately 12 % of the world's population, with a high recurrence rate in both men (70–80 %) and women (47–60 %). Standard medications used for the prevention and treatment of urolithiasis, such as uricosuric, diuretic, and hypoazotemic agents, do not always show universal effectiveness and may cause side effects [41]. Recent experimental and clinical studies have confirmed that biologically active compounds, such as flavonoids, phenolic acids, phytosterols, saponins, furanochromones, alkaloids, and terpenoids can effectively suppress the formation of calcium-oxalate urinary calculi (the most common kidney stones) [[41], [42], [43]]. These compounds found in the chemical composition of various plants, demonstrate litholytic, lithokinetic, antispasmodic, diuretic, antimicrobial, antioxidant, and anti-inflammatory properties [43,44], which can inhibit lithogenesis in the urinary system, and in particular, the processes of crystallization, nucleation and aggregation of crystals [44,45]. Based on their systematic review of 163 original articles retrieved from the PubMed database, A. Khan et al. concluded that the antiurolytic effect of herbal preparations stems from their antioxidant, anti-inflammatory, and/or diuretic effects [46].
D. styracifolium has also been reported to reduce calcium oxalate deposits in the kidneys [50] and to alleviate the effects of tissue damage by crystals due to its anti-inflammatory and antioxidant properties [51]. J. Mi et al. attributed the beneficial effects of D. styracifolium as a means of preventing the formation of oxalate kidney stones to an increase in urinary citrate excretion, a decrease in calciumuria, an increase in diuresis, and an antioxidant effect [52].
Currently, there are numerous plant-derived phytotherapeutic agents with antiurolytic properties, including preparations from plants such as Aerva lanata (Linn), Ammi visnaga (L.), Argemone mexicana L., Berberis trifoliata Hartw. ex Lindl., Chenopodium album L., Costus spicatus Jacq., Duranta erecta L., Eysenhardtia polystachya (Ortega) Sarg., Selaginella lepidophylla (Hook. & Grev.), and Taraxacum officinale L., among others [42,43,[46], [47], [48], [49]].
Desmodium styracifolium has also demonstrated potential in reducing calcium oxalate deposits in the kidneys [50] and mitigating the effects of tissue damage caused by crystals due to its anti-inflammatory and antioxidant properties [51]. According to J. Mi et al., the beneficial effects of D. styracifolium in preventing the formation of oxalate kidney stones can be attributed to increased urinary citrate excretion, decreased calciumuria, increased diuresis, and an antioxidant effect [52].
It is noteworthy that pharmacokinetic studies of 5 active ingredients of D. styracifolium (shaftoside, vicenin-1, vicenin-2, vicenin-3, and isovitexin) have shown that their plasma levels significantly increase in rats exposed to modelled urolithiasis [53].
However, only recently some reports elucidating the specific molecular targets and therapeutic mechanisms of D. styracifolium have been published. Through a proteomic analysis of kidney cells from C57BL/6 mice and Sprague-Dawley rats, as well as the HK-2 cell line following experimental kidney damage induced by oxalate, these studies have demonstrated that the administration of D. styracifolium extract significantly reduces the increased expression of cathepsin D, p38 mitogen-activated protein kinase (p38 MAPK), and cyclin-dependent kinase 2 (CDK-2) in the experimental models [28]. These changes can be attributed to the action of flavonoids and isoflavonoids (luteolin, apigenin, and genistein).
The reduced expression of cathepsin D and p38 MAPK is indicative of the inhibition of autophagy leading to a reduction in the deposition of calcium oxalate crystals and subsequent development of oxidative damage in rat kidneys. Other researchers have also confirmed that D. styracifolium flavones facilitate apoptosis and autophagy of HK-2 cells through the p38 MAPK-dependent signalling pathway [54]. Autophagy activation and oxidative stress associated with p38 MAPK play an important role in the mechanisms of crystal-induced damage to renal tubular epithelial cells [55,56]. Lysosomal degradation involving cathepsin D is also considered to be a key process in autophagy [57]. Additionally, inhibiting cathepsin D expression can reduce renal fibrosis and slow down the progression of obstructive nephropathy [58]. Increased activity of CDK-2, which controls the cell cycle, has also been previously identified in renal failure [59]. Therefore, the active components of D. styracifolium serve as reliable protectors of tubular cells against oxalate-induced renal damage.
The literature reports that certain chemical constituents of D. styracifolium exhibit antiurolytic actions. For example, soyasaponin I isolated from D. styracifolium, when administered at a daily dose of 0.6 mg/kg for 3 weeks in a rat model of calcium-oxalate lithogenesis, significantly reduced the formation of urinary stones by 29 % compared to the control group (81 %). Researchers have attributed the inhibition of calculus formation in the kidneys of rats to increased diuresis, decreased calcium excretion, and increased urinary citrate excretion resulting from soyasaponin I administration [5].
The administration of apigenin to rats also reduces the formation of urinary stones in experimental urolithiasis [60]. Furthermore, apigenin has been shown to reduce the formation of malondialdehyde, a secondary product of lipid peroxidation, and enhance the activity of antioxidant enzymes in kidney homogenate. This leads to a significant decrease in the number of calcium oxalate crystals in urine samples from rats with ethylene glycol-induced hyperoxaluria, as confirmed by pathological studies. Although the main mechanism of apigenin action is still unclear, the authors suggest that it can reduce the content of stone-forming components, increase antioxidant activity, and suppress the expression of transforming growth factor β [60].
Similarly, another flavonoid, kaempferol, has been found to reduce the deposition of calcium oxalate crystals in renal tubules [61]. Moreover, kaempferol can also lessen crystal-mediated cell damage, oxidative stress, inflammation, and overexpression of the matrix glycoprotein osteopontin and CD44 in the kidneys, while suppressing androgen receptor expression in tubular epithelial cells. The authors have identified the mechanism by which kaempferol can reduce calcium oxalate crystal deposition and the resulting oxidative and inflammatory damage to the kidneys through negative regulation of the androgen receptor-leukocyte NADPH oxidase (Nox 2) signalling pathway.
Researchers consider quercetin as a promising medicine able to suppress the formation of oxalate calculi resulting from hyperoxaluria, particularly in patients with recurrent urolithiasis. In rats exposed to reproducible hyperoxaluria, the administration of quercetin has been found to reduce malondialdehyde formation, increase catalase and superoxide dismutase activity [62,63], and inhibit signalling pathways that initiate oxidative processes and lead to the formation of kidney stones, particularly p38 MAPK [64].
This evidence of the anti-urolithic effect of individual polyphenols aligns with studies on the D. styracifolium bioflavonoid complex's impact on lithogenesis processes in the kidneys (Fig. 1). The complex of these compounds has been shown to effectively inhibit the formation of calcium oxalate crystals in rat kidneys by combining antioxidant, anti-inflammatory effects, alkalinization of urine, and a decrease in the concentration of components that form urinary stones [65]. All of these findings have significant clinical implications for preventing oxidative damage to nephrocytes and, ultimately, the formation of kidney stones. The authors' results support the use of D. styracifolium bioflavonoids for therapy of urolithiasis and suggest this plant species as a potential source of new anti-urolithic medication.
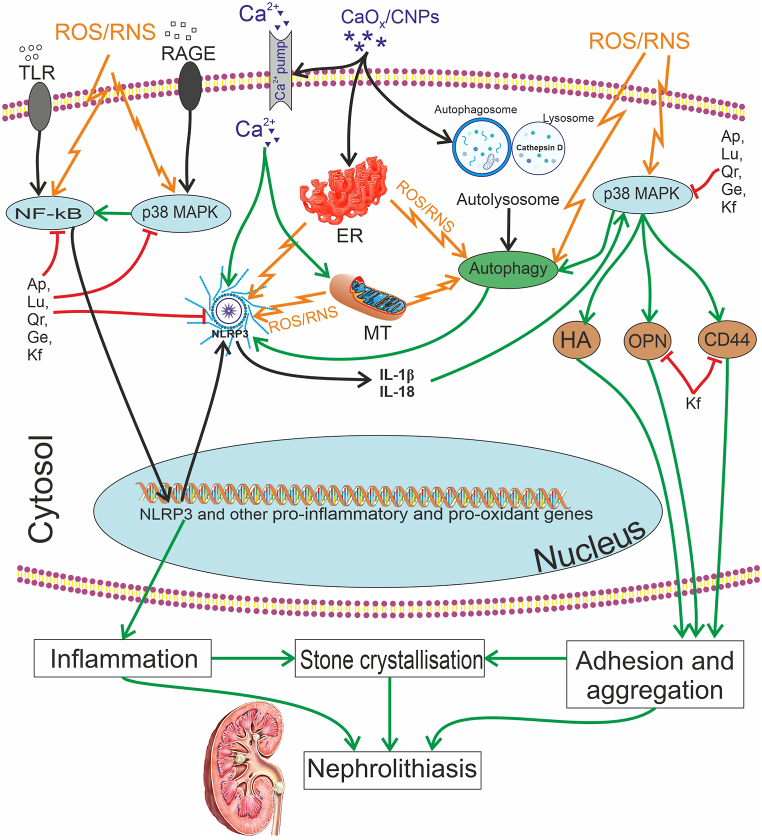
Fig. 1.
Molecular mechanisms of the anti-urolithic effect of D. styracifolium polyphenols. These compounds influence the key links of nephrolithiasis pathogenesis: they reduce the ROS/RNS-dependent nuclear translocation of NF-κB and p38 MAPK activation, as well as inhibit the NLRP3 inflammasome, both directly and by suppressing the autophagy signalling pathway. As a result, the expression of pro-oxidant and pro-inflammatory genes (Cyp7b, Cyp2C11, Cyp2E1, gp91phox, inducible NO-synthase, tumour necrosis factor-α, interleukins 1β and 6, chemokines, cathepsins, phospholipase A2, cyclooxygenase 2, etc.) decreases, along with the attenuation of inflammasome-dependent inflammatory cytokine activation. This, in turn, interrupts positive feedback loops that amplify the production of ROS/RNS and other inducers of NF-κB, p38 MAPK, and autophagy. Moreover, several polyphenols have the ability to inhibit p38 MAPK and its dependent expression of hyaluronic acid, osteopontin, and CD44, thereby altering the adhesion of renal tubular epithelial cells to calcium oxalate crystals and lowering intensity of crystallization and nephrolithiasis. Note: CaOx – Calcium oxalate; CNP – Calcifying nanoparticles; ER – endoplasmic reticulum; HA - Hyaluronic acid; IL – Interleukin; MT-mitochondrion; NF-κB – Nuclear Factor κB; NLRP3 – NOD-like receptor pyrin domain-containing protein 3; OPN – Osteopontin; ROS – Reactive Oxygen Species; RNS – Reactive Nitrogen Species. Polyphenols: Ap – Apigenin; Ge – Genistein; Kf – Kaemferol; Lu – Luteolin; Qr – Quercetin.
Several experimental studies confirmed the potential of D. styracifolium to influence mineral metabolism, to decrease the concentration of constituents involved in the formation of urinary calculi, and to reduce mineral encrustation in the urinary tract, as well as to alleviate the damage caused by crystal structures. This beneficial impact is achieved through a combination of antioxidant and anti-inflammatory actions, along with urine alkalinization. The molecular mechanisms of D. styracifolium's antiureolytic action consist in the inhibition of autophagy in renal cells that is accompanied with a decrease in the deposition of calcium oxalate crystals and subsequent oxidative damage. Chemical constituents of D. styracifolium, such as flavones (luteolin, apigenin, vicenin, shaftoside, and isovitexin), flavonoids (kaempferol and quercetin), isoflavones (such as genistein), and terpenoids (soyasaponin) have been shown to possess antiureolytic effects.
5. Cholelytic, cholagogic and hepatoprotective actions
Based on data from phytochemical studies of D. styracifolium, the presence of various polyphenolic compounds suggests that D. styracifolium may possess cholelytic activity. Additionally, considering the other properties of polyphenolic compounds, it can be assumed that preparations derived from D. styracifolium will demonstrate hepatoprotective properties as well.
Recent studies have highlighted the therapeutic potential of shaftoside, a flavonoid isolated from D. styracifolium, in the treatment of cholelithiasis that is attributed to its capacity to inhibit the formation of cholesterol stones in the gallbladder [66,67]. In a study by M. Liu et al., mice treated with shaftoside appeared to be protected against gallstones and demonstrated increased levels of bile salts in bile and decreased cholesterol concentration that led to lower cholesterol saturation index [67]. Furthermore, the serum concentrations of triacylglycerols and cholesterol in animals decreased, and the expression of mRNA of liver Xα receptor (LXRα), ATP-binding cassette transporter 5/8 (ABCG5/8), bile acid binding protein (IBABP), farnesoid X receptor (FXR), and bile salt export protein (BSEP) in the liver and ileum was significantly increased (Fig. 2). According to the researchers, these findings are attributed to the ability of shaftoside to inhibit the formation of cholesterol gallstones through the activation of LXRα and FXR. Taken together, these results suggest that the flavonoid shaftoside isolated from D. styracifolium possesses cholelytic, cholagogic, and hepatoprotective activities and could be a potential treatment for gallstone disease.
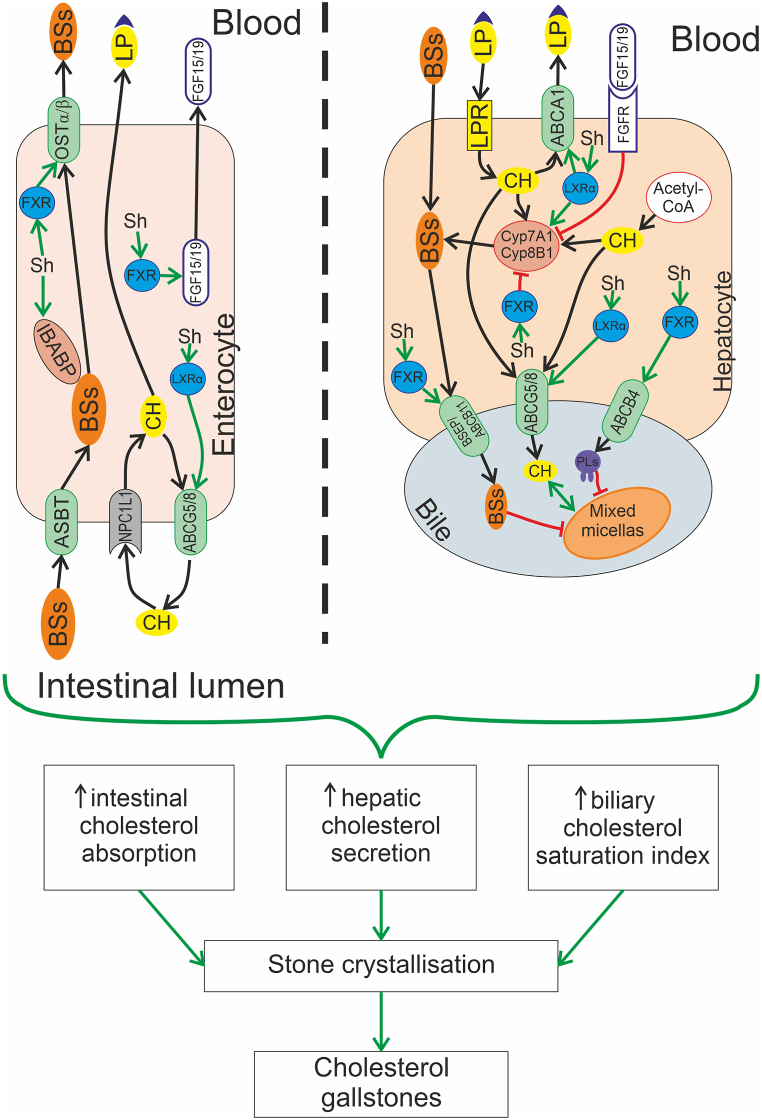
Fig. 2.
Molecular mechanisms of the protective action of flavonoid shaftoside, isolated from D. styracifolium, in cholelithiasis. This action of shaftoside (Sh) is associated with its ability to activate nuclear receptors in the liver and the small intestine, such as farnesoid X receptor (FXR) and liver Xα receptor (LXRα). Activation of FXR up-regulates bile salts (BSs) concentration by promoting the expression of OSTα/β transporter and fibroblast growth factor 15/19 (FGF15/19) in the ileum, as well as increasing the expression of bile salt export protein (BSEP)/ABCB11 and ABCB4 transporter in the liver. Activation of LXRα enhances cholesterol efflux from the gut and the liver by stimulating ABCG5/8 and ABCA1 transporters. Additionally, LXRα activates Cyp7A1/Cyp8B1, increasing the liver's production of BSs. Thus, shaftoside affects critical steps in the development of cholelithiasis: it reduces intestinal cholesterol absorption and hepatic cholesterol secretion. These changes, along with an increase in BSs or phospholipids (PLs) contents in the bile, lead to a decrease in the biliary cholesterol saturation index and prevention of cholesterol precipitation and crystallization. As a result of this action, shaftoside impedes the formation of cholesterol stones in the gallbladder.
LXRα belongs to the hepatic X receptor family of transcription factors, which play a central role in regulating cholesterol homeostasis and lipid synthesis [68,69]. These receptors control hepatic lipogenesis mainly by mediating the expression of SREBP-1 (sterol regulatory element-binding protein 1) and its target genes for synthesizing fatty acids, including acetyl-CoA carboxylase, fatty acid synthase, and stearoyl-CoA desaturase-1 [70]. In the liver, LXRα directly induces cytochrome 7A1 (CYP7A1), promoting the conversion of cholesterol to bile acids [71]. As a result, hepatic X receptors could be promising targets for treating common human diseases, such as obesity, diabetes mellitus, neurodegenerative diseases, and chronic inflammatory conditions [72,73]. However, synthetic agonists developed for LXRα activation induce hepatic lipogenic enzymes that results in increased synthesis and accumulation of triacylglycerols. This phenomenon diminishes the effectiveness of pharmacotherapy for cardiovascular diseases [71]. Natural flavonoids, such as shaftoside isolated from D. styracifolium, do not cause such negative effects [74].
Histological studies have confirmed the hepatoprotective properties of D. styracifolium [67]. This plant is an effective therapeutic agent for acetaminophen-induced hepatotoxicity through modulating oxidative stress and inflammation, which is also associated with FXR activation by shaftoside [75]. FXR is a therapeutic target for the treatment of non-alcoholic fatty liver disease due to its regulatory role in lipid metabolism. Shaftoside reduces liver cell damage and lipid accumulation induced by a high-fat diet, as indicated by a decrease in aspartate aminotransferase activity, cholesterol and triacylglycerols in the blood serum, and by the concentration of the latter in liver tissue. Gene expression profiles demonstrated that shaftoside prevents the diet-induced decrease in FXR expression in a dose-dependent manner [74]. It is known that activation of FXR (e.g., with ursodeoxycholic acid) is one of the ways to ensure secretory choleresis, as FXR regulates proliferation, differentiation and secretory activity of cholangiocytes [76].
To summarise, recent studies have experimentally confirmed the cholelitholytic, choleretic and hepatoprotective effects of D. styracifolium plants, primarily associated with the activation of the hepatic Xα receptor and the bile acid receptor, farnesoid X receptor, by the flavonoid shaftoside.
6. Antioxidant, anti-inflammatory and anti-diabetic effects
The recent discovery of polyphenols in different parts of the D. styracifolium plant, which have been confirmed to possess antioxidant, anti-inflammatory, and anti-diabetic properties, indicates their potential effectiveness in treating pro-oxidant and inflammatory conditions, as well as type 2 diabetes mellitus. These polyphenols act through specific intracellular signalling pathways, as supported by in vitro study results.
The 95 % alcohol extract of D. styracifolium has demonstrated significant antioxidant action as assessed by the photometric method. The extract was found to scavenge 60.7 % of hydroxyl radicals, 87.5 % of superoxide anion radicals, 21.1 % of cigarette smoke free radicals, and inhibit lipid peroxidation by 31.6 % [77]. Additionally, extracts from D. styracifolium seeds have shown strong potential for antioxidant activity, particularly in scavenging hydroxyl and superoxide radicals [78].
The extract of D. styracifolium has an inhibitory effect on lipid peroxidation that is comparable to ascorbic acid. However, its ability to scavenge hydroxyl radicals and reduce Fe (III) is inferior to that of ascorbate, as stated in Xia et al. [79]. Although phenolic compounds are present in the extract, their reducing capacity is low. Therefore, it is evident that the antioxidant properties of the extract may be attributed to other compounds, such as bioflavonoids. The quantitative and qualitative composition of bioflavonoids in the extract is higher than that of phenolic acids.
Recent studies have demonstrated that most flavonoids have the ability to inhibit the activation of nuclear factor-kappa B (NF-κB) by modulating the IκB-kinase complex (IKK)/IκB/NF-κB inhibitory protein axis (Fig. 3). This inhibition leads to a reduction in oxidative-nitrosative stress in various organs, as indicated by research findings [[80], [81], [82], [83], [84]]. The activation of NF-κB is responsible for the upregulation of a number of genes encoding pro-oxidant and pro-inflammatory proteins, such as gp91 phox, xanthine oxidoreductase, cyclooxygenase 2, lipoxygenase 5 and 12, monooxygenases Cyp7b, Cyp2E1, Cyp2C11, inducible and neuronal isoforms of NO synthase, histolytic enzymes such as collagenase, stromelysin, gelatinase B, among others [85,86].
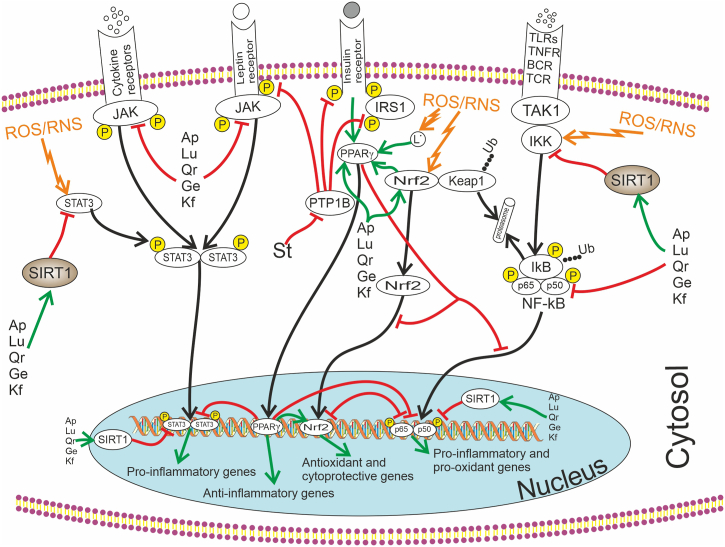
Fig. 3.
The molecular mechanisms of the antioxidant, anti-inflammatory, and anti-diabetic effects of certain chemical components present in D. styracifolium. On one hand, all phenolic compounds have the ability to scavenge reactive oxygen and nitrogen species, thereby preventing their activating influence on redox-sensitive transcription factors (NF-κB, STAT3, Nrf2, etc.). Flavonoids and isoflavonoids (Ap – Apigenin; Ge – Genistein; Kf – Kaemferol; Lu – Luteolin; Qr – Quercetin) inhibit NF-κB both through IκBα and by activating SIRT1, Nrf2, and PPARγ signalling. Influence of polyphenols on the pro-inflammatory and pro-oxidant cascades is mitigated by their ability to suppress the JAK-STAT3 signalling. Induction of Nrf2 and PPARγ enhances the expression of antioxidant, anti-inflammatory, and cytoprotective genes. Styracifoline (St) exhibits anti-inflammatory and anti-diabetic effects by binding to the leptin receptor and its dephosphorylation, and inhibiting PTP1B, which is responsible for insulin resistance through IRS-1 blocking. This alkaloid activates STAT3 as well. Note: BCR – B Cell Receptor: IκB – NF-κB Inhibitory Protein; IKK – IκB kinase; IRS-1 – Insulin Receptor Substrate 1; JAK – Janus Kinase; Keap1 – Kelch like ECH Associated Protein 1; NF-κB – Nuclear Factor κB; Nrf2 – Nuclear Factor Erythroid 2-Related Factor 2; P – Phosphorylation; PPARγ – Peroxisome Proliferator-Activated Receptor γ; PTP1B – Protein Tyrosine Phosphatase 1B; ROS – Reactive Oxygen Species; RNS – Reactive Nitrogen Species; SIRT1 – Sirtuin 1; STAT3 – Signal Transducer and Activator of Transcription 3; TAK1 – Transforming Growth factor-β-Activated Kinase; TCR – T Cell Receptor: TLRs – Toll-Like Receptors; TNFR –Tumour Necrosis Factor Receptor; Ub – Ubiquitination.
Moreover, flavonoids possess the ability to modulate the activation of the SIRT1 cascade, which in turn, blocks the activation of NF-κB [87], and modulate NF-κB activation through Toll-like receptor (TLR) type 4 [81]. The inhibitory effect of flavonoids on oxidative and nitrosative stress in various organs can also be attributed to their ability to inhibit the Janus kinase (JAK) transcriptional cascade – signal transducers and activators of transcription (STAT) [88,89], as well as their ability to induce the Nrf2/antioxidant-responsive element (ARE) signalling pathway [[90], [91], [92]]. Additionally, flavonoids are able to activate the antioxidant response of the cell through peroxisome proliferator-activated receptors (PPAR) γ [93].
It has been shown that the inhibitory effect of quercetin on the concentration of pro-inflammatory cytokines such as interleukins 1β, 6, 8, and tumour necrosis factor α, is associated with its effect on transcription factors such as the inhibition of NF-κB and the induction of Nrf2 [94,95], rather than with a direct inhibitory effect [96]. Moreover, the direct antioxidant effect of quercetin can be attributed to its ability to form chelated compounds with metals of variable valence, as well as its ability to inhibit pro-oxidant enzymes, such as lipoxygenase, cyclooxygenase, and xanthine oxidase [97].
Thus, the presence of potent antioxidant and anti-inflammatory compounds in D. styracifolium raw materials determines its effectiveness in treating acute and chronic inflammatory processes, including rheumatic diseases, fever, parasitic diseases, wound healing, hepatitis, cholecystitis, colitis, stomatitis, laryngitis, etc. Additionally, the plant's antioxidants can prevent papillary and intra-tubular calcification in the kidneys [98].
The ability of D. styracifolium components to modulate signalling pathways associated with NF-κB and JAK-STAT, SIRT1, Nrf2/ARE and PPARγ seems as very promising and beneficial in the treatment of diseases associated with the development of a systemic inflammatory response, because these signalling cascades are of central importance in the mechanisms of its development [[99], [100], [101], [102], [103], [104]]. The systemic inflammatory response underlies the pathogenesis of such common diseases as metabolic syndrome, cardiovascular disease, type 2 diabetes mellitus, inflammatory kidney disease, steatohepatitis, periodontitis, sialoadenitis, neurodegenerative diseases, osteoarthritis, autoimmune and allergic diseases [[105], [106], [107], [108], [109]].
A new alkaloid, styracifoline, has been recently identified in the aerial part of D. styracifolium. Studies have shown that styracifoline is able to inhibit the enzyme protein tyrosine phosphatase 1B (PTP1B) [14], which is an important regulator of carbohydrate and energy metabolism and therefore can be seen as a promising target for therapeutic intervention in type 2 diabetes and obesity [110].
PTP1B is responsible for blocking the insulin receptor substrate 1 (IRS-1) and dephosphorylating phosphotyrosine residues thereby causing insulin resistance or even cessation of intracellular insulin signalling. PTP1B also binds and dephosphorylates the leptin receptor, which determines the interaction with JAK2 and causes an energy imbalance. This also activates the pro-inflammatory transcription factor STAT3 [111].
Thus, modern literature sources emphasise the presence of antiradical and antiperoxide effects of extracts obtained from the above-ground parts and seeds of D. styracifolium. Special attention is focused on the potential therapeutic applications of flavonoids derived from D. styracifolium for diseases associated with the development of chronic inflammation and systemic response, emphasizing the ability of flavonoids to exert antioxidant and anti-inflammatory effects by acting directly and through the modulation of transcription factors such as NF-κB, JAK-STAT, SIRT1, Nrf2/ARE, and PPARγ. Styracifoline, a protein tyrosine phosphatase 1B inhibitor, found in the above-ground part of D. styracifolium opens up prospects for further research of D. styracifolium-based medicines as pathogenetic therapy for metabolic syndrome, type 2 diabetes mellitus, and obesity.
7. Conclusions
Current scientific studies elucidate an important role of modulation of signalling pathway associated with p38 MAPK and transcription factors (NF-κB, STAT3, Nrf2, liver Xα receptor, farnesoid X receptor, etc.) in providing a number of pharmacological effects (antiurolytic, cholelytic/cholagogic, hepatoprotective, antioxidant, anti-inflammatory and anti-diabetic).
Desmodium styracifolium (leave/stem, seeds, aerial parts, whole plant) is a valuable source of polyphenols and alkoloids providing these effects.
Literature sources report detailed characterictics of effects produced by extracts of this plant in in vitro experiments, but at present there is only sporadic information on the creation of clinically tested preparations derived from D. styracifolium. At the same time, there are no published results of randomised multicenter clinical trials.
Concomitantly, preparations of D. styracifolium may be potentially effective in the treatment of various diseases including urolithiasis, cholelithiasis, type 2 diabetes mellitus, metabolic syndrome, acute and chronic inflammatory processes, and more, as their pathogenesis involves the signalling pathway associated with p38 MAPK and transcription factors (NF-κB, STAT3, Nrf2, liver Xα receptor, farnesoid X receptor, etc.).
New strategies for the prevention and treatment of these diseases may rely on the promising development of dosage forms of D. styracifolium with their subsequent pre-clinical and clinical trials.
Data availability statement
Data will be made available on request.
CRediT authorship contribution statement
Valentyna Opryshko: Writing – original draft, Methodology, Investigation, Formal analysis. Anna Prokhach: Investigation, Formal analysis, Data curation, Conceptualization. Oleh Akimov: Writing – review & editing, Writing – original draft, Visualization, Methodology, Investigation, Formal analysis, Conceptualization. Mykola Riabushko: Software, Project administration, Methodology, Investigation, Conceptualization. Heorhii Kostenko: Writing – original draft, Visualization, Methodology, Investigation. Viktoriia Kostenko: Writing – review & editing, Writing – original draft, Methodology, Formal analysis. Artur Mishchenko: Methodology, Investigation, Formal analysis, Conceptualization. Natalia Solovyova: Writing – original draft, Visualization, Methodology, Investigation. Vitalii Kostenko: Writing – review & editing, Writing – original draft, Validation, Supervision, Project administration, Formal analysis, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Abufaraj M., Al Karmi J., Yang L. Prevalence and trends of urolithiasis among adults. Curr. Opin. Urol. 2022 Jul 1;32(4):425–432. doi: 10.1097/MOU.0000000000000994. [DOI] [PubMed] [Google Scholar]
- 2.Wagner C.A. Etiopathogenic factors of urolithiasis. Arch. Esp. Urol. 2021 Jan;74(1):16–23. [PubMed] [Google Scholar]
- 3.Tzelves L., Türk C., Skolarikos A. European association of urology urolithiasis guidelines: where are we going? Eur Urol Focus. 2021 Jan;7(1):34–38. doi: 10.1016/j.euf.2020.09.011. [DOI] [PubMed] [Google Scholar]
- 4.Swarne E., Srikanth M.S., Shreyas A., Desai S., Mehdi S., Gangadharappa H.V., Suman Krishna KL. Recent advances, novel targets and treatments for cholelithiasis; a narrative review. Eur. J. Pharmacol. 2021 Oct 5;908 doi: 10.1016/j.ejphar.2021.174376. [DOI] [PubMed] [Google Scholar]
- 5.Ma X., Zheng C., Hu C., Rahman K., Qin L. The genus Desmodium (Fabaceae)-traditional uses in Chinese medicine, phytochemistry and pharmacology. J. Ethnopharmacol. 2011;138(2) doi: 10.1016/j.jep.2011.09.053. 314-132. [DOI] [PubMed] [Google Scholar]
- 6.Liguo F., Tanqing C., Kaiyong L., Hong T., Qi Lin. Qingdao Publishing House; Qingdao: 2008. Higher Plants of China; p. 152. [Google Scholar]
- 7.Wagner H., Bauer R., Melchart D., Xiao P.G., Staudinger A. In: Chromatographic Fingerprint Analysis of Herbal Medicines; III. Wagner H., Bauer R., Melchart D., Xiao P.G., Staudinger A., editors. Springer; 2015. Herba desmodii styracifolii – guangjinqiancao; pp. 159–160. [DOI] [Google Scholar]
- 8.Zhonghua Bencao (Chinese Herbal Medicine) Shanghai Science and Technology Publishing House; Shanghai: 1996. Editorial Committee of Zhonghua Bencao National Traditional Chinese Herb Administration; pp. 3120–3343. [Google Scholar]
- 9.Pharmacopoeia of the People's Republic of China. English edition, Vol. I. China Medical Science Press; Beijing: 2010. [Google Scholar]
- 10.Hempen C.-H., Fischer T. 1st Engl. ed. Churchill Livingstone, Elsevier; 2009. A Materia Medica for Chinese Medicine: Plants, Minerals and Animal Products; p. 1016. [Google Scholar]
- 11.Xiong Y., Wang J., Deng J. Comparison between lysimachiae herba and desmodii styracifolii herba in pharmacological activities. China J. Chin. Mater. Med. 2015;40(11):2106–2111. doi: 10.4268/cjcmm20151108. [DOI] [PubMed] [Google Scholar]
- 12.Cueva-Chamba A., Bustamante-Pacheco F., Vanegas D., Peñaherrera E. Traditional medicinal uses and biological activities of species of the genus Desmodium: a literature review. Bol Latinoam Caribe Plant Med Aromat. 2023;22(6):700–746. doi: 10.37360/blacpma.23.22.6.51. [DOI] [Google Scholar]
- 13.Innovative TCM Desmodium styracifolium flavonoids capsule approved for marketing. National Medical Products Newsletter. 2022;4:4–5. http://regional.chinadaily.com.cn/pdf/nmpanewsletter202204.pdf [Google Scholar]
- 14.Tran T.D., Bui T.Q., Le T.A., Nguyen M.T., Hai N.T.T., Pham N.H., Phan M.N., Healy P.C., Pham N.B., Quinn R.J., PhT Quy, Nth Triet, Nguyen H.N., Le N.H., Phung T.V., Nhung N.T.A. Styracifoline from the Vietnamese plant Desmodium styracifolium: a potential inhibitor of diabetes-related and thrombosis-based proteins. ACS Omega. 2021;6(36):23211–23221. doi: 10.1021/acsomega.1c02840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ning G., Tianhua L., Xin Y., He P. Constituents in Desmodium blandumand their antitumor activity. Chin. Tradit. Herb. Drugs. 2009;40(6):852–856. [Google Scholar]
- 16.Kurian G.A., Paddikkala J. Oral delivery of insulin with Desmodium gangeticum root aqueous extract protects rat hearts against ischemia reperfusion injury in streptozotocin induced diabetic rats. Asian Pac. J. Tropical Med. 2010;3(2):94–100. doi: 10.1016/S1995-7645(10)60043-0. [DOI] [Google Scholar]
- 17.Kurian G.A., Suryanarayanan S., Raman A., Padikkala J. Antioxidant effects of ethyl acetate extract of Desmodium gangeticum root on myocardial ischemia reperfusion injury in rat hearts. Chin. Med. 2010 Jan 22;5:3. doi: 10.1186/1749-8546-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu Z.Z., Ma K.J., Ran X., Zhang H., Zheng C.J., Han T., Zhang Q.Y., Qin L.P. Analgesic, anti-inflammatory and antipyretic activities of the petroleum ether fraction from the ethanol extract of Desmodium podocarpum. J. Ethnopharmacol. 2011 Feb 16;133(3):1126–1131. doi: 10.1016/j.jep.2010.11.042. [DOI] [PubMed] [Google Scholar]
- 19.Muanda F.N., Bouayed J., Djilani A., Yao C., Soulimani R., Dicko A. Chemical composition and, cellular evaluation of the antioxidant activity of Desmodium adscendens leaves. Evid Based Complement Alternat Med. 2011;2011 doi: 10.1155/2011/620862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khan Z.R., Pickett J.A., Wadhams L.J., Hassanali A., Midega C.A.O. Combined control of Striga hermonthica and stemborers by maize-Desmodium spp. intercrops. Crop Protect. 2006;25:989–995. doi: 10.1016/j.cropro.2006.01.008. [DOI] [Google Scholar]
- 21.Gan N., Yang X., Li T.H., He P. Studies on constituents of rootsanel leaves from Desmodium blandum and their cytotoxic activity against growth of several tumor cells. Zhongguo Zhongyao Zazhi. 2008;33(18):2077–2080. [PubMed] [Google Scholar]
- 22.Joshi B.R., Hakim M.M., Patel I.C. The biological active compounds and biological activities of Desmodium species from Indian region: a review. Beni-Suef Univ J Basic Appl Sci. 2023;12(1):1. doi: 10.1186/s43088-022-00339-4. [DOI] [Google Scholar]
- 23.Yang J.S., Su Y.L., Wang Y.L. Studies on the chemical constituents of Desmodium styracifolium (Osbeck) Merr. Yao Xue Xue Bao. 1993;28(3):197–201. [PubMed] [Google Scholar]
- 24.Fenglian C., Shilling W., Honghua X. vol. 22. Guangzhou University of Chinese Medicine; 2005. pp. 302–303. (Analysis of the Volatile Oil from Desmodium Styracifolium (Osbeck) Merr. By Gas Chromatography-Mass Spectrometry). [Google Scholar]
- 25.Yang Q., Cheng X.X., Guo C.C., Tang X.M., Zhang C.R., Huang L.Q. Chemical constituents from seeds of Desmodium styracifolium and their scavenging activity of DPPH free radicals. Zhongguo Zhongyao Zazhi. 2015;46:2517–2521. doi: 10.7501/j.issn.0253-2670.2015.17.004. [DOI] [Google Scholar]
- 26.Li X.L., Wang H., Liu G., Zhang X., Ye W., Zhao Sh. Study on chemical constituents from Desmodium styracifolium. Zhong Yao Cai. 2007;30(7):802–805. [PubMed] [Google Scholar]
- 27.Zhao M., Duan J.-A., Che C.-T. Isoflavanones and their O-glycosides from Desmodium styracifolium. Phytochemistry. 2007;68(10):1471–1479. doi: 10.1016/j.phytochem.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 28.Hou J., Chen W., Lu H., Zhao H., Gao S., Liu W., Dong X., Guo Zh. Exploring the therapeutic mechanism of Desmodium styracifolium on oxalate crystal-induced kidney injuries using comprehensive approaches based on proteomics and network pharmacology. Front. Pharmacol. 2018;9:620. doi: 10.3389/fphar.2018.00620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li X., Liu C., Liang J., Zhou L., Li J., Chen H., Jiang T., Guan Y., Khoo H.E. Antioxidative mechanisms and anticolitic potential of Desmodium styracifolium (Osb.) Merr. in DSS-induced colitic mice. J. Funct.Foods. 2022;93 doi: 10.1016/j.jff.2022.105077. [DOI] [Google Scholar]
- 30.Zhou C., Luo J.G., Kong L.Y. Quality evaluation of Desmodium styracifolium using high-performance liquid chromatography with photodiode array detection and electrospray ionisation tandem mass spectrometry. Phytochem. Anal. 2012 May-Jun;23(3):240–247. doi: 10.1002/pca.1349. [DOI] [PubMed] [Google Scholar]
- 31.Chen L.Y., Cheng X.X., Tang X.M., Yang Q. Quality evaluation of Desmodium styracifolium by fingerprint, pattern recognition combined with quantitative analysis of multi-components by single marker. 2018;43(16):3322–3328. doi: 10.19540/j.cnki.cjcmm.2018.0097. [DOI] [PubMed] [Google Scholar]
- 32.Sun X., Tang X., Yang Q. An effective quantitative fingerprint method for evaluating the quality consistency of Desmodium styracifolium. Pharmazie. 2018;73(10):579–584. doi: 10.1691/ph.2018.8590. [DOI] [PubMed] [Google Scholar]
- 33.Chen L., Tang X., Yang Q., Cheng X. Quantitative and chemical fingerprint analysis of Desmodium styracifolium by high-performance liquid chromatography combined with chemometrics. J. Chromatogr. Sci. 2020;58(4):294–302. doi: 10.1093/chromsci/bmz112. [DOI] [PubMed] [Google Scholar]
- 34.Zhuo L., Yan D., Ning W., Nan W., Jin-hui W., Xian L. Chemical studies on the constituents of Desmodium styracifolium (Osb.) Merr. J. Shenyang Pharm. Univ. 2005;6:422–425. [Google Scholar]
- 35.Della Greca M., Fiorentino A., Monaco P., Previtera L. Cycloartane triterpenes from Juncus effuses. Phytochemistry. 1994;35(4):1017–1022. doi: 10.1016/S0031-9422(00)90659-9. [DOI] [PubMed] [Google Scholar]
- 36.Kubo T., Kajimoto T., Nohara T., Hirayama H., Ikegami K., Irino N.J. Extraction of flavonoids from Desmodium styracifolium for prevention of kidney stones. Jpn Kokai Tokkyo Koho. 1989;6 [Google Scholar]
- 37.Ai-dong M. Research progress of medicinal plant Desmodium styracifolium (Osbeck) Merr. J. Guangxi Acad. Sci. 2008;24:148–151. [Google Scholar]
- 38.Kubo T., Hamada S., Nohara T., Wang Z.R., Hirayama H., Ikegami K., Yasukawa K., Takido M. Study on the constituents of Desmodium styracifolium. Chem. Pharm. Bull. (Tokyo) 1989;37:2229–2231. doi: 10.1248/cpb.37.2229. [DOI] [PubMed] [Google Scholar]
- 39.Phan M.G., Phan T.S., Matsunami K., Otsuka H. Flavonoid compounds from Desmodium styracifolium of Vietnamese origin. Chem. Nat. Compd. 2010;V.46:671–798. doi: 10.1007/s10600-010-9746-7. [DOI] [Google Scholar]
- 40.Guo P., Yan W., Han Q., Wang Ch, Zhang Z. Simultaneous quantification of 25 active constituents in the total flavonoids extract from Herba Desmodii Styracifolii by high-performance liquid chromatography with electrospray ionization tandem mass spectrometry. J. Separ. Sci. 2015;38(7):1156–1163. doi: 10.1002/jssc.201401360. [DOI] [PubMed] [Google Scholar]
- 41.Ahmed S., Hasan M.M., Khan H., Mahmood Z.A., Patel S. The mechanistic insight of polyphenols in calcium oxalate urolithiasis mitigation. Biomed. Pharmacother. 2018;106:1292–1299. doi: 10.1016/j.biopha.2018.07.080. [DOI] [PubMed] [Google Scholar]
- 42.Zeng X., Xi Y., Jiang W. Protective roles of flavonoids and flavonoid-rich plant extracts against urolithiasis: a review. Crit. Rev. Food Sci. Nutr. 2019;59(13):2125–2135. doi: 10.1080/10408398.2018.1439880. [DOI] [PubMed] [Google Scholar]
- 43.Sansores-España D., Pech-Aguilar A.G., Cua-Pech K.G., Medina-Vera I., Guevara-Cruz M., Gutiérrez-Solis A.L., Reyes-García J.G., Avila-Nava A. Plants used in Mexican traditional medicine for the management of urolithiasis: a review of preclinical evidence, bioactive compounds, and molecular mechanisms. Molecules. 2022;27(6):2008. doi: 10.3390/molecules27062008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nirumand M.C., Hajialyani M., Rahimi R., Farzaei M.H., Zingue S., Nabavi S.M., Bishayee A. Dietary plants for the prevention and management of kidney stones: preclinical and clinical evidence and molecular mechanisms. Int. J. Mol. Sci. 2018;19:765. doi: 10.3390/ijms19030765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pedro R.N., Aslam A.U., Bello J.O., Bhatti K.H., Philipraj J., Sissoko I., Vasconcellos G.S., Trinchieri A., Buchholz N. Nutrients, vitamins, probiotics and herbal products: an update of their role in urolithogenesis. Urolithiasis. 2020;48:285–301. doi: 10.1007/s00240-020-01182-x. [DOI] [PubMed] [Google Scholar]
- 46.Khan A., Bashir S., Khan S.R. Antiurolithic effects of medicinal plants: results of in vivo studies in rat models of calcium oxalate nephrolithiasis – a systematic review. Urolithiasis. 2021;49(2):95–122. doi: 10.1007/s00240-020-01236-0. [DOI] [PubMed] [Google Scholar]
- 47.Agawane S.B., Gupta V.S., Kulkarni M.J., Bhattacharya A.K., Koratkar S.S., Rao V.K. Patho-physiological evaluation of Duranta erecta for the treatment of urolithiasis. J. Ayurveda Integr. Med. 2019;10(1):4–11. doi: 10.1016/j.jaim.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moreno K.G.T., Gasparotto Junior A., Dos Santos A.C., Palozi R.A.C., Guarnier L.P., Marques A.A.M., Romão P.V.M., Lorençone B.R., Cassemiro N.S., Silva D.B., Tirloni C.A.S., de Barros M.E. Nephroprotective and antilithiatic activities of Costus spicatus (Jacq.) sw.: ethnopharmacological investigation of a species from the dourados region, mato grosso do sul state, Brazil. J. Ethnopharmacol. 2021;266 doi: 10.1016/j.jep.2020.113409. [DOI] [PubMed] [Google Scholar]
- 49.Mandal B., Madan S., Ahmad S., Sharma A.K., Ansari M.H.R. Antiurolithic efficacy of a phenolic rich ethyl acetate fraction of the aerial parts of Aerva lanata (Linn) Juss. ex Schult. in ethylene glycol induced urolithic rats. J. Pharm. Pharmacol. 2021;73(4):560–572. doi: 10.1093/jpp/rgaa071. [DOI] [PubMed] [Google Scholar]
- 50.Rodgers A.L., Webber D., Ramsout R., Gohel M.D. Herbal preparations affect the kinetic factors of calcium oxalate crystallization in synthetic urine: implications for kidney stone therapy. Urolithiasis. 2014;42(3):221–225. doi: 10.1007/s00240-014-0654-3. [DOI] [PubMed] [Google Scholar]
- 51.Xiang S., Zhou J., Li J., Wang Q., Zhang Q., Zhao Zh, Zhang L., Chen Zh, Wang Sh. Antilithic effects of extracts from different polarity fractions of Desmodium styracifolium on experimentally induced urolithiasis in rats. Urolithiasis. 2015;43(5):433–439. doi: 10.1007/s00240-015-0795-z. [DOI] [PubMed] [Google Scholar]
- 52.Mi J., Duan J., Zhang J., Lu Jianzhong, Wang H., Wang Zh. Evaluation of antiurolithic effect and the possible mechanisms of Desmodium styracifolium and Pyrrosiae petiolosa in rats. Urol. Res. 2012;40(2):151–161. doi: 10.1007/s00240-011-0401-y. [DOI] [PubMed] [Google Scholar]
- 53.Li X., Chen C., Zhang T., Ding N., Zheng P., Yang M. Comparative pharmacokinetic studies of five C-glycosylflavones in normal and urolithiasis model rats following administration of total flavonoids from Desmodium styracifolium by liquid chromatography-tandem mass spectrometry. J. Separ. Sci. 2022;45(15):2901–2913. doi: 10.1002/jssc.202200010. [DOI] [PubMed] [Google Scholar]
- 54.Xie H., Li J., Gao H., Wang J., Li Ch, Xu Y., Liu Ch. Total flavone of Desmodium styracifolium relieved apoptosis and autophagy of COM-induced HK-2 cells by regulating KIM-1 via p38/MAPK pathway. Mol. Cell. Biochem. 2018;442(1–2):169–175. doi: 10.1007/s11010-017-3201-z. [DOI] [PubMed] [Google Scholar]
- 55.Liu Y., Li D., He Z., Liu Q., Wu J., Guan X., Tao Zh, Deng Y. Inhibition of autophagy-attenuated calcium oxalate crystal-induced renal tubular epithelial cell injury in vivo and in vitro. Oncotarget. 2017;9(4):4571–4582. doi: 10.18632/oncotarget.23383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Duan X., Kong Z., Mai X., Lan Y., Liu Y., Yang Zh, Zhao Zh, Deng T., Zeng T., Cai Ch, Li Sh, Zhong W., Wu W., Zeng G. Autophagy inhibition attenuates hyperoxaluria-induced renal tubular oxidative injury and calcium oxalate crystal depositions in the rat kidney. Redox Biol. 2018;16:414–425. doi: 10.1016/j.redox.2018.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Song X.B., Liu G., Liu F., Yan ZhG., Wang ZhY., Liu Z.P., Wang L. Autophagy blockade and lysosomal membrane permeabilization contribute to lead-induced nephrotoxicity in primary rat proximal tubular cells. Cell Death Dis. 2017;8(6) doi: 10.1038/cddis.2017.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fox C., Cocchiaro P., Oakley F., Howarth R., Callaghan K., Leslie J., Luli S., Wood K.M., Genovese F., Sheerin N.S., Moles A. Inhibition of lysosomal protease cathepsin D reduces renal fibrosis in murine chronic kidney disease. Sci. Rep. 2016;6 doi: 10.1038/srep20101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang Q.H., Liu D.W., Long Y., Liu H.Z., Chai W.Z., Wang X.T. Acute renal failure during sepsis: potential role of cell cycle regulation. J. Infect. 2009;58(6):459–464. doi: 10.1016/j.jinf.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 60.Azimi A., Eidi A., Mortazavi P., Rohani A.H. Protective effect of apigenin on ethylene glycol-induced urolithiasis via attenuating oxidative stress and inflammatory parameters in adult male Wistar rats. Life Sci. 2021;279 doi: 10.1016/j.lfs.2021.119641. [DOI] [PubMed] [Google Scholar]
- 61.Yuan P., Sun X., Liu X., Hutterer G., Pummer K., Hager B., Ye Z., Chen Z. Kaempferol alleviates calcium oxalate crystal-induced renal injury and crystal deposition via regulation of the AR/NOX2 signaling pathway. Phytomedicine. 2021;86 doi: 10.1016/j.phymed.2021.153555. [DOI] [PubMed] [Google Scholar]
- 62.Park H.K., Jeong B.C., Sung M.K., Park M.Y., Choi E.Y., Kim B.S., Kim H.H., Kim J.I. Reduction of oxidative stress in cultured renal tubular cells and preventive effects on renal stone formation by the bioflavonoid quercetin. J. Urol. 2008;179(4):1620–1626. doi: 10.1016/j.juro.2007.11.039. [DOI] [PubMed] [Google Scholar]
- 63.Zhu W., Xu Y.F., Feng Y., Peng B., Che J., Liu M., Zheng J. Prophylactic effects of quercetin and hyperoside in a calcium oxalate stone forming rat model. Urolithiasis. 2014;42(6):519–526. doi: 10.1007/s00240-014-0695-7. [DOI] [PubMed] [Google Scholar]
- 64.Guzel A., Yunusoglu S., Calapoglu M., Candan I.A., Onaran I., Oncu M., Ergun O., Oksay T. Protective effects of quercetin on oxidative stress-induced tubular epithelial damage in the experimental rat hyperoxaluria model. Medicina (Kaunas). 2021;57(6):566. doi: 10.3390/medicina57060566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou J., Jin J., Li X., Zhao Z., Zhang L., Wang Q., Li J., Zhang Q., Xiang S. Total flavonoids of Desmodium styracifolium attenuates the formation of hydroxy-L-proline-induced calcium oxalate urolithiasis in rats. Urolithiasis. 2018;46(3):231–241. doi: 10.1007/s00240-017-0985-y. [DOI] [PubMed] [Google Scholar]
- 66.Wang Z., Yu F., Shi D., Wang Y., Xu F., Zeng S. Selection and validation of reference genes for RT-qPCR analysis in Desmodium styracifolium Merr. Biotech. 2021;11(9):403. doi: 10.1007/s13205-021-02954-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu M., Liu C., Chen H., Huang X., Zeng X., Zhou J., Mi S. Prevention of cholesterol gallstone disease by schaftoside in lithogenic diet-induced C57BL/6 mouse model. Eur. J. Pharmacol. 2017;815:1–9. doi: 10.1016/j.ejphar.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 68.Hong C., Tontonoz P. Liver X receptors in lipid metabolism: opportunities for drug discovery. Nat. Rev. Drug Discov. 2014 Jun;13(6):433–444. doi: 10.1038/nrd4280. [DOI] [PubMed] [Google Scholar]
- 69.Ducheix S., Montagner A., Theodorou V., Ferrier L., Guillou H. The liver X receptor: a master regulator of the gut-liver axis and a target for non alcoholic fatty liver disease. Biochem. Pharmacol. 2013;86(1):96–105. doi: 10.1016/j.bcp.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 70.Heckmann B.L., Zhang X., Saarinen A.M., Schoiswohl G., Kershaw E.E., Zechner R., Liu J. Liver X receptor α mediates hepatic triglyceride accumulation through upregulation of G0/G1 Switch Gene 2 expression. JCI Insight. 2017;2(4) doi: 10.1172/jci.insight.88735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.De Marino S., Carino A., Masullo D., Finamore C., Marchianò S., Cipriani S., Di Leva F.S., Catalanotti B., Novellino E., Limongelli V., Fiorucci S., Zampella A. Hyodeoxycholic acid derivatives as liver X receptor α and G-protein-coupled bile acid receptor agonists. Sci. Rep. 2017;7 doi: 10.1038/srep43290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hong C., Tontonoz P. Liver X receptors in lipid metabolism: opportunities for drug discovery. Nat. Rev. Drug Discov. 2014;13(6):433–444. doi: 10.1038/nrd4280. [DOI] [PubMed] [Google Scholar]
- 73.Tice C.M., Noto P.B., Fan K.Y., Zhuang L., Lala D.S., Singh S.B. The medicinal chemistry of liver X receptor (LXR) modulators. J. Med. Chem. 2014;57(17):7182–7205. doi: 10.1021/jm500442z. [DOI] [PubMed] [Google Scholar]
- 74.Liu M., Zhang G., Wu S., Song M., Wang J., Cai W., Mi S., Liu Ch. Schaftoside alleviates HFD-induced hepatic lipid accumulation in mice via upregulating farnesoid X receptor. J. Ethnopharmacol. 2020;255 doi: 10.1016/j.jep.2020.112776. [DOI] [PubMed] [Google Scholar]
- 75.Liu M., Zhang G., Song M., Wang J., Shen Ch, Chen Z., Huang X., Gao Y., Zhu C., Lin C., Mi S., Liu C. Activation of farnesoid X receptor by schaftoside ameliorates acetaminophen-induced hepatotoxicity by modulating oxidative stress and inflammation. Antioxid. Redox Signal. 2020;33(2):87–116. doi: 10.1089/ars.2019.7791. [DOI] [PubMed] [Google Scholar]
- 76.Mueller M., Thorell A., Claudel T., Jha P., Koefeler H., Lackner C., Hoesel B., Fauler G., Stojakovic T., Einarsson C., Marschall H.U., Trauner M. Ursodeoxycholic acid exerts farnesoid X receptor-antagonistic effects on bile acid and lipid metabolism in morbid obesity. J. Hepatol. 2015;62(6):1398–1404. doi: 10.1016/j.jhep.2014.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cong L. Journal of Yunnan University; 2007. Studies on Antioxidation Activity of Three Plants of Desmodium. [Google Scholar]
- 78.Cheng X., Tang X., Guo C., Zhang C., Yang Q. New flavonol glycosides from the seeds of Desmodium styracifolium. Chem. Nat. Compd. 2018;54(5):846–850. doi: 10.1007/s10600-018-2496-7. [DOI] [Google Scholar]
- 79.Xia L., Chengxin L., Jun L., Zhou L., Li J., Chen H., Jiang T., Guan Y., Khoo E.H. Antioxidative mechanisms and anticolitic potential of Desmodium styracifolium (Osb.) Merr. in DSS-induced colitic mice. J. Funct.Foods. 2022;93 doi: 10.1016/j.jff.2022.105077. [DOI] [Google Scholar]
- 80.Kostenko V., Akimov O., Gutnik O., Kostenko H., Kostenko V., Romantseva T., Morhun Y., Nazarenko S., Taran O. Modulation of redox-sensitive transcription factors with polyphenols as pathogenetically grounded approach in therapy of systemic inflammatory response. Heliyon. 2023 Apr;9(5) doi: 10.1016/j.heliyon.2023.e15551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Choy K.W., Murugan D., Leong X.F., Abas R., Alias A., Mustafa M.R. Flavonoids as natural anti-inflammatory agents targeting nuclear factor-kappa B (NFκB) signaling in cardiovascular diseases: a mini review. Front. Pharmacol. 2019 Oct 31;10:1295. doi: 10.3389/fphar.2019.01295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yelins’ka A.M., Kostenko V.O. Synergistic effect of quercetin and epigallocatechin-3-gallate as a agents for correction of connective tissue disruption in rats' periodontium under systemic and local administration of lipopolisaccharide of Salmonella typhi. Probl Ekol Med. 2019;23(5–6):42–44. doi: 10.31718/mep.2019.23.5-6.07. [DOI] [Google Scholar]
- 83.Yelins’ka A.M., Liashenko L.I., Kostenko V.O. Quercetin potentiates antiradical properties of epigallocatechin-3-gallate in periodontium of rats under systemic and local administration of lipopolisaccharide of Salmonella typhi. Wiad. Lek. 2019;72(8):1499–1503. [PubMed] [Google Scholar]
- 84.Yavtushenko I.V., Nazarenko S.M., Katrushov O.V., Kostenko V.O. Quercetin limits the progression of oxidative and nitrosative stress in the rats' tissues after experimental traumatic brain injury. Wiad. Lek. 2020;73(10):2127–2132. [PubMed] [Google Scholar]
- 85.Morgan M.J., Liu Z.G. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011;21(1):103–115. doi: 10.1038/cr.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lingappan K. NF-κB in oxidative stress. Curr. Opin. Toxicol. 2018;7:81–86. doi: 10.1016/j.cotox.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sayed A.M., Hassanein E.H.M., Salem S.H., Hussein O.E., Mahmoud A.M. Flavonoids-mediated SIRT1 signaling activation in hepatic disorders. Life Sci. 2020;259 doi: 10.1016/j.lfs.2020.118173. [DOI] [PubMed] [Google Scholar]
- 88.Yin Q., Wang L., Yu H., Chen D., Zhu W., Sun C. Pharmacological effects of polyphenol phytochemicals on the JAK-STAT signaling pathway. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.716672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zalpoor H., Nabi-Afjadi M., Forghaniesfidvajani R., Tavakol C., Farahighasreaboonasr F., Pakizeh F., Dana V.G., Seif F. Quercetin as a JAK-STAT inhibitor: a potential role in solid tumors and neurodegenerative diseases. Cell. Mol. Biol. Lett. 2022 Jul 26;27(1):60. doi: 10.1186/s11658-022-00355-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kozaeva R., Klymenko M.O., Katrushov O.V., Kostenko V.O. Bioflavonoids as agents for correcting nitro-oxidative stress and salivary gland functions in rats exposed to alcohol during modeled lipopolysaccharide-induced systemic inflammatory response. Wiad. Lek. 2022;75(3):685–690. doi: 10.36740/WLek202203121. [DOI] [PubMed] [Google Scholar]
- 91.Suraweera T.L., Rupasinghe H.P.V., Dellaire G., Xu Z. Regulation of nrf2/ARE pathway by dietary flavonoids: a friend or foe for cancer management? Antioxidants. 2020;9(10):973. doi: 10.3390/antiox9100973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Frenkel’ Y.D., Zyuzin V.O., Cherno V.S., Kostenko V.O. Effect of epigallocatechin-3-gallate and quercetin on the production of reactive oxygen and nitrogen species in liver of rats exposed to round-the-clock light and kept on carbohydrate-lipid diet. Fiziol. Zh. 2022;68(1):20–27. doi: 10.15407/fz68.01.020. [DOI] [Google Scholar]
- 93.Feng X., Weng D., Zhou F., Owen Y.D., Qin H., Zhao J., WenYu, Huang Y., Chen J., Fu H., Yang N., Chen D., Li J., Tan R., Shen P. Activation of PPARγ by a natural flavonoid modulator, apigenin ameliorates obesity-related inflammation via regulation of macrophage polarization. EBioMedicine. 2016 Jul;9:61–76. doi: 10.1016/j.ebiom.2016.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kang C.H., Choi Y.H., Moon S.K., Kim W.J., Kim G.Y. Quercetin inhibits lipopolysaccharide-induced nitric oxide production in BV2 microglial cells by suppressing the NF-κB pathway and activating the Nrf2-dependent HO-1 pathway. Int Immunopharmacol. 2013 Nov;17(3):808–813. doi: 10.1016/j.intimp.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 95.Lai W.W., Hsu S.C., Chueh F.S., Chen Y.Y., Yang J.S., Lin J.P., Lien J.C., Tsai C.H., Chung J.G. Quercetin inhibits migration and invasion of SAS human oral cancer cells through inhibition of NF-κB and matrix metalloproteinase-2/-9 signaling pathways. Anticancer Res. 2013;33(5):1941–1950. [PubMed] [Google Scholar]
- 96.Min Y.D., Choi C.H., Bark H., Son H.Y., Park H.H., Lee S., Park J.W., Park E.K., Shin H.I., Kim S.H. Quercetin inhibits expression of inflammatory cytokines through attenuation of NF-kappaB and p38 MAPK in HMC-1 human mast cell line. Inflamm. Res. 2007;56(5):210–215. doi: 10.1007/s00011-007-6172-9. [DOI] [PubMed] [Google Scholar]
- 97.Moybenko A.A., editor. Bioflavonoids as Organoprotectors (Quercetin, Corvitin, Quertin) Naukova dumka; Kyiv: 2012. p. 274. [Google Scholar]
- 98.Grases F., Prieto R.M., Gomila I., Sanchis P., Costa-Bauzá A. Phytotherapy and renal stones: the role of antioxidants. A pilot study in Wistar rats. Urol. Res. 2009;37(1):35–40. doi: 10.1007/s00240-008-0165-1. [DOI] [PubMed] [Google Scholar]
- 99.Kauppinen A., Suuronen T., Ojala J., Kaarniranta K., Salminen A. Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell. Signal. 2013 Oct;25(10):1939–1948. doi: 10.1016/j.cellsig.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 100.Akimov O.Y., Kostenko V.O. Role of NF-κB transcriptional factor activation during chronic fluoride intoxication in development of oxidative-nitrosative stress in rat's gastric mucosa. J. Trace Elem. Med. Biol. 2020;61 doi: 10.1016/j.jtemb.2020.126535. [DOI] [PubMed] [Google Scholar]
- 101.Bharadwaj U., Kasembeli M.M., Robinson P., Tweardy D.J. Targeting Janus kinases and signal transducer and activator of transcription 3 to treat inflammation, fibrosis, and cancer: rationale, progress, and caution. Pharmacol. Rev. 2020;72(2):486–526. doi: 10.1124/pr.119.018440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Saha S., Buttari B., Panieri E., Profumo E., Saso L. An overview of Nrf2 signaling pathway and its role in inflammation. Molecules. 2020;25(22):5474. doi: 10.3390/molecules25225474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Frenkel YuD., Cherno V.S., Kostenko V.O. Nrf2 induction alleviates metabolic disorder and systemic inflammatory response in rats under a round-the-clock lighting and high-carbohydrate-lipid diet. Romanian Journal of Diabetes, Nutrition and Metabolic Diseases. 2022;29(2):194–201. doi: 10.46389/rjd-2022-1092. [DOI] [Google Scholar]
- 104.Frenkel ' YD., Cherno V.S., Kostenko V.O. Effect of NF-κB and Nrf2 transcription factor modulators on indicators of oxidative–nitrosative stress in skeletal muscles of rats under chronic hypomelatoninemia and carbohydrate-lipid diet. Fiziol. Zh. 2023;69(2):11–18. doi: 10.15407/fz69.02.011. [DOI] [Google Scholar]
- 105.Franceschi C., Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. Biol. Sci Med. Sci. 2014;69(Suppl 1):S4–S9. doi: 10.1093/gerona/glu057. [DOI] [PubMed] [Google Scholar]
- 106.Minihane A.M., Vinoy S., Russell W.R., Baka A., Roche H.M., Tuohy K.M., Teeling J.L., Blaak E.E., Fenech M., Vauzour D., McArdle H.J., Kremer B.H., Sterkman L., Vafeiadou K., Benedetti M.M., Williams C.M., Calder P.C. Low-grade inflammation, diet composition and health: current research evidence and its translation. Br. J. Nutr. 2015;114(7):999–1012. doi: 10.1017/S0007114515002093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Matulewicz N., Karczewska-Kupczewska M. Insulin resistance and chronic inflammation. Postepy Hig. Med. Dosw. 2016;70(0):1245–1258. doi: 10.5604/17322693.1226662. [DOI] [PubMed] [Google Scholar]
- 108.Robinson W.H., Lepus C.M., Wang Q., Raghu H., Mao R., Lindstrom T.M., Sokolove J. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016;12(10):580–592. doi: 10.1038/nrrheum.2016.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen Y., Liu S., Leng S.X. Chronic low-grade inflammatory phenotype (CLIP) and senescent immune dysregulation. Clin Ther. 2019 Mar;41(3):400–409. doi: 10.1016/j.clinthera.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 110.Gurzov E.N., Stanley W.J., Brodnicki T.C., Thomas H.E. Protein tyrosine phosphatases: molecular switches in metabolism and diabetes. Trends Endocrinol Metab. 2015;26(1):30–39. doi: 10.1016/j.tem.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 111.Lund I.K., Hansen J.A., Andersen H.S., Møller N.P., Billestrup N. Mechanism of protein tyrosine phosphatase 1B-mediated inhibition of leptin signalling. J. Mol. Endocrinol. 2005;34(2):339–351. doi: 10.1677/jme.1.01694. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.