Abstract
Misfolded proteins are recognized in the endoplasmic reticulum (ER), transported back to the cytoplasm and degraded by the proteasome. Processing intermediates of N-linked oligosaccharides on incompletely folded glycoproteins have an important role in their folding/refolding, and also in their targeting to proteolytic degradation. In Saccharomyces cerevisiae, we have identified a gene coding for a non-essential protein that is homologous to mannosidase I (HTM1) and that is required for degradation of glycoproteins. Deletion of the HTM1 gene does not affect oligosaccharide trimming. However, deletion of HTM1 does reduce the rate of degradation of the mutant glycoproteins such as carboxypeptidase Y, ABC-transporter Pdr5-26p and oligosaccharyltransferase subunit Stt3-7p, but not of mutant Sec61-2p, a non-glycoprotein. Our results indicate that although Htm1p is not involved in processing of N-linked oligosaccharides, it is required for their proteolytic degradation. We propose that this mannosidase homolog is a lectin that recognizes Man8GlcNAc2 oligosaccharides that serve as signals in the degradation pathway.
INTRODUCTION
Most secretory and membrane proteins are co-translationally translocated into the lumen of the endoplasmic reticulum (ER) (Rapoport et al., 1996) where they acquire N-linked oligosaccharides, catalyzed by oligosaccharyltransferase. Also in the ER, secretory and membrane proteins attain their correct three-dimensional structure by the concerted action of chaperones, isomerases and folding mediators (Ellgaard et al., 1999). The N-linked oligosaccharides are processed by glucosidase I, glucosidase II and mannosidase I (Herscovics, 1999a). It has been shown that oligosaccharide processing and protein folding are linked processes coordinating protein folding, ER retention/retrieval and protein degradation (Ellgaard et al., 1999; Herscovics, 1999b; Jakob and Burda, 1999).
Mechanisms that discern misfolded secretory and membrane proteins have been discovered in the past few years (Bonifacino and Weissman, 1998). Misfolded proteins in the ER lumen are specifically recognized by as yet unknown mechanisms and subjected to a cytosolically located proteolytic process termed ER-associated degradation (ERAD). The targeted proteins are transported back into the cytoplasm by a mechanism that is formed, at least partially, by constituents of the ER import machinery (Römisch, 1999). Many of the retrotranslocated proteins are polyubiquitin modified by the action of the cytosolically located enzymes Ubc6p, Ubc7p and Hrd1/Der3p, followed by degradation by the proteasome (Brodsky and McCracken, 1997; Kopito, 1997; Sommer and Wolf, 1997; Bays et al., 2001; Deak and Wolf, 2001).
The degradation of misfolded mutant carboxypeptidase Y [CPY* (prc1-1); Finger et al., 1993] has been shown to be N-glycan dependent (Knop et al., 1996) and, moreover, the structure of this oligosaccharide plays an important role in rapid protein degradation (Jakob et al., 1998). Specifically, glycoproteins carrying Man8GlcNAc2 sugars are more rapidly degraded than others. This finding supported the ‘mannose timer hypothesis’ (Su et al., 1993; Helenius et al., 1997) and has led to the proposal that the ER lumen contains a Man8GlcNAc2-binding protein that initiates glycoprotein ERAD (Liu et al., 1997; Jakob et al., 1998; Yang et al., 1998).
In the yeast genome we have identified a good candidate for this Man8GlcNAc2-specific lectin (Htm1p) that is homologous to mannosidase I (Camirand et al., 1991). We show that strains deleted for HTM1 are defective in the degradation of a mutant soluble protein (CPY*), and also two mutant membrane proteins (Pdr5-26p, Stt3-7p). Our data strongly suggest that the Htm1 protein is the putative Man8GlcNAc2-binding lectin.
RESULTS AND DISCUSSION
HTM1 codes for a gene homologous to mannosidase and is involved in protein degradation
We searched the Saccharomyces cerevisiae genome for open reading frames with homology to ER mannosidase since we hypothesized that a ‘degradation lectin’ might contain similar domains. The reading frame YHR204w (termed gene homologous to mannosidase; HTM1) was identified, showing a 40% amino acid sequence similarity to yeast mannosidase.
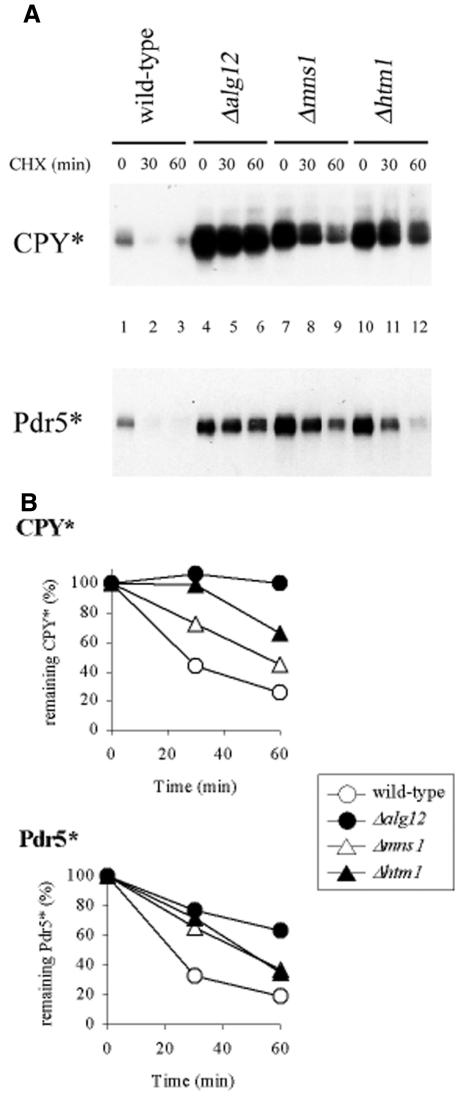
To test whether HTM1 was involved in protein degradation, we deleted the gene in a yeast strain containing CPY* (Wolf and Fink, 1975) and Pdr5* [mutant Pdr5 protein (pdr5-26); Egner et al., 1998], two substrates that have previously been shown to undergo ubiquitin-dependent proteasomal degradation. As controls, we used mutant strains that were deficient in the biosynthesis of complete oligosaccharide chains (Δalg12) or in trimming of the oligosaccharides (Δmns1). These mutations prevent the biosynthesis of Man8GlcNAc2 oligosaccharide or its formation by processing, respectively, and stabilize misfolded CPY* (Jakob et al., 1998). Equal numbers of cells were inoculated and de novo protein synthesis was stopped by the addition of cycloheximide; the levels of CPY* and Pdr5* were determined by immunoblotting and quantified at different time points. The CPY* degradation rate in Δalg12 and Δmns1 cells was reduced in comparison to ‘wild-type’ cells, but also in the HTM1-deficient cells (Figure 1). In addition, we also found a higher steady-state level of CPY* in Δalg12, Δmns1 and Δhtm1 cells as compared with wild-type cells (time point 0; Figure 1A, compare lanes 1, 4, 7 and 10). The mutant membrane protein Pdr5* showed a similar change in its degradation: steady-state levels of Pdr5* were increased in Δalg12 and Δmns1, and also Δhtm1 cells (Figure 1), and degradation rates were significantly reduced (Figure 1).
Fig. 1. Oligosaccharide-dependent degradation of the mutant proteins CPY* and Pdr5*. (A) Isogenic cells were grown to mid-log phase. Equal cell numbers were then incubated with 100 µg/ml cycloheximide and aliquots removed at given time points. Crude protein extract was separated by SDS–PAGE and analyzed by immunoblotting using anti-CPY (CPY*) and anti-HA (Pdr5*) antisera. (B) Protein amounts from at least two independent experiments were quantified and plotted against time, setting the quantity of time point 0 (steady-state level) to 100%.
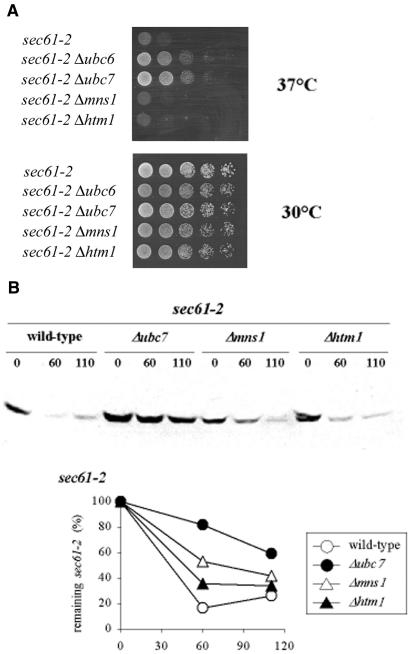
To test whether HTM1 was involved in general protein degradation, we studied the mutant Sec61-2 protein, a membrane protein with no N-glycosylation sites. The sec61-2 mutant cells display a temperature-sensitive growth phenotype. Biederer et al. (1996) showed that Sec61-2 protein was degraded at restrictive temperature. Impairing protein degradation by deleting the UBC6 and UBC7 genes allowed the cells to survive restrictive growth conditions (Figure 2A). We deleted the MNS1 and HTM1 genes in the sec61-2 mutants and tested whether these two genes influenced degradation of the Sec61-2 protein by cell growth at selective temperature and by western blot analysis. Neither of the two mutant cells (sec61-2 Δmns1 and sec61-2 Δhtm1) improved growth on plates at restrictive temperature (Figure 2A) nor reduced the degradation rate of Sec61-2 protein (Figure 2B).
Fig. 2. The HTM1 gene does not influence the degradation of mutant Sec61-2 protein. (A) Δmns1 and Δhtm1 do not suppress temperature-sensitive growth of sec61-2 mutant cells. Isogenic sec61-2 single- and double-mutant cells were plated in serial dilutions on rich medium and grown at 30 and 37°C for 3 days. (B) Degradation of Sec61-2 protein is not affected in mns1- and htm1-deficient cells. Cells were grown at 30°C to mid-log phase and shifted to 37°C for 2 h. Equal cell numbers were incubated with 100 µg/ml cycloheximide, aliquots were removed and cells broken at given time points. Extracted proteins were separated by SDS–PAGE, and analyzed and quantified by immunoblotting using anti-Sec61 antiserum. The protein quantity of time point 0 (steady-state level) was set to 100%.
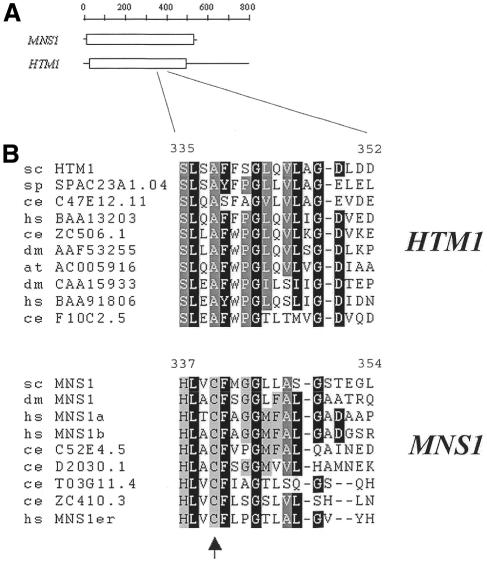
These results support the hypothesis that the HTM1 gene is required for efficient secretory and membrane glycoprotein degradation. Degradation of proteins lacking N-glycans was not affected. Glycoprotein degradation might require a specific lectin that recognizes N-linked Man8GlcNAc2 oligosaccharides (Jakob et al., 1998). For this we reasoned that such a lectin might be similar to ER mannosidase I. The HTM1 gene codes for a polypeptide of 796 amino acids with significant sequence similarity to yeast mannosidase I (Figure 3A), and amino acid sequences similar to Htm1 protein are found in fungal, plant, insect, worm and mammal genomes. Phylogenetic analysis of these sequences revealed two distinct sequence groups, of which one contained all class I α1,2-mannosidases and the other proteins homologous to Htm1p (data not shown). Many amino acids reported to be involved in oligosaccharide binding, catalysis and Ca2+ coordination in Mns1p (Vallee et al., 2000) are conserved in Htm1 protein and its homologs. One of the characteristic features of the Htm1 protein family group was the absence of the conserved cysteine residues 340 and 385 (position given for Mns1p of S. cerevisiae), which form a disulfide bond. These two cysteine residues are conserved in α1,2-mannosidase family members and are most likely to be important for maintaining the correct structure of processing mannosidases (Lipari and Herscovics, 1996) (Figure 3B).
Fig. 3. Similarity of Mns1p and Htm1 proteins. (A) Areas of homology of Mns1p and Htm1p. Areas of high amino acid similarity are drawn as boxes. (B) Htm1 family proteins lack a highly conserved cysteine residue. Amino acid sequences of α-mannosidases and Htm1 family proteins of plant, mammalian, insect, worm and yeast cells were aligned with Clustal_W. The sequences were grouped by sequence similarity. The arrowhead indicates the position of an alanine residue in Htm1 proteins at the position of Cys340 (in S. cerevisiae) conserved in and most likely essential for maintaining the correct structure of α1,2-mannosidases. The first two letters indicate the species: at, Arabidopsis thaliana; ce, Caenorhabditis elegans; dm, Drosophila melanogaster; hs, Homo sapiens; sc, S. cerevisiae; sp, Schizosaccharomyces pombe. The following numbers refer to the enzyme name or DDBJ/EMBL/GenBank accession No.
HTM1 is not involved in processing of N-linked oligosaccharides
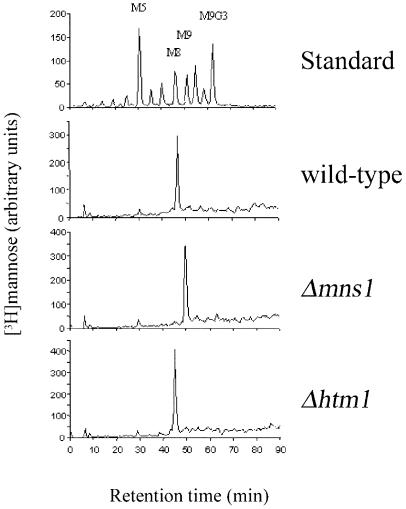
Processing of N-glycans to create the Man8GlcNAc2 glycan is required for efficient degradation in yeast (Jakob et al., 1998 and see above). We determined whether altered processing of oligosaccharides was the reason for reduced glycoprotein degradation in Δhtm1 cells. For this, oligosaccharides were metabolically labeled in vivo. N-linked sugars were extracted and released from the protein by peptide N-glycosidase F (PNGase F) treatment and analyzed by HPLC. Attention was focused on sugars that were not processed by Golgi mannosyltransferases (Figure 4) and, as expected, wild-type cells contained Man8GlcNAc2 oligosaccharides, and Δmns1 cells contained Man9GlcNAc2 (Figure 4). However, oligosaccharides obtained from cells deleted for HTM1 showed Man8GlcNAc2 but no Man9GlcNAc2 oligosaccharides. These results confirm that normal processing of N-glycans occurs in Δhtm1 cells and, therefore, the Htm1 protein is not a processing mannosidase. Although Htm1p displays sequence similarity to class I mannosidases, it is not involved in the specific trimming of N-glycans in the ER, which is the only process that requires mannosidase activity in yeast (Dean, 1999; Gemmill and Trimble, 1999; Herscovics, 1999a). This is consistent with our conclusion that Htm1p is not a mannosidase, and with our inference that it may be a lectin.
Fig. 4. Normal N-linked oligosaccharide processing in htm1-deficient yeast cells. N-linked oligosaccharides of isogenic strains were metabolically labeled with [3H]mannose, extracted, released with PNGase F and separated by HPLC.
There have been several reports that indicate a distinct function of N-glycan trimming by mannosidase I in glycoprotein degradation (Liu et al., 1997, 1999; Yang et al., 1998; Cabral et al., 2000; Mancini et al., 2000; Wang and Androlewicz, 2000; Wang and White, 2000). These reports are in good agreement with results found in S. cerevisiae, where a specific Man8GlcNAc2 glycan is required for efficient protein degradation in the ER (Knop et al., 1996; Jakob et al., 1998). This specific oligosaccharide on degradation substrates might be recognized by the Htm1 protein and by this facilitate glycoprotein degradation.
Degradation of the Stt3-7 protein is oligosaccharide specific and mediated by the ubiquitin–proteasomal pathway
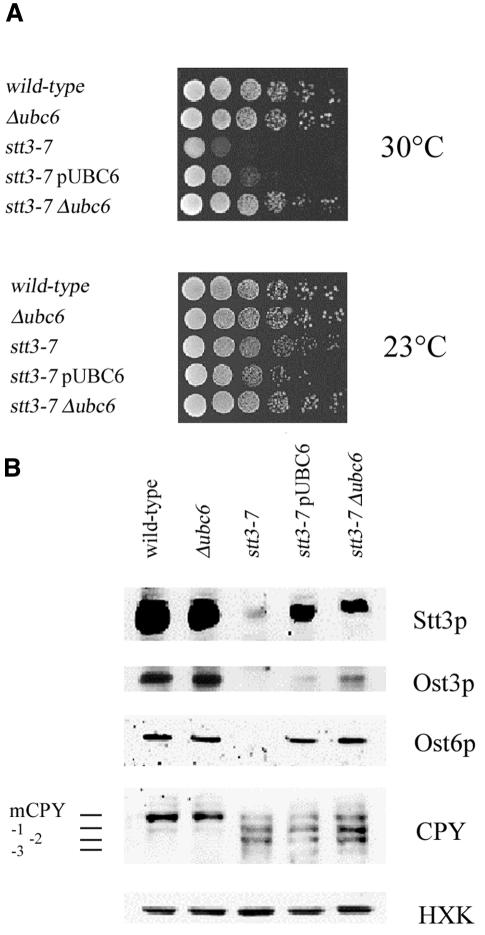
In yeast, oligosaccharyltransferase (OST), the enzyme complex that transfers oligosaccharides from lipid-linked precursors to nascent polypeptides at N-X-S/T, is composed of nine membrane proteins (Knauer and Lehle, 1999), of which Stt3p is the largest subunit and is also essential for the N-linked glycosylation process in vivo (Zufferey et al., 1995). Assembly of the OST complex is a multistep process, and integration of either the Ost3p or Ost6p subunit is dependent upon the presence of Stt3p (U. Spirig, D. Bodmer, M. Wacker and M. Aebi, in preparation). The stt3-7 conditional mutant has been shown to severely impair OST activity, leading to incomplete N-glycosylation of nascent glycoproteins at restrictive temperature (Spirig et al., 1997). We observed that overexpression or deletion of the UBC6 gene could suppress the temperature-sensitive phenotype of mutant stt3-7 strains (Figure 5A).
Fig. 5. UBC6-dependent degradation of stt3-7 protein. (A) Overexpression or deletion of the UBC6 gene suppressed the temperature-sensitive growth phenotype of stt3-7 mutant cells. Isogenic stt3-7 single- and double-mutant cells were plated in serial dilutions on rich medium and grown at 23 and 30°C for 3 days. (B) Overexpression or deletion of the UBC6 gene stabilized the mutated Stt3-7 protein. Cells were shifted to 37°C and protein was extracted. Steady-state protein levels and CPY glycosylation were determined by western analysis using the indicated antisera. Hexokinase was used as the control for equal protein loading.
UBC6 encodes the E2 ubiquitin-conjugating enzyme situated on the cytosolic face of the ER membrane and plays a pivotal role in the degradation of misfolded proteins in the ER (Sommer and Wolf, 1997). Overexpression or deletion of the UBC6 gene increased the steady-state level of the mutant Stt3-7 protein as well as levels of the OST subunits Ost3p and Ost6p, as shown by western blot analysis (Figure 5B). N-linked glycosylation was strongly affected in stt3-7 mutant cells (visualized by the presence of hypoglycosylated CPY) and was only slightly improved by reducing protein degradation using UBC6 mutants (Figure 5B). Our finding shows that mutant Stt3-7 protein is degraded by the ubiquitin–proteasome pathway at restrictive temperature. We speculated that reducing protein degradation by deleting the UBC6 gene increased the steady-state level of Ost3p and Ost6p as well as mutant Stt3-7 protein, permitting the integration of Ost3p, Ost6p and Stt3-7p into a partially functional oligosaccharyltransferase complex. This partially restored enzymatic activity then increases N-linked glycosylation to allow mutant cells to survive at restrictive temperature.
Deletion of a gene (HTM1) homologous to mannosidases suppresses the stt3-7 mutation and stabilizes misfolded protein in the ER
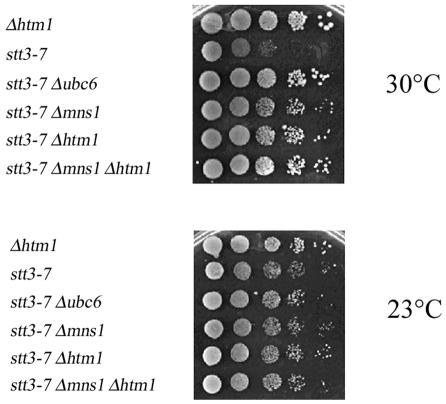
UBC6-dependent growth of stt3-7 strains is a convenient method to monitor the effect of mutant genes in this process. If reduced protein degradation can rescue these mutant cells, we reasoned that we might find similar results upon creating the stt3-7 Δmns1 or stt3-7 Δhtm1 double mutants since Stt3 protein is N-glycosylated. In validation of this assay, Δmns1 suppressed the temperature sensitivity of stt3-7 cells at 30°C, indicating a direct role of N-glycan trimming in the degradation of Stt3-7 protein (Figure 6). Moreover, the stt3-7 Δhtm1 double mutant could grow at elevated temperature, similar to the stt3-7 Δmns1 strain (Figure 6). This result further supported the interpretation that Htm1 protein plays an important role in efficient degradation of the mutant glycoprotein Stt3-7p.
Fig. 6. Oligosaccharide-dependent suppression of conditional growth of stt3-7 by deletion of MNS1 and HTM1 genes. Isogenic stt3-7 single- and double-mutant cells were plated in serial dilutions on rich medium and grown at 23 and 30°C for 3 days.
Our phenotypic analysis of Δhtm1 mutant strains is compatible with the idea that Htm1 protein is in fact the lectin involved in this process. Detailed biochemical studies will be required to confirm this hypothesis.
METHODS
Materials. Yeast strains used are detailed in Table I. The strains harboring the prc1-1 allele were described previously (Jakob et al., 1998). The pRS313 HA-pdr5-26 plasmid was kindly provided by Dr D.H. Wolf (University of Stuttgart, Germany). For western blot analysis, antisera raised against CPY, Sec61p, Ost3p, Ost6p (Dr R. Knauer, University of Regensburg, Germany), hexokinase (Dr S. Schröder, Göttingen, Germany) and anti-hemagglutinin (anti-HA) (Santa Cruz, CA) were used.
Table I. Yeast strains used.
| Strain | Genotype | Reference |
|---|---|---|
| SS328 | MATα ade2-101 ura3-52 his3Δ200 lys2-801 | Vijayraghavan et al. (1989) |
| YG543 | MATa ade2-101 ura3-52 his3Δ200 lys2-801 leu2 stt3-7 | Spirig et al. (1997) |
| YG885 | MATα ade2-101 ura3-52 his3Δ200 lys2-801 Δubc6::HIS3 | this study |
| YG950 | MATα ade2-101 ura3-52 his3Δ200 lys2-801 stt3-7 Δmns1::KanMX | this study |
| YG894 | MATα ade2-101 ura3-52 his3Δ200 lys2-801 leu2 stt3-7 Dubc6::HIS3 | this study |
| YG618 | MATα ade2-101 ura3-52 his3Δ200 lys2-801 prc1-1 | Jakob et al. (1998) |
| YG777 | MATa ade2-101 ura3-52 his3Δ200 tyr1 Δmns1::KanMX prc1-1 | Jakob et al. (1998) |
| YG807 | MATα ade2-101 ura3-52 his3Δ200 lys2-801 Δalg12::KanMX prc1-1 | Jakob et al. (1998) |
| YG1211 | MATα ade2-101 ura3-52 his3Δ200 lys2-801 Δmns1::KanMX prc1-1 | this study |
| YCJ1 | MATα ade2-101 ura3-52 his3Δ200 lys2-801 Δhtm1::KanMX prc1-1 | this study |
| YCJ82 | MATa ade2-101 ura3-52 his3Δ200 lys2-801 stt3-7 Dhtm1::KanMX | this study |
| YCJ88 | MATa ade2-101 ura3-52 his3Δ200 lys2-801 stt3-7 Dhtm1::KanMX | this study |
| RSY533 | MATα sec61-2 ade2 leu2-3 leu2-112 pep4-3 ura3-52 | R. Schekman |
| YG1350 | MATα sec61-2 ade2 leu2-3 leu2-112 pep4-3 ura3-52 Δubc6::KanMX | this study |
| YG1351 | MATα sec61-2 ade2 leu2-3 leu2-112 pep4-3 ura3-52 Δubc7::KanMX | this study |
| YG1352 | MATα sec61-2 ade2 leu2-3 leu2-112 pep4-3 ura3-52 Δmns1::KanMX | this study |
| YG1353 | MATα sec61-2 ade2 leu2-3 leu2-112 pep4-3 ura3-52 Δhtm1::KanMX | this study |
Yeast manipulations. Standard protocols were followed for yeast manipulation (Guthrie and Fink, 1991). Cells were grown at permissive temperature in either YPD (2% bactopeptone, 1% yeast extract, 2% glucose) or, for maintaining plasmids, in minimal medium (0.67% yeast nitrogen base, 2% glucose, with the appropriate supplements to allow growth). Whole-cell protein was extracted as previously described (Jakob et al., 1998). Extracts containing the HA-tagged Pdr5* gene were incubated at 50°C for 15 min instead of boiled. The cycloheximide chase was performed as described by Plemper et al. (1998). Serial dilutions of cells for growth assays, starting at 5 × 105 cells, were spotted on YPD plates and incubated at the given temperatures for 3 days.
Construction of yeast strains. The loci YHR204w/HTM1, UBC6 and UBC7 were deleted by replacing the major part of the gene with the KanMX cassette (Wach et al., 1994) using the primers given in Table II. The correct integration was verified by PCR. Deletion of the MNS1 gene was described previously (Jakob et al., 1998).
Table II. Primers for gene deletions.
| Deletion | Name | Sequence 5′ → 3′a |
|---|---|---|
| HTM1 | HR204forKan | tcgatcatgtagcatgcgaagacgatgcgtactcattcacttctatcgatgaattcgagctc |
| HR204revKan | gccattggaagtgagcacaggactatgtttcttgatttgtacaccgtacgctgcaggtcgac | |
| HR204–90u | gcaataaagaaggacgcg | |
| HR204+350L | ccggtcttccagtatagc | |
| UBC6 | UBC6 forKan | gatggtggaaaaccctccaccatatattcttgctcgccccaacgaagattcgatgaattcgagctc |
| UBC6revKan | tgaagaactatcattaggttctttgccatctttcttggaattattttctgcgtacgctgcaggtcgac | |
| UBC6–180u | actaccatcgcatatcgc | |
| UBC6+130L | gttatcattctgatagccg | |
| UBC7 | UBC7forKan | ctcctcaaggagcttcaacagttaattaaagattctccacctggtatatcgatgaattcgagctc |
| UBC7revKan | ctcactcaacatgctcataacacttaatagaattttttctacactttgcgtacgctgcaggtcgac | |
| UBC7–70u | ggcgtttagcgtacgaag | |
| UBC7+130L | agcgtatggcgtatctgg | |
| All | KanMXu | gtattgatgttggacgag |
aBold letters represent sequences specific to plasmid pFA6aKanMX4 for the amplification of the KanMX resistance gene (Wach et al., 1994).
Labeling of N-linked oligosaccharides. The procedures for the metabolic labeling, recovery of glycoproteins and release of N-glycans by treatment with PNGase F (Roche Diagnostics, Rotkreuz, Switzerland) were as described previously (Jakob et al., 1998).
Acknowledgments
ACKNOWLEDGEMENTS
We are greatly indebted to Dr D.H. Wolf (pRS313-HA-pdr5-26 plasmid), Dr R. Knauer (anti-Ost6p) and Dr S. Schröder (anti-hexokinase). The authors would like to thank Dr Charles J. Waechter for critically reading the manuscript. This work has been supported by the Swiss National Science Foundation (fellowship 81ZH-054340 to C.A.J.; grants 31-57082.99 to M.A.), and grants from the Canadian Institutes for Health Research (to D.Y.T. and J.J.M.B.).
References
- Bays N.W., Gardner, R.G., Seelig, L.P., Joazeiro, C.A. and Hampton, R.Y. (2001) Hrd1p/Der3p is a membrane-anchored ubiquitin ligase required for ER-associated degradation. Nature Cell Biol., 3, 24–29. [DOI] [PubMed] [Google Scholar]
- Biederer T., Volkwein, C. and Sommer, T. (1996) Degradation of subunits of the Sec61p complex, an integral component of the ER membrane, by the ubiquitin-proteasome pathway. EMBO J., 15, 2069–2076. [PMC free article] [PubMed] [Google Scholar]
- Bonifacino J.S. and Weissman, A.M. (1998) Ubiquitin and the control of protein fate in the secretory and endocytic pathways. Annu. Rev. Cell Dev. Biol., 14, 19–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky J.L. and McCracken, A.A. (1997) ER-associated and proteasome-mediated degradation: how two topologically restricted events came together. Trends Cell Biol., 7, 151–155. [DOI] [PubMed] [Google Scholar]
- Cabral C.M., Choudhury, P., Liu, Y. and Sifers, R.N. (2000) Processing by endoplasmic reticulum mannosidases partitions a secretion-impaired glycoprotein into distinct disposal pathways. J. Biol. Chem., 275, 25015–25022. [DOI] [PubMed] [Google Scholar]
- Camirand A., Heysen, A., Grondin, B. and Herscovics, A. (1991) Glycoprotein biosynthesis in Saccharomyces cerevisiae. Isolation and characterization of the gene encoding a specific processing α-mannosidase. J. Biol. Chem., 266, 15120–15127. [PubMed] [Google Scholar]
- Deak P.M. and Wolf, D.H. (2001) Membrane topology and function of Der3/Hrd1p as an ubiquitin-protein ligase (E3) involved in endoplasmic reticulum degradation. J. Biol. Chem., 276, 10663–10669. [DOI] [PubMed] [Google Scholar]
- Dean N. (1999) Asparagine-linked glycosylation in the yeast Golgi. Biochim. Biophys. Acta, 1426, 309–322. [DOI] [PubMed] [Google Scholar]
- Egner R., Rosenthal, F.E., Kralli, A., Sanglard, D. and Kuchler, K. (1998) Genetic separation of FK506 susceptibility and drug transport in the yeast Pdr5 ATP-binding cassette multidrug resistance transporter. Mol. Biol. Cell, 9, 523–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellgaard L., Molinari, M. and Helenius, A. (1999) Setting the standards: quality control in the secretory pathway. Science, 286, 1882–1888. [DOI] [PubMed] [Google Scholar]
- Finger A., Knop, M. and Wolf, D.H. (1993) Analysis of two mutated vacuolar proteins reveals a degradation pathway in the endoplasmic reticulum or a related compartment in yeast. Eur. J. Biochem., 218, 565–574. [DOI] [PubMed] [Google Scholar]
- Gemmill T.R. and Trimble, R.B. (1999) Overview of N- and O-linked oligosaccharide structures found in various yeast species. Biochim. Biophys. Acta, 1426, 227–237. [DOI] [PubMed] [Google Scholar]
- Guthrie C. and Fink, G.R. (1991) Guide to yeast genetics and molecular biology. Methods Enzymol., 194, 1–270. [PubMed] [Google Scholar]
- Helenius A., Trombetta, E.S., Hebert, D.N. and Simons, J.F. (1997) Calnexin, calreticulin and the folding of glycoproteins. Trends Cell Biol., 7, 193–200. [DOI] [PubMed] [Google Scholar]
- Herscovics A. (1999a) Processing glycosidases of Saccharomyces cerevisiae. Biochim. Biophys. Acta, 1426, 275–285. [DOI] [PubMed] [Google Scholar]
- Herscovics A. (1999b) Importance of glycosidases in mammalian glycoprotein biosynthesis. Biochim. Biophys. Acta, 1473, 96–107. [DOI] [PubMed] [Google Scholar]
- Jakob C.A. and Burda, P. (1999) Quality control in biosynthetic pathways of N-linked glycoproteins in the yeast endoplasmic reticulum. Protoplasma, 207, 1–7. [Google Scholar]
- Jakob C.A., Burda, P., Roth, J. and Aebi, M. (1998) Degradation of misfolded endoplasmic reticulum glycoproteins in Saccharomyces cerevisiae is determined by a specific oligosaccharide structure. J. Cell Biol., 142, 1223–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauer R. and Lehle, L. (1999) The oligosaccharyltransferase complex from yeast. Biochim. Biophys. Acta, 1426, 259–273. [DOI] [PubMed] [Google Scholar]
- Knop M., Hauser, N. and Wolf, D. (1996) N-glycosylation affects ER degradation of a mutated derivative of carboxypeptidase yscY in yeast. Yeast, 12, 1229–1238. [DOI] [PubMed] [Google Scholar]
- Kopito R.R. (1997) ER quality control: the cytoplasmic connection. Cell, 88, 427–430. [DOI] [PubMed] [Google Scholar]
- Lipari F. and Herscovics, A. (1996) Role of the cysteine residues in the α1,2-mannosidase involved in N-glycan biosynthesis in Saccharomyces cerevisiae—the conserved Cys(340) and Cys(385) residues form an essential disulfide bond. J. Biol. Chem., 271, 27615–27622. [DOI] [PubMed] [Google Scholar]
- Liu Y., Choudhury, P., Cabral, C.M. and Sifers, R.N. (1997) Intracellular disposal of incompletely folded human α1-antitrypsin involves release from calnexin and post-translational trimming of asparagine-linked oligosaccharides. J. Biol. Chem., 272, 7946–7951. [DOI] [PubMed] [Google Scholar]
- Liu Y., Choudhury, P., Cabral, C.M. and Sifers, R.N. (1999) Oligosaccharide modification in the early secretory pathway directs the selection of a misfolded glycoprotein for degradation by the proteasome. J. Biol. Chem., 274, 5861–5867. [DOI] [PubMed] [Google Scholar]
- Mancini R., Fagioli, C., Fra, A.M., Maggioni, C. and Sitia, R. (2000) Degradation of unassembled soluble Ig subunits by cytosolic proteasomes: evidence that retrotranslocation and degradation are coupled events. FASEB J., 14, 769–778. [DOI] [PubMed] [Google Scholar]
- Plemper R.K., Egner, R., Kuchler, K. and Wolf, D.H. (1998) Endoplasmic reticulum degradation of a mutated ATP-binding cassette transporter Pdr5 proceeds in a concerted action of Sec61 and the proteasome. J. Biol. Chem., 273, 32848–32856. [DOI] [PubMed] [Google Scholar]
- Rapoport T.A., Jungnickel, B. and Kutay, U. (1996) Protein transport across the eukaryotic endoplasmic reticulum and bacterial inner membranes. Annu. Rev. Biochem., 65, 271–303. [DOI] [PubMed] [Google Scholar]
- Römisch K. (1999) Surfing the Sec61 channel: bidirectional protein translocation across the ER membrane. J. Cell Sci., 112, 4185–4191. [DOI] [PubMed] [Google Scholar]
- Sommer T. and Wolf, D.H. (1997) Endoplasmic reticulum degradation: reverse protein flow of no return. FASEB J., 11, 1227–1233. [DOI] [PubMed] [Google Scholar]
- Spirig U., Glavas, M., Bodmer, D., Reiss, G., Burda, P., Lippuner, V., te Heesen, S. and Aebi, M. (1997) The STT3 protein is a component of the yeast oligosaccharyltransferase complex. Mol. Gen. Genet., 256, 628–637. [DOI] [PubMed] [Google Scholar]
- Su K., Stoller, T., Rocco, J., Zemsky, J. and Green, R. (1993) Pre-Golgi degradation of yeast prepro-α-factor expressed in a mammalian cell. Influence of cell type-specific oligosaccharide processing on intracellular fate. J. Biol. Chem., 268, 14301–14309. [PubMed] [Google Scholar]
- Vallee F., Lipari, F., Yip, P., Sleno, B., Herscovics, A. and Howell, P.L. (2000) Crystal structure of a class I α1,2-mannosidase involved in N-glycan processing and endoplasmic reticulum quality control. EMBO J., 19, 581–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayraghavan U., Company, M. and Abelson, J. (1989) Isolation and characterization of pre-mRNA splicing mutants of Saccharomyces cerevisiae. Genes Dev., 3, 1206–1216. [DOI] [PubMed] [Google Scholar]
- Wach A., Brachat, A., Pöhlmann, R. and Philippsen, P. (1994) New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. Yeast, 10, 1793–1808. [DOI] [PubMed] [Google Scholar]
- Wang Y. and Androlewicz, M.J. (2000) Oligosaccharide trimming plays a role in the endoplasmic reticulum-associated degradation of tyrosinase. Biochem. Biophys. Res. Commun., 271, 22–27. [DOI] [PubMed] [Google Scholar]
- Wang J. and White, A.L. (2000) Role of calnexin, calreticulin, and endoplasmic reticulum mannosidase I in apolipoprotein(a) intracellular targeting. Biochemistry, 39, 8993–9000. [DOI] [PubMed] [Google Scholar]
- Wolf D.H. and Fink, G.R. (1975) Proteinase C (carboxypeptidase Y) mutant of yeast. J. Bacteriol., 123, 1150–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M., Omura, S., Bonifacino, J.S. and Weissman, A.M. (1998) Novel aspects of degradation of T cell receptor subunits from the endoplasmic reticulum (ER) in T cells: importance of oligosaccharide processing, ubiquitination, and proteasome-dependent removal from ER membranes. J. Exp. Med., 187, 835–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zufferey R., Knauer, R., Burda, P., Stagljar, I., te Heesen, S., Lehle, L. and Aebi, M. (1995) STT3, a highly conserved protein required for yeast oligosaccharyltransferase activity in vitro. EMBO J., 14, 4949–4960. [DOI] [PMC free article] [PubMed] [Google Scholar]