Abstract
Adhesion to extracellular matrix (ECM) induces intracellular signals that modulate cell proliferation, survival and differentiation. To study signalling events triggered by cell–ECM interactions in vivo we used transgenic mice exhibiting reduced mammary epithelial cell proliferation and increased apoptosis rates during the growth phase in pregnancy and lactation due to expression of a β1-integrin dominant-negative mutant in the mammary gland epithelium. Here we show that ERK and JNK MAPKs were markedly less activated in lactating transgenic glands thereby accounting for the growth defects. The FAK pathway was not affected suggesting a mechanism of activation additional to the ECM signal. On the contrary, the significant decrease of Shc phosphorylation, Grb2 recruitment and the reduced phosphorylation level of Akt Thr308 and Akt substrates FKHR and Bad detected in transgenic glands show that activation of the Shc and the Akt pathways require intact cell–ECM interactions. These results provide an insight into the mechanisms of growth control by integrin-mediated adhesion that operate in vivo.
INTRODUCTION
Binding of integrins, the transmembrane adhesive receptors, to extracellular matrix (ECM) ligands generates intracellular signals that modulate cell proliferation, survival and differentiation (for review see Giancotti and Ruoslahti, 1999). Integrins co-operate with growth factor receptors in activating intracellular signalling pathways, which have been dissected and characterized in numerous studies carried out in cell culture systems. However, in a particular tissue context, cells interact with a specifically organized ECM and are exposed to a complex mixture of growth factors and hormones. The concerted action of all these factors can hardly be modelled in an in vitro system. Therefore, to study the β1-integrin function in vivo, we generated transgenic mice, referred to hereafter as MMTV-β1-cyto (Faraldo et al., 1998). These mice express, in mammary gland epithelium, under the control of the MMTV promoter, a chimeric molecule consisting of the β1-integrin cytoplasmic and transmembrane domains and the extracellular domain of the T-cell differentiation antigen, CD4, which does not bind to ECM proteins. In cultured cells, the chimera acts as a dominant-negative mutant, as it localizes to focal adhesions and interferes with cell spreading, migration and matrix assembly, presumably by competing with the cytoplasmic domain of the endogenous β1-integrin for the cytoskeletal and signalling ligands (LaFlamme et al., 1994; Lukashev et al., 1994; Fenczik et al., 1997). The MMTV-β1-cyto females presented significant mammary gland growth defects during pregnancy and lactation (Faraldo et al., 1998). Here, we identify the signalling pathways perturbed by the expression of the β1-integrin dominant-negative mutant in the lactating mammary gland epithelium. This is the first study dedicated to the analysis of the ensemble of the adhesion-dependent signalling events in a concrete physiological context, in vivo.
RESULTS AND DISCUSSION
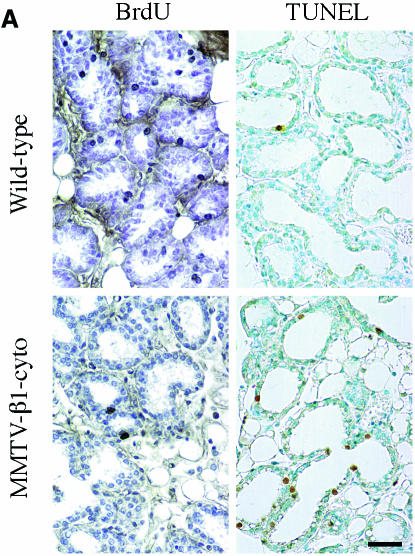
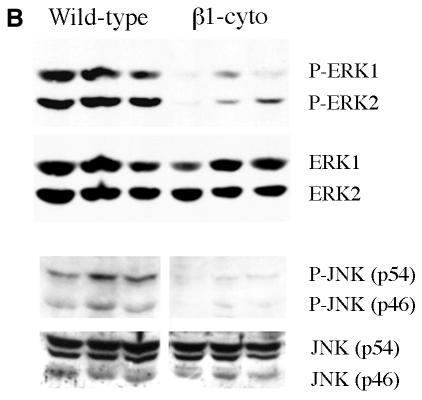
Perturbation of β1-integrin function in mouse mammary gland resulted in reduced epithelial cell proliferation and increased apoptosis rates during the growth phase in pregnancy and lactation (Figure 1A). Therefore, we have investigated the phosphorylation status of extracellular signal-regulated kinases (ERK1 and ERK2) and the Jun N-terminal kinase (JNK), the MAPKs, known to be responsible for the control of cell cycle progression in response to adhesion-induced signals (Giancotti and Ruoslahti, 1999). The level of phosphorylation of ERK1 and ERK2, and of both JNK forms, p46 and p54, was markedly lower in the mammary glands of transgenic mice (Figure 1B).
Fig. 1. Growth defects and low levels of MAPK phosphorylation in mammary gland epithelium from 2-day-lactating MMTV-β1-cyto transgenic mice. (A) BrdU incorporation and TUNEL assay. The microphotographs show representative fields from sections of 2-day-lactating glands from transgenic and wild-type mice subjected to BrdU incorporation analysis to detect proliferating cells and TUNEL assay to detect apoptotic cells. BrdU-positive nuclei are dark grey to black, the tissue was slightly counterstained with haematoxylin; apoptotic nuclei are brown, the corresponding tissue sections were counterstained with methyl green. Bar, 40 µm. (B) Comparison of MAPK phosphorylation levels in 2-day-lactating wild-type and MMTV-β1-cyto glands. At first, phosphorylated ERK1 and ERK2 (upper panel) and phosphorylated JNKs, p46 and p54, (lower panel) were detected in the gland extracts by western blotting using corresponding antibodies. The blots were then stripped and reprobed for total ERK and total JNK. An additional non-specific band of 50–52 kDa was detected with the antibody against total JNK. Each lane corresponds to a mammary gland extract from an individual animal (three wild-type, followed by three transgenic). An aliquot of protein extract (100 µg per lane) was loaded.
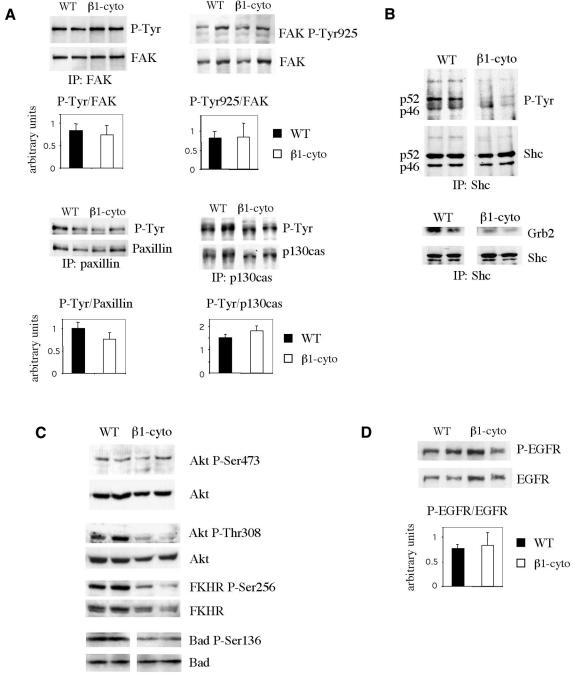
Integrins may activate the MAPK cascade via two major pathways, one, involving focal adhesion kinase (FAK) and the other, the adaptor protein Shc. A recent study has clearly demonstrated that Shc and FAK signalling pathways are activated independently and function in parallel (Barberis et al., 2000). FAK is phosphorylated upon the binding of most integrins to ECM ligands and is involved in activation of the ERK (Renshaw et al., 1999) and JNK cascades (Oktay et al., 1999). Upon activation by ECM, FAK is autophosphorylated at Tyr-397, creating a binding site for Src kinase. Src can then phosphorylate FAK at Tyr-925 creating a binding site for the Grb2–SOS complex, which transmits the signal to Ras, Raf and the ERK cascade. We have immunoprecipitated FAK from extracts of 2-day-lactating mammary gland, and determined P-Tyr-FAK and total FAK content in the obtained immunoprecipitate by western blotting. The level of FAK phosphorylation in transgenic animals was similar to that in wild-type animals (Figure 2A). We also used antibodies that specifically recognized FAK phosphorylated at Tyr397 (not shown) or Tyr925 (Figure 2A), to analyse the levels of phosphorylation of individual Tyr residues, but we found no significant difference between wild-type and transgenic glands.
Fig. 2. Analysis of the phosphorylation status of the signalling molecules implicated in MAPK activation. Each lane corresponds to a mammary gland extract from an individual animal; representative data for two wild-type and two transgenic glands are shown in each case. (A) Tyrosine phosphorylation levels of FAK, paxillin and p130cas. Immunoprecipitation of FAK, paxillin and p130cas was performed by incubating 2 mg of mammary gland protein extract with the corresponding antibodies, and the immunoprecipitates were analysed by western blotting, first with an anti-P-Tyr-antibody and, after stripping, with anti-FAK, anti-paxillin and anti-p130cas antibodies. Bars in the diagrams represent P-Tyr levels relative to FAK, paxillin and p130cas, respectively. The phosphorylation level of FAK Tyr-925 was estimated by western blotting using 100 µg of gland protein extract. In the diagram, levels relative to the total amount of FAK are presented. The bars show the mean values for three or four animals ± SEM. (B) Analysis of Shc phosphorylation levels and Grb2 recruitment. Shc was immunoprecipitated from lactating mammary gland extracts (0.5 mg of protein) with anti-Shc mAb, and the amounts of P-Tyr-Shc, total Shc, and co-precipitating Grb2 in the immunoprecipitate were determined by western blotting. (C) Analysis of the phosphorylation status of PI3K signalling pathway components. First, phosphorylation levels of Akt Ser 473 and Thr 308, of FKHR Ser256, and of Bad Ser136 were analysed by western blotting using the corresponding specific antibodies; blots were then stripped and reprobed for total Akt, FKHR and Bad. A 100 µg aliquot of 2-day-lactating mammary gland extract was loaded per lane. (D) Analysis of EGFR phosphorylation. The phosphorylation levels of EGFR were estimated by western blotting using 100 µg of gland protein extract per lane. The blots were first probed with the antibody specific for phosphorylated EGFR, then stripped and probed for total EGFR. The bars indicate the values obtained for phosphorylated EGFR relative to the total amount of EGFR. Mean values for three or four animals ± SEM are shown.
FAK activation is followed by the phosphorylation of a number of cytoskeletal and signalling molecules, in particular, the docking protein p130cas and paxillin. We have found that the level of phosphorylation of paxillin and p130cas in transgenic glands did not differ significantly from that in wild-type animals (Figure 2A). These results confirm that FAK activity is unaffected in transgenic glands and suggest that the FAK pathway cannot account for the observed decrease in ERK and JNK activation in mammary glands expressing the β1-integrin mutant.
The adaptor protein Shc is an important intermediate of MAPK activation by integrins (Wary et al., 1996). Shc–/– fibroblasts exhibit a decrease in activation of ERK in response to ECM and altered organization of focal adhesions and actin stress fibers (Lai and Pawson, 2000). Upon engagement of certain integrins, Shc is phosphorylated at Tyr317, resulting in recruitment of the Grb2–SOS complex. Shc was immunoprecipitated from extracts of 2-day-lactating glands, and its phosphorylation status and the amount of recruited Grb2 in the immunoprecipitates were determined by western blotting. As shown in Figure 2B, two Shc isoforms, p46 and p52, were detected. The phosphorylation level of p46 was too low to be estimated, whereas the p52 isoform was clearly less phosphorylated in the transgenic glands, and less Grb2 was co-precipitated with Shc. Thus, perturbation of β1-integrin function in the lactating mammary gland led to a decrease in Shc phosphorylation resulting in failure to recruit Grb2 and, consequently, to activate the Ras pathway.
Phosphoinositol 3-kinase (PI3K) is known to be involved in the activation of MAPK by integrins, whereas PI3K-mediated activation of the serine/threonine kinase Akt is necessary for transmission of the cell survival signal triggered by integrin mediated adhesion (reviewed in Datta et al., 1999). Activation of PI3K leads to the phosphorylation of Akt at Thr308 and Ser473. Using antibodies against these specific Akt phosphoamino acids, we found that Ser473 phosphorylation was not altered in transgenic glands, whereas Akt Thr308 phosphorylation levels were very low (Figure 2C). Phosphorylation of FKHR, a member of the Forkhead family of transcription factors, at Ser256, by activated Akt has been shown to promote cell survival (Datta et al., 1999). In the transgenic glands, FKHR Ser256 phosphorylation levels were found to be low (Figure 2C). Bad, a member of Bcl-2 family promoting cell death, is another target of Akt. Its phosphorylation by Akt at Ser136 results in inactivation and cell survival, whereas dephosphorylated Bad binds to pro-survival Bcl-2 family member Bcl-XL to inactivate it and to cause cell death (reviewed in Datta et al., 1999). Phosphorylation level of Bad was reduced in transgenic glands (Figure 2C).
β1-integrin binding to ECM can induce ligand-independent EGF receptor (EGFR) activation (Moro et al., 1998). Therefore, we investigated whether the perturbation of β1-integrin function affected EGFR activation by analysing its phosphorylation level in MMTV-β1-cyto glands. No significant difference in EGFR phosphorylation was observed between wild-type and transgenic animals (Figure 2D), suggesting that the activation of EGFR was not affected by transgene expression.
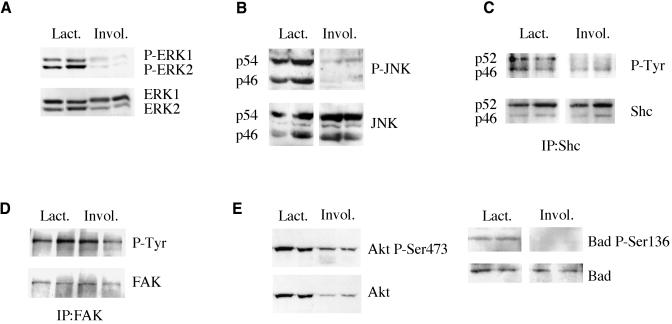
To assess the physiological significance of the observed correlation between growth defects characteristic of transgenic glands and reduced phosphorylation of the intracellular signalling molecules, we have compared their phosphorylation levels in lactating and involuting wild-type mammary glands. At day 2 of lactation, mammary epithelial cells (MECs) proliferate and apoptotic cells are rare (Figure 1A), whereas in involution, numerous MECs enter in apoptosis. In agreement with these physiological changes, in involuting glands, phosphorylation levels of ERK and JNK MAPKs were very low if compared to those in lactation (Figure 3A and B). Similarly, Shc p52 phosphorylation level was markedly decreased at day 2 of involution (Figure 3C). Total amount of FAK was rather low in involuting glands making impossible estimation of the individual Tyr phosphorylation levels. However, relative phospho-Tyr content of FAK at day 2 of involution remained equal to that observed in lactation (Figure 3D). Similarly to FAK, total content of Akt was markedly reduced in involuting gland, whereas its Ser473 phosphorylation level remained very close to that found in lactation (Figure 3E). Thr308 phosphorylation level could not be estimated due to low Akt content. Bad was less phosphorylated in involuting glands (Figure 3E), while FKHR amount was too low to examine its phosphorylation status (not shown). These results clearly demonstrate that similarly to MMTV-β1-cyto lactating glands, a low level/absence of proliferation and an induction of MEC apoptosis in wild-type involuting glands correlated with a lack of ERK, JNK and Shc activation, as well as with reduced phosphorylation level of Bad. The decrease of FAK and Akt content observed in wild-type involuting glands may be explained by their degradation in numerous apoptotic MECs (Wen et al., 1997; Widmann et al., 1998). It should be mentioned that apoptosis rates observed in MMTV-β1-cyto lactating glands even though being markedly elevated compared to those in wild-type lactating animals (1.1 and 0.3%, respectively, Faraldo et al., 1998), are still much lower than in 2–4-day involuting glands (5%; M.M. Faraldo, data not shown). This may account for a lack of any significant decrease in the signalling molecule content in MMTV-β1-cyto lactating glands.
Fig. 3. Comparison of the phosphorylation status of the signalling molecules in lactating and involuting wild-type glands. (A and B) ERK and JNK phosphorylation levels were determined by western blotting of 2-day-lactating and 4-day-involuting wild-type glands with the phospho-protein specific antibodies, as described in the legend to Figure 1. (C and D) Shc and FAK phospho-Tyr levels were determined in the immunoprecipates obtained with the corresponding antibodies. Protein extracts (0.5 mg) of 2-day-lactating and 2-day-involuting glands were used to immunoprecipitate Shc, whereas 0.5 mg of 2-day-lactating and 1 mg of 2-day-involuting gland extracts were used to immunoprecipitate FAK. (E) Phosphorylation of Akt at Ser473 was analysed by western blotting of 2-day-lactating and 4-day involuting gland extracts (100 µg). Throughout the figure, each lane corresponds to a mammary gland extract from an individual animal; three or four animals were analysed in each case.
We have used the schematic diagram summarizing the data on integrin and growth factor-controlled signalling pathways (Giancotti and Ruoslahti, 1999) to illustrate the results of this study (Figure 4). Overall, perturbation of β1-integrin function in the mammary gland epithelium led to lower levels of phosphorylation of MAPK ERKs and JNKs, indicating a lower level of activation of these molecules, thereby accounting for the growth defects observed in the mammary glands of MMTV-β1-cyto mice. Original studies performed with β1-integrin-CD4 or similar β1-integrin-cytoplasmic tail-containing chimeras in cultured cells, suggested that the molecule might interfere with the FAK signalling pathway (Lukashev et al., 1994). However, the data presented here show that neither the phosphorylation of FAK itself, nor that of the downstream components of this pathway, were altered in transgenic glands. Recent studies have shown that FAK can be phosphorylated by activated EGFR at the autophosphorylation site, Tyr397, (Sieg et al., 2000). Thus the FAK phosphorylation detected in vivo may result not just from adhesion signal, but also from growth factor activity. Importantly, our data suggest that in MECs, activation of the FAK pathway per se is not sufficient to stimulate MAPKs to the level necessary for normal gland growth.
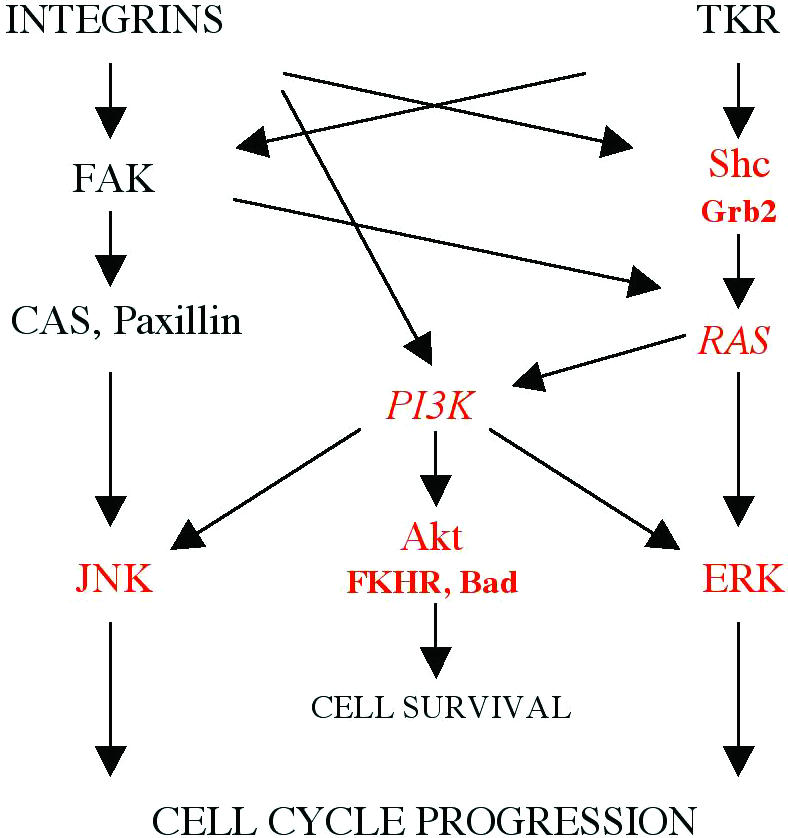
Fig. 4. Signalling pathways regulated by integrins in concert with Tyr-kinase growth factor receptors (from Giancotti and Ruoslahti, 1999, with modification). The molecules whose activation was affected by perturbation of β1-integrin-mediated adhesion in the lactating mammary gland epithelium are shown in red. PI3K and RAS are shown in italic as their activation was not directly estimated in this study, although the data presented herein suggest their impaired activity.
We have found that perturbation of β1-integrin function interfered with the Shc pathway. Only a few integrin dimers were found to be able to recruit and to activate Shc (Wary et al., 1996). The integrins of the β1-family expressed in luminal MECs (α2β1, α3β1 and α6β1) do not belong to the Shc-activating subgroup. However, the list of integrins identified to date in the mammary gland cannot be considered as exhaustive. On the other hand, two recent reports demonstrate that conditional ablation of β1-integrin in the epidermis resulted in a perturbation of expression or loss of α6β4 integrin in basal keratinocytes (Brakebusch et al., 2000; Raghavan et al., 2000). These results suggest that the perturbation of β1-integrin function may subsequently perturb the function and signalling events associated with α6β4, one of the major laminin receptors expressed by MECs. Indeed, although overall organization of the mammary tissue was not altered by the transgene expression, we have observed an abnormal localization of the β4-integrin chain at the lateral surfaces of the alveolar epithelial cells in the MMTV-β1-cyto lactating glands (Faraldo et al., 1998). Engagement of α6β4 results in activation of Shc followed by phosphorylation and activation of ERK and in activation of JNK MAPKs by a mechanism involving Ras and PI3K (Mainiero et al., 1997). Thus, reduced activation of signalling molecules observed in the transgenic glands may result from the perturbation of cell–ECM interactions mediated either by β1-integrin and α6β4 integrins in concert, or essentially by α6β4.
In contradiction with our results, expression of β1-integrin-IL2R chimera in NIH 3T3 fibroblasts did not interfere with ERK activation in adherent cells and, moreover, allowed ERK activation in response to serum in suspended cells (Renshaw et al., 1999). On the contrary, in primary keratinocytes, in agreement with our data, expression of β1-integrin-CD8 chimera led to reduced ERK activation upon adhesion, without affecting FAK phosphorylation (Zhu et al., 1999). Thus, even though major intracellular signalling intermediates are similar in various cell types, particular mechanisms as well as relative contribution of distinct signalling pathways into MAPK activation may be different in fibroblasts and epithelial cells. Clearly distinct signalling characteristics of α6β4 integrin (Mainiero et al., 1997) expressed exclusively by epithelial cells provide a potential background for this diversity.
We found that in transgenic mice, Akt was poorly phosphorylated at Thr308, whereas phosphorylation of Ser473 was unaffected. The precise in vivo mechanism of Akt phosphorylation remains to be elucidated (reviewed in Datta et al., 1999). However, our observation favours the hypothesis that in vivo, Akt Thr308 and Ser473 are phosphorylated by different kinases. Thr308 lies within the Akt kinase domain activation loop, and when non-phosphorylated negatively regulates the kinase activity of Akt (Datta et al., 1999). Coordinately, we have detected that in transgenic animals, Akt targets, FKHR and Bad presented reduced phosphorylation levels presumably accounting for the observed increase in apoptosis.
Finally, it should be considered that perturbation of cell–ECM interactions in MMTV-β1-cyto glands may interfere directly or indirectly with growth factor receptor signalling pathways. We, however, have not observed any alteration in EGFR phosphorylation suggesting that EGFR activation was not altered in transgenic glands. In any case, the results of this study show that in MECs, Shc and Akt activation, unlike that of FAK, although also dependent on growth factor-controlled pathways, require intact cell–ECM interactions. In conclusion, the data presented here, provide the first insight into the mechanisms of growth control by integrin-mediated cell–ECM interactions that operate in MECs in vivo.
METHODS
Transgenic mice, proliferation and apoptosis assays. Transgenic mouse line #17, expressing a dominant-negative mutant of β1-integrin under the control of the MMTV promoter (MMTV-β1-cyto) has been described elsewhere (Faraldo et al., 1998). In all cases, 2-day-lactating females with litters of 7 or 8 pups were analysed. For the cell proliferation test, mice were injected with 0.25 mg/g body weight of BrdU 2 h before being killed. BrdU detection and TUNEL analysis were performed using the ‘Cell Proliferation Kit’ (Amersham) and ‘ApopTag Kit’ (Oncor) according to the manufacturer’s instructions.
Antibodies. Mouse monoclonal antibodies (mAb) against FAK (Upstate Biotech. Inc.), paxillin (Transduction Lab.) and Shc (Santa Cruz Biotech.) and rabbit polyclonal antibody against p130cas, sc-20 (Santa Cruz Biotech.) were used for immunoprecipitation. mAbs against p130cas, paxillin, FAK, Bad, Grb2 (Transduction Labs.), phospho-Tyr 4G10 (UBI), and rabbit polyclonal antibodies against Shc (UBI), ERK1/ERK2, JNK, Akt, FKHR, phospho-ERK1/ERK2, JNK, Akt Ser473, Akt Thr308, FKHR Ser256, Bad Ser136 (New England Biolabs), FAK Tyr397, FAK Tyr925 (Biosource Int.), and Tyr (Zymed) were used for immunoblotting.
Protein extracts, immunoprecipitation and immunoblotting. Protein extracts were prepared from the fourth and fifth mammary gland as follows. Gland tissue (125 mg/ml) was homogenized in lysis buffer (40 mM Tris-HCl, pH 8.0, 276 mM NaCl, 20% glycerol, 2% NP-40, 4 mM EDTA, 20 mM NaF, 2 mM sodium orthovanadate, 40 µg/ml phenylmethylsulfonyl fluoride, 10 µg/ml aprotinin, 10 µg/ml leupeptin). The extracts were cleared by centrifugation at 10 000 g at 4°C for 15 min.
For immunoprecipitation assays, aliquots (0.5–2 mg) of protein extracts were preadsorbed with protein A–Sepharose (Pharmacia-Biotech) or protein G–agarose beads (Santa Cruz Biotech.) for 2 h at 4°C on a vertical rotator, incubated with 1–2 µg of immunoprecipitating antibody for 1 h, and, finally, with protein A–Sepharose or protein G–agarose beads for 2 h. The beads were spun down, washed three times with lysis buffer, resuspended in the sample buffer for electrophoresis, boiled for 3 min, spun briefly, and the supernatants were loaded on a 10% SDS–PAGE. After electrophoresis, proteins were transferred to PVDF membranes (Millipore). To prevent non-specific protein binding, membranes were incubated in 5% BSA in TBST (10 mM Tris, pH 8.0, 150 mM NaCl, 0.1% Tween 20) for 1 h at room temperature and treated overnight at 4°C with primary antibodies diluted in TBST. After washing with TBST, membranes were incubated for 1 h at room temperature with horse radish peroxidase-conjugated anti-mouse or anti-rabbit IgG (Amersham) diluted 1:5000. Immunoblots were developed using the ECL detection system (Amersham). Quantitative analysis was performed using the ImageQuant program.
Acknowledgments
ACKNOWLEDGEMENTS
We are especially grateful to Dr I. Cerutti, and to the personnel of the Service d’Expérimentation Animale et de Transgenèse, Villejuif, particularly to R. Duchâteau and A. Loeuillet, for taking care of transgenic mice. We greatly appreciate the criticism and advice of Drs F. Giancotti, G. Tarone, A.M. Valles, M. Arpin and B. Elliot. This work was supported by the Association pour la Recherche contre le Cancer (ARC 5495). M.M.F. was supported by a fellowship from Institut Curie. M.-A.D. is a Chargé de Recherche, and M.A.G. is a Directeur de Recherche at the INSERM.
References
- Barberis L., Wary, K.K., Fiucci, G., Liu, F., Brancaccio, M., Altruda, F., Tarone, G. and Giancotti, F.G. (2000) Distinct roles of the adaptor protein Shc and focal adhesion kinase in integrin signaling to ERK. J. Biol. Chem., 275, 36532–36540. [DOI] [PubMed] [Google Scholar]
- Brakebusch C. et al. (2000) Skin and hair follicle integrity is crucially dependent on β1 integrin expression on keratinocytes. EMBO J., 19, 3990–4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S.R., Brunet, A. and Greenberg, M.E. (1999) Cellular survival: a play in three Akts. Genes Dev., 13, 2905–2927. [DOI] [PubMed] [Google Scholar]
- Faraldo M.M., Deugnier, M.A., Lukashev, M., Thiery, J.P. and Glukhova, M.A. (1998) Perturbation of β1-integrin function alters the development of murine mammary gland. EMBO J., 17, 2139–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenczik C.A., Sethi, T., Ramos, J.W., Hughes, P.E. and Ginsberg, M.H. (1997) Complementation of dominant suppression implicates CD98 in integrin activation. Nature, 390, 81–85. [DOI] [PubMed] [Google Scholar]
- Giancotti F.G. and Ruoslahti, E. (1999) Integrin signaling. Science, 285, 1028–1032. [DOI] [PubMed] [Google Scholar]
- LaFlamme S.E., Thomas, L.A., Yamada, S.S. and Yamada, K.M. (1994) Single subunit chimeric integrins as mimics and inhibitors of endogenous integrin functions in receptor localization, cell spreading and migration, and matrix assembly. J. Cell Biol., 126, 1287–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai K.M. and Pawson, T. (2000) The ShcA phosphotyrosine docking protein sensitizes cardiovascular signaling in the mouse embryo. Genes Dev., 14, 1132–1145. [PMC free article] [PubMed] [Google Scholar]
- Lukashev M.E., Sheppard, D. and Pytela, R. (1994) Disruption of integrin function and induction of tyrosine phosphorylation by the autonomously expressed β1 integrin cytoplasmic domain. J. Biol. Chem., 269, 18311–18314. [PubMed] [Google Scholar]
- Mainiero F., Murgia, C., Wary, K.K., Curatola, A.M., Pepe, A., Blumemberg, M., Westwick, J.K., Der, C.J. and Giancotti, F.G. (1997) The coupling of α6β4 integrin to Ras-MAP kinase pathways mediated by Shc controls keratinocyte proliferation. EMBO J., 16, 2365–2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro L., Venturino, M., Bozzo, C., Silengo, L., Altruda, F., Beguinot, L., Tarone, G. and Defilippi, P. (1998) Integrins induce activation of EGF receptor: role in MAP kinase induction and adhesion-dependent cell survival. EMBO J., 17, 6622–6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oktay M., Wary, K.K., Dans, M., Birge, R.B. and Giancotti, F.G. (1999) Integrin-mediated activation of focal adhesion kinase is required for signaling to Jun NH2-terminal kinase and progression through the G1 phase of the cell cycle. J. Cell Biol., 145, 1461–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan S., Bauer, C., Mundschau, G., Li, Q. and Fuchs, E. (2000) Conditional ablation of β1 integrin in skin. Severe defects in epidermal proliferation, basement membrane formation, and hair follicle invagination. J. Cell Biol., 150, 1149–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renshaw M.W., Price, L.S. and Schwartz, M.A. (1999) Focal adhesion kinase mediates the integrin signaling requirement for growth factor activation of MAP kinase. J. Cell Biol., 147, 611–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieg D.J., Hauck, C.R., Ilic, D., Klingbeil, C.K., Schaefer, E., Damsky, C.H. and Schlaepfer, D.D. (2000) FAK integrates growth-factor and integrin signals to promote cell migration. Nature Cell Biol., 2, 249–256. [DOI] [PubMed] [Google Scholar]
- Wary K.K., Mainiero, F., Isakoff, S.J., Marcantonio, E.E. and Giancotti, F.G. (1996) The adaptor protein Shc couples a class of integrins to the control of cell cycle progression. Cell, 87, 733–743. [DOI] [PubMed] [Google Scholar]
- Wen L.P., Fahrni, J.A., Troie, S., Guan, J.L., Orth, K. and Rosen, G.D. (1997) Cleavage of focal adhesion kinase by caspases during apoptosis. J. Biol. Chem., 272, 26056–26061. [DOI] [PubMed] [Google Scholar]
- Widmann C., Gibson, S. and Johnson, G.L. (1998) Caspase-dependent cleavage of signaling proteins during apoptosis. A turn-off mechanism for anti-apoptotic signals. J. Biol. Chem., 273, 7141–7147. [DOI] [PubMed] [Google Scholar]
- Zhu A.J., Haase, I. and Watt, F.M. (1999) Signaling via β1 integrins and mitogen-activated protein kinase determines human epidermal stem cell fate in vitro. Proc. Natl Acad. Sci. USA, 96, 6728–6733. [DOI] [PMC free article] [PubMed] [Google Scholar]