Abstract
The implementation of artificial intelligence (AI) and machine learning (ML) techniques in healthcare has garnered significant attention in recent years, especially as a result of their potential to revolutionize personalized medicine. Despite advances in the treatment and management of asthma, a significant proportion of patients continue to suffer acute exacerbations, irrespective of disease severity and therapeutic regimen. The situation is further complicated by the constellation of factors that influence disease activity in a patient with asthma, such as medical history, biomarker phenotype, pulmonary function, level of healthcare access, treatment compliance, comorbidities, personal habits, and environmental conditions. A growing body of work has demonstrated the potential for AI and ML to accurately predict asthma exacerbations while also capturing the entirety of the patient experience. However, application in the clinical setting remains mostly unexplored, and important questions on the strengths and limitations of this technology remain. This review presents an overview of the rapidly evolving landscape of AI and ML integration into asthma management by providing a snapshot of the existing scientific evidence and proposing potential avenues for future applications.
Keywords: Asthma, Algorithm, Artificial intelligence, Exacerbation, Machine learning
Key Summary Points
| Asthma exacerbation events remain difficult to avert and approaches for personalized medicine are lacking. |
| Each patient’s asthma journey and disease severity are influenced by a range of factors including medical history, biomarker phenotype, pulmonary function, level of healthcare system support, compliance to prescribed therapy, comorbidities, personal habits, and environmental conditions. |
| Machine learning (ML) uses mathematical and statistical methods to detect patterns across large datasets including electronic health records (EHR). |
| ML has the potential to augment clinical decision-making and provide appropriate treatment to improve both asthma prognosis and the overall quality of life. |
| In this review, we summarize recent studies that have demonstrated the ability to predict asthma exacerbations using different algorithms and propose a few next steps for this field. |
Introduction
Asthma exacerbations are difficult to predict on the basis of available data of patients at risk. Most medical practitioners usually rely on the patient’s medical history, pulmonary function test (PFT) results, levels of asthma control, and treatments; however, it is unclear as to what proportion of clinicians follow asthma guidelines for the assessment of risk and prevention of exacerbations or use biomarkers [1, 2]. With such multiple variables, it is unsurprising that up to 10 million asthma exacerbations are reported annually in the USA, constituting the majority of expenditures devoted to asthma care [3].
Additionally, management of asthma is a complex process. Clinically, many patients can have poor asthma control and/or poor PFT results; however, they may not seek (or find) medical help and/or comply with treatments until they develop severe bronchoconstriction. Other patients with mild or even asymptomatic asthma can deteriorate suddenly and end up in the emergency department (ED), become hospitalized, or even suffer from fatal asthma attacks [4]. Biomarkers can help pinpoint which patients are at risk of exacerbations [5–7]; however, the endotypes may not be stable because of spontaneous, environmentally triggered, or treatment-induced variability [7–9]. Furthermore, healthcare systems can vary by region, and lack of access is a major challenge that can lead to undertreatment and deterioration of health [10–12]. Patient habits and/or lack of compliance to medications can also influence the development of exacerbations [13–15]. Lastly, environmental air quality is a critical determinant of airway inflammation, bronchospasm, and asthma deterioration [16, 17]. All of these factors can be assessed in the clinic via questionnaires, physical examinations, PFTs, and/or laboratory evaluations. However, once these initial evaluations are completed, there is a need for accurate prediction of the probability of future exacerbations for each individual patient. As clinical factors evolve, subsequent predictive modeling may be beneficial for effective disease management in said patients.
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
There are at least two studies that have used multivariable logistic regression to pinpoint features which may predict exacerbations. Blakey et al. extracted longitudinal medical records of 118,981 patients with actively treated asthma from the Optimum Patient Care Research Database, seeking to predict the number of exacerbations in a 2-year period. After selecting predictors with significant univariable association, the authors trained multiple multivariate logistic regression for recurrent asthma attacks prediction. The strongest features associated with future attacks included the following: history of exacerbations, lung function, smoking status, blood eosinophilia, age, sex and history of rhinitis, nasal polyps, eczema, gastroesophageal reflux disease, and obesity. The final validated models utilized 19 and 16 risk factors for two or more (area under the receiver operating characteristic curve (AUC) 0.785) and four or more (AUC 0.867) attacks over 2 years, respectively [18].
In addition, Noble et al. trained a multivariable logistic regression to predict asthma events (accident and emergency attendance, hospitalization, or death) on a dataset of 61,861 people with asthma extracted from England and Scotland using the Clinical Practice Research Datalink. The fitted model was validated on 174,240 patients with asthma from Wales and had AUC of 0.71. Older and underweight patients who smoked and had history of blood eosinophilia and past asthmatic attacks had the highest probability of experiencing asthma-related crisis events [19]. Since clinicians are not as well suited to the implementation of complex algorithms as machines, assessment of the aforementioned features may not be sufficient to alert patients to their risk of asthma exacerbation. Machines may be unable to implement complex algorithms because data may be inconsistently captured or maintained in an unstructured format [20]. Indeed, additional factors beyond past medical history, current symptoms, PFTs, and even biomarkers may be required for predicting future exacerbations. The patient’s access to medical care, genetic makeup and expression, habits, and environmental exposures may also impact their asthma prognosis. Thus, there is a need to integrate the many characteristics of the “asthmatic patient” using algorithms trained and tested in large, independent datasets to arrive at an accurate prognosis of the disease and potentially augment our ability to predict exacerbations for each patient individually.
Machine learning (ML) and deep learning (DL) are fields within artificial intelligence (AI) that are increasingly being used in medicine [21, 22]. However, one of the challenges in ML is the “no free lunch” theorem, which states that there is no single algorithm that will fit all datasets or solve all questions [23]. There have been efforts to—at a minimum—correlate symptoms and airway function with asthma exacerbations using ML [24]. Although the analysis of lung images was one of the first areas of respiratory medicine to demonstrate increased diagnostic accuracy with the use of neural networks [25], the prediction of acute medical events has become more recently a major unmet need, particularly during the COVID-19 pandemic [26]. This need to establish an accurate prognosis of serious conditions, such as asthma exacerbations, still persists today. In this review, we summarize recent studies that have demonstrated the ability to predict asthma exacerbations using different algorithms and propose a few next steps for this field.
Complexity of Exacerbations
More than 10 million asthma exacerbations occurred in the USA in 2020 [3]. There a need not only to accurately predict who is at risk of exacerbations [27] but also to avoid overdiagnosis and overtreatment of asthma [28]. Asthma exacerbations are defined by symptoms of increased severity that require treatment with systemic corticosteroids for at least three consecutive days [29]. Short bursts of systemic corticosteroids are effective to treat acute asthma, but they tend to be overprescribed and can cause serious side effects [28]. Furthermore, patients with poorly controlled asthma can progress to develop exacerbations due to lack of access to medical care and proper therapies [30], or lack of compliance to prescribed treatments [14]. Indeed, providing proper medications has been shown to reduce the incidence of exacerbations in some geographies [31–33]. However, when applied broadly, such therapeutic approaches incorrectly assume that every person with asthma is at risk of exacerbations, resulting in overtreatment, which in turn can lead to the buildup of unnecessary costs and an increased incidence of treatment-emergent adverse events. Incidentally, among well-treated patients with asthma, exacerbations are ultimately triggered by environmental insults (isolated or in combination) such as respiratory infections, allergens, and air pollution [34, 35].
Asthma exacerbations are complex and multidimensional phenomena, and ML can analyze and predict such medical events if proper inputs (including intrinsic and external factors such as patient features and environmental insults, respectively) are provided to train (or develop), validate, and test said algorithms. One of the key challenges when predicting asthma exacerbations is that the analysis of small sample sizes and few determinants of asthma severity may lead to less accurate predictions and limited conclusions [36, 37]. For example, there are patients with daily symptoms and/or low forced expiratory volume in 1 s (FEV1) who do not exacerbate; conversely, there are patients who seem clinically stable but may go on to develop an acute asthma attack when several dimensions of the asthma milieu occur in concert [38]. Several studies have analyzed levels of asthma biomarkers, such as increased eosinophils in peripheral blood/sputum and increased fractional exhaled nitric oxide (FeNO) [5, 6], yet given the multidimensional aspects of asthma [39], ML approaches will likely be required in the future to augment the accuracy of predictions.
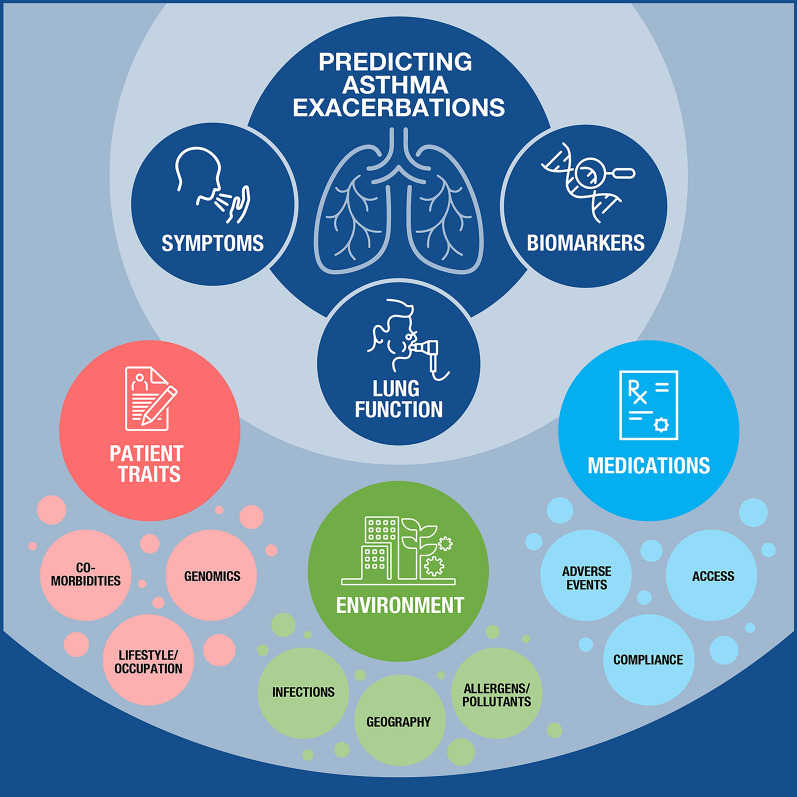
Another potential consequence of acute asthma is disease relapse in some patients who are treated in the ED and then sent back to the ambulatory setting. Although the reported percentage varies among studies, median 2-week relapse rate is considered to be between 4% and 17%, with nearly two-thirds of the patients experiencing a relapse within the first few weeks of discharge [40, 41]. Factors associated with relapse are the same as in any exacerbation [40], with the additional consideration that some patients may be discharged without full recovery of airflow obstruction and underlying inflammation [42], and/or may suffer from psychosocial challenges making recovery outside of the hospital difficult [43, 44]. Even in the best-case scenario, considering only the patient’s past medical history, asthma symptoms, PFT results, biomarkers, and treatments, the number of exacerbations remains constant. Thus, there continues to be a need to increase our knowledge of asthma and/or consider including more features of the “asthmatic” circumstances to predict exacerbations with greater accuracy using AI (Fig. 1).
Fig. 1.
The constellation of factors influencing the asthmatic patient’s journey that may be analyzed using artificial intelligence to augment clinical decision-making. Patients with asthma present a unique collection of factors that influence their disease severity and outcome. The cornerstones of traditional patient management consist of assessments of pulmonary function (i.e., spirometry measuring lung capacity and FEV1), biomarker levels (e.g., blood eosinophils, IgE, FeNO), and patient-reported symptoms (e.g., cough, wheezing, shortness of breath, sleep). However, a myriad of factors together influence the individual patient’s risk of asthma exacerbation and disease progression, including both intrinsic factors such as genomics, comorbidities, and allergic status, and extrinsic factors such as occupational and environmental exposures, access to the healthcare system and medications, and geography. Because asthma exacerbations are complex and multidimensional phenomena, the use of artificial intelligence may better predict such events if proper inputs (including intrinsic and extrinsic factors) are provided to train, validate, and test next-generation treatment algorithms. IgE immunoglobulin E, FeNO fractional exhaled nitric oxide, FEV1 forced expiratory volume in 1 s
Machine Learning and Deep Learning
ML is a subset of AI that allows a machine to automatically learn from data without explicit programming. ML consists of algorithms (a series of steps) that must be trained (developed), validated, and tested before being deployed for general use [45]. There exist many algorithms that can be used in ML (Table 1), which are mainly classified into three categories: (i) supervised learning, (ii) unsupervised learning, and (iii) reinforcement learning.
Table 1.
Commonly used machine learning algorithms (with Python and R codes)
| Algorithms | Objectives | References |
|---|---|---|
| Linear regression |
Used to estimate real values (independent variables such as predicted FEV1) based on continues variables (dependent variable such as height, age, etc.) [46] It can be simple or multiple if there is more than one independent variable |
Frank E. Harrell Jr Regression modeling strategies: with applications to linear models, logistic and ordinal regression, and survival analysis Springer International; 2015 |
| Logistic regressiona |
A classification algorithm used to estimate discrete values (yes/no, true/false, etc.) [46] Since it predicts the probability of developing a certain condition, the values are between 0 and 1 |
Frank E. Harrell Jr Regression modeling strategies: with applications to linear models, logistic and ordinal regression, and survival analysis Springer International; 2015 |
| Support vector machine (SVM) |
A classification algorithm in which the value of each feature is plotted in a particular coordinate (known as support vectors) [47] Data are split by a line (called classifier) equidistant to the closest point from each group |
Ingo Steinwart, Andreas Christmann Support vector machines Springer New York; 2008 |
| Naïve Bayes |
A classification algorithm that assumes independence between predictors [48] It is simple, useful in large datasets, and known to outperform sophisticated classification methods |
Thomas Mitchell Machine learning McGraw-Hill Education; 1997 |
| Decision tree |
A supervised learning algorithm mostly used for the classification of problems with both categorical and continuous dependent variables [49] It splits populations into homogenous sets based on significant attributes (independent variables) |
Clinton Sheppard Tree-based machine learning algorithms: decision trees, random forests, and boosting CreateSpace; 2017 |
| Random foresta |
An ensemble of decision trees It classifies a new object on the basis of attributes and the tree “votes” with forest choosing the classification having the most votes Combining trees improves the accuracy of the model [50] |
Chris Smith, Mark Koning Decision trees and random forests: a visual introduction for beginners Blue Windmill Media; 2017 |
| Gradient boosting machines (GBM)a |
Has high prediction power and is used on large datasets By combining learning algorithms, gradient boosting works well with scientific data XGBoost has high predictive power and accuracy; regularized boosting helps reduce overfitting [51] LightGBM is a faster algorithm that uses tree-based algorithms [52] |
Corey Wade, Kevin Glynn Hands-on gradient boosting with XGBoost and Scikit-learn: perform accessible machine learning and extreme gradient boosting with Python Packt; 2020 Ke G, Meng Q, Finley T, et al. Lightgbm: a highly efficient gradient boosting decision tree Adv Neural Inf Process Syst 2017;30:3149–3157 |
| Recurrent neural networks (RNN)a |
Designed to process sequential data such as time series Have been used to predict hospital readmissions [53] |
Ralf C. Staudemeyer, Eric Rothstein Morris Understanding LSTM – a tutorial into long short-term memory recurrent neural networks arXiv 1909.09586 [preprint]. 2019 |
| Long short-term memory (LSTM)a |
A type of RNN designed to handle long-term dependencies in sequential data [53] Has been used to predict disease progression |
Ralf C. Staudemeyer, Eric Rothstein Morris Understanding LSTM – a tutorial into long short-term memory recurrent neural networks arXiv 1909.09586 [preprint]. 2019 |
| K-nearest neighbors (KNN) |
Can be used for classification or regression problems In classification, it stores all cases and classifies new cases by a majority vote of k-neighbors measured by a distance function [54] The sum of the square of the difference between the centroid and the data points within a cluster determines the value of k for that cluster |
Antonio Mucherino, Petraq J. Papajorgji, Panos M. Pardalos Data mining in agriculture Springer Science & Business Media; 2009 |
| K-means clustering | An unsupervised algorithm that solves clustering problems by picking a k number known as centroid, and each data point forms a cluster with the closest centroids [55] |
Swati Patel K-means clustering algorithm: implementation and critical analysis Scholars; 2019 |
| Dimensionality reduction | It identifies highly significant variables from vast data sets where the data are unstructured or in great detail [56] |
Benyamin Ghojogh, Mark Crowley, Fakhri Karray, Ali Ghodsi Elements of dimensionality reduction and manifold learning Springer Nature; 2023 |
aMost commonly used to predict medical events
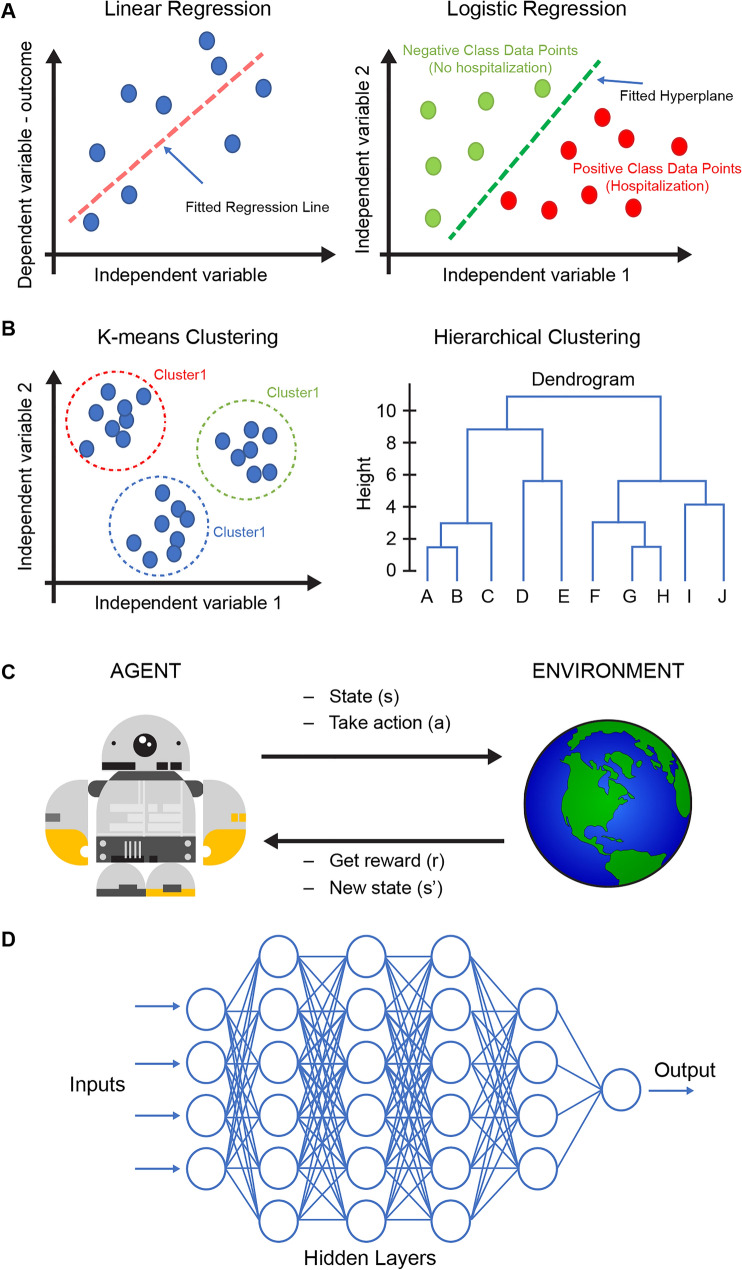
In supervised learning, data are labeled, and the ML model learns how to best map feature vectors (input) to labels (output), allowing for the prediction of outcomes for unforeseen data [45]. This process is called training and gives the model the ability to produce accurate predictions when it is exposed to new and unlabeled datasets. Supervised learning is categorized into two algorithms: regression and classification. In regression algorithms, the output is a continuous variable (i.e., FEV1), while in classification algorithms, the output is a category (i.e., hospitalization). Examples of such algorithms include linear regression and logistic regression (Fig. 2a). In unsupervised learning, the algorithm acts without guidance as data points are not labeled or classified. The algorithm discovers patterns in the data that allow for the classification of similar datapoints together into clusters. Unsupervised learning algorithms are categorized into clustering and associations. Examples of such algorithms are K-means clustering and hierarchical clustering (Fig. 2b). In reinforcement learning, the algorithm (also called “agent”) learns by trial and error, i.e., through interaction with and feedback from the environment in a manner resembling the learning process in animals and humans. Reinforcement learning is used to train the agent to perform more complicated tasks and receive a reward, or not (Fig. 2c). The latter has not been used in medicine yet, as far as we know.
Fig. 2.
Supervised, unsupervised, reinforcement, and deep learning algorithms. a Linear regression and logistic regression are among many supervised learning algorithms and are trained on labeled data. The label can be either a continuous outcome (linear regression) or a category (logistic regression). b K-means clustering and hierarchical clustering are examples of unsupervised learning algorithms and use unlabeled data to discover hidden patterns and data clusters. The output of the supervised learning can be the clusters’ information, a dendrogram, or other visuals that highlight notable patterns within the data. c Reinforcement learning algorithms train an agent to find the optimal succession of actions that optimize a given task. The agent acts within an environment, according to defined rules and a reward system, and through trial and error, the agent explores the whole solution space and eventually identifies the optimal solution. d Deep learning models are composed of multiple layers of artificial neural networks and can be trained to predict continuous and categorical variables, using both structured (i.e., tables) and unstructured data (i.e., images, time series)
Deep learning is a type of ML that involves training artificial neural networks (ANN) containing several hidden layers on a large dataset. These ANNs learn to predict the outcome variable, which can be either continuous or categorical, on the basis of the input. In asthma, DL models have been trained on patient data for demographics, medical history, asthma phenotype, environmental factors, biomarkers, as well as PFT results. The trained model can then be used to predict the likelihood of asthma exacerbations in the near future on the basis of the patient’s data in the baseline period. This approach holds the potential for application in personalized medicine in asthma (Fig. 2d).
Studies Using Machine Learning to Predict Asthma Exacerbations
According to the “no free lunch theorem” there is no single algorithm that can be applied to all datasets homogeneously, making algorithm selection a key decision when applying ML [57, 58]. Thus, the use of ML to analyze features of asthma creates the possibility to uncover interactions between all potential features that can produce clinical outcomes and that may have not been considered until now, thereby leading to better asthma care without increasing the burden on patients and healthcare practitioners.
Original research efforts in ML and asthma were done in small clinical datasets and/or using only genomics data. Results were compared with predictions made by clinicians [59], or those made in genome-wide association studies (GWAS) [60], resulting in ML models with lower accuracy in predicting asthma exacerbations than those based on physicians’ opinions. In 2017, however, Finkelstein and Jeong interrogated data from 7001 self-reported records during a 7-day home telemonitoring window and found that ML algorithms, such as naïve Bayesian classifier, adaptive Bayesian network, and support vector machines (SVM), respectively, were able to predict asthma exacerbations on day 8 with a sensitivity of 0.80, 1.00, and 0.84; a specificity of 0.77, 1.00, and 0.80; and an accuracy of 0.77, 1.00, and 0.80 [61]. Predictive modeling included asthma symptoms, self-reported medication consumption, asthma trigger exposure, and lung function assessed by peak expiratory flow (PEF). Although using these parameters demonstrated promising results, future predictive models for asthma exacerbation should include other potentially relevant predictive factors such as genomic, clinical, sociodemographic, behavioral, and environmental.
Additional studies have also used ML to predict hospitalizations in children and adults with acute asthma [62, 63]. One study in children with acute asthma seen in the ED compared four ML approaches using features from 29,392 patients (mean age ± SD, 7 ± 4.2 years) [62]. Data used to predict hospitalizations included clinical data available at the time of triage, weather reports, neighborhood characteristics, and viruses circulating in the community. Four models were trained and validated, including decision trees, least absolute shrinkage and selection operator (LASSO) logistic regression, random forests, and gradient boosting machines (GBMs), and the area under the receiver operating characteristic curve (AUC) was calculated for each model. AUCs for each model included decision tree, 0.72 (95% confidence interval [CI] 0.66–0.77); logistic regression, 0.83 (95% CI 0.82–0.83); random forests, 0.82 (95% CI 0.81–0.83); and GBM, 0.84 (95% CI 0.83–0.85). Patient vital signs and acuity, age, and weight, followed by socioeconomic status and weather-related features, were the most important features predicting hospitalizations, with GBM identified as the best predictor of hospitalization in the study.
In another study, Goto et al. tested four ML algorithms (LASSO logistic regression, random forest, GBM, and deep neural network [DNN]) on data from 3206 adults with asthma and chronic obstructive pulmonary disease (COPD) exacerbations who visited the ED [63]. In both patient populations (asthma and COPD), GBM was the best-performing approach and achieved the highest discriminative ability (C-statistics 0.80 vs 0.68), reclassification improvement (net reclassification improvement [NRI] 53%; P = 0.002), and sensitivity (0.79 vs 0.53) over the reference model. For the prediction of hospitalizations, random forest provided the highest discriminative ability (C-statistics 0.83 vs 0.64), reclassification improvement (NRI 92%; P < 0.001), and sensitivity (0.75 vs 0.33).
Zein et al. analyzed data extracted from electronic health records (EHRs) of 60,302 patients with asthma and tested three different models (logistic regression, random forests, and GBM) to predict three different outcomes occurring in 19,772 patients with acute asthma [64]. Of the three asthma outcomes, 32.8% required oral glucocorticoid bursts, 2.9% required ED visits, and 1.5% required hospitalizations. The three outcomes were predicted best by light gradient boosting machine, with an AUC of 0.88 (95% CI 0.86–0.89). Risk factors for all three outcomes included age and use of long-acting β-agonists, high-dose inhaled glucocorticoids, or chronic oral glucocorticoid therapies. In a subgroup analysis of 9448 patients with spirometry data, adding results from PFTs did not improve the models’ predictive performances.
More recently, a predictive model of asthma exacerbations used data downloaded from ProAir Digihaler® together with clinical and demographic information [65]. The generated model predicted an impending exacerbation over the following 5 days with high diagnostic accuracy (AUC 0.83). The most significant factors contributing to the model were features based on the mean number of inhaler puffs during the 4 days before prediction. Similarly, Zhang et al. analyzed 728,535 records of daily peak expiratory flow and asthma symptoms in 2010 patients [24]. The authors used logistic regression, decision tree, naïve Bayes, and perceptron algorithms to assess the primary outcome of exacerbation detection on the same day, or up to 3 days in the future. The best model used logistic regression, which showed an AUC of 0.85 with a sensitivity of 90% and specificity of 83% for severe asthma exacerbations.
Tong et al. published results from an accurate model using data from patients with asthma treated at Intermountain Healthcare between 2011 and 2018 [66]. The authors inputted 234 candidate features leading to the analysis of 82,888 data instances to predict asthma exacerbations in the following 12 months. The XGBoost algorithm was used because it deals with missing values and calculates the importance of each feature according to its contribution to the model. The final model, using XGBoost and 71 features in descending order of their importance, yielded an AUC of 0.90 with an accuracy of 91%, sensitivity of 70%, and specificity of 91%. The top 10 features associated with exacerbations were all related to loss of asthma control and African American race. To calculate the probability of an asthma exacerbation occurring in children, Overgaard et al. developed a model that yielded an AUC of 0.80 using logistic regression, whereas random forest showed an AUC of 0.79 and perceptron showed an AUC of 0.78 [67]. Their purpose was to further conduct a clinical trial to assess safety, clinical outcomes, care quality, care cost, and satisfaction of end users (e.g., clinicians, patients, and caregivers) comparing ML algorithms versus standard care for clinical decision-making.
ML has also been used to assess the influence of environmental exposures on asthma exacerbations using conditional random forest, conditional tree, and generalized linear models. Conditional random forest and conditional tree identified five features (prednisone use, race, particulate matter exposure, obesity, and gender) to be of significant importance in the association with exacerbations. The same features (except for gender) were replicated using a generalized linear model [68]. In addition, Haque et al. used a DNN regression (DNNR) model to predict asthma exacerbations on the basis of Asthma Control Test (ACT) scores, weather triggers (temperature, humidity, pressure, and windspeed) and demographic data, with their approach achieving an accuracy of 94% [69].
Another aspect of acute asthma is the number of readmissions that can occur after the initial treatment of acute asthma in the ED. In a study of 81 patients with asthma, Halner et al. developed multivariate random forest models to identify predictors of treatment failure defined as the need for additional systemic corticosteroids and/or antibiotics, hospital readmission, or death within 30 days of the initial ED visit [70]. Forty-three patients failed treatment, which was predicted by an 11-variable random forest model (including medication, history of exacerbations, symptoms, and quality of life) with an AUC of 0.81. In this regard, de Hond et al. recently used home monitoring of peak flow rates and symptoms in 266 patients to adjust their asthma medications and avert exacerbations [71]. These authors compared ML algorithms (XGBoost vs one-class SVM) with a clinical rule and logistic regression. The AUCs were 0.85 for XGBoost and 0.88 for logistic regression. Clinical application, however, remains a challenge owing to the low incidence of events, which may be overly sensitive and create false alarms [72]. Additionally, because asthma is the result of the patient interacting with the environment, both patient activity and environmental data should be included in any ML approach to augment clinical decision-making.
The ML algorithms used in the aforementioned studies found that the most common clinical features associated with asthma exacerbations were loss of asthma control and poor air quality or weather changes; intake of medications and use of PFTs were less clearly linked to asthma exacerbations with their relevance depending on the patient populations and settings. Table 2 contains a concise summary of the studies described above.
Table 2.
Summary of studies employing ML to predict asthma outcomes
| References | Algorithms implemented | Predictive features | Notes |
|---|---|---|---|
| Farion et al. [59] | Naïve Bayes | Not assessed | Predictions made by physicians were more accurate than those made with the Naïve Bayes model or based on PRAM score |
| Xu et al. [60] | Random forest | 160 SNPs | Highest predictive power was obtained by incorporating the 160 most significant SNPs |
| Finkelstein et al. [61] |
Bayesian classifier, Adaptive Bayesian network, Support vector machines |
Not assessed | Prediction accuracy drops by decreasing the prediction time windows (from day 7 to day 1) |
| Blakey et al. [18] | Logistic regression | History of asthma-related events (acute oral corticosteroid courses, emergency visits), frequency of healthcare utilization, lung function, smoking status, blood eosinophilia, rhinitis, nasal polyps, eczema, gastroesophageal reflux disease, obesity, age, and sex | |
| Patel et al. [62] |
Decision trees, Lasso logistic regression, Random forests, Gradient boosting machines |
Oxygen saturation, respiratory rate, triage acuity, pulse rate, weight-for-age z-score, age, socioeconomic status, and weather variables | Gradient boosting produced the highest predictive power |
| Goto et al. [63] |
Lasso regression, Random forest, XGBoost, Deep neural network |
Critical care outcome: arrhythmia, respiratory rate, congestive heart failure, temperature, oxygen saturation, arrival mode (ambulance vs walk-in), asthma status Hospitalization outcome: age, congestive heart failure, arrival mode, asthma status, COPD status, oxygen saturation, respiratory rate |
XGBoost and random forest were the best-performing algorithms for critical care and hospitalization prediction, respectively |
| Zein et al. [64] |
Logistic regression, Random forest, LightGBM |
Non-severe exacerbation outcome: history of sinusitis, treatment with combination iCS and LABA or with HDiCS, and leukotriene inhibitors, high BMI, eosinophilia, low blood albumin Emergency department visit outcome: age, Black/African American race, a history of non-severe exacerbations, history of severe asthma, eosinophilia, low blood albumin Hospitalization outcome: a history of non-severe exacerbations, low hemoglobin, high BMI |
LightGBM generated the best predictions for non-severe exacerbation, emergency department visit, and hospitalization |
| Noble et al. [19] | Logistic regression | Previous hospitalization, older age, being underweight, smoking, history of asthma attacks and blood eosinophilia | |
| Lugogo et al. [65] | Gradient boosting machines | Mean number of daily albuterol inhalations during the 4 days prior to the prediction, inhalation parameters in the 4 days prior to prediction (PIF, inhalation volume, and inhalation duration), and comparison to the baseline values for these inhalation parameters | |
| Zhang et al. [24] |
Logistic regression, Decision tree, Naïve Bayes, Perceptron algorithms |
Not assessed | Logistic regression was the best-performing model |
| Tong et al. [66] | XGBoost | Features related to prior emergency department visits and asthma medications, race | |
| Overgaard et al. [67] |
Logistic regression, Support vector machine, Random forest, Gaussian Naïve Bayes, Perceptron |
Not assessed | Three best-performing algorithms were logistic regression, random forest, and perceptron |
| Lan et al. [68] |
Conditional random forest, Conditional tree, Generalized linear model |
Top four predictive features were prednisone usage, race, daily particulate matter exposure, and obesity | |
| Haque et al. [69] | Deep neural network regression | Not assessed | Model was trained to predict ACT score |
| Halner et al. [70] | Random forest |
Top five predictors were breathlessness, sputum purulence, use of long-acting muscarinic antagonist, number of unscheduled primary care and emergency department visits in the previous 12 months, and ICS use |
Outcome was treatment failure, defined as the need for additional SCS and/or antibiotics, hospital readmission or death within 30 days of initial emergency department visit |
| de Hond et al. [71] |
XGBoost, Support vector machines, Logistic regression |
Not assessed | Logistic regression provided the best predictive performance |
FEV1 forced expiratory volume in 1 s, PRAM pediatric respiratory assessment measure, SNPs single nucleotide polymorphisms, iCS inhaled corticosteroids, LABA long-acting beta agonists, HDiCS high-dose inhaled corticosteroids, ACT Asthma Control Test, SCS systemic corticosteroids
Pros and Cons of Using Machine Learning to Predict Asthma Exacerbations
There are clear advantages to using ML approaches to predict asthma exacerbations with greater accuracy than it is being done today. Such advantages include the more accurate prediction of asthma exacerbation and prognosis through the analyses of large databases (as mentioned in the studies discussed above). ML approaches are faster and less expensive than clinical trials as they reduce the need for manual data entry and analyses, and detect patterns that may be more difficult for human experts to detect [73, 74]. The ability to automatically integrate the different dimensions that are part of the asthma syndrome and its timeline of deterioration leading to exacerbations can only be achieved using ML owing to its lesser scope for human error. In this regard, new features or data patterns that can predict asthma exacerbations could be detected (as has been done in other areas of medical care). For instance, studies have demonstrated that ML can prevent adverse events such as healthcare-associated infections, adverse drug events, venous thromboembolism, surgical complications, pressure ulcers, falls, decompensation, and diagnostic errors [75]. Another advantage of using ML is the ability to implement therapeutic measures by predicting the future risk of deterioration at the point-of-care [76]. Finally, ML may also be applied retrospectively in specific patient subgroups to understand/identify likely clinical outcomes depending on treatment selected, sometimes described as real-world evidence (RWE). There exist some ethical challenges that need to be taken into consideration when planning to use AI in healthcare. First, informed consent must explain when (and if) AI has been used to provide a therapeutic recommendation or to inform prognosis and risk of future events. Explaining to the patient the type of AI used and how it has augmented the accuracy of the prediction is needed, while “black box” algorithms (when the developer cannot share details of the algorithm) should be avoided [77]. Black box approaches are those in which the inner workings of the system are not easily explained, but they are often used in ML algorithms that are trained on large amounts of data. These algorithms can be very accurate at making predictions but can also be difficult to interpret, meaning that it is not always clear why they make the predictions they do. Therefore, there are several reasons why black box approaches should be avoided in healthcare. First, they can lead to a lack of trust between patients and healthcare providers. Second, black box approaches can make it difficult to identify and address bias in healthcare algorithms. Third, black box approaches can make it difficult to improve healthcare algorithms over time. If it is not clear how an algorithm works, it can be difficult to identify the factors that contribute to its accuracy and to make changes that can improve its performance. This can lead to algorithms that are less accurate and less effective over time.
Additionally, the data currently available for use in AI are often unstructured, simply not in any documented form, and/or disputed. High-quality, large, annotated databases may prove to be quite fruitful in minimizing patient harm in the future (caused by potentially incorrect predictions) [78]. Until this happens, patients must be informed of potential pitfalls when implementing AI in healthcare decisions. Because all AI input is developed by humans, it is subject to human bias. This can occur when algorithms are trained in limited demographic groups leading to a lack of generalizability and unintended social bias. If the data are biased, the algorithm will perpetuate these biases, leading to incorrect predictions [79]. Finally, with regards to data privacy, EHRs contain confidential patient information; searching such data using algorithms may constitute a breach of patient privacy.
Conclusion
Despite the use of inhaled corticosteroids, bronchodilators, leukotriene modifiers, and biologics for T2-high and T2-low asthma, some patients with asthma continue to suffer from acute exacerbations. Predicting asthma exacerbations with better accuracy using disease management programs and RWE can significantly reduce asthma-related morbidity and healthcare costs.
Each patient’s asthma journey and disease severity are influenced by a constellation of factors, including their medical history, biomarker phenotype, pulmonary function, level of healthcare system support, compliance to prescribed therapy, comorbidities, personal habits, and environmental conditions. Such aspects of the patient’s experience can be analyzed using ML to augment clinical decision-making and provide appropriate treatment to improve both asthma prognosis and the overall quality of life.
It must be noted that there are two metrics used to evaluate performance of an ML model in binary classification tasks: precision and recall. These measure how accurate and how complete the model’s positive predictions are, respectively. In general, it is desirable to have both high precision and high recall. However, in a clinical diagnosis system, one may prioritize recall over precision because it is more important to identify all patients at risk of exacerbations, even if some of the patients with less severe asthma may present with comparatively lower risk of exacerbations.
Additionally, while ML works well in very large and/or well-labeled datasets, it may be difficult to apply ML directly in smaller and/or less defined datasets. In such circumstances, an initial step of natural language processing (NLP) could help provide a curated dataset as input to the ML model. Clinical NLP can be applied to clinician notes to extract clinical features such as clinic visits, hospital treatments, and PFTs among other features in the patient timeline that can serve as ML inputs. Furthermore, the interpretability of ML algorithms by patients and healthcare professionals can be improved by using simpler algorithms that can explain their predictions in plain language, or by visualizing the algorithm’s decision-making process that can then be audited by humans.
Acknowledgments
Medical Writing and Editorial Assistance
Medical writing support was provided by Meenakshi Mukherjee, PhD, CMPP (Cactus Life Sciences, part of Cactus Communications) with funding from Amgen Inc.
Author Contributions
Conception or design of the work: Nestor A Molfino. Acquisition, analysis, or interpretation of data for the work: Nestor A Molfino and Gianluca Turcatel. Drafting the work or reviewing it critically for important intellectual content: Nestor A Molfino, Gianluca Turcatel, and Daniel Riskin. Final approval of the version to be published: Nestor A Molfino, Gianluca Turcatel, and Daniel Riskin.
Funding
This study was sponsored by Amgen Inc, including the funding of the journal’s Rapid Service Fee and Open Access Fee.
Data Availability
Qualified researchers may request data from Amgen clinical studies. Complete details are available at https://wwwext.amgen.com/science/clinical-trials/clinical-data-transparency-practices/.
Declarations
Conflict of Interest
NA Molfino and G Turcatel are employees and stockholders of Amgen Inc. D Riskin is the Founder and Chief Executive Officer of Verantos Inc.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
References
- 1.Baldacci S, Simoni M, Maio S, et al. Prescriptive adherence to GINA guidelines and asthma control: an Italian cross sectional study in general practice. Respir Med. 2019;146:10–7. [DOI] [PubMed] [Google Scholar]
- 2.Cloutier MM, Salo PM, Akinbami LJ, et al. Clinician agreement, self-efficacy, and adherence with the guidelines for the diagnosis and management of asthma. J Allergy Clin Immunol Pract. 2018;6:886-894.e884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Asthma: Most recent national asthma data. 2022. https://www.cdc.gov/asthma/most_recent_national_asthma_data.htm.
- 4.O’Byrne P, Fabbri LM, Pavord ID, Papi A, Petruzzelli S, Lange P. Asthma progression and mortality: the role of inhaled corticosteroids. Eur Respir J. 2019;54:1900491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buhl R, Korn S, Menzies-Gow A, et al. Prospective, single-arm, longitudinal study of biomarkers in real-world patients with severe asthma. J Allergy Clin Immunol Pract. 2020;8:2630–2639.e2636. [DOI] [PubMed] [Google Scholar]
- 6.Peters MC, Mauger D, Ross KR, et al. Evidence for exacerbation-prone asthma and predictive biomarkers of exacerbation frequency. Am J Respir Crit Care Med. 2020;202:973–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Porpodis K, Tsiouprou I, Apostolopoulos A, et al. Eosinophilic asthma, phenotypes-endotypes and current biomarkers of choice. J Pers Med. 2022;12(7):1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuruvilla ME, Lee FE, Lee GB. Understanding asthma phenotypes, endotypes, and mechanisms of disease. Clin Rev Allergy Immunol. 2019;56:219–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaur R, Chupp G. Phenotypes and endotypes of adult asthma: moving toward precision medicine. J Allergy Clin Immunol. 2019;144:1–12. [DOI] [PubMed] [Google Scholar]
- 10.Zachary CY, Scott TA, Foggs M, Meadows JA. Asthma: an illustration of health care disparities. Ann Allergy Asthma Immunol. 2020;124:148–9. [DOI] [PubMed] [Google Scholar]
- 11.Gaffney AW, Hawks L, Bor D, et al. National trends and disparities in health care access and coverage among adults with asthma and COPD: 1997–2018. Chest. 2021;159:2173–82. [DOI] [PubMed] [Google Scholar]
- 12.Nadeem MF, Kaiser LR. Disparities in health care delivery systems. Thorac Surg Clin. 2022;32:13–21. [DOI] [PubMed] [Google Scholar]
- 13.Stern L, Berman J, Lumry W, et al. Medication compliance and disease exacerbation in patients with asthma: a retrospective study of managed care data. Ann Allergy Asthma Immunol. 2006;97:402–8. [DOI] [PubMed] [Google Scholar]
- 14.Engelkes M, Janssens HM, de Jongste JC, Sturkenboom MC, Verhamme KM. Medication adherence and the risk of severe asthma exacerbations: a systematic review. Eur Respir J. 2015;45:396–407. [DOI] [PubMed] [Google Scholar]
- 15.Chan A, De Simoni A, Wileman V, et al. Digital interventions to improve adherence to maintenance medication in asthma. Cochrane Database Syst Rev. 2022;6:CD013030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poole JA, Barnes CS, Demain JG, et al. Impact of weather and climate change with indoor and outdoor air quality in asthma: a Work Group Report of the AAAAI Environmental Exposure and Respiratory Health Committee. J Allergy Clin Immunol. 2019;143:1702–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tiotiu AI, Novakova P, Nedeva D, et al. Impact of air pollution on asthma outcomes. Int J Environ Res Public Health. 2020;17:6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blakey JD, Price DB, Pizzichini E, et al. Identifying risk of future asthma attacks using UK medical record data: a respiratory effectiveness group initiative. J Allergy Clin Immunol Pract. 2017;5:1015–1024.e8. [DOI] [PubMed] [Google Scholar]
- 19.Noble M, Burden A, Stirling S, et al. Predicting asthma-related crisis events using routine electronic healthcare data: a quantitative database analysis study. Br J Gen Pract. 2021;71:e948–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martínez-García M, Hernández-Lemus E. Data integration challenges for machine learning in precision medicine. Front Med (Lausanne). 2022;8:784455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Topol EJ. High-performance medicine: the convergence of human and artificial intelligence. Nat Med. 2019;25:44–56. [DOI] [PubMed] [Google Scholar]
- 22.Acosta JN, Falcone GJ, Rajpurkar P, Topol EJ. Multimodal biomedical AI. Nat Med. 2022;28:1773–84. [DOI] [PubMed] [Google Scholar]
- 23.Atkinson MK, Saghafian S. Who should see the patient? On deviations from preferred patient-provider assignments in hospitals. Health Care Manag Sci. 2023;26:165–99. [DOI] [PubMed] [Google Scholar]
- 24.Zhang O, Minku LL, Gonem S. Detecting asthma exacerbations using daily home monitoring and machine learning. J Asthma. 2021;58:1518–27. [DOI] [PubMed] [Google Scholar]
- 25.Akhter Y, Singh R, Vatsa M. AI-based radiodiagnosis using chest X-rays: a review. Front Big Data. 2023;6:1120989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sarmiento Varón L, González-Puelma J, Medina-Ortiz D, et al. The role of machine learning in health policies during the COVID-19 pandemic and in long COVID management. Front Public Health. 2023;11:1140353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Couillard S, Petousi N, Smigiel KS, Molfino NA. Toward a predict and prevent approach in obstructive airway diseases. J Allergy Clin Immunol Pract. 2023;11:704–12. [DOI] [PubMed] [Google Scholar]
- 28.Price D, Castro M, Bourdin A, Fucile S, Altman P. Short-course systemic corticosteroids in asthma: striking the balance between efficacy and safety. Eur Respir Rev. 2020;29: 190151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343–73. [DOI] [PubMed] [Google Scholar]
- 30.Codispoti CD, Greenhawt M, Oppenheimer J. The role of access and cost-effectiveness in managing asthma: a systematic review. J Allergy Clin Immunol Pract. 2022;10:2109–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Comaru T, Pitrez PM, Friedrich FO, Silveira VD, Pinto LA. Free asthma medications reduces hospital admissions in Brazil (Free asthma drugs reduces hospitalizations in Brazil). Respir Med. 2016;121:21–5. [DOI] [PubMed] [Google Scholar]
- 32.Koltermann V, Friedrich FO, Fensterseifer AC, Ongaratto R, Pinto LA. Cost-benefit impact of free asthma medication provision for the pediatric population. Respir Med. 2020;164:105915. [DOI] [PubMed] [Google Scholar]
- 33.Haahtela T, Tuomisto LE, Pietinalho A, et al. A 10 year asthma programme in Finland: major change for the better. Thorax. 2006;61:663–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.May L, Carim M, Yadav K. Adult asthma exacerbations and environmental triggers: a retrospective review of ED visits using an electronic medical record. Am J Emerg Med. 2011;29:1074–82. [DOI] [PubMed] [Google Scholar]
- 35.McIntyre A, Busse WW. Asthma exacerbations: the Achilles heel of asthma care. Trends Mol Med. 2022;28:1112–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Puranik S, Forno E, Bush A, Celedón JC. Predicting severe asthma exacerbations in children. Am J Respir Crit Care Med. 2017;195:854–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fleming L. Asthma exacerbation prediction: recent insights. Curr Opin Allergy Clin Immunol. 2018;18:117–23. [DOI] [PubMed] [Google Scholar]
- 38.Albanna AS, Atiah AK, Alamoudi SM, Khojah OM, Alajmi RS, Dabroom AA. Treatment response among asthmatic patients with and without reversible airflow limitations. J Taibah Univ Med Sci. 2021;16:950–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Han YY, Zhang X, Wang J, et al. Multidimensional assessment of asthma identifies clinically relevant phenotype overlap: a cross-sectional study. J Allergy Clin Immunol Pract. 2021;9:349–362.e318. [DOI] [PubMed] [Google Scholar]
- 40.Hill J, Arrotta N, Villa-Roel C, Dennett L, Rowe BH. Factors associated with relapse in adult patients discharged from the emergency department following acute asthma: a systematic review. BMJ Open Respir Res. 2017;4: e000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nowak RM, Parker JM, Silverman RA, et al. A randomized trial of benralizumab, an antiinterleukin 5 receptor α monoclonal antibody, after acute asthma. Am J Emerg Med. 2015;33:14–20. [DOI] [PubMed] [Google Scholar]
- 42.Hasegawa K, Craig SS, Teach SJ, Camargo CA Jr. Management of asthma exacerbations in the emergency department. J Allergy Clin Immunol Pract. 2021;9:2599–610. [DOI] [PubMed] [Google Scholar]
- 43.Grant T, Croce E, Matsui EC. Asthma and the social determinants of health. Ann Allergy Asthma Immunol. 2022;128:5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schyllert C, Lindberg A, Hedman L, et al. Socioeconomic inequalities in asthma and respiratory symptoms in a high-income country: changes from 1996 to 2016. J Asthma. 2023;60:185–94. [DOI] [PubMed] [Google Scholar]
- 45.Sarker IH. Machine learning: Algorithms, real-world applications and research directions. SN Comput Sci. 2021;2:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harrell FE. Regression modeling strategies: with applications to linear models, logistic and ordinal regression, and survival analysis. Springer International; 2015. [Google Scholar]
- 47.Steinwart I, Christmann A. Support vector machines. New York: Springer; 2008. [Google Scholar]
- 48.Mitchell TM. Machine learning. McGraw-Hill Education; 1997. [Google Scholar]
- 49.Sheppard C. Tree-based machine learning algorithms: decision trees, random forests, and boosting. Create Space; 2017. [Google Scholar]
- 50.Koning M, Smith C. Decision trees and random forests: a visual introduction for beginners: a simple guide to machine learning with decision trees. Blue Windmill; 2017. [Google Scholar]
- 51.Wade C, Glynn K. Hands-on gradient boosting with XGBoost and Scikit-learn: perform accessible machine learning and extreme gradient boosting with Python. Packt; 2020. [Google Scholar]
- 52.Ke G, Meng Q, Finley T, et al. Lightgbm: a highly efficient gradient boosting decision tree. Adv Neural Inf Process Syst. 2017;30:3149–57. [Google Scholar]
- 53.Staudemeyer RC, Morris ER. Understanding LSTM: a tutorial into long short-term memory recurrent neural networks. ArXiv Preprint. 2019. 10.48550/arXiv.1909.09586. [Google Scholar]
- 54.Mucherino A, Papajorgji P, Pardalos PM. Data mining in agriculture. Springer Science and Business Media; 2009. [Google Scholar]
- 55.Patel S. K-means clustering algorithm: implementation and critical analysis. Cham: Scholars; 2019. [Google Scholar]
- 56.Ghojogh B, Crowley M, Karray F, Ghodsi A. Elements of dimensionality reduction and manifold learning. Springer Nature; 2023. [Google Scholar]
- 57.Magdon-Ismail M. No free lunch for noise prediction. Neural Comput. 2000;12:547–64. [DOI] [PubMed] [Google Scholar]
- 58.Almas B, Mujtaba H, Khan KU. EHHR: an efficient evolutionary hyper-heuristic based recommender framework for short-text classifier selection. Cluster Comput. 2023;26:1425–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Farion KJ, Wilk S, Michalowski W, O’Sullivan D, Sayyad-Shirabad J. Comparing predictions made by a prediction model, clinical score, and physicians: pediatric asthma exacerbations in the emergency department. Appl Clin Inform. 2013;4:376–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu M, Tantisira KG, Wu A, et al. Genome wide association study to predict severe asthma exacerbations in children using random forests classifiers. BMC Med Genet. 2011;12:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Finkelstein J, Jeong IC. Machine learning approaches to personalize early prediction of asthma exacerbations. Ann N Y Acad Sci. 2017;1387:153–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Patel SJ, Chamberlain DB, Chamberlain JM. A machine learning approach to predicting need for hospitalization for pediatric asthma exacerbation at the time of emergency department triage. Acad Emerg Med. 2018;25:1463–70. [DOI] [PubMed] [Google Scholar]
- 63.Goto T, Camargo CA Jr, Faridi MK, Yun BJ, Hasegawa K. Machine learning approaches for predicting disposition of asthma and COPD exacerbations in the ED. Am J Emerg Med. 2018;36:1650–4. [DOI] [PubMed] [Google Scholar]
- 64.Zein JG, Wu CP, Attaway AH, Zhang P, Nazha A. Novel machine learning can predict acute asthma exacerbation. Chest. 2021;159:1747–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lugogo NL, DePietro M, Reich M, et al. A predictive machine learning tool for asthma exacerbations: results from a 12-week, open-label study using an electronic multi-dose dry powder inhaler with integrated sensors. J Asthma Allergy. 2022;15:1623–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tong Y, Messinger AI, Wilcox AB, et al. Forecasting future asthma hospital encounters of patients with asthma in an academic health care system: Predictive model development and secondary analysis study. J Med Internet Res. 2021;23:e22796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Overgaard SM, Peterson KJ, Wi CI, et al. A technical performance study and proposed systematic and comprehensive evaluation of an ML-based CDS solution for pediatric asthma. AMIA Jt Summits Transl Sci Proc. 2022;2022:25–35. [PMC free article] [PubMed] [Google Scholar]
- 68.Lan B, Haaland P, Krishnamurthy A, et al. Open application of statistical and machine learning models to explore the impact of environmental exposures on health and disease: an asthma use case. Int J Environ Res Public Health. 2021;18:11398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Haque R, Ho S, Chai I, Abdullah A. Optimised deep neural network model to predict asthma exacerbation based on personalised weather triggers. F1000Research. 2021;10:911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Halner A, Beer S, Pullinger R, Bafadhel M, Russell REK. Predicting treatment outcomes following an exacerbation of airways disease. PLoS ONE. 2021;16:e0254425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.de Hond AAH, Kant IMJ, Honkoop PJ, Smith AD, Steyerberg EW, Sont JK. Machine learning did not beat logistic regression in time series prediction for severe asthma exacerbations. Sci Rep. 2022;12:20363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Winters BD, Cvach MM, Bonafide CP, et al. Technological distractions (part 2): a summary of approaches to manage clinical alarms with intent to reduce alarm fatigue. Crit Care Med. 2018;46:130–7. [DOI] [PubMed] [Google Scholar]
- 73.Jain AK. Data clustering: 50 years beyond K-means. Pattern Recogn Lett. 2010;31:651–66. [Google Scholar]
- 74.Gulshan V, Peng L, Coram M, et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA. 2016;316:2402–10. [DOI] [PubMed] [Google Scholar]
- 75.Hong N, Liu C, Gao J, et al. State of the art of machine learning-enabled clinical decision support in intensive care units: literature review. JMIR Med Inform. 2022;10:e28781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rajkomar A, Oren E, Chen K, et al. Scalable and accurate deep learning with electronic health records. NPJ Digit Med. 2018;1:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lipton ZC. The mythos of model interpretability: in machine learning, the concept of interpretability is both important and slippery. Queue. 2018;16:31–57. [Google Scholar]
- 78.Doshi-Velez F, Kim B. Towards a rigorous science of interpretable machine learning. ArXiv Preprint. 2017. 10.48550/arXiv.1702.08608. [Google Scholar]
- 79.O’Neil C. Weapons of math destruction: how big data increases inequality and threatens democracy. Penguin; 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Qualified researchers may request data from Amgen clinical studies. Complete details are available at https://wwwext.amgen.com/science/clinical-trials/clinical-data-transparency-practices/.