Abstract
Staphylococcus aureus and Staphylococcus epidermidis both recognize and bind the human iron-transporting glycoprotein, transferrin, via a 42-kDa cell surface protein receptor. In an iron-deficient medium, staphylococcal growth can be promoted by the addition of human diferric transferrin but not human apotransferrin. To determine whether the staphylococcal transferrin receptor is involved in the removal of iron from transferrin, we employed 6 M urea–polyacrylamide gel electrophoresis, which separates human transferrin into four forms (diferric, monoferric N-lobe, and monoferric C-lobe transferrin and apotransferrin). S. aureus and S. epidermidis but not Staphylococcus saprophyticus (which lacks the transferrin receptor) converted diferric human transferrin into its apotransferrin form within 30 min. During conversion, iron was removed sequentially from the N lobe and then from the C lobe. Metabolic poisons such as sodium azide and nigericin inhibited the release of iron from human transferrin, indicating that it is an energy-requiring process. To demonstrate that this process is receptor rather than siderophore mediated, we incubated (i) washed staphylococcal cells and (ii) the staphylococcal siderophore, staphyloferrin A, with porcine transferrin, a transferrin species which does not bind to the staphylococcal receptor. While staphyloferrin A removed iron from both human and porcine transferrins, neither S. aureus nor S. epidermidis cells could promote the release of iron from porcine transferrin. In competition binding assays, both native and recombinant N-lobe fragments of human transferrin as well as a naturally occurring human transferrin variant with a mutation in the C-lobe blocked binding of 125I-labelled transferrin. Furthermore, the staphylococci removed iron efficiently from the iron-loaded N-lobe fragment of human transferrin. These data demonstrate that the staphylococci efficiently remove iron from transferrin via a receptor-mediated process and provide evidence to suggest that there is a primary receptor recognition site on the N-lobe of human transferrin.
One common factor among the complex interactions which occur between a bacterial pathogen and its host is the ability of the invading pathogen to multiply in host tissues. In extracellular mammalian body fluids, the iron transport proteins transferrin and lactoferrin maintain the level of free ionic iron at a level (about 10−18 M) which is far too low to sustain bacterial growth (6, 43). In spite of this, pathogenic bacteria clearly multiply successfully in vivo to establish an infection. Since all known bacterial pathogens need iron to multiply, it can be argued that they must be able to adapt to the severely iron-restricted extracellular environment usually found in vivo and develop mechanisms for assimilating transferrin- or lactoferrin-bound iron. Such high-affinity iron-scavenging mechanisms capable of removing iron from transferrins have been intensively investigated in gram-negative bacteria, where they depend either on the synthesis and secretion of low-molecular-mass iron chelators (siderophores) or, alternatively, on direct contact between the host transferrin and a surface receptor (for reviews, see references 9, 18, and 43). While siderophores remove iron from transferrin irrespective of its origin, bacterial transferrin receptors exhibit significant specificity for the transferrins of their natural hosts. For example, Haemophilus influenzae, Neisseria meningitidis, and Neisseria gonorrhoeae exhibit a marked preference for human transferrin (18, 43) while the porcine pathogen Actinobacillus (Haemophilus) pleuropneumoniae is able to bind and use pig but not human transferrin as an iron source (17, 32, 33). Furthermore, gonococcal transferrin receptor mutants are incapable of causing experimental urethritis in human male volunteers, demonstrating for the first time that an iron acquisition system is an essential virulence factor for human infection (11).
In contrast, the mechanism(s) by which the gram-positive staphylococci acquire iron from transferrin has not been fully elucidated. Early work by Schade (37) indicated that Staphylococcus aureus grows in human serum which contains transferrin, implying that staphylococci are able to utilize glycoprotein-bound iron. Staphylococci have been shown to secrete siderophores such as staphyloferrin A and staphyloferrin B which, chemically, are carboxylate-type siderophores (20, 25, 29). They have also been reported to employ primary metabolites such as α-ketoacids and α-hydroxyacids as siderophores (23). More recently, a new S. aureus siderophore termed “aureochelin” has been identified and partially characterized (12). Whether these siderophores are capable of removing iron from transferrin has not been determined. However, Lindsay et al. (27) reported that S. aureus but not Staphylococcus epidermidis could remove iron from 55Fe-labelled transferrin via a process which did not require transferrin-staphylococcus cell surface contact and which therefore was assumed to be siderophore mediated.
Previously we identified a saturable specific receptor for transferrin on the surface of S. aureus and a number of different species of coagulase-negative staphylococci, including S. epidermidis (30). This receptor involves a 42-kDa cell wall transferrin-binding protein which, in common with the gram-negative bacterial transferrin binding proteins, exhibits considerable transferrin species specificity. Human, rabbit, and rat serum transferrins but not bovine or porcine serum transferrins or hen ovotransferrin compete efficiently with the 125I-labelled human transferrin for the S. aureus and S. epidermidis transferrin receptor (30). Furthermore, staphylococci recovered without subculture from an implanted peritoneal chamber in rats are coated with surface-bound transferrin and express the 42-kDa transferrin-binding protein (31). The presence of a cell surface transferrin-binding protein, which, in many staphylococci, is iron regulated (30) suggests that this receptor may contribute to virulence by facilitating the acquisition of transferrin-bound iron.
Human transferrin is an approximately 80-kDa monomeric protein consisting of 679 amino acid residues with two N-linked glycan chains and can be divided into two homologous domains, referred to as the N and C lobes, each of which contains an iron-binding site (2, 42). Each site binds one ferric ion atom coordinated with a bicarbonate anion. Amino acid sequencing of the two transferrin lobes has revealed that they have a high degree of homology and may have arisen by gene duplication (42). Approximately 42% of amino acids in the N lobe have an identical counterpart in the C lobe which also contains both glycan chains (21). It is therefore possible that, since both human apotransferrin and diferric human transferrin have similar affinities for the staphylococcal receptor (30), the primary receptor-binding site on transferrin is located within a single domain, as the two domains within a given lobe undergo substantial conformational changes upon iron binding (19).
In the present work, we explore the contribution of the staphylococcal receptor to the acquisition of iron from transferrin and provide evidence that there is a primary receptor recognition site on the N lobe of human transferrin.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
S. aureus BB was obtained from J. P. Arbuthnott (University of Strathclyde, Glasgow, United Kingdom); S. epidermidis 138 and Staphylococcus saprophyticus 907 were isolated from the peritoneal dialysis fluid of infected patients undergoing continuous ambulatory peritoneal dialysis (41). Staphylococci were grown in an iron-depleted, serum-free tissue culture medium (RPMI 1640; Sigma) statically for 18 h at 37°C in air enriched with 5% CO2. Iron was removed from the RPMI by batch incubation with 6% (wt/vol) Chelex 100 (Sigma) for 18 h as described before (22). After removal of the resin, calcium chloride (10 μM) and magnesium sulfate (100 μM) were added and the medium was filter sterilized. For some experiments, iron-depleted RPMI was supplemented with either diferric human transferrin (250 μM protein) or with ferric chloride (25 μM). Growth of staphylococci was followed by measuring the optical density at 600 nm at 1-h intervals for 24 h.
Preparation of transferrins.
Human transferrin was isolated from outdated plasma by ammonium sulphate precipitation and ion-exchange chromatography. Porcine transferrin was purchased from First Link, Briarley Hill, West Midlands, United Kingdom. Transferrins were made iron saturated by the addition of iron(III) nitrilotriacetate to transferrin dissolved in 1 M NaHCO3. Unbound iron was removed by gel filtration on Sephadex G-25 equilibrated with 0.05 M NH4HCO3, and the protein-containing fractions were collected and lyophilized (15). The iron saturation of the protein was confirmed by electrophoresis on a 6 M urea–polyacrylamide gel (see below). The C-lobe human transferrin variant from a subject heterozygous for an abnormal transferrin (14) was isolated by immunoaffinity chromatography and ion-exchange chromatography on a Pharmacia LKB (Uppsala, Sweden) HP Q-Sepharose column as previously described (15). The N-terminal lobe of human transferrin was prepared as described by Evans et al. (15) by digestion of the diferric protein in 0.1 M NaHCO3, pH 8.3, with subtilisin (Sigma) for 6 h at 37°C at an enzyme/protein ratio of 1/30 (wt/wt). The digest was fractionated by gel filtration on a Sephacryl S-200 column (2.4 by 120 cm) equilibrated with 0.1 M NH4HCO3. The 36-kDa fragment obtained had the characteristic absorption band of iron transferrin at 470 nm and lacked carbohydrate. The recombinant N lobe of human transferrin was expressed in baby hamster kidney cells and purified from culture fluid by immunoaffinity and ion-exchange chromatography as described by Zak et al. (47).
Preparation of 125I-labelled human transferrin.
Two hundred microliters of a 100-μg/ml solution of Iodogen (Pierce and Warriner, Chester, United Kingdom) in dichloromethane was added to a 4-ml test tube, and the dichloromethane was allowed to evaporate by rotating in a water bath at 37°C. Three hundred micrograms of iron-saturated human transferrin (Sigma) and approximately 6 MBq of carrier-free 125I-labelled sodium iodide (Amersham International plc, Little Chalfont, United Kingdom) were added to each Iodogen-coated tube in 300 μl of phosphate-buffered saline (PBS), pH 7.4. The mixture was incubated with agitation at room temperature for 15 min, and the unincorporated 125I was removed by passing the 125I-transferrin down a Sephadex G-25 column (Pharmacia) preequilibrated with PBS containing 0.25% (wt/vol) transferrin.
Transferrin binding assays.
Competitive binding assays were carried out essentially as described by Modun et al. (30). Briefly, iron-depleted staphylococci (108 CFU) were washed, resuspended in 1 ml of PBS and incubated with diferric human 125I-transferrin (4 nM) in the presence of 700 nM of unlabelled diferric human (normal or C-lobe variant) or porcine transferrin or the monoferric transferrin N lobe (subtilisin generated or recombinant). After 30 min of incubation, bacteria were pelleted and washed three times in PBS and transferred to a fresh microcentrifuge tube and the amount of cell-associated 125I-transferrin was determined with an LKB 1282 Compugamma counter (Pharmacia LKB). Specific binding was defined as the difference between the amounts of 125I-human transferrin bound in the absence and presence of a 100-fold excess of the unlabelled ligand.
6 M urea–polyacrylamide gel electrophoresis.
Iron removal from transferrins by staphylococcal cells and by the siderophore staphyloferrin A (kindly provided by G. Winkelmann, Tübingen, Germany) was monitored over time by 6 M urea–polyacrylamide gel electrophoresis (13, 28). S. epidermidis, S. aureus, and S. saprophyticus grown in iron-depleted RPMI were washed and resuspended in PBS before incubation with 250 μg of diferric human transferrin or the recombinant monoferric N lobe of human transferrin in the presence or absence of glucose (0.2% wt/vol) at 37°C for intervals of 5, 10, 20, 30, and 60 min. Bacterial cells were centrifuged, and the supernatant containing transferrin was loaded onto a 6 M urea–polyacrylamide gel prepared by the method of Evans and Williams (13). The pH of the supernatant was monitored during each experiment to ensure that loss of iron from the protein was not a direct result of acidification of the medium through the metabolism of glucose. In addition, supernatants were assayed for the presence of siderophores by using the universal chrome azurol S assay system of Schwyn and Neilands (38). For some experiments, the staphylococcal siderophore staphyloferrin A (250 μg/ml) was used instead of whole cells. Electrophoresis was performed at 100 V for 4 h in Tris-borate-EDTA buffer (pH 7.4), and the gel was stained with Coomassie brilliant blue. To determine whether the release of iron from transferrin by whole staphylococcal cells is an energy-requiring process, the metabolic poison sodium azide or nigericin (both from Sigma) was included in the reaction mixtures at concentrations ranging from 0 to 6 mM (26, 36).
RESULTS
Utilization of iron-bound transferrin.
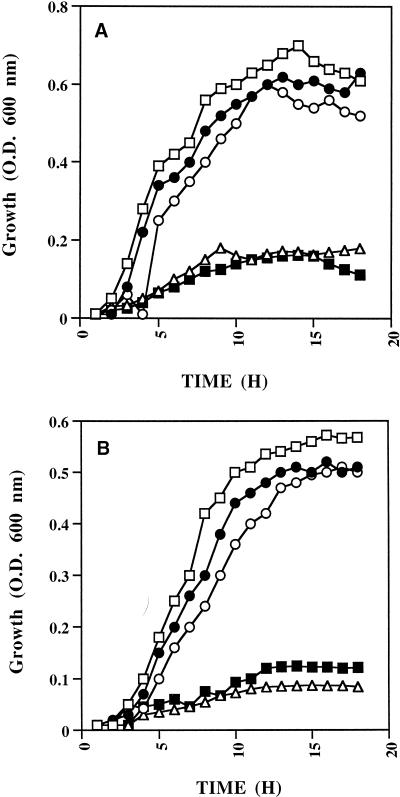
Figure 1A shows that in iron-depleted RPMI, the growth of S. aureus is not supported unless the medium is supplemented with an iron source such as ferric chloride. Growth was also promoted by diferric human transferrin but not by apotransferrin. Similar results were obtained for S. epidermidis (Fig. 1B).
FIG. 1.
Utilization of transferrin-bound iron by S. aureus (A) and S. epidermidis (B). Staphylococci were grown in RPMI (□), iron-depleted RPMI (▵), iron-depleted RPMI supplemented with human diferric transferrin (○), human apotransferrin (■), or ferric chloride (•). Growth was monitored by measuring optical density (O.D.) at 600 nm at 1-h intervals over 18 h.
Removal of iron from human transferrin.
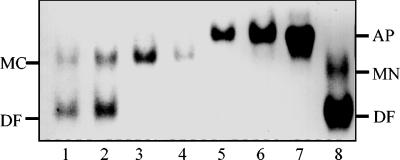
Since transferrin has two binding sites for iron, we used 6 M urea–polyacrylamide gel electrophoresis to follow the release of iron from diferric human transferrin. This technique is capable of resolving a partially iron-saturated sample of human transferrin into four forms, apotransferrin, C-terminal monoferric transferrin, N-terminal monoferric transferrin, and diferric transferrin (13, 16, 28, 44). When incubated in PBS supplemented with glucose, S. aureus converted diferric human transferrin into its apotransferrin form within 30 min (Fig. 2). During this time, the pH of the incubation buffer decreased from pH 7.4 to 6.6 after 60 min of incubation. However, such a small reduction does not influence the release of iron from transferrin. Furthermore, iron is clearly removed sequentially, first from the N lobe of diferric transferrin, as shown by the accumulation of the monoferric C lobe (Fig. 2). This monoferric transferrin is then converted to the apoprotein (Fig. 2). Similar results were obtained for S. epidermidis (data not shown). In contrast, S. saprophyticus, which lacks the transferrin receptor and 42-kDa transferrin-binding protein (30), was unable to remove iron from human transferrin (data not shown).
FIG. 2.
Time course experiment showing (by 6 M urea–polyacrylamide gel electrophoresis) the removal of iron from diferric human transferrin by S. aureus. Staphylococci (108 CFU/ml) were incubated in PBS buffer plus glucose with 250 μg of diferric human transferrin at 37°C for 0 (lane 1), 5 (lane 2), 10 (lane 3), 20 (lane 4), 30 (lane 5), and 60 (lane 6) min. Cells were pelleted by centrifugation, and the supernatant was loaded onto a 6 M urea–polyacrylamide gel. Human apotransferrin (lane 7) and a mixture of human diferric and monoferric N-lobe transferrin (lane 8) are shown as controls. The positions of diferric (DF), monoferric N-lobe (MN), and monoferric C-lobe (MC) transferrins and apotransferrins (AP) are indicated on the right- and left-hand sides of the figure.
Removal of iron from transferrin is an energy-requiring process.
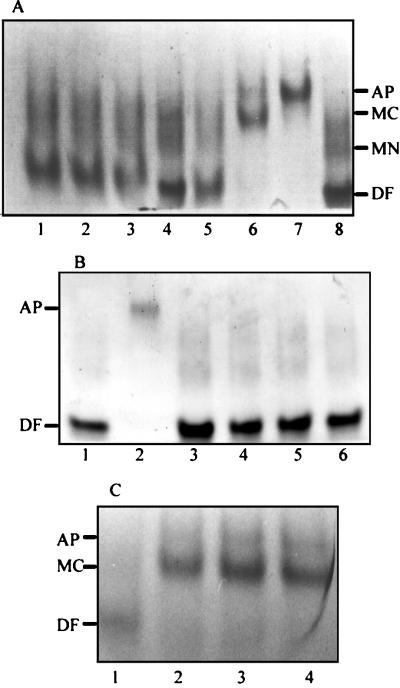
When incubated in PBS without glucose, neither S. aureus (Fig. 3A) nor S. epidermidis (data not shown) was able to remove iron from diferric human transferrin. Similarly, when incubated in PBS plus glucose in the presence of a range of concentrations of sodium azide or nigericin, we observed that S. aureus was unable to promote the release of transferrin-bound iron at a concentration of 6 mM sodium azide (Fig. 3B) or 4 mM nigericin (data not shown). By reducing the azide or nigericin concentration to 2 mM, the removal of iron from transferrin stalled at the monoferric C-lobe form (Fig. 3C).
FIG. 3.
Gels (6 M urea–polyacrylamide) showing the removal of iron from diferric human transferrin by S. aureus in the presence and absence of glucose (A) and the effect of sodium azide on the receptor-mediated removal of iron from human transferrin by S. aureus (B and C). (A) Staphylococci were incubated in PBS without (lanes 1 to 3) or with (lanes 5 to 7) glucose at 37°C for 0 (lanes 1 and 5), 20 (lanes 2 and 6), and 60 (lanes 3 and 7) min and pelleted by centrifugation, and the supernatant was loaded onto a urea-polyacrylamide gel. As controls, lanes 4 and 8 contain diferric human transferrin. The positions of each of the four forms of transferrin are indicated on the right-hand side. (B) Staphylococci (108 CFU/ml) were incubated in PBS plus glucose at 37°C with diferric human transferrin in the presence of 6 mM sodium azide for 0 (lane 3), 5 (lane 4), 20 (lane 5), and 60 (lane 6) min. Cells were pelleted, and the supernatant was loaded onto the urea-polyacrylamide gel. Diferric human transferrin (lane 1) and human apotransferrin (lane 2) were loaded as controls. (C) Staphylococci (108 CFU/ml) were incubated in PBS plus glucose at 37°C with diferric human transferrin in the presence of 2 mM sodium azide for 0 (lane 1), 5 (lane 2), 20 (lane 3), and 60 (lane 4) min. Cells were pelleted, and the supernatant was loaded onto the urea-polyacrylamide gel. The positions of diferric and monoferric C transferrins and apotransferrins are indicated on the left-hand side. Abbreviations: AP, human apotransferrin; DF, diferric human transferrin; MC, monoferric C-lobe transferrin; MN, monoferric N-lobe transferrin.
Receptor-mediated removal of iron from transferrin.
To confirm that the removal of iron from transferrin by staphylococcal cells was receptor rather than siderophore mediated, we exploited our previous observation that staphylococci exhibit a preference for certain transferrins (30). Although porcine transferrin is unable to block the binding of human transferrin to the staphylococcal transferrin receptor (30), siderophores do not exhibit such transferrin species specificity (43). When incubated with porcine transferrin, washed S. aureus cells are unable to remove iron from porcine transferrin over a 60-min period; the same results were obtained for S. epidermidis (data not shown). In addition, by using the universal chrome azurol S siderophore assay (38), we were unable to detect any iron-chelating activity in the PBS plus glucose incubation buffer during the time course of the experiment. However, when incubated with the purified staphylococcal siderophore staphyloferrin A, both human and porcine diferric transferrins were converted to their respective apotransferrin forms (Fig. 4).
FIG. 4.
Gels (6 M urea–polyacrylamide) showing the removal of iron from human (A) and porcine (B) transferrin by the staphylococcal siderophore staphyloferrin A. The siderophore was incubated in PBS plus glucose at 37°C for 60 min. (A) Samples were removed at 0 (lane 3), 5 (lane 4), 20 (lane 5), and 60 (lane 6) min. Lanes 1 and 2 contain diferric transferrin and human apotransferrin as controls. (B) Samples were removed after incubation with porcine transferrin for 0 (lane 1), 20 (lane 2), and 60 (lane 3) min. The positions of diferric transferrin (DF) and apotransferrin (AP) are indicated on the left-hand side.
Receptor-mediated recognition of human transferrin by the staphylococcal transferrin receptor.
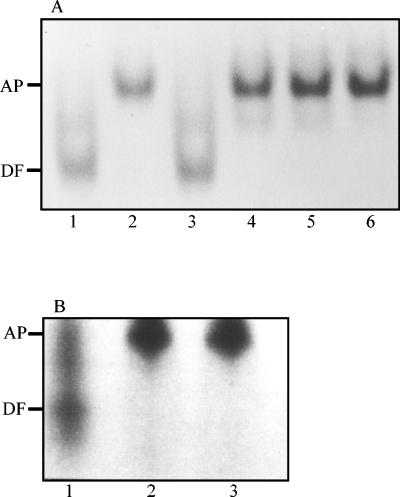
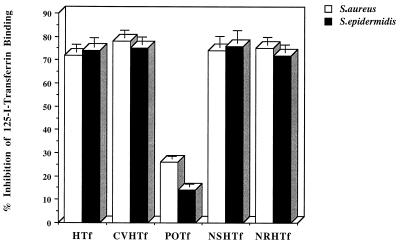
Since both S. aureus and S. epidermidis removed iron initially from the N lobe of intact human transferrin, we sought to determine whether any receptor binding sites were located within the transferrin N lobe. In competitive binding assays, the binding of 125I-labelled diferric human transferrin was blocked to the same extent by unlabelled transferrin and both the subtilisin-generated and recombinant N lobes of human transferrin (Fig. 5). In addition, a naturally occurring human transferrin variant with a mutation in the C lobe (14) which perturbs the iron-binding site and the conformation of the C lobe (15) also efficiently blocked the binding of intact normal transferrin (Fig. 5).
FIG. 5.
Whole-cell competition binding assay showing the inhibition of binding of human 125I-transferrin to staphylococci by the subtilisin-generated and recombinant N lobe of human transferrin. Staphylococci (108 CFU/ml) were incubated with 125I-transferrin (4 nM) in the presence of 700 nM of one of the following unlabelled transferrins: human (HTf), human C-lobe variant (CVHTf), porcine (POTf), subtilisin generated human N lobe (NSHTf), and recombinant human N lobe (NRHTf). After 30 min at 37°C, bacteria were pelleted and the amount of cell-associated 125I-transferrin was determined. Data presented are the means of three independent experiments + standard deviations (error bars).
Removal of iron from the N lobe of human transferrin.
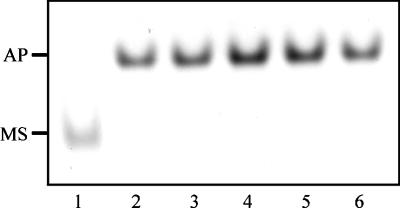
To determine whether the staphylococci could remove iron from the single-sited N lobe of human transferrin alone, we performed 6 M urea–polyacrylamide gel electrophoresis on the supernatants obtained following the incubation of S. aureus or S. epidermidis with the recombinant iron-binding protein. Figure 6 shows that S. aureus efficiently removes iron from the single-sited protein within 5 min of incubation; similar results were obtained with S. epidermidis (data not shown).
FIG. 6.
Gel (6 M urea–polyacrylamide) showing the removal of iron from the N-lobe fragment of human transferrin. S. aureus (108 CFU/ml) were incubated in PBS plus glucose and 250 μg of the recombinant monoferric N lobe for intervals of 0 (lane 1), 5 (lane 2), 10 (lane 3), 20 (lane 4), 30 (lane 5), and 60 (lane 6) min. Bacterial cells were pelleted, and the supernatant was loaded onto the urea gel. The positions of the iron-loaded N lobe (MS) and apoprotein (AP) are indicated on the left-hand side.
DISCUSSION
The present study was initiated to determine whether the staphylococcal transferrin receptor identified previously as a 42-kDa transferrin binding protein (30) contributes to the acquisition of iron from human transferrin. In an iron-depleted medium, the growth of both S. aureus BB and S. epidermidis 138 was supported by the addition of human diferric transferrin but not apotransferrin. For S. aureus this finding is consistent with the uptake of 55Fe from radiolabelled transferrin as observed by Lindsay et al. (27). S. epidermidis, however, was reported to be unable to acquire 55Fe from transferrin. In contrast, Brock et al. (5) reported that both S. aureus and S. epidermidis were able to access 55Fe from transferrin but only inefficiently. In these experiments, the bacterial cells were separated from the radiolabelled transferrin by a dialysis membrane. As a consequence, no direct interaction between the bacterial cell surface and the iron-binding glycoprotein could occur, which may account for the inefficient uptake of transferrin-bound iron observed by Brock et al. (5).
Since, both S. aureus and S. epidermidis grew well in RPMI medium containing diferric human transferrin as the sole iron source, we used 6 M urea–polyacrylamide gel electrophoresis to investigate the contribution of the transferrin receptor to the acquisition of iron. Using this approach, we have presented evidence for a receptor-mediated, siderophore-independent iron uptake mechanism in both S. aureus and S. epidermidis. This receptor-mediated mechanism of iron acquisition is clearly absent in S. saprophyticus, which lacks the transferrin receptor (30). Further confirmation was obtained by replacing human transferrin with porcine transferrin, since the latter does not bind to the staphylococcal receptor. While neither S. aureus nor S. epidermidis was able to remove iron from porcine transferrin, the staphylococcal siderophore staphyloferrin A efficiently removed iron from both mammalian transferrins. These data demonstrate unequivocally that S. aureus and S. epidermidis are both able to remove transferrin-bound iron either via receptor- or siderophore-mediated processes.
By following the time course of iron removal from human transferrin, we observed that iron was removed first from the N lobe and then from the C lobe. Although the ligands involved in transferrin iron binding are generally assumed to be equivalent because of similarities in their spectroscopic, thermodynamic, and functional properties, the iron-binding sites on each lobe are not identical (2, 21). In particular, the two sites differ in their affinity for iron and in their acid lability. Iron is lost from the N lobe of transferrin at pH values between 6 and 5.5, whereas the C-lobe site is more acid stable, losing its iron only between pH 5.5 and 4. The physiological significance of such differences has been the matter for much speculation, especially since the predominant transferrin species in plasma are the monoferric transferrin and apotransferrin forms (21, 45). Furthermore, more iron appears to be associated with the N- rather than the C-lobe site of monoferric transferrin (45). However, despite their physical and chemical differences, the two sites appear to behave as functionally equivalent (2, 21), and human transferrin labelled in either domain has been shown to donate iron equally well to immature human erythrocytes (40). Whether the gram-negative transferrin receptor-expressing pathogens take iron sequentially from the N or C lobe of diferric transferrin has not been reported. However, studies of the transferrin domain preference of the enterobacterial siderophores aerobactin and enterobactin (enterochelin) revealed that the hydoxamate, aerobactin, removes iron preferentially from the thermodynamically more stable C lobe whereas the phenolate, enterobactin, exhibits a preference for the N lobe (16).
Since the removal of iron from human transferrin by S. aureus and S. epidermidis requires an energy source, it was important to establish whether this process involved active transport. Metabolic poisons such as sodium azide, which is a potent inhibitor of S. aureus membrane-associated ATPase activity (26), at 6 mM inhibited the conversion of diferric transferrin to its apotransferrin form. With 2 mM sodium azide, the removal of iron from diferric human transferrin stalled at the monoferric C form; i.e., iron was removed from the N-lobe site only. This may be a consequence of the reduced thermodynamic stability of the N lobe compared with the C lobe (21). Similar results were obtained with nigericin, which disrupts membrane potential (36). The receptor-mediated acquisition of iron from transferrin by pathogenic Neisseria is also an energy-requiring process, in which receptor energization is a necessary prerequisite for ligand release (4, 10, 39). However, the Neisseria are gram-negative bacteria, and iron released from surface-bound transferrin must cross the outer membrane, the periplasm, and the inner membrane prior to internalization. Iron from receptor-bound transferrin is probably transferred via a gated pore in transferrin binding protein A (TbpA) to the periplasmic iron-binding protein, FbpA (7). Intriguingly, FbpA is structurally and functionally homologous to transferrin itself and reversibly binds one ferric ion per protein molecule (34, 35). In the both Neisseria (1) and H. influenzae (24), FbpA is part of an ATP-dependent transporter necessary for the internalization of iron from receptor-bound transferrin. How iron is released from transferrin bound to the surface of staphylococci is not yet known, but in these gram-positive bacteria it is likely to be mechanistically different. Recently we have cloned and sequenced an iron-regulated staphylococcal ABC transporter incorporating a 32-kDa lipoprotein (8) which could conceivably function in a manner analogous to FbpA by acting as an acceptor for iron released from surface-bound transferrin.
Since the staphylococci exhibit a preference for iron from the N-lobe binding site, we sought to determine whether the binding of the intact transferrin molecule could be blocked by the monoferric N-lobe fragment. Both recombinant and subtilisin-generated N lobes inhibited binding. In addition, a naturally occurring human transferrin variant with a mutation in the C lobe (14, 15) also efficiently blocked the binding of intact normal human transferrin. Taken together these results suggest that the primary receptor-binding site is located within the N-terminal lobe of the protein. Furthermore, when the iron-loaded N lobe of human transferrin was incubated with S. aureus, there was rapid removal of the iron. These results contrast with those obtained for the human transferrin receptor (46) and many gram-negative bacterial transferrin receptors (18), the primary recognition sites for which are located in the transferrin C-terminal lobe. Furthermore, only the C fragment was found capable of donating iron or binding to the transferrin surface receptors of hepatoma-derived HuH-7 cells and leukemic K562 cells (46). Neither the isolated C or N lobes nor a mixture is capable of supporting the growth of Haemophilus paragallinarum (which recognizes both N and C lobe sites on ovotransferrin) or N. meningitidis (which recognizes a C-lobe binding site only) (3, 18).
In conclusion, we have now extended our earlier work (30) and demonstrated that both S. aureus and S. epidermidis express a functional transferrin receptor which is involved in the acquisition of transferrin-bound iron. Competitive transferrin binding studies and iron-uptake experiments indicate that a primary receptor recognition site lies within the N-terminal lobe. With this information and knowledge of receptor specificity, using site-directed mutagenesis we can now begin to define the precise residues involved in the transferrin-receptor interaction.
ACKNOWLEDGMENTS
This work was supported by grants from the Medical Research Council (to P.W.), the Special Trustees for St. Thomas’ Hospital (to R.W.E. and C.L.I.), and the Wellcome Trust (to R.W.E.).
We thank Gunther Winkelmann for his kind donation of staphyloferrin A.
REFERENCES
- 1.Adhikari P, Berish S A, Nowalk A J, Veraldi K L, Morse S A, Mietzner T A. The fbpABC locus of Neisseria gonorrhoeae functions in the periplasm-to-cytosol transport of iron. J Bacteriol. 1996;178:2145–2149. doi: 10.1128/jb.178.7.2145-2149.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aisen P. Physical biochemistry of the transferrins: update, 1984–1988. Phys Bioinorg Chem Ser. 1989;5:353–371. [Google Scholar]
- 3.Alcantara J, Schryvers A B. Transferrin binding protein 2 interacts with both the N-lobe and C-lobe of ovotransferrin. Microb Pathog. 1996;209:73–85. doi: 10.1006/mpat.1996.0007. [DOI] [PubMed] [Google Scholar]
- 4.Archibald F S, DeVoe I W. Iron acquisition by Neisseria meningitidis in vitro. Infect Immun. 1980;27:322–334. doi: 10.1128/iai.27.2.322-334.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brock J H, Williams P H, Liceaga J, Woolridge K G. Relative availability of transferrin-bound iron and cell-derived iron to aerobactin-producing and enterochelin-producing strains of Escherichia coli and to other microorganisms. Infect Immun. 1991;59:3185–3190. doi: 10.1128/iai.59.9.3185-3190.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bullen J J. The significance of iron in infection. Rev Infect Dis. 1981;3:1127–1137. doi: 10.1093/clinids/3.6.1127. [DOI] [PubMed] [Google Scholar]
- 7.Chen C-J, Berish S A, Morse S A, Meitzner T A. The ferric binding protein of pathogenic Neisseria spp. functions as a periplasmic transport protein in iron acquisition from human transferrin. Mol Microbiol. 1993;10:311–318. doi: 10.1111/j.1365-2958.1993.tb01957.x. [DOI] [PubMed] [Google Scholar]
- 8.Cockayne, A., P. J. Hill, N. J. Powell, K. Bishop, C. Sims, and P. Williams. Molecular cloning of a 32-kilodalton lipoprotein component of a novel iron-regulated Staphylococcus epidermidis ABC transporter. Infect. Immun. 66:3767–3774. [DOI] [PMC free article] [PubMed]
- 9.Cornelissen C N, Sparling P F. Iron piracy: acquisition of transferrin-bound iron by bacterial pathogens. Mol Microbiol. 1994;14:843–850. doi: 10.1111/j.1365-2958.1994.tb01320.x. [DOI] [PubMed] [Google Scholar]
- 10.Cornelissen C N, Anderson J E, Sparling P F. Energy-dependent changes in the gonococcal transferrin receptor. Mol Microbiol. 1997;26:25–35. doi: 10.1046/j.1365-2958.1997.5381914.x. [DOI] [PubMed] [Google Scholar]
- 11.Cornelissen C N, Kelley M, Hobbs M M, Anderson J E, Cannon J G, Cohen M S, Sparling P F. The transferrin receptor expressed by gonococcal strain FA1090 is required for the experimental infection of human male volunteers. Mol Microbiol. 1998;27:611–616. doi: 10.1046/j.1365-2958.1998.00710.x. [DOI] [PubMed] [Google Scholar]
- 12.Courcol R J, Trivier D, Bissinger M C, Martin G R, Brown M R W. Siderophore production by Staphylococcus aureus and identification of iron-regulated proteins. Infect Immun. 1997;65:1944–1948. doi: 10.1128/iai.65.5.1944-1948.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evans R W, Williams J. Studies on the binding of different iron donors to human serum transferrin and isolation of iron-binding fragments from the N- and C-terminal regions of the protein. Biochem J. 1978;173:543–552. doi: 10.1042/bj1730543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans R W, Williams J, Moreton K. A variant of transferrin with abnormal properties. Biochem J. 1982;201:19–26. doi: 10.1042/bj2010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evans R W, Crawley J B, Garratt R C, Grossman J G, Neu M, Aitken A, Patel K J, Meilak A, Wong C, Singh J, Bomford A, Hasnain S S. Characterization and structural analysis of a functional human serum transferrin variant and implications for receptor recognition. Biochemistry. 1994;33:12512–12520. doi: 10.1021/bi00207a019. [DOI] [PubMed] [Google Scholar]
- 16.Ford S, Cooper R A, Evans R W, Hider R C, Williams P H. Domain preference in iron removal from human transferrin by the bacterial siderophores aerobactin and enterochelin. Eur J Biochem. 1988;178:477–481. doi: 10.1111/j.1432-1033.1988.tb14473.x. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez G C, Caamana D L, Schryvers A B. Identification and characterization of a porcine-specific transferrin receptor in Actinobacillus pleuropneumoniae. Mol Microbiol. 1990;4:1173–1179. doi: 10.1111/j.1365-2958.1990.tb00692.x. [DOI] [PubMed] [Google Scholar]
- 18.Gray-Owen S D, Schryvers A B. Bacterial transferrin and lactoferrin receptors. Trends Microbiol. 1996;4:185–190. doi: 10.1016/0966-842x(96)10025-1. [DOI] [PubMed] [Google Scholar]
- 19.Grossman J G, Neu M, Evans R W, Lindley P F, Appel H, Hasnain S S. Metal-induced conformational changes in transferrins. J Mol Biol. 1993;229:585–590. doi: 10.1006/jmbi.1993.1063. [DOI] [PubMed] [Google Scholar]
- 20.Haag H, Fiedler H P, Meiwes J, Zahner H. Isolation and characterisation of staphyloferrin B, a compound with siderophore activity from staphylococci. FEMS Microbiol Lett. 1994;115:125–130. doi: 10.1111/j.1574-6968.1994.tb06626.x. [DOI] [PubMed] [Google Scholar]
- 21.Harris D C, Aisen P. Physical biochemistry of transferrins. Loehr (ed.), Iron carriers and proteins. Phys Bioinorg Chem Ser. 1989;5:239–351. [Google Scholar]
- 22.Hasan A A, Holland J, Smith A, Williams P. Elemental iron does repress transferrin, haemopexin and haemoglobin receptor expression in Haemophilus influenzae. FEMS Microbiol Lett. 1997;150:19–26. doi: 10.1111/j.1574-6968.1997.tb10344.x. [DOI] [PubMed] [Google Scholar]
- 23.Hueck D, Beer W, Reissbrodt R. Iron supply of staphylococci and of micrococci by α-ketoacids. J Med Microbiol. 1995;43:26–32. doi: 10.1099/00222615-43-1-26. [DOI] [PubMed] [Google Scholar]
- 24.Kirby S D, Gray-Owen S D, Schryvers A B. Characterization of a ferric-binding protein mutant in Haemophilus influenzae. Mol Microbiol. 1997;25:979–987. doi: 10.1111/j.1365-2958.1997.mmi535.x. [DOI] [PubMed] [Google Scholar]
- 25.Konetschny-Rapp S, Jung G, Meiwes J, Zahner H. Staphyloferrin A: a structurally new siderophore from staphylococci. Eur J Biochem. 1991;191:65–74. doi: 10.1111/j.1432-1033.1990.tb19094.x. [DOI] [PubMed] [Google Scholar]
- 26.Kubak B, Yotis W. Staphylococcus aureus adenosine triphosphatase: inhibitor sensitivity and the release from membrane. J Bacteriol. 1981;146:385–390. doi: 10.1128/jb.146.1.385-390.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lindsay J A, Riley T V, Mee B J. Staphylococcus aureus but not Staphylococcus epidermidis can acquire iron from transferrin. Microbiology. 1995;141:197–203. doi: 10.1099/00221287-141-1-197. [DOI] [PubMed] [Google Scholar]
- 28.Makey D G, Seal U S. The detection of four molecular forms of human transferrin during the iron-binding process. Biochim Biophys Acta. 1976;453:250–256. doi: 10.1016/0005-2795(76)90270-1. [DOI] [PubMed] [Google Scholar]
- 29.Meiwes J, Fiedler H P, Haag H, Zahner H, Konetschny-Rapp S, Jung G. Isolation and characterisation of staphyloferrin A, a compound with siderophore activity from Staphylococcus hyicus DSM 20459. FEMS Microbiol Lett. 1990;67:201–206. doi: 10.1111/j.1574-6968.1990.tb13863.x. [DOI] [PubMed] [Google Scholar]
- 30.Modun B, Kendall D, Williams P. Staphylococci express a receptor for human transferrin: identification of a 42-kilodalton cell wall transferrin-binding protein. Infect Immun. 1994;62:3850–3858. doi: 10.1128/iai.62.9.3850-3858.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Modun B, Cockayne A, Finch R G, Williams P. The Staphylococcus aureus and Staphylococcus epidermidis transferrin-binding proteins are expressed in vivo during infection. Microbiology. 1998;144:1005–1012. doi: 10.1099/00221287-144-4-1005. [DOI] [PubMed] [Google Scholar]
- 32.Morton D J, Williams P. Utilisation of transferrin-bound iron by Haemophilus species of human and porcine origin. FEMS Microbiol Lett. 1989;65:123–128. doi: 10.1016/0378-1097(89)90378-9. [DOI] [PubMed] [Google Scholar]
- 33.Morton D J, Williams P. Siderophore-independent acquisition of transferrin-bound iron by Haemophilus influenzae type b. J Gen Microbiol. 1990;136:927–933. doi: 10.1099/00221287-136-5-927. [DOI] [PubMed] [Google Scholar]
- 34.Nowalk A J, Tencza S B, Mietzner T A. Co-ordination of iron by the ferric iron-binding protein of pathogenic Neisseria is homologous to the transferrins. Biochemistry. 1994;33:12769–12775. doi: 10.1021/bi00209a007. [DOI] [PubMed] [Google Scholar]
- 35.Nowalk A J, Vaughan K G, Day B W, Tencza S B, Mietzner T A. Metal-dependent conformers of the periplasmic ferric-binding protein. Biochemistry. 1997;36:13054–13059. doi: 10.1021/bi971413o. [DOI] [PubMed] [Google Scholar]
- 36.Rottenberg H, Scarpa A. Calcium uptake and membrane potential in mitochondria. Biochemistry. 1974;13:4811–4817. doi: 10.1021/bi00720a020. [DOI] [PubMed] [Google Scholar]
- 37.Schade A L. Significance of serum iron for the growth, biological characteristics, and metabolism of Staphylococcus aureus. Biochem Z. 1963;388:140–148. [PubMed] [Google Scholar]
- 38.Schwyn B, Neilands J B. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 1987;160:47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- 39.Simonson C, Brener D, DeVoe I W. Expression of a high-affinity mechanism for the acquisition of transferrin iron by Neisseria meningitidis. Infect Immun. 1982;36:107–113. doi: 10.1128/iai.36.1.107-113.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van der Heul C, Kroos M J, van Noort W L, van Eijk H G. No functional difference of the two iron-binding sites of human transferrin in vitro. Clin Sci. 1981;60:185–190. doi: 10.1042/cs0600185. [DOI] [PubMed] [Google Scholar]
- 41.Wilcox M H, Williams P, Smith D G E, Finch R G, Denyer S P. Variation in the expression of cell envelope proteins of coagulase-negative staphylococci cultured under iron-restricted conditions in human peritoneal dialysate. J Gen Microbiol. 1991;137:2561–2570. doi: 10.1099/00221287-137-11-2561. [DOI] [PubMed] [Google Scholar]
- 42.Willams J. The evolution of transferrin. Trends Biochem Sci. 1982;7:394–397. [Google Scholar]
- 43.Williams P, Griffiths E. Bacterial transferrin receptors—structure, function and contribution to virulence. Med Microbiol Immunol. 1992;181:301–322. doi: 10.1007/BF00191543. [DOI] [PubMed] [Google Scholar]
- 44.Wolz C, Hohloch K, Ocaktan A, Poole K, Evans R W, Rochel N, Albrecht-Gary A-M, Abdallah M A, Döring G. Iron release from transferrin by pyoverdin and elastase from Pseudomonas aeruginosa. Infect Immun. 1994;62:4021–4027. doi: 10.1128/iai.62.9.4021-4027.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zak O, Aisen P. Non-random distribution of iron in circulating human transferrin. Blood. 1986;68:157–161. [PubMed] [Google Scholar]
- 46.Zak O, Trinder D, Aisen P. Primary receptor recognition site of human transferrin is in the C-terminal lobe. J Biol Chem. 1994;269:7110–7114. [PubMed] [Google Scholar]
- 47.Zak O, Aisen P, Crawley J B, Joannou C J, Patel K J, Rafiq M, Evans R W. Iron release from recombinant N-lobe and mutants of human transferrin. Biochemistry. 1995;34:14428–14434. doi: 10.1021/bi00044a020. [DOI] [PubMed] [Google Scholar]