Abstract
Thyroid hormone T3/T4 is a major regulator of energy metabolism in vertebrates, and defects in thyroid status are frequently associated with changes in body weight. It is demonstrated here that thyroid hormone regulates in vivo and in vitro the tub gene, which when mutated in tubby mice causes obesity, insulin resistance and sensory deficits. Hypothyroidism in rats altered tub mRNA and protein in discrete brain areas. These changes could be attributed to thyroid hormone deficiency since T3/T4 treatment restored normal tub expression. T3 also upregulated tub mRNA within 4–6 h in neuronal cells in culture, suggesting that T3 is a positive regulator of tub gene expression. Thus, these results establish a novel pathway of T3 action and provide an important molecular link between thyroid status and the tubby-associated syndrome.
INTRODUCTION
Thyroid hormone (tri-iodothyronine, T3, and its precursor thyroxine, T4) plays an important role in development and physiology of vertebrate organisms, and altered T3/T4 levels manifest themselves in pleiotropic defects, e.g. in energy metabolism, body growth, neuronal development and in the cardiovascular system (Forrest, 1994; Freake and Oppenheimer, 1995). T3 exerts its activity through binding to specific high affinity nuclear receptors (thyroid hormone receptors, TRs; Gronemeyer and Laudet, 1995; Mangelsdorf et al., 1995). TRs are ligand-dependent transcription factors that regulate gene expression by interacting with specific response elements present in T3-responsive promoters. In mammals two TR genes exist, encoding TRα and TRβ, respectively. TRα and TRβ have been inactivated in mice by gene targeted mutations, leading to defects e.g. in growth, bone maturation, heart rate, intestine development, body temperature and sensory functions (Forrest and Vennström, 2000; Ng et al., 2001).
tub was initially identified in mouse as the gene involved in the tubby syndrome that includes late-onset obesity, insulin resistance, hearing loss and retinal degeneration (Kleyn et al., 1996; Noben-Trauth et al., 1996 and references therein). tub represents the founding member of a recently discovered multigene family that includes tubby-like protein 1, 2 and 3 (tulp-1, -2, -3) whose products contain a highly conserved C-terminal domain that shows no homology to other known proteins (Kleyn et al., 1996; Noben-Trauth et al., 1996; North et al., 1997; Nishina et al., 1998). Although highly homologous and exhibiting an overlapping expression profile, Tub and Tulp proteins seem to serve non-redundant roles in vertebrate organisms as demonstrated by gene inactivation experiments (Ikeda et al., 2000; Stubdal et al., 2000). Initial evidence has implicated Tub as an adapter protein in intracellular signaling of insulin receptor, while another study indicated that Tub might act as a transcription factor (Boggon et al., 1999; Kapeller et al., 1999). Additionally, targeted gene inactivation in mice demonstrated that the tubby phenotype results from the loss-of-function mutation of the tub gene (Stubdal et al., 2000). Finally, the phenotype observed in tubby mutant and tub–/– mice resembles certain human syndromes such as Usher, Alström and Bardet–Biedl (Kleyn et al., 1996; Noben-Trauth et al., 1996; Barsh et al., 2000 and references therein), and mutations in the tulp-1 gene in man have been implicated in retinitis pigmentosa, a hereditary disease of retina degeneration (see Ikeda et al., 2000 for references).
A screen for T3-regulated genes by differential display identified tub as a potential TR target gene (see Supplementary data available at EMBO reports Online). This is interesting given the fact that T3 increases energy expenditure and that hypothyroidism increases the propensity to weight gain and eventually obesity in humans (Freake and Oppenheimer, 1995; Lowell and Spiegelman, 2000). Here we demonstrate that T3 regulates tub gene expression in vivo and in vitro, and thus provide a novel molecular link between thyroid status and the tubby-associated syndrome.
RESULTS AND DISCUSSION
Thyroid hormone regulates tub in vivo
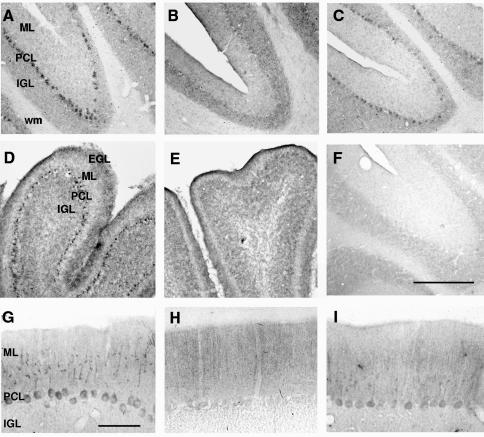
To determine whether TR regulates tub gene expression in vivo, we analysed the effects of thyroid hormone administration and deprivation on tub expression in rat brain by in situ hybridization. These experiments showed that tub mRNA is expressed in many regions of the adult rat brain, predominantly in neuronal populations (Figures 1 and 2, and data not shown). In line with previous reports in mice (Kleyn et al., 1996; Sahly et al., 1998), highest expression was found in cerebral cortex layers II and V, retrosplenial and piriform cortices, hippocampus, subiculum, hypothalamus and Purkinje cells of the cerebellum. In hypothyroid rats the regional pattern of expression was similar to that of control animals. However, most significantly in Purkinje cells, tub expression was lower in hypothyroid rats than in control (Figure 1A and B). To confirm that these differences were related to reduced levels of T3, a group of hypothyroid animals was treated with the pro-hormone thyroxine (T4), the major hormone produced in the thyroid gland, which crosses the blood–brain barrier more efficiently than T3 and is converted into the active T3 inside the cells. After four daily T4 injections, tub expression in Purkinje cells was restored and reached nearly the same level as in control (Figure 1C). Accordingly, immunohistochemical analysis of Purkinje cells revealed that Tub protein levels were reduced in hypothyroid animals and following T4 treatment restored to nearly normal levels (Figure 1G, H and I).
Fig. 1. Thyroid hormone regulates tub expression in Purkinje cells of cerebellum in adult and neonatal rats. In situ hybridization micrographs of coronal cerebellar sections of control (A), hypothyroid (B) and T4-treated hypothyroid (C) adult animals, and of control (D) and hypothyroid (E) 10-day-old (P10) animals. Only Purkinje cells are labeled. (F) Background signal obtained by hybridizing sections of control adult brain with a sense riboprobe. EGL, external granular layer; ML, molecular layer; PCL, Purkinje cell layer; IGL, internal granular layer; wm, white matter. Bar in (F) corresponds to 200 µm for (A)–(C) and (F), and 500 µm for (D) and (E). Immunostaining of coronal cerebellar sections of control (G), hypothyroid (H) and T4-treated hypothyroid (I) adult animals with Tub-specific antibody. Bar in (G) corresponds to 50 µm for (G)–(I).
Fig. 2. Effect of thyroid hormone deprivation and of in vivo hormone treatment on tub expression in adult rat brain. In situ hybridization micrographs of coronal brain sections of control (A, D and G), hypothyroid (B, E and H) and T4-treated hypothyroid (C, F and I) animals. (A)–(C) Micrographs showing tub RNA expression in the subicular area (S), retrosplenial cortex (RS) and dentate gyrus (DG) of the hippocampus; fmj, forceps major corpus callosum. (D)–(F) Image of the somatosensory cortex. Note the higher signal in layers II and V, and subplate (sp) in hypothyroid animals (E) versus control (D) or T4-treated hypothyroid animals (F). (G)–(I) Labeled cells in the compact dorsomedial hypothalamic nucleus (DMC), surrounded by unlabeled cells in the diffuse dorsomedial hypothalamic nucleus (DMD). Bar in (D) corresponds to 500 µm for (A)–(C) and to 200 µm for (D)–(I).
Interestingly, T3 deprivation had an opposite effect on tub expression levels in other brain areas. In hypothyroid animals, tub expression was higher in subiculum and retrosplenial cortex (Figure 2A and B) and in cortical layers II and V (Figure 2D and E). T4 treatment restored normal tub expression in all these areas (Figure 2C and F). The same result was obtained when Tub protein expression was analysed by immunohistochemistry (data not shown). There were only marginal differences in tub expression in ventral and dorsomedial hypothalamic nuclei (Figure 2G, H and I), which play a role in body weight control and feeding behavior (Kleyn et al., 1996; Schwartz et al., 2000). No differences in tub mRNA and protein expression were found in other regions such as the piriform cortex or the hippocampus.
Importantly, tub is one of the few genes found to be under thyroid hormone control in the adult brain while most target genes identified so far are exclusively thyroid dependent in the early postnatal period (reviewed in Bernal and Guadano-Ferraz, 1998). Consequently, we next analysed the influence of congenital hypothyroidism on tub expression. The major effect was found in the cerebellum. Like adult rats, hypothyroid 10-day-old animals showed reduced tub expression in Purkinje cells (Figure 1D and E). Differences in other brain regions were similar to but less apparent than those observed in adult rats. Taken together, these results indicated that tub expression in Purkinje cells is upregulated by thyroid hormone in vivo while in other brain areas tub is regulated in a complex regional and temporal-specific fashion.
T3 upregulates tub expression in vitro
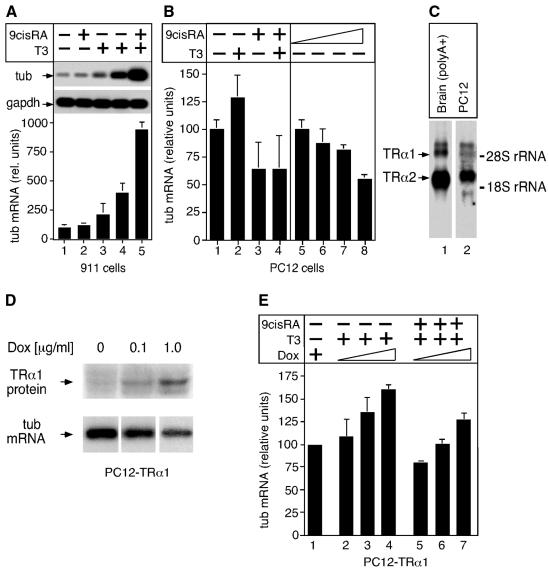
To gain further insight into the regulation of tub by T3, a panel of cell lines and primary cells representing neuronal and glial cell types was screened for tub expression. Rat PC12 cells, representing neuronal/chromaffin progenitor cells, and human embryonic retina cells (911 cells; Fallaux et al., 1996) were found to express tub (Figure 3 and data not shown), which is in accordance with the prominent tub expression in adrenal medulla and retina, respectively, in mice (Sahly et al., 1998).
Fig. 3. T3 upregulates tub expression. (A) tub expression in 911 cells was analysed by RT–PCR. tub-specific signals after treatment with 9cRA (lane 2; 6 h), T3 (lane 3, 4 h; lane 4, 6 h) or T3 plus 9cRA (lane 5, 6 h) were quantified by Phosphoimager, normalized for GAPDH control and plotted relative to untreated cells that were arbitrarily set as 100 (lane 1). Average values of three experiments with standard deviation are shown. (B) Northern blot analysis of tub expression in PC12 cells in response to T3 and 9cRA as indicated (lanes 5–8; 0.001, 0.01, 0.1 and 1 µM 9cRA, respectively). Signals were quantified as in (A) and average values of two independent experiments with duplicate values are shown. (C) Northern blot analysis of TRα1 and TRα2 expression in PC12 cells (total RNA). Control, brain poly(A)+ RNA. Probe was a 1 kb XbaI fragment of TRα cDNA that detects both TRα1 and TRα2. Exposure times were 24 h and 4 days for brain and PC12 cells, respectively. (D) TRα1 expression in PC12-TRα1 cells is induced by doxycyclin treatment (16 h) as revealed by western blotting using TR-specific antibody. No TRα1 induction was seen in unmodified PC12 Tet-On cells (not shown). PC12-TRα1 cells were also analysed for tub expression by northern blotting as indicated; equal amounts of total RNA were loaded per lane. (E) TRα1 expression was induced in PC12-TRα1 cells as in (D) followed by T3 or T3 plus 9cRA treatment as indicated, and tub expression was analysed by northern blotting. Signals were quantified and plotted relative to untreated cells as in (A).
PC12 and 911 cells were then analysed for tub expression in response to T3. Cells were treated with T3 to activate TR or with T3 plus 9-cis-retinoic acid (9cRA) to also activate RXR, the obligate heterodimeric partner of TR, and tub mRNA levels were monitored by RT–PCR and northern blot hybridization. 911 cells express TRα1 and low levels of tub mRNA and protein (Figure 3A and data not shown), and tub expression in response to hormone was determined by RT–PCR. Importantly, T3 effectively induced tub expression within 4–6 h, which was even further enhanced by T3 plus 9cRA treatment (Figure 3A). In PC12 cells, tub mRNA levels remained virtually unaffected by T3 treatment (Figure 3B). This is most likely because of low levels of TRα1 and TRβ, and high expression of the non-hormone-binding TRα2 isoform (Figure 3C and data not shown; Muñoz et al., 1993), which is believed to counteract TR function (Forrest, 1994). In addition, 9cRA caused a dose-dependent downregulation of tub, possibly through formation of TRα2–RXR heterodimers that might repress tub expression (Figure 3B). This downregulation by 9cRA was also seen in the presence of T3, indicating that the low level of endogenous hormone-activated TRα1 is unable to relieve tub repression. Therefore, to increase TRα1 activity, PC12 cells were generated that conditionally express TRα1 from a tetracyclin-dependent expression vector.
PC12 Tet-On cells expressing the tetracyclin-controlled transactivator were infected with retrovirus containing TRα1 under the control of a tetracyclin response element. PC12-TRα1 cells effectively expressed TRα1 in response to the tetracyclin derivative doxycyclin in a dose-dependent fashion (Figure 3D). Additionally, unliganded TRα1 repressed tub transcription, in line with the tub downregulation observed in Purkinje cells of hypothyroid rats (Figure 1B and E, and Figure 3D). Downregulation of transcription by unliganded TR has been reported in several systems (see Bartunek and Zenke, 1998 and references therein). PC12-TRα1 cells were then treated with T3 or T3 plus 9cRA, and 6 h later tub expression was analysed by northern blotting. Interestingly, increasing levels of hormone-activated TRα1 elevated tub mRNA (Figure 3E). Ligand-activated TRα1 also relieved the 9cRA-induced downregulation of tub (Figure 3B and E) possibly by alleviating the effect of TRα2. Collectively, these results demonstrate that T3 upregulates tub expression.
Regulation of tub by T3 is interesting for several reasons: (i) TR and tub are coexpressed in various regions in the brain (Mellström et al., 1991; Strait et al., 1991; Forrest, 1994; Kleyn et al., 1996; Sahly et al., 1998) and, as also demonstrated here, in neuronal cells. (ii) TR–/– mice and the tubby mutant or tub–/– mice exhibit an overlapping phenotype. For example, TRβ–/– mice show sensory defects in vision and hearing as do tubby mutant or tub–/– mice (Forrest et al., 1996; Kleyn et al., 1996; Noben-Trauth et al., 1996; Stubdal et al., 2000; Ng et al., 2001). This indicates that TR and tub may be interconnected and/or part of the same biochemical pathway(s). (iii) Defects in T3 metabolism and/or function (e.g. hypothyroidism) often manifest themselves in obesity as does inactivation of tub in mice.
Tub protein is specifically phosphorylated in response to insulin, thus supporting a role of Tub in signaling from insulin receptor (Kapeller et al., 1999; Schwartz et al., 2000). Our results suggests that T3, by affecting Tub expression, might influence insulin signaling. Yet another study suggested that Tub acts as a transcription factor that directly affects gene expression (Boggon et al., 1999). Our observation that in PC12 and NIH 3T3 cells Tub protein is found both at the cytoplasmic membrane and in the nucleus (see Supplementary data) would be compatible with Tub acting as an adapter molecule in insulin receptor signaling and/or as a transcription factor.
tub levels were very low in Purkinje cells of adult hypothyroid animals and induced by T3/T4 treatment as demonstrated here on the RNA and protein level. Thus, tub expression and its regulation by T3 in these cells might be important for tub-associated functions. Interestingly, recent evidence demonstrated that Purkinje cells express leptin receptor (Udagawa et al., 2000); yet the importance of these two obesity-related pathways in Purkinje cells is presently unknown.
While T3/T4 upregulated tub expression in vivo and in vitro in some cells (Purkinje and 911 cells, respectively) its effects on tub in other cell types and brain areas were more complex. A distinct regional and temporal-specific regulation of genes by T3 is not without precedence and has been reported previously (Bernal and Guadano-Ferraz, 1998; Alvarez-Dolado et al., 1999). This might be because T3 cooperates with other factors in regulating TR target gene expression, or because hormone action is modulated by region- and cell-specific proteins. Different expression levels of coactivators and corepressors that mediate TR transcriptional activity or the expression of TR isoforms (like TRα2 in PC12 cells) are candidates to modulate T3 effects (Forrest, 1994). Another possible factor regulating hormonal effects is the selective expression of enzymes that are responsible for the generation of T3 from T4 and their catabolism to inactive metabolites in different brain regions (Escamez et al., 1999 and references therein).
The impact of thyroid hormone on energy metabolism is well established and deficiencies in thyroid status are frequently associated with increased body weight. The finding reported here that T3 regulates the tub gene uncovers a novel pathway of how thyroid hormone might regulate body weight and might help to devise new strategies for treatment of the obesity syndrome.
METHODS
Induction of hypothyroidism.
Adult-onset hypothyroidism in rats was induced by surgical thyroidectomy on P40 followed by treatment with 2-mercapto-1-methylimidazole (MMI, 0.02%; Sigma; 20 days), and animals were sacrificed on P60. At least three animals per experimental group were analysed. Thyroxine (T4; Sigma) was administered as single daily intraperitoneal injections of 1.8 µg/100 g body weight for 4 days and rats were killed 24 h after the last T4 injection. Neonatal hypothyroidism was induced as described (Alvarez-Dolado et al., 1999).
In situ hybridization and immunohistochemistry.
Coronal brain sections (25 µm) were analysed by in situ hybridization as described (Alvarez-Dolado et al., 1999). Rat tub cDNA (DDBJ/EMBL/GenBank accession No. U92546) was used as template for riboprobes. For immunohistochemistry, tissue sections were incubated overnight with anti-Tub antibody (Santa Cruz Biotechnology Inc.), or as control, goat IgG, at 4°C. Sections were then incubated with a secondary biotinylated rabbit anti-goat antibody followed by ABC peroxidase detection system (Vector Laboratories).
Cells and cell culture.
PC12 cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) with 5% horse serum and 5% fetal calf serum (FCS), 2 mM glutamine and 100 U/ml penicillin/streptomycin. 911 cells (Fallaux et al., 1996) were grown in DMEM plus 10% FCS but no antibiotics. Ligand treatments were carried out with serum samples that were extensively deprived from T3 and retinoids (Bartunek and Zenke, 1998).
Analysis of tub expression by northern blotting and RT–PCR.
TRα1 cDNA (Sap et al., 1986) was introduced into pRev-TRE vector (Clontech), virus stocks were generated in Phoenix-ECO packaging cells and used for infection of PC12 Tet-On cells (Clontech). After hygromycin selection PC12-TRα1 cells were treated with doxycyclin (16 h, 0.1 or 1 µg/ml; Sigma) followed by treatment with T3 (0.1 µM; Sigma) or T3 plus 9cRA (0.1 µM and 1 µM, respectively; Sigma). Total RNA was extracted and analysed by northern blotting with rat tub cDNA probe.
tub expression in 911 cells was analysed by semiquantitative RT–PCR (27 cycles) with tub-specific primers (positions 1290–1622). GAPDH-specific cDNA was amplified accordingly (20 cycles). PCR fragments were then analysed by Southern blotting using rat tub or mouse GAPDH as probes.
Supplementary data.
Supplementary data are available at EMBO reports Online.
Supplementary Material
Acknowledgments
ACKNOWLEDGEMENTS
We thank Introgen, Leiden, The Netherlands for 911 cells; R. Förster for confocal microscopy; P. Bartunek, D. Forrest, G.P. Nolan and B. Vennström for reagents; and S. Knespel and I. Gallagher for excellent assistance. N.P.K. and M.A.-D. were supported by a Max-Delbrück postdoctoral fellowship and EMBO short-term fellowship, respectively. M.F.H. received a fellowship from the Erasmus program. Supported in part by the Comision Interministerial de Ciencia y Tecnología of Spain (No. SAF98-0060 to A.M.).
REFERENCES
- Alvarez-Dolado M. et al. (1999) Thyroid hormone regulates reelin and dab1 expression during brain development. J. Neurosci., 19, 6979–6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsh G.S., Farooqi, I.S. and O’Rahilly, S. (2000) Genetics of body-weight regulation. Nature, 404, 644–651. [DOI] [PubMed] [Google Scholar]
- Bartunek P. and Zenke, M. (1998) Retinoid X receptor and c-erbA/thyroid hormone receptor regulate erythroid cell growth and differentiation. Mol. Endocrinol., 12, 1269–1279. [DOI] [PubMed] [Google Scholar]
- Bernal J. and Guadano-Ferraz, A. (1998) Thyroid hormone and the development of the brain. Curr. Opin. Endocr. Diabetes, 5, 296–304. [Google Scholar]
- Boggon T.J., Shan, W.S., Santagata, S., Myers, S.C. and Shapiro, L. (1999) Implication of tubby proteins as transcription factors by structure-based functional analysis. Science, 286, 2119–2125. [DOI] [PubMed] [Google Scholar]
- Escamez M.J., Guadano-Ferraz, A., Cuadrado, A. and Bernal, J. (1999) Type 3 iodothyronine deiodinase is selectively expressed in areas related to sexual differentiation in the newborn rat brain. Endocrinology, 140, 5443–5446. [DOI] [PubMed] [Google Scholar]
- Fallaux F.J., Kranenburg, O., Cramer, S.J., Houweling, A., Van Ormondt, H., Hoeben, R.C. and Van Der Eb, A.J. (1996) Characterization of 911: a new helper cell line for the titration and propagation of early region 1-deleted adenoviral vectors. Hum. Gene Ther., 7, 215–222. [DOI] [PubMed] [Google Scholar]
- Forrest D. (1994) The erbA/thyroid hormone receptor genes in development of the central nervous system. Semin. Cancer Biol., 5, 167–176.8061332 [Google Scholar]
- Forrest D. and Vennström, B. (2000) Functions of thyroid hormone receptors in mice. Thyroid, 10, 41–52. [DOI] [PubMed] [Google Scholar]
- Forrest D., Erway, L.C., Ng, L., Altschuler, R. and Curran, T. (1996) Thyroid hormone receptor β is essential for development of auditory function. Nature Genet., 13, 354–357. [DOI] [PubMed] [Google Scholar]
- Freake H.C. and Oppenheimer, J.H. (1995) Thermogenesis and thyroid function. Annu. Rev. Nutr., 15, 263–291. [DOI] [PubMed] [Google Scholar]
- Gronemeyer H. and Laudet, V. (1995) Transcription factors 3: nuclear receptors. Protein Profile, 2, 1173–1308. [PubMed] [Google Scholar]
- Ikeda S. et al. (2000) Retinal degeneration but not obesity is observed in null mutants of the tubby-like protein 1 gene. Hum. Mol. Genet., 9, 155–163. [DOI] [PubMed] [Google Scholar]
- Kapeller R. et al. (1999) Tyrosine phosphorylation of Tub and its association with Src homology 2 domain-containing proteins implicate Tub in intracellular signaling by insulin. J. Biol. Chem., 274, 24980–24986. [DOI] [PubMed] [Google Scholar]
- Kleyn P.W. et al. (1996) Identification and characterization of the mouse obesity gene tubby: a member of a novel gene family. Cell, 85, 281–290. [DOI] [PubMed] [Google Scholar]
- Lowell B.B. and Spiegelman, B.M. (2000) Towards a molecular understanding of adaptive thermogenesis. Nature, 404, 652–660. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf D.J. et al. (1995) The nuclear receptor superfamily: the second decade. Cell, 83, 835–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellström B., Naranjo, J.R., Santos, A., Gonzalez, A.M. and Bernal, J. (1991) Independent expression of the α and β c-erbA genes in developing rat brain. Mol. Endocrinol., 5, 1339–1350. [DOI] [PubMed] [Google Scholar]
- Muñoz A., Wrighton, C., Seliger, B., Bernal, J. and Beug, H. (1993) Thyroid hormone receptor/c-erbA: control of commitment and differentiation in the neuronal/chromaffin progenitor line PC12. J. Cell Biol., 121, 423–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng L., Hurley, J.B., Dierks, B., Srinivas, M., Salto, C., Vennström, B., Reh, T.A. and Forrest, D. (2001) A thyroid hormone receptor that is required for the development of green cone photoreceptors. Nature Genet., 27, 94–98. [DOI] [PubMed] [Google Scholar]
- Nishina P.M., North, M.A., Ikeda, A., Yan, Y. and Naggert, J.K. (1998) Molecular characterization of a novel tubby gene family member, TULP3, in mouse and humans. Genomics, 54, 215–220. [DOI] [PubMed] [Google Scholar]
- Noben-Trauth K., Naggert, J.K., North, M.A. and Nishina, P.M. (1996) A candidate gene for the mouse mutation tubby. Nature, 380, 534–538. [DOI] [PubMed] [Google Scholar]
- North M.A., Naggert, J.K., Yan, Y., Noben-Trauth, K. and Nishina, P.M. (1997) Molecular characterization of TUB, TULP1, and TULP2, members of the novel tubby gene family and their possible relation to ocular diseases. Proc. Natl Acad. Sci. USA, 94, 3128–3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahly I. et al. (1998) Prominent neuronal-specific tub gene expression in cellular targets of tubby mice mutation. Hum. Mol. Genet., 7, 1437–1447. [DOI] [PubMed] [Google Scholar]
- Sap J., Muñoz, A., Damm, K., Goldberg, Y., Ghysdael, J., Leutz, A., Beug, H. and Vennström, B. (1986) The c-erb-A protein is a high-affinity receptor for thyroid hormone. Nature, 324, 635–640. [DOI] [PubMed] [Google Scholar]
- Schwartz M.W., Woods, S.C., Porte, D., Jr, Seeley, R.J. and Baskin, D.G. (2000) Central nervous system control of food intake. Nature, 404, 661–671. [DOI] [PubMed] [Google Scholar]
- Silva J.E. (1995) Thyroid hormone control of thermogenesis and energy balance. Thyroid, 5, 481–492. [DOI] [PubMed] [Google Scholar]
- Strait K.A., Schwartz, H.L., Seybold, V.S., Ling, N.C. and Oppenheimer, J.H. (1991) Immunofluorescence localisation of thyroid hormone receptor protein β1 and variant α2 in selected tissues: cerebellar Purkinje cells as a model for β1 receptor-mediated developmental effects of thyroid hormone in brain. Proc. Natl Acad. Sci. USA, 88, 3887–3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubdal H. et al. (2000) Targeted deletion of the tub mouse obesity gene reveals that tubby is a loss-of-function mutation. Mol. Cell. Biol., 20, 878–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udagawa J., Hatta, T., Naora, H. and Otani, H. (2000) Expression of the long form of leptin receptor (Ob-Rb) mRNA in the brain of mouse embryos and newborn mice. Brain Res., 868, 251–258. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.