Abstract
Polymicrobial sepsis induced by cecal ligation and puncture (CLP) reproduces many of the pathophysiologic features of septic shock. In this study, we demonstrate that mRNA for a broad range of pro- and anti-inflammatory cytokine and chemokine genes are temporally regulated after CLP in the lung and liver. We also assessed whether prophylactic administration of monophosphoryl lipid A (MPL), a nontoxic derivative of lipopolysaccharide (LPS) that induces endotoxin tolerance and attenuates the sepsis syndrome in mice after CLP, would alter tissue-specific gene expression post-CLP. Levels of pulmonary interleukin-6 (IL-6), tumor necrosis factor alpha (TNF-α), granulocyte colony-stimulating factor (G-CSF), IL-1 receptor antagonist (IL-1ra), and IL-10 mRNA, as well as hepatic IL-1β, IL-6, gamma interferon (IFN-γ), G-CSF, inducible nitric oxide synthase, and IL-10 mRNA, were reduced in MPL-pretreated mice after CLP compared to control mice. Chemokine mRNA expression was also profoundly mitigated in MPL-pretreated mice after CLP. Specifically, levels of pulmonary and hepatic macrophage inflammatory protein 1α (MIP-1α), MIP-1β, MIP-2, and monocyte chemoattractant protein-1 (MCP-1) mRNA, as well as hepatic IFN-γ-inducible protein 10 and KC mRNA, were attenuated in MPL-pretreated mice after CLP. Attenuated levels of IL-6, TNF-α, MCP-1, MIP-1α, and MIP-2 in serum also were observed in MPL-pretreated mice after CLP. Diminished pulmonary chemokine mRNA production was associated with reduced neutrophil margination and pulmonary myeloperoxidase activity. These data suggest that prophylactic administration of MPL mitigates the sepsis syndrome by reducing chemokine production and the recruitment of inflammatory cells into tissues, thereby attenuating the production of proinflammatory cytokines.
The development of sepsis in surgical, burn, and trauma patients continues to be a substantial cause of morbidity and the leading cause of mortality in intensive care units (10). Sepsis-related mortality frequently results from multiple-organ failure, which is characterized by impaired pulmonary function (ARDS), hepatic failure, cardiac dysfunction, acute renal failure, and disseminated intravascular coagulation (10). During sepsis, neutrophils are sequestered in the lungs and liver (27, 72) and monocytes are retained in the lungs (17, 59). It is the activation of recruited neutrophils and monocytes, as well as resident tissue macrophages, and their subsequent overproduction of proinflammatory mediators that is thought to lead to the pulmonary and hepatic damage that precedes multiple-organ failure. Neutrophil sequestration during polymicrobial sepsis and endotoxemia has been associated with increased chemokine production, augmented expression of the β2-integrin CD11b/CD18, and upregulation of selectins such as vascular cell adhesion molecule, intracellular adhesion molecule, and E-selectin (19, 33, 53, 57, 64). Chemokine production promotes the recruitment of inflammatory cells, while increased expression of adhesion molecules facilitates leukocyte-endothelial cell interactions. The importance of neutrophil-endothelial cell interactions and chemokine production in the development of lung and liver injury during sepsis and/or endotoxemia is illustrated by the ability of treatment with Ab to cellular adhesion molecules and chemokines like MIP-1α and CINC to attenuate PMN accumulation and tissue damage (19, 20, 33, 53, 57).
MPL is a nontoxic derivative of the lipid A moiety of LPS that was developed in part as a prophylactic drug for septic shock (44). MPL administration, like pretreatment with sublethal doses of LPS, induces a state of endotoxin tolerance (30) such that pretreatment of experimental animals with MPL not only increases survival rates following shock induced by LPS or TNF-α but also protects against peritonitis and some infections by gram-negative and -positive bacteria (2, 4, 44). The protective effects of prophylactic MPL treatment has been associated with attenuated fever, reduced levels of circulating proinflammatory cytokines (e.g., TNF-α, IFN-γ, IL-6, and IL-8), attenuated development of disseminated intravascular coagulation, and reduced levels of circulating transaminases in serum, a measure of liver damage (25, 30, 44, 72). While levels of circulating cytokines have been analyzed in models of MPL-induced tolerance, whether prophylactic administration of MPL alters tissue-specific gene expression has yet to be addressed.
Polymicrobial sepsis induced by CLP is a model of sepsis which reproduces many of the inflammatory and pathological sequelae that are observed clinically (58, 61). Following CLP, animals develop bacteremia, hypothermia, hypotension, and damage to multiple organ systems (1, 43, 60, 61). The lungs develop pathological changes indicative of ARDS, which is fatal. Since the recruitment of inflammatory cells and overproduction of proinflammatory cytokines mediate the tissue damage leading to multiple-organ failure, we sought to examine the tissue-specific regulation of chemokine and cytokine gene expression early after the induction of polymicrobial sepsis. We also assessed this panel of genes in mice that were given MPL prophylactically prior to CLP and report that MPL pretreatment reduced the in vivo expression of the genes encoding a number of proinflammatory and anti-inflammatory cytokines. Interestingly, the subset of cytokine mRNAs that was reduced by MPL pretreatment differed between the lungs and liver, indicating that tolerance can be organ and gene specific. Moreover, some of the most profound reductions in in vivo mRNA and protein expression in MPL-pretreated mice after CLP were observed among the chemokines. Reduced levels of chemokine expression in MPL-pretreated mice after CLP were associated with reductions in neutrophil accumulation in the lungs.
MATERIALS AND METHODS
Abbreviations used in this paper.
Ab, antibody; ARDS, adult respiratory distress syndrome; CINC, cytokine-induced neutrophil chemoattractant; CLP, cecal ligation and puncture; CSF, colony-stimulating factor; ELISA, enzyme-linked immunosorbent assay; G-CSF, granulocyte-CSF; GM-CSF, granulocyte macrophage-CSF; IFN, interferon; IL, interleukin; IL-1ra, IL-1 receptor antagonist; iNOS, inducible nitric oxide synthase; i.p., intraperitoneal; IP-10, IFN-γ-inducible protein 10; LPS, lipopolysaccharide; M-CSF, macrophage-CSF; MCP, monocyte chemoattractant protein; MIP, macrophage inflammatory protein; MPL, monophosphoryl lipid A; MPO, myeloperoxidase; PMN, polymorphonuclear leukocyte; RANTES; SEM, standard error of the mean; TNF, tumor necrosis factor.
Mice.
C57BL/6J mice (Jackson Laboratories, Bar Harbor, Maine) were housed in cages with filter tops in a laminar-flow hood and fed food and acid water ad libitum. Sepsis was induced by CLP. The mice were anesthetized, and the cecum was ligated below the ileocecal junction; intestinal continuity was maintained. The cecum was punctured twice with a 20-gauge needle, and a small amount of cecal contents was expressed through the punctures. The incision was closed, and 1 ml of sterile saline was administered subcutaneously. By 4 to 6 h after CLP, all of the mice had developed the early clinical signs of sepsis, including lethargy, piloerection, and diarrhea. A survival rate of ∼90% was observed 24 h after CLP, a mortality rate which was comparable to that reported for other mouse strains (6, 62). After CLP, a 90 to 100% mortality rate has been reported, with death occurring between days 1 to 5 CLP (6, 60, 61, 64). In tolerance experiments, mice were injected i.p. with 100 μg of MPL (RIBI ImmunoChemical, Inc., Hamilton, Mont.) or saline 48 h prior to CLP. This concentration of MPL and timing of MPL pretreatment have been shown previously by our laboratory and others to reduce the levels of circulating cytokines and protect against CLP-, LPS-, and Escherichia coli-mediated death (38, 44). Both untreated and sham-operated mice served as controls.
The experiments reported herein were conducted according to the principles set forth in the Guide for the Care and Use of Laboratory Animals, Institute of Laboratory Animal Resources, National Research Council (DHEW Publication NIH 85-23).
ELISAs for cytokine and chemokine levels in serum.
Mice were bled through the retro-orbital sinus, and the serum was collected and stored at −70°C. Serum was assayed for IL-6, MCP-1, MIP-1α, MIP-2, and TNF-α by ELISA as specified by the manufacturer. The lower limits of sensitivity for the IL-6, MCP-1, MIP-1α, and MIP-2 ELISAs (R & D Systems, Minneapolis, Minn.) were 3.1, 2, 1.5, and 1.5 pg/ml, respectively. The lower limit of sensitivity for the TNF-α ELISA (Genzyme, Cambridge, Mass.) was 15 pg/ml.
Histological testing and MPO assay.
To assess migration of inflammatory cells into the lungs, histological testing and an MPO assay were performed. Mice were pretreated with saline or MPL, and sepsis was induced by CLP, as described above. At 6 h later, one lung lobe was fixed in phosphate-buffered formalin for histological evaluation and the remaining lobes were used in the MPO assay. For histological testing, the lobes were embedded in paraffin, sectioned, and stained with hematoxylin and eosin. Histopathological changes were evaluated by the Pathobiology Division, Naval Medical Research Institute, Bethesda, Md. For the MPO assay, the lobes were washed in cold saline, blotted dry, weighted, and homogenized in 50 mM sodium phosphate (pH 6). The homogenate was centrifuged at 35,000 × g (13 min at 4°C), and the supernatant was stored at −70°C. The pellet was resuspended in 0.5% hexadecyltrimethylammonium bromide in 50 mM sodium phosphate (pH 6), homogenized on ice with a Polytron homogenizer, freeze-thawed (20 min at −70°C), and centrifuged at 35,000 × g (13 min at 4°C). The resulting supernatant was frozen at −70°C. MPO activity was assessed in the supernatant by spectrophotometry with a kinetic computer program. Briefly, test samples were mixed with 2.9 ml of 100 mM potassium phosphate (pH 6) containing 0.17 mg of o-dianisidine dihydrochloride per ml and 0.0005% hydrogen peroxide. The change in optical density at 460 nm was measured for 1 to 2 min in a Cary 3 UV/Vis spectrophotometer. Data were derived by linear regression and are expressed as the optical density at 460 nm per minute per gram of tissue.
Analysis of tissue mRNA by reverse transcription-PCR.
At the indicated times after CLP, the liver and lungs were removed from each animal and frozen at −70°C. The tissues were homogenized in RNA Stat 60 (Tel-Test, Inc., Friendswood, Tex.), and total RNA was isolated as specified by the manufacturer. Relative quantities of mRNA for each gene of interest were determined by a coupled reverse transcription-PCR as described previously (29, 30). The primers (sense [S] and antisense [AS]) and probe [P] for the chemokines were as follows: IP-10, 5′-GTGTTGACATCATTGCCACG (S), 5′-GCTTACAGTACAGAGCTAGG (AS), and 5′-GAATCTAAGACCATCAAGAAG (P); KC, 5′-AACGGAGAAAGAAGACAGACTGCT (S), 5′-GACGAGACCAGGAGAAACAGGG (AS), and 5′-GTGAACGCTGGCTTCTGACA (P); MCP-1, 5′-GGAAAAATGGATCCACACCTTGC (S), 5′-TCTCTTCCTCCACCACCATGCAG (AS), and 5′-CTCATTCACCAGCAAGATGA (P); MCP-5, 5′-AGCTTTCATTTCGAAGTCTTTG (S), 5′-CTCCTTATCCAGTATGGTCC (AS), and 5′-CAGTCCTCAGGTATTGGCTGG (P); MIP-1α, 5′-CCCAGCCAGGTGTCATTTTCC (S), 5′-GCATTCAGTTCCAGGTCAGTG (AS), and 5′-TGCGCTGACTCCAAAGAGAC (P); MIP-1β, 5′-CCCTCTCTCTCCTCTTGCTCGT (S), 5′-TTCAACTCCAAGTCACTCATGTACTCA (AS), and 5′-AAAGAGGCAGACAGATCTGTGCTAAC (P); MIP-2, 5′-TGGGTGGGATGTAGCTAGTTCC (S), 5′-AGTTTGCCTTGACCCTGAAGCC (AS), and 5′-CCTGATGTGCCTCGCTGTCTG (P); and RANTES, 5′-GCGGGTACCATGAAGATCTCTG (S), 5′-CACTTCTTCTCTGGGTTGGCAC (AS), and 5′-GCAGTCGTGTTTGTCACTCGAA (P). The primers and probes for all other genes assessed in this study have been published previously (22, 23, 42, 52, 70). The optimum number of cycles for each organ and gene was determined empirically and was defined as the number of cycles that resulted in detectable PCR-amplified product under nonsaturating conditions.
Detection and quantitation of PCR products.
Amplified products were electrophoresed and transferred to Hybond N+ membranes (Amersham, Arlington Heights, Ill.) in 10× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) by standard southern blotting techniques. DNA was cross-linked by exposure to UV light, baked onto the nylon membrane, and hybridized with an internal oligonucleotide probe. Labeling of the probe and subsequent detection of bound probe were carried out with an enhanced chemiluminescence system (Amersham). Chemiluminescent signals were quantified with a scanner (Datacopy GS plus; Xerox Imaging Systems, Sunnyvale, Calif.). To determine the magnitude of change in gene expression, cDNA from a sample known to be positive for the transcript of interest was used to generate a standard curve by serial dilution of the positive control and simultaneous amplification. The signal of each band was plotted and fit to a standard curve by linear regression. The equation from this line was used to calculate the relative expression levels in test samples. Data were individually normalized for the relative quantity of mRNA by comparison to hypoxanthine-guanine phosphoribosyltransferase. Mean fold changes and SEM were calculated from the signal obtained from at least six mice analyzed at each time point. For each organ, means are expressed as fold induction relative to the response of untreated controls (t = 0), which was arbitrarily assigned a value of 1; this precludes the comparison of basal gene expression between organs.
Statistics.
Results were analyzed by Student’s t test for comparisons between two groups. P < 0.05 were accepted as the level of significance. All experiments were repeated two or three times with similar results.
RESULTS
Kinetic analysis of proinflammatory cytokine and iNOS mRNA expression.
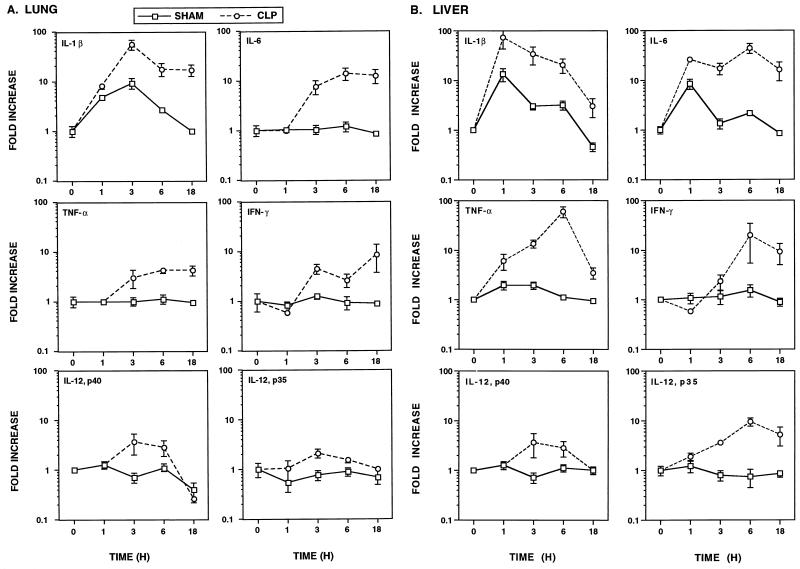
Peak levels of circulating cytokines like TNF-α and IL-6 can be detected during the first few hours after CLP (62). It is the overproduction of these and other proinflammatory cytokines that leads to the tissue damage observed during polymicrobial sepsis. Both severe ARDS (61) and hepatic injury (60) have been found by 24 h after CLP. Thus, in initial experiments, we established the kinetics of pulmonary and hepatic mRNA expression for the cytokines IL-1β, IL-6, TNF-α, IFN-γ, IL-12 (p35 and p40), G-CSF, M-CSF, and GM-CSF at 1, 3, 6, and 18 h after CLP. As shown in Fig. 1A, IL-1β, IL-6, TNF-α, and IFN-γ mRNA levels in the lungs were increased over those in sham-operated controls by 3 h after CLP and remained heightened for 18 h. In contrast to the slightly slower gene induction observed in the lungs, substantial increases in IL-1β, IL-6, and TNF-α mRNA levels in the liver, compared with those in sham-operated controls, were observed by 1 h after CLP (Fig. 1B). Even 18 h after CLP, IL-1β, IL-6, and TNF-α mRNA expression in the liver remained heightened over that in sham-operated controls. IFN-γ mRNA was not induced in the liver until 6 h after CLP, and its level remained elevated at 18 h. In contrast to IL-12 p35 mRNA in the lungs, which was weakly modulated (≤twofold), IL-12 p35 mRNA in the liver was induced ∼10-fold at 6 and 18 h after CLP. Interestingly, levels of IL-12 p40 mRNA in both the lungs and liver were poorly modulated following CLP, with some mice failing to respond with increased IL-12 p40 mRNA expression.
FIG. 1.
Regulation of proinflammatory cytokine mRNA expression in the lungs (A) and liver (B) following CLP. Data are expressed as the mean ± SEM from at least six individual mice. Means are expressed relative to the response of untreated mice (t = 0), which was arbitrarily assigned a value of 1. Sham-operated mice served as controls. When not visible, SEM bars are smaller than the symbol.
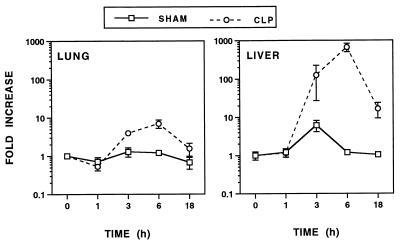
Hypotension develops after CLP and the ensuing bacteremia. Recently, iNOS knockout mice were used to demonstrate an iNOS-independent pathway of LPS-induced hypotension (37). Increased levels of iNOS mRNA in the lungs and liver, compared with those in sham-operated controls, were not observed until 3 h after CLP and peaked by 6 h after CLP (Fig. 2). A more dramatic increase in the level of iNOS mRNA was observed in the liver (>200-fold) than in the lungs (∼7-fold). While iNOS mRNA expression in the lungs had declined to near basal levels by 18 h, the level in the liver remained elevated (∼15-fold) over that in sham-operated controls.
FIG. 2.
Modulation of iNOS mRNA expression in the lungs and liver following CLP. Data were obtained as described in the legend to Fig. 1.
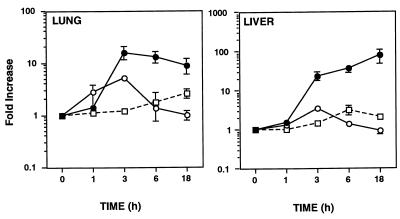
While pretreatment with G-CSF or GM-CSF protects against death in rodent models of peritonitis (5, 41), whether CSF mRNA expression is modulated during polymicrobial sepsis has not been addressed. G-CSF mRNA was strongly induced (≥10-fold) by 3 h in both the lungs and liver and remained at heightened levels 18 h after CLP (Fig. 3). In striking contrast to G-CSF mRNA, only a slight increase in M-CSF and GM-CSF mRNA (between two- and fourfold) were observed in the liver and lungs following CLP.
FIG. 3.
Temporal regulation of G-CSF (•), M-CSF (○), and GM-CSF (□) mRNA expression in the lungs and liver following CLP. CSF mRNA expression was not modulated in sham-operated mice control mice (data not shown). Data were obtained as described in the legend to Fig. 1.
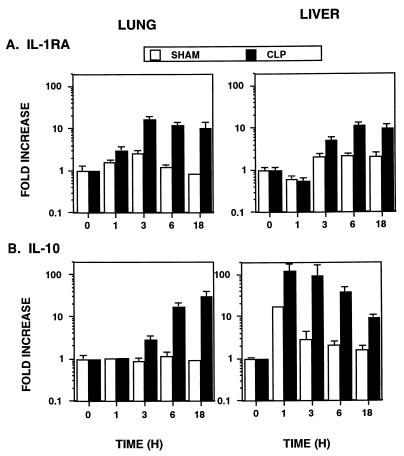
Regulation of anti-inflammatory cytokine mRNA expression during sepsis.
IL-10 and IL-1ra protect against CLP-mediated death and/or sepsis (1, 34, 58). Recently, IL-11 was shown to down-regulate the expression of many LPS-induced cytokines, including TNF-α, IL-1β, and IFN-γ, in vivo (55). Therefore, we next examined the temporal expression of potential negative regulators of the proinflammatory cytokine cascade that is induced during sepsis. IL-1ra mRNA was induced by 1 h in the lungs after CLP and by 3 h in the liver after CLP (Fig. 4). The peak IL-1ra mRNA level (∼10-fold increase) occurred 3 to 6 h after CLP and was sustained for 18 h. IL-10 mRNA in the lungs was induced by 3 h after CLP, while the IL-10 mRNA level in the liver was increased over that in sham-operated controls by 1 h. Interestingly, the IL-10 mRNA level in the lungs continued to increase, while the level in the liver slowly declined by 18 h. IL-11 mRNA expression in the lungs and liver was not modulated during sepsis (data not shown).
FIG. 4.
Regulation of anti-inflammatory cytokine mRNA expression following CLP. Data were obtained as described in the legend to Fig. 1.
Temporal analysis of chemokine mRNA expression during sepsis.
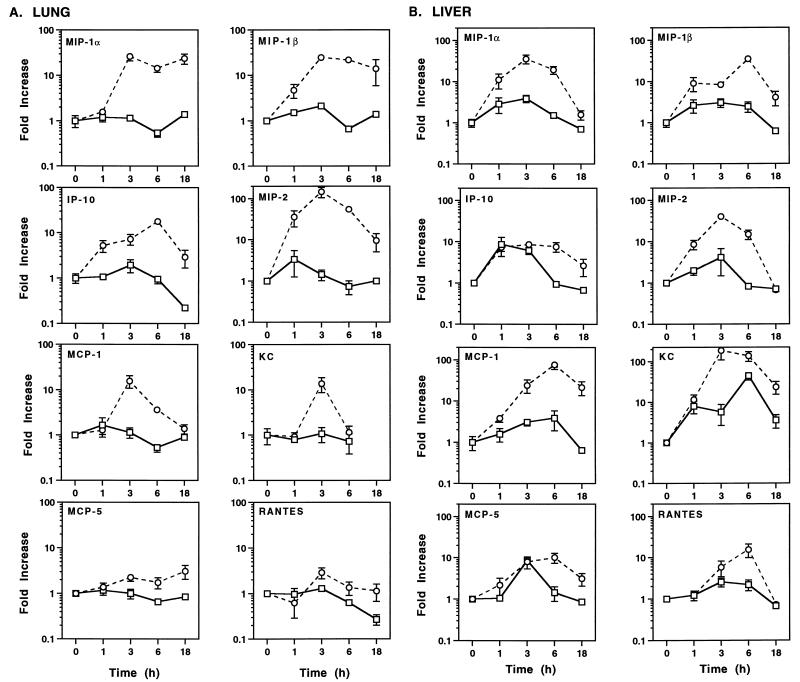
During sepsis, neutrophils and monocytes accumulate in the lungs and liver (17, 27, 59, 72). To address the role of chemoattractant production in mediating the influx of inflammatory cells, chemokine mRNA induction during polymicrobial sepsis was next investigated. As shown in Fig. 5A, MIP-1α, MIP-1β, MIP-2, and IP-10 mRNA levels in the lungs were strongly induced by 1 to 3 h after CLP, peaking at ∼10- to 100-fold-higher levels than those observed in sham-operated controls 3 to 6 h after CLP. mRNA expression remained heightened (∼10-fold) over that in sham-operated controls 18 h after CLP. In contrast, MCP-1 and KC mRNA levels in the lungs peaked (∼10-fold) at 3 h after CLP and declined to basal levels by 18 h. MCP-5 and RANTES mRNA levels in the lungs were poorly modulated (<two- to threefold) over those in sham-operated controls during sepsis.
FIG. 5.
Temporal regulation of chemokine mRNA expression in the lungs and liver following CLP (○) or sham operation (□). Data were obtained as described in the legend to Fig. 1.
A slightly different pattern of chemokine mRNA expression was observed in the liver. Not only MIP-1α, MIP-1β, and MIP-2 mRNA, but also MCP-1 and KC mRNA, were strongly induced (∼10- to 100-fold) during sepsis (Fig. 5B). Moreover, the subset of chemokine mRNA levels that remained heightened in the liver 18 h after CLP differed. Specifically, MIP-1β, MCP-1, and KC mRNA levels, but not MIP-1α or MIP-2 mRNA levels, remained heightened (∼5- to 20-fold) 18 h after CLP. Finally, IP-10, MCP-5, and RANTES mRNA levels in the liver were increased later (6 h) and to a much lower extent than for the other chemokine mRNAs in the liver. By 18 h after CLP, IP-10, MCP-5, and RANTES mRNA levels approached those exhibited by controls.
MPL pretreatment reduces cytokine, iNOS, and chemokine mRNA expression during sepsis.
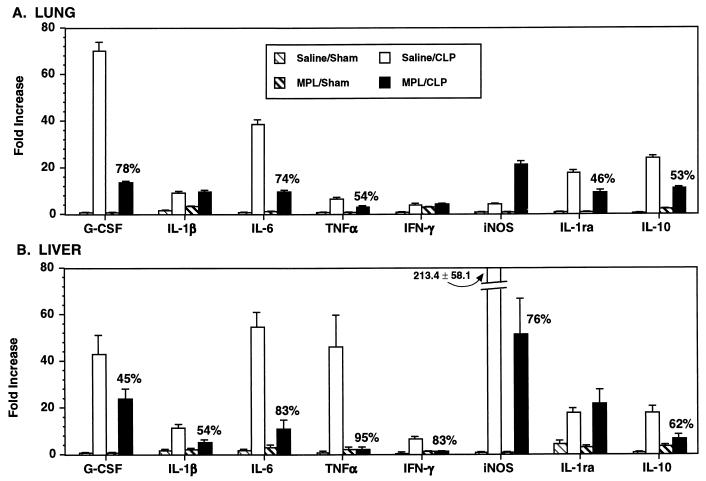
We and others have demonstrated that pretreatment with MPL induces endotoxin tolerance in vivo, reducing the levels of circulating cytokines like TNF-α, IFN-γ, and IL-6 following LPS challenge, as well as protecting against CLP-, LPS-, and E. coli-mediated death (4, 30, 38, 44). Thus, we next examined whether MPL-induced tolerance modulated proinflammatory cytokine, anti-inflammatory cytokine, and chemokine mRNA expression in the lungs and liver during polymicrobial sepsis. Mice were pretreated with 100 μg of MPL or saline 48 h prior to either CLP or sham operation, and the levels of cytokine and chemokine mRNA were assessed 6 h later. Only genes which had been modulated >twofold during CLP (Fig. 1 to 5) were examined in the tolerance experiments. G-CSF, IL-6, TNF-α, IL-1ra, and IL-10 mRNA levels in the lungs were reduced 46 to 78% in MPL-pretreated mice after CLP compared to those in saline-pretreated mice after CLP (Fig. 6A). G-CSF, IL-1β, IL-6, TNF-α, IFN-γ, iNOS, and IL-10 mRNA levels in the liver were reduced 45 to 95% in MPL-pretreated mice after CLP compared to those in saline-pretreated mice after CLP (Fig. 6B). Interestingly, IL-1β, IFN-γ, and iNOS mRNA levels in the lungs and the IL-1ra mRNA level in the liver were not down-regulated in MPL-pretreated mice after CLP. Finally, the level of mRNA for the anti-inflammatory cytokine IL-11 was not modulated in MPL-pretreated mice after CLP (data not shown).
FIG. 6.
Effect of MPL-pretreatment on cytokine and iNOS mRNA expression in the lungs (A) and liver (B) during sepsis. Mice were injected i.p. with either saline or MPL (100 μg) 48 h prior to CLP or sham operation. The lungs and liver were removed 6 h after CLP. Data are expressed as the mean ± SEM from six individual mice and are expressed relative to the response of untreated mice (data not shown), which were arbitrarily assigned a value of 1. When not visible, SEM bars are smaller than the symbol. Data in parentheses are the fold decrease in mRNA expression observed in MPL-pretreated (tolerized) mice after CLP compared to saline-pretreated (nontolerized) mice after CLP.
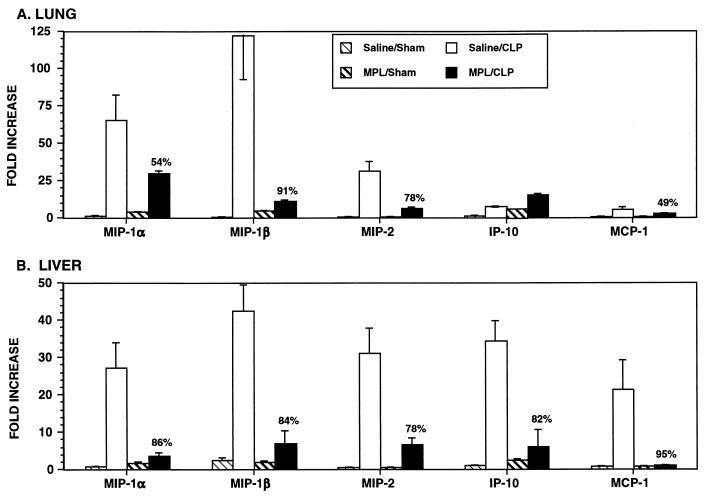
The tolerizing effect of MPL on CLP-induced chemokine gene expression was even more profound (Fig. 7). In the lungs, MIP-1α, MIP-1β, MIP-2, and MCP-1 mRNA levels were reduced 49 to 91% in MPL-pretreated mice after CLP. Moreover, in the liver, MIP-1α, MIP-1β, MIP-2, IP-10, and MCP-1 levels were reduced 78 to 95% in MPL-pretreated mice after CLP compared to those in saline-pretreated mice after CLP. Interestingly, expression of a subset of chemokine genes that included IP-10 mRNA in the lungs and KC, MCP-5, and RANTES mRNA in the liver (Fig. 7 and data not shown) was not reduced in MPL-pretreated mice after CLP.
FIG. 7.
MPL pretreatment reduces chemokine mRNA expression in the lungs (A) and liver (B) during sepsis. Data were obtained as described in the legend to Fig. 6.
MPL pretreatment reduces the levels of circulating cytokines and chemokines during CLP-induced sepsis.
We next assessed whether reduced levels of mRNA expression in the tissues of MPL-pretreated mice after CLP were associated with a corresponding decrease in circulating cytokine and/or chemokine levels. Levels of IL-6, TNF-α, MCP-1, MIP-1α, and MIP-2 in serum were quantified by ELISA from the same mice assessed for mRNA expression in Fig. 6 and 7. As shown in Table 1, MPL-pretreated mice had substantially reduced levels (≥fivefold) of IL-6, TNF-α, MCP-1, MIP-1α, and MIP-2 in serum after CLP than did saline-pretreated mice after CLP.
TABLE 1.
Cytokine and chemokine levels in serum are reduced in MPL-pretreated micea
| Cytokine or chemokine | Cytokine or chemokine level (pg/ml) inb:
|
|||
|---|---|---|---|---|
| Saline pretreated mice after:
|
MPL pretreated mice after:
|
|||
| Sham operation | CLP | Sham operation | CLP | |
| IL-6 | 220 ± 62 | 69,819 ± 27,350 | 74 ± 7 | 13,878 ± 7,314c |
| TNF-α | <45 | 2,026 ± 1,173 | <45 | 149 ± 85c |
| MCP-1 | 256 ± 44 | 37,099 ± 11,698 | 291 ± 8 | 5,245 ± 1,758c |
| MIP-1α | <5 | 2,067 ± 1,056 | <5 | 74.9 ± 55d |
| MIP-2 | 48 ± 11 | 36,345 ± 14,840 | 19 ± 6 | 3,885 ± 1,572c |
Mice were injected i.p. with saline or 100 μg of MPL 48 h prior to CLP or sham operation. Serum was collected 6 h after CLP or sham operation.
Mean ± SEM from six mice.
Data from MPL-pretreated mice after CLP are significantly lower (P < 0.05) than data from saline-pretreated mice after CLP.
Comparison of MPL-pretreated mice after CLP to saline-pretreated mice after CLP yielded P = 0.061.
Neutrophil margination and MPO activity are reduced during MPL-induced tolerance.
Since MPL-pretreated mice exhibited substantially lower levels of chemokine mRNA in their lungs, as well as reduced levels of circulating chemokines, after CLP than did saline-pretreated mice, histopathological changes and MPO activity in the lungs were assessed in saline- and MPL-pretreated mice 6 h after CLP. Normal lung tissue was observed in both saline- and MPL-pretreated sham-operated control mice. Increased neutrophil margination along pulmonary venules was observed in four of five saline-pretreated mice and only in two of five MPL-pretreated mice. Moreover, MPO activity, which has been used as a more sensitive, quantitative measure of neutrophil sequestration (27, 66), was reduced (∼threefold [P = 0.002]) in the lungs of MPL-pretreated mice after CLP compared to that in saline-pretreated mice after CLP (Table 2).
TABLE 2.
Effect of MPL pretreatment on CLP-induced neutrophil sequestration in the lungs
| Groupa | MPO activity (U/g of tissue)b |
|---|---|
| Saline/sham | 5.3 ± 0.4 |
| Saline/CLP | 36.4 ± 5.5 |
| MPL/sham | 4.0 ± 0.4 |
| MPL/CLP | 11.4 ± 1.4c |
Mice were injected i.p. with saline or 100 μg of MPL 48 h prior to CLP or sham operation.
Data are expressed as the mean ± SEM from five mice.
Significantly different (P = 0.002) from the result for saline/CLP by Student’s two-tailed t test.
DISCUSSION
In the present study, we addressed the in vivo kinetics of a large panel of proinflammatory and anti-inflammatory cytokines, as well as chemokines, in the lungs and liver following CLP. This allowed us, as sepsis progressed, to monitor gene expression in two organ systems that are typically involved in the multiple-organ dysfunction syndrome observed clinically. The genes used in this study were chosen for their potential involvement as mediators of the overwhelming inflammatory response and the resulting pathological sequelae observed during sepsis. To date, mRNA expression following CLP had been monitored for only a few genes, including those encoding TNF-α, IL-1β, iNOS, IL-10, and CINC (a rat analog of KC) (15, 26, 35, 58, 67). Our data demonstrate that IL-1β, TNF-α, IL-6, and iNOS mRNAs are strongly induced following CLP, with generally greater increases and faster kinetics in the liver than in the lungs. A striking observation was the profound increase in TNF-α mRNA expression observed in the liver after CLP. This was unexpected since the high levels of circulating TNF-α observed during endotoxemia in mice (26, 45, 48) are not typically observed after CLP (Table 1) (26, 27). Moreover, only modest increases in TNF-α mRNA expression in tissues have been observed after LPS challenge (48, 56). This discordance between tissue-specific TNF-α mRNA production and protein levels in serum between models of endotoxicity and sepsis suggests that locally synthesized TNF-α does not necessarily reach the circulation and that the levels of a particular cytokine in serum are not always an accurate reflection of events in the tissue microenvironment (48). Moreover, the data also suggest fundamental differences between LPS and CLP with regard to TNF induction. This is supported by the observation that anti-TNF Abs protect against death during endotoxicity but not during polymicrobial sepsis induced by CLP (8, 18).
IFN-γ enhances both the CLP- and LPS-induced mortality rate (28, 39). In many systems, including endotoxicity, IL-12 appears to be both proximal to and required for IFN-γ production (29, 71). While IFN-γ mRNA expression was induced in both the lungs and liver following CLP, IL-12 p40 mRNA levels in the lungs and liver and IL-12 p35 mRNA levels in the lungs were only weakly modulated. The IL-12 p40 mRNA data obtained in the CLP model are in sharp contrast to the profound induction of hepatic IL-12 p40 mRNA (>100-fold) observed after LPS challenge (48) and suggest that IL-12 may not contribute to the induction of IFN-γ in the CLP model to the same extent as has been observed in LPS-injected mice. Recently, IL-18 also has been demonstrated to up-regulate IFN-γ and anti-IL-18 Ab prevented liver damage in Proprionibacterium acnes-sensitized, LPS-challenged mice (40). Whether IL-18 is involved in up-regulating IFN-γ production during sepsis is unknown. Of note, IL-12 p35 mRNA was induced ∼10-fold in the liver after CLP, as observed after LPS injection (48); typically, IL-12 p40 mRNA production exceeds that of IL-12 p35 (13, 29, 48). While excess IL-12 p40 is secreted and functions as a receptor antagonist (36), IL-12 p35 secretion has not been observed (14).
The CSFs are a family of growth factors that promote the proliferation and differentiation of myeloid precursors and the activation of neutrophils and monocytes. G-CSF, GM-CSF, and M-CSF enhance chemotaxis and phagocytosis and upregulate β2-integrin and selectin expression on phagocytic and endothelial cells (12, 24, 50, 65). While pretreatment with G-CSF or GM-CSF protects against death in rodent models of peritonitis (5, 41), whether CSF mRNA expression was modulated during sepsis was previously unknown. We observed a striking increase in G-CSF mRNA levels in both the lungs and liver and a modest increase in the M-CSF mRNA level in the lungs following CLP. In contrast, the M-CSF mRNA level in the liver and the GM-CSF mRNA levels in the lungs and liver were weakly induced. During endotoxemia, large increases in G-CSF, GM-CSF, and M-CSF mRNA levels in the liver (20- to 100-fold) are observed (48, 49), once again illustrating that substantial differences in tissue-specific gene induction are observed between models of polymicrobial sepsis and endotoxemia.
IL-10 is a potent inhibitor of LPS-induced gene transcription, down-regulating a broad range of cytokines and chemokines (7, 16, 46), and endogenous production of IL-10 during sepsis is protective (58, 63). In this study, IL-10 mRNA was induced rapidly in the liver but its induction in the lungs was delayed. These data are in agreement with IL-10 mRNA data published by van der Poll et al. (58). More recently, IL-11 was shown to inhibit TNF-α, IL-1β, and IFN-γ in serum following LPS injection (55). In contrast to IL-10, we observed no in vivo modulation of IL-11 mRNA after CLP, indicating that while IL-11 has efficacy in down-regulating LPS-induced cytokines, it may not be endogenously produced during septic shock.
Disrupting the recruitment of inflammatory cells with Abs to either PMNs, MIP-1α, or MIP-2 attenuates tissue damage during endotoxemia and peritonitis (32, 51, 53, 66), and illustrates the importance of chemokine production and inflammatory cell recruitment in the pathological findings associated with sepsis. A diverse array of chemokines were induced in the lungs and liver during polymicrobial sepsis. The most profound up-regulation (>150-fold) of chemokine mRNA expression was observed for PMN chemoattractants, i.e., MIP-2 in the lungs and KC in the liver. These data support and extend the work of others, who found MIP-2 in serum and organ homogenates and both CINC/KC mRNA and protein in the liver (15, 64). Recently, anti-MIP-2 Ab was shown to reduce only partially the number of infiltrating neutrophils in peritoneal fluid after CLP (64). The results of this study, in combination with our in vivo data, suggest that other PMN chemoattractants, like KC, which has roughly equivalent potency to MIP-2 (69), and G-CSF, which also has chemotactic activity (12), and possibly others, contribute to PMN recruitment during sepsis. Large increases in the levels of monocyte chemoattractants were also observed after CLP. In the liver, MCP-1, MIP-1α, and MIP-1β mRNA levels were up-regulated ∼80-, 35-, and 30-fold, respectively, while production of MIP-1α, MIP-1β, and IP-10 predominated in the lungs. In vitro, MCP-1 is a potent monocyte chemoattractant with an activity comparable to that of IL-8 activity for PMNs, while MIP-1α and MIP-1β are progressively less potent than MCP-1 (11).
In the lungs, increased PMN infiltration precedes increased macrophage numbers after LPS injection (53, 59), and an analogous situation presumably occurs after CLP. However, no overt differences in the kinetics of PMN and monocyte chemoattractants in either the lungs or the liver were observed. In the lungs, for example, MIP-1β, IP-10, and MIP-2 were induced 1 h after CLP, indicating that both monocyte and PMN recruitment begins quite early. Finally, of all the chemokines examined, only pulmonary MCP-5 and RANTES were poorly modulated (<threefold). Of interest, these chemokines were up-regulated to a greater extent in the lungs following LPS challenge (47).
Prophylactic administration of MPL protects against CLP-, E. coli-, and LPS-mediated death and is associated with ameliorated production of circulating cytokines and attenuated liver damage (2, 4, 25, 30, 38, 44). These studies demonstrate for the first time that MPL pretreatment profoundly alters tissue-specific mRNA expression in tissues that are associated with organ failure. The levels of tissue mRNA and/or serum protein for proinflammatory mediators like TNF-α, IL-1β, IL-6, IFN-γ, and iNOS were reduced, and in some cases ablated, in MPL-pretreated mice after CLP. These agents mediate the adhesive properties of phagocytic cells, vascular dysfunction, tissue damage and/or potentiate death during sepsis (9, 21, 35, 39, 54, 68). Noteworthy was the tissue-specific difference in genes that were rendered tolerant by MPL pretreatment. Specifically, IL-1β, IFN-γ, and iNOS mRNA levels in the liver, but not the lungs, were reduced in MPL-pretreated mice after CLP. Anti-inflammatory cytokine (IL-10 and IL-1ra) levels also were reduced in MPL-pretreated mice after CLP. This would suggest that reduced levels of proinflammatory mediators observed in tolerized subjects rendered septic are not likely to be due to the overproduction of anti-inflammatory cytokines with broad negative regulatory activity, such as IL-10 and IL-11.
In these studies, we observed profound reductions in chemokine mRNA production in the lungs and liver of MPL-pretreated mice after CLP, as well as in their serum. While not all the chemokine genes examined were affected, a broad range of neutrophil and monocyte chemoattractants showed reduced levels. Moreover, reduction in the chemokine mRNA level corresponded to decreased leukocyte margination and MPO activity in the lungs, indicating that endotoxin tolerance attenuates neutrophil accumulation in vivo. These findings support and extend the work by Yao et al. (72), who demonstrated reduced MPO activity in the lungs, liver, kidneys, and heart of MPL-pretreated rats challenged with LPS. In toto, these data suggest that prophylactic administration of MPL attenuates chemokine production, thereby reducing the number of inflammatory cells migrating into tissue. This is concomitant with a reduced production of proinflammatory mediators in tissues, attenuating organ damage and increasing survival. The reduced level of proinflammatory mediators in a particular organ is probably due to not only decreased numbers of activated recruited cells but also reduced cytokine production by both resident and recruited cells. To this end, macrophages (cells central to the host response to LPS [48]) that have been tolerized with MPL prior to LPS treatment have suppressed levels of TNFα, IL-1β, and IP-10 (31). Finally, the IL-8 level in serum is decreased in human volunteers prophylactically administered MPL prior to LPS challenge (3).
In summary, we have demonstrated that mRNA expression for a broad range of proinflammatory and anti-inflammatory cytokines and chemokines is up-regulated during the course of polymicrobial sepsis. In general, cytokine and chemokine mRNA expression was induced earlier in the liver than in the lungs. Prophylactic administration of MPL reduced the level of tissue-specific mRNA for a number of cytokines and chemokines. A concomitant diminution of cytokine and chemokine protein levels was observed in the serum. Attenuated levels of chemokine expression were associated with reductions in pulmonary neutrophil accumulation. The ability of prophylactic MPL treatment to mitigate the recruitment of inflammatory cells into organs during the course of an inflammatory event like sepsis under the conditions used in this study, along with the concomitant reduction in the levels of proinflammatory mediators, very probably contributes to the capacity of MPL to ameliorate tissue damage (25) and to provide nonspecific resistance to a diverse array of agents including TNF-α, gram-positive and negative bacteria and their products, and other infectious agents (44).
ACKNOWLEDGMENTS
We thank J. Terry Ulrich, RIBI ImmunoChemical Research, Inc., for providing the MPL used in these studies, and we thank Diana Miller for technical assistance.
This work was supported by NMRDC protocol 63706NM0095.001.9401 and NIH grant AI-18797 (to S.N.V.).
REFERENCES
- 1.Alexander H R, Doherty G M, Venzon D J, Merino M J, Fraker D L, Norton J A. Recombinant interleukin-1 receptor antagonist (IL-1ra): effective therapy against Gram-negative sepsis in rats. Surgery. 1992;112:188–194. [PubMed] [Google Scholar]
- 2.Astiz M E, Galera A, Saha D C, Carpati C, Rackow E C. Monophosphoryl lipid A protects against gram-positive sepsis and tumor necrosis factor. Shock. 1994;2:271–274. doi: 10.1097/00024382-199410000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Astiz M E, Rackow E C, Still J G, Howell S T, Cato A, Von Eschen K B, Ulrich J T, Rudbach J A, McMahon G, Vargas R, Stern W. Pretreatment of normal humans with monophosphoryl lipid A induces tolerance to endotoxin: a prospective, double-blind, randomized, controlled trial. Crit Care Med. 1995;23:9–17. doi: 10.1097/00003246-199501000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Astiz M E, Saha D C, Carpati C M, Rackow E C. Induction of endotoxin tolerance with monophosphoryl lipid A in peritonitis: importance of localized therapy. J Lab Clin Med. 1994;123:89–93. [PubMed] [Google Scholar]
- 5.Austin O M, Redmond H P, Watson W G, Cunney R J, Grace P A, Bouchier-Hayes D. The beneficial effects of immunostimulation in posttraumatic sepsis. J Surg Res. 1995;59:446–449. doi: 10.1006/jsre.1995.1189. [DOI] [PubMed] [Google Scholar]
- 6.Baker C C, Chaudry I H, Gaines H O, Baue A E. Evaluation of factors affecting mortality rate after sepsis in a murine cecal ligation and puncture model. J Surg. 1983;94:331–335. [PubMed] [Google Scholar]
- 7.Berkman N, John M, Roesems G, Jose P J, Barnes P J, Chung K F. Inhibition of macrophage inflammatory protein-1 alpha expression by IL-10. Differential sensitivities in human blood monocytes and alveolar macrophages. J Immunol. 1995;155:4412–4418. [PubMed] [Google Scholar]
- 8.Beutler B, Milsark I W, Cerami A C. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985;229:869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- 9.Bevilacqua M P, Pober J S, Wheeler M E, Cotran R S, Gimbrone M A., Jr Interleukin 1 acts on cultured human vascular endothelium to increase the adhesion of polymorphonuclear leukocytes, monocytes, and related leukocyte cell lines. J Clin Invest. 1985;76:2003–2011. doi: 10.1172/JCI112200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bone R C. Gram-negative sepsis: a dilemma of modern medicine. Clin Microbiol Rev. 1993;6:57–68. doi: 10.1128/cmr.6.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cross A K, Richardson V, Ali S A, Palmer I, Taub D D, Rees R C. Migration responses of human monocytic cell lines to alpha- and beta-chemokines. Cytokine. 1997;9:521–528. doi: 10.1006/cyto.1996.0196. [DOI] [PubMed] [Google Scholar]
- 12.Dale D C, Liles W C, Summer W R, Nelson S. Granulocyte colony-stimulating factor—role and relationships in infectious diseases. J Infect Dis. 1995;172:1061–1075. doi: 10.1093/infdis/172.4.1061. [DOI] [PubMed] [Google Scholar]
- 13.D’Andrea A, Aste-Amezaga M, Valiante N M, Ma X, Kubin M, Trinchieri G. Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J Exp Med. 1993;178:1041–1048. doi: 10.1084/jem.178.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D’Andrea A, Rengaraju M, Valiante N M, Chehimi J, Kubin M, Aste M, Chan S H, Kobayashi M, Young D, Nickbarg E, Chizzonite R, Wolf S F, Trinchieri G. Production of natural killer cell stimulatory factor (interleukin 12) by peripheral blood mononuclear cells. J Exp Med. 1992;176:1387–1398. doi: 10.1084/jem.176.5.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deutschman C S, Haber B A, Andrejko K, Cressman D E, Harrison R, Elenko E, Taub R. Increased expression of cytokine-induced neutrophil chemoattractant in septic rat liver. Am J Physiol. 1996;271:R593–R600. doi: 10.1152/ajpregu.1996.271.3.R593. [DOI] [PubMed] [Google Scholar]
- 16.de Waal Malefyt R, Abrams J, Bennett B, Figdor C G, de Vries J E. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doherty D E, Downey G P, Schwab III B, Elson E, Worthen G S. Lipolysaccharide-induced monocyte retention in the lung. Role of monocyte stiffness, actin assembly, and CD18-dependent adherence. J Immunol. 1994;153:241–255. [PubMed] [Google Scholar]
- 18.Echtenacher B, Falk W, Mannell D N, Krammer P H. Requirement of endogenous tumor necrosis factor/cachectin for recovery from experimental peritonitis. J Immunol. 1990;148:3762–3766. [PubMed] [Google Scholar]
- 19.Essani N A, Bajt M L, Farhood A, Vonderfecht S L, Jaeschke H. Transcriptional activation of vascular cell adhesion molecule-1 gene in vivo and its role in the pathophysiology of neutrophil-induced liver injury in murine endotoxin shock. J Immunol. 1997;158:5941–5948. [PubMed] [Google Scholar]
- 20.Essani N A, Fisher M A, Farhood A, Manning A M, Smith C W, Jaeschke H. Cytokine-induced upregulation of hepatic intercellular adhesion molecule-1 messenger RNA expression and its role in the pathophysiology of murine endotoxin shock and acute liver failure. Hepatology. 1995;21:1632–1639. [PubMed] [Google Scholar]
- 21.Fischer E, Marano M A, Van Zee K J, Rock C S, Hawes A S, Thompson W A, DeForge L, Kenney J S, Remick D G, Bloedow D C, Thompson S F, Moldawer L L. Interleukin-1 receptor blockade improves survival and hemodynamic performance in Escherichia coli septic shock, but fails to alter host responses to sublethal endotoxemia. J Clin Invest. 1992;89:1551–1557. doi: 10.1172/JCI115748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fultz M J, Barber S A, Dieffenbach C W, Vogel S N. Induction of IFN-gamma in macrophages by lipopolysaccharide. Int Immunol. 1993;5:1383–1392. doi: 10.1093/intimm/5.11.1383. [DOI] [PubMed] [Google Scholar]
- 23.Gazzinelli R T, Eltoum I, Wynn T A, Sher A. Acute cerebral toxoplasmosis is induced by in vivo neutralization of TNF-α and correlates with the down-regulated expression of inducible nitric oxide synthase and other markers of macrophage activation. J Immunol. 1993;151:3672–3681. [PubMed] [Google Scholar]
- 24.Goebeler M, Roth J, Kunz M, Sorg C. Expression of intercellular adhesion molecule-1 by murine macrophages is up-regulated during differentiation and inflammatory activation. Immunobiology. 1993;188:159–171. doi: 10.1016/S0171-2985(11)80495-X. [DOI] [PubMed] [Google Scholar]
- 25.Gustafson G L, Rhodes M J, Hegel T. Monophosphoryl lipid A as a prophylactic for sepsis and septic shock. In: Levin J, Alving C R, Munford R S, Redl H, editors. Bacterial endotoxins: lipopolysaccharides from genes to therapy. New York, N.Y: Wiley-Liss; 1995. pp. 567–579. [PubMed] [Google Scholar]
- 26.Hadjiminas D J, McMasters K M, Peyton J C, Cheadle W G. Tissue tumor necrosis factor mRNA expression following cecal ligation and puncture or intraperitoneal injection of endotoxin. J Surg Res. 1994;56:549–555. doi: 10.1006/jsre.1994.1088. [DOI] [PubMed] [Google Scholar]
- 27.Hadjiminas D J, McMasters K M, Peyton J C, Cook M D, Cheadle W G. Passive immunization against tumor necrosis factor and interleukin-1 fails to reduce lung neutrophil sequestration in chronic sepsis. Shock. 1994;2:376–380. doi: 10.1097/00024382-199411000-00013. [DOI] [PubMed] [Google Scholar]
- 28.Heinzel F P. The role of IFN-γ in the pathology of experimental endotoxemia. J Immunol. 1990;145:2920–2924. [PubMed] [Google Scholar]
- 29.Heinzel F P, Rerko R M, Ling P, Hakimi J, Schoenhaut D S. Interleukin 12 is produced in vivo during endotoxemia and stimulates synthesis of gamma interferon. Infect Immun. 1994;62:4244–4249. doi: 10.1128/iai.62.10.4244-4249.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henricson B E, Benjamin W R, Vogel S N. Differential cytokine induction by doses of lipopolysaccharide and monophosphoryl lipid A that result in equivalent early endotoxin tolerance. Infect Immun. 1990;58:2429–2437. doi: 10.1128/iai.58.8.2429-2437.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Henricson B E, Manthey C L, Perera P Y, Hamilton T A, Vogel S N. Dissociation of lipopolysaccharide (LPS)-inducible gene expression in murine macrophages pretreated with smooth LPS versus monophosphoryl lipid A. Infect Immun. 1993;61:2325–2333. doi: 10.1128/iai.61.6.2325-2333.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hewett J A, Schultze A E, VanCise S, Roth R A. Neutrophil depletion protects against liver injury from bacterial endotoxin. Lab Invest. 1992;66:347–361. [PubMed] [Google Scholar]
- 33.Jaeschke H, Farhood A, Smith C W. Neutrophil-induced liver cell injury in endotoxin shock is a CD11b/CD18-dependent mechanism. Am J Physiol. 1991;261:G1051–1056. doi: 10.1152/ajpgi.1991.261.6.G1051. [DOI] [PubMed] [Google Scholar]
- 34.Kato T, Murata A, Ishida H, Toda H, Tanaka N, Hayashida H, Monden M, Matsuura N. Interleukin 10 reduces mortality from severe peritonitis in mice. Antimicrob Agents Chemother. 1995;39:1336–1340. doi: 10.1128/aac.39.6.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li S, Fan S X, McKenna T M. Role of nitric oxide in sepsis-induced hyporeactivity in isolated rat lungs. Shock. 1996;5:122–129. doi: 10.1097/00024382-199602000-00007. [DOI] [PubMed] [Google Scholar]
- 36.Ling P, Gately M K, Gubler U, Stern A S, Lin P, Hollfelder K, Su C, Pan Y C, Hakimi J. Human IL-12 p40 homodimer binds to the IL-12 receptor but does not mediate biologic activity. J Immunol. 1995;154:116–127. [PubMed] [Google Scholar]
- 37.MacMicking J D, Nathan C, Hom G, Chartrain N, Fletcher D S, Trumbauer M, Stevens K, Xie Q W, Sokol K, Hutchinson N, Chen H, Mudgett J S. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell. 1995;81:641–650. doi: 10.1016/0092-8674(95)90085-3. [DOI] [PubMed] [Google Scholar]
- 38.Madonna G S, Peterson J E, Ribi E E, Vogel S N. Early-phase endotoxin tolerance: induction by a detoxified lipid A derivative, monophosphoryl lipid A. Infect Immun. 1986;52:6–11. doi: 10.1128/iai.52.1.6-11.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miles R H, Paxton T P, Dries D J, Gamelli R L. Interferon-gamma increases mortality following cecal ligation and puncture. J Trauma. 1994;36:607–611. doi: 10.1097/00005373-199405000-00001. [DOI] [PubMed] [Google Scholar]
- 40.Okamura H, Tsutsi H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, Torigoe K, Okura T, Nukada Y, Hattori K, Akita K, Namba M, Tanabe F, Konishi K, Fukuda F, Kurimoto M. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature. 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 41.O’Reilly M, Silver G M, Greenhalgh D G, Gamelli R L, Davis J H, Hebert J C. Treatment of intra-abdominal infection with granulocyte colony-stimulating factor. J Trauma. 1992;33:679–682. doi: 10.1097/00005373-199211000-00014. [DOI] [PubMed] [Google Scholar]
- 42.Peterson V M, Adamovicz J J, Elliott T B, Moore M M, Madonna G S, Jackson III W E, Ledney G D, Gause W C. Gene expression of hematoregulatory cytokines is elevated endogenously after sublethal gamma irradiation and is differentially enhanced by therapeutic administration of biologic response modifiers. J Immunol. 1994;153:2321–2330. [PubMed] [Google Scholar]
- 43.Remick D, Manohar P, Bologos G, Rodriguez J, Moldawar L, Wollenberg G. Blockada of tumor necrosis factor reduces lipopolysaccharide, but not the lethality of cecal ligation and puncture. Shock. 1995;4:89–95. doi: 10.1097/00024382-199508000-00002. [DOI] [PubMed] [Google Scholar]
- 44.Rudbach J A, Myers K R, Rechtman D J, Ulrich J T. Prophylactic use of monophosphoryl lipid A in patients at risk for sepsis. In: Levin J, van Deventer S J H, van der Poll T, Sturk A, editors. Bacterial endotoxins. Basic science to anti-sepsis strategies. New York, N.Y: Wiley-Liss, Inc.; 1994. pp. 107–124. [PubMed] [Google Scholar]
- 45.Salkowski C A, Detore G, McNally R, van Rooijen N, Vogel S N. Regulation of inducible nitric oxide synthase messenger RNA expression and nitric oxide production by lipopolysaccharide in vivo. The roles of macrophages, endogenous IFN-gamma, and TNF receptor-1-mediated signaling. J Immunol. 1997;158:905–912. [PubMed] [Google Scholar]
- 46.Salkowski C A, Detore G R, Vogel S N. Lipopolysaccharide and monophosphoryl lipid A differentially regulate interleukin-12, gamma interferon, and interleukin-10 mRNA production in murine macrophages. Infect Immun. 1997;65:3239–3247. doi: 10.1128/iai.65.8.3239-3247.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salkowski C A, Koplydowski K, Franks A, Falk M, Vogel S N. Abstracts of The Society for Leukocyte Biology 32nd Annual Meeting. Baltimore, Md: Society for Leukocyte Biology; 1997. In vivo chemokine mRNA expression during endotoxemia, peritonitis and tolerance, abstr. S5. [Google Scholar]
- 48.Salkowski C A, Neta R, Wynn T A, Strassmann G, van Rooijen N, Vogel S N. Effect of liposome-mediated macrophage depletion on LPS-induced cytokine gene expression and radioprotection. J Immunol. 1995;155:3168–3179. [PubMed] [Google Scholar]
- 49.Salkowski, C. A., and S. N. Vogel. Unpublished data.
- 50.Savage C O, Brooks C J, Adu D, Richards G, Howie A J. Cell adhesion molecule expression within human glomerular and kidney organ culture. J Pathol. 1997;181:111–115. doi: 10.1002/(SICI)1096-9896(199701)181:1<111::AID-PATH698>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 51.Schmal H, Shanley T P, Jones M L, Friedl H P, Ward P A. Role for macrophage inflammatory protein-2 in lipopolysaccharide-induced lung injury in rats. J Immunol. 1996;156:1963–1972. [PubMed] [Google Scholar]
- 52.Sivo J, Salkowski C A, Politis A D, Vogel S N. Differential regulation of LPS-induced IL-1β and IL-1 receptor antagonist mRNA by IFNα and IFNγ in murine peritoneal macrophages. J Endotoxin Res. 1994;1:30–37. [Google Scholar]
- 53.Standiford T J, Kunkel S L, Lukacs N W, Greenberger M J, Danforth J M, Kunkel R G, Strieter R M. Macrophage inflammatory protein-1α mediates lung leukocyte recruitment, lung capillary leak, and early mortality in murine endotoxemia. J Immunol. 1995;155:1515–1524. [PubMed] [Google Scholar]
- 54.Stephens K E, Ishizaka A, Larrick J W, Raffin T A. Tumor necrosis factor causes increased pulmonary permeability and edema. Comparison to septic acute lung injury. Am Rev Respir Dis. 1988;137:1364–1370. doi: 10.1164/ajrccm/137.6.1364. [DOI] [PubMed] [Google Scholar]
- 55.Trepicchio W L, Bozza M, Pedneault G, Dorner A J. Recombinant human IL-11 attenuates the inflammatory response through down-regulation of proinflammatory cytokine release and nitric oxide production. J Immunol. 1996;157:3627–3634. [PubMed] [Google Scholar]
- 56.Ulich T R, Guo K, del Castillo J. Endotoxin-induced cytokine gene expression in vivo. I. Expression of tumor necrosis factor mRNA in visceral organs under physiologic conditions and during endotoxemia. Am J Pathol. 1989;134:11–14. [PMC free article] [PubMed] [Google Scholar]
- 57.Ulich T R, Howard S C, Remick D G, Wittwer A, Yi E S, Yin S, Guo K, Welply J K, Williams J H. Intratracheal administration of endotoxin and cytokines. VI. Antiserum to CINC inhibits acute inflammation. Am J Physiol. 1995;268:L245–250. doi: 10.1152/ajplung.1995.268.2.L245. [DOI] [PubMed] [Google Scholar]
- 58.van der Poll T, Marchant A, Buurman W A, Berman L, Keogh C V, Lazarus D D, Nguyen L, Goldman M, Moldawer L L, Lowry S F. Endogenous IL-10 protects mice from death during septic peritonitis. J Immunol. 1995;155:5397–5401. [PubMed] [Google Scholar]
- 59.VanOtteren G M, Strieter R M, Kunkel S L, Paine III R, Greenberger M J, Danforth J M, Burdick M D, Standiford T J. Compartmentalized expression of RANTES in a murine model of endotoxemia. J Immunol. 1995;154:1900–1908. [PubMed] [Google Scholar]
- 60.Villa P, Demitri M, Meazza C, Sironi M, Gnocchi P, Ghezzi P. Effects of methyl palmitate on cytokine release, liver injury and survival in mice with sepsis. Eur Cytokine Network. 1996;7:765–769. [PubMed] [Google Scholar]
- 61.Villa P, Meassa M, Sironi M, Bianchi M, Ulrich P, Botchkina G, Tracey K J, Ghezzi P. Protection against lethal polymicrobial sepsis by CNI-1493, an inhibitor of pro-inflammatory cytokine synthesis. J Endotoxin Res. 1997;4:197–204. [Google Scholar]
- 62.Villa P, Sartor G, Angelini M, Sironi M, Conni M, Gnocchi P, Isetta A M, Grau G, Burrman W, van Tits L J, Ghezzi P. Pattern of cytokines and pharmacomodulation in sepsis induced by cecal ligation and puncture compared with that induced by endotoxin. Clin Diagn Lab Immunol. 1995;2:549–553. doi: 10.1128/cdli.2.5.549-553.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Walley K R, Lukacs N W, Standiford T J, Strieter R M, Kunkel S L. Balance of inflammatory cytokines related to severity and mortality of murine sepsis. Infect Immun. 1996;64:4733–4738. doi: 10.1128/iai.64.11.4733-4738.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Walley K R, Lukacs N W, Standiford T J, Strieter R M, Kunkel S L. Elevated levels of macrophage inflammatory protein 2 in severe murine peritonitis increase neutrophil recruitment and mortality. Infect Immun. 1997;65:3847–3851. doi: 10.1128/iai.65.9.3847-3851.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang J M, Chen Z G, Colella S, Bonilla M A, Welte K, Bordignon C, Mantovani A. Chemotactic activity of recombinant human granulocyte colony-stimulating factor. Blood. 1988;72:1456–1460. [PubMed] [Google Scholar]
- 66.Wickel D J, Cheadle W G, Mercer-Jones M A, Garrison R N. Poor outcome from peritonitis is caused by disease acuity and organ failure, not recurrent peritoneal infection. Ann Surg. 1997;225:744–753. doi: 10.1097/00000658-199706000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wilson M A, Chou M C, Spain D A, Downard P J, Qian Q, Cheadle W G, Garrison R N. Fluid resuscitation attenuates early cytokine mRNA expression after peritonitis. J Trauma. 1996;41:622–627. doi: 10.1097/00005373-199610000-00005. [DOI] [PubMed] [Google Scholar]
- 68.Witthaut R, Farhood A, Smith C W, Jaeschke H. Complement and tumor necrosis factor-α contribute to Mac-1 (CD11b/CD18) up-regulation and systemic neutrophil activation during endotoxemia in vivo. J Leukocyte Biol. 1994;55:105–111. doi: 10.1002/jlb.55.1.105. [DOI] [PubMed] [Google Scholar]
- 69.Wuyts A, Haelens A, Proost P, Lenaerts J P, Conings R, Opdenakker G, Van Damme J. Identification of mouse granulocyte chemotactic protein-2 from fibroblasts and epithelial cells. Functional comparison with natural KC and macrophage inflammatory protein-2. J Immunol. 1996;157:1736–1743. [PubMed] [Google Scholar]
- 70.Wynn T A, Eltoum I, Cheever A W, Lewis F A, Gause W C, Sher A. Analysis of cytokine mRNA expression during primary granuloma formation induced by eggs of Schistosoma mansoni. J Immunol. 1993;151:1430–1440. [PubMed] [Google Scholar]
- 71.Wysocka M, Kubin M, Vieira L Q, Ozmen L, Garotta G, Scott P, Trinchieri G. Interleukin-12 is required for interferon-γ production and lethality in lipopolysaccharide-induced shock in mice. Eur J Immunol. 1995;25:672–676. doi: 10.1002/eji.1830250307. [DOI] [PubMed] [Google Scholar]
- 72.Yao Z, Foster P A, Gross G J. Monophosphoryl lipid A protects against endotoxic shock via inhibiting neutrophil infiltration and preventing disseminated intravascular coagulation. Circ Shock. 1994;43:107–114. [PubMed] [Google Scholar]