Abstract
Recent studies identified YidC as a novel membrane factor that may play a key role in membrane insertion of inner membrane proteins (IMPs), both in conjunction with the Sec-translocase and as a separate entity. Here, we show that the type II IMP FtsQ requires both the translocase and, to a lesser extent, YidC in vivo. Using photo-crosslinking we demonstrate that the transmembrane (TM) domain of the nascent IMP FtsQ inserts into the membrane close to SecY and lipids, and moves to a combined YidC/lipid environment upon elongation. These data are consistent with a crucial role for YidC in the lateral transfer of TM domains from the Sec translocase into the lipid bilayer.
INTRODUCTION
Most Escherichia coli inner membrane proteins (IMPs) use the signal recognition particle (SRP) and its receptor FtsY for co-translational membrane targeting (reviewed in Herskovits et al., 2000). In contrast, secreted proteins usually require binding to SecB in a post-translational or late co-translational targeting reaction (reviewed in Fekkes and Driessen, 1999).
Crosslinking and in vivo depletion studies suggest that at least a subset of IMPs inserts at the translocase that was originally identified as the protein-conducting pore which receives and translocates secretory proteins (reviewed in Bernstein, 2000). This led to the concept of converging SRP- and SecB-targeting routes at the membrane (Valent et al., 1998; Beck et al., 2000). The core translocase consists of the integral IMPs SecY, SecE and SecG, which constitute an oligomeric complex, homologous to the Sec61 channel complex in the endoplasmic reticulum (ER) (reviewed in Manting and Driessen, 2000). The peripheral membrane ATPase SecA, which is unique to bacteria, catalyzes the actual polypeptide transfer through the translocase. It has been proposed that the role of SecA in IMP biogenesis is restricted to the translocation of large periplasmic domains (Beck et al., 2000; Scotti et al., 2000).
Recently, a novel factor, YidC, has been identified that might play a key role in the biogenesis of IMPs. Site-specific photo-crosslinking revealed that YidC interacts specifically with the transmembrane (TM) sequences of the nascent IMPs (Houben et al., 2000; Scotti et al., 2000). Moreover, YidC was found associated with purified translocase, suggesting that YidC is a component of the translocase (Scotti et al., 2000). In vivo depletion studies subsequently showed that YidC is essential for both Sec-dependent and Sec-independent IMP insertion, pointing to a role of YidC independent of the Sec-translocase (Samuelson et al., 2000). Interestingly, YidC is homologous to the yeast mitochondrial IMP Oxa1p and to the chloroplast thylakoid membrane protein Albino3, which have been implicated in novel Sec-independent pathways of membrane protein biogenesis (Bernstein, 2000).
In this paper we have studied the biogenesis of FtsQ, a type II IMP, in vivo. Furthermore, we have studied the consecutive interactions of short nascent FtsQ in the membrane in vitro. Our data suggest that the initial insertion of the TM takes place close to SecY and lipids. As the nascent chain grows, the contact with SecY is lost and replaced by an interaction with YidC. These data are consistent with a role for YidC in the transfer of TM domains from the Sec-translocase into the lipid phase.
RESULTS AND DISCUSSION
Efficient in vivo assembly of FtsQ requires SecYEG, SecA and YidC
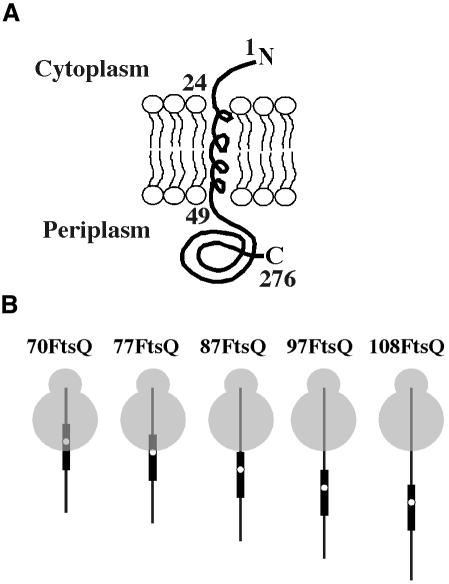
FtsQ is used as a model protein to study the biogenesis of simple IMPs. FtsQ is a single-spanning type II IMP (Nin–Cout topology) that plays a role in cell division (Figure 1A). Previous crosslinking experiments suggested interactions of nascent FtsQ with the translocase components SecY, SecA and YidC (Scotti et al., 2000; see also below).
Fig. 1. Schematic representation of the model protein FtsQ. (A) Topology of FtsQ in the inner membrane. (B) Nascent FtsQ species used in this study. The TM region is represented by a thick line with a white dot at the position of the photo-crosslinking probe.
We have now investigated whether these factors are required for membrane assembly of full-length FtsQ in vivo by analyzing the proteinase K accessibility of its large periplasmic domain in spheroplasts prepared from conditional strains. Upon depletion of SecA or SecE, the assembly of FtsQ was severely affected (Figure 2A and B). The SecE depletion strain was used since it enables the most efficient inactivation of the SecYEG core translocase (Traxler and Murphy, 1996). The assembly of FtsQ is also affected upon the depletion of YidC, but less severely (∼15%; Figure 2C). These experiments were verified by studying the assembly of FtsQ fused to the biotinylatable PBST domain (see Supplementary data, available at EMBO reports Online) as described by Beckwith and coworkers (Tian et al., 2000). Together, the data confirm the involvement of SecA, SecYEG and, to a lesser extent, YidC in the proper assembly of FtsQ into the inner membrane.
Fig. 2. Assembly of FtsQ is dependent on SecA, SecE and YidC. (A) Proteinase K accessibility of FtsQ (top) and OmpA/bandX (bottom) in BA13 (SecA depletion strain) and DO251 (control strain) spheroplasts, made from cells cultured at 41°C. Cells were labeled and processed as described in Methods. OmpA and band X are periplasmic and cytoplasmic control proteins, respectively. (B) Proteinase K accessibility in CM124 (SecE depletion strain) spheroplasts not depleted and depleted for SecE. (C) Proteinase K accessibility in JS7131 (YidC depletion strain) spheroplasts not depleted and depleted for YidC.
Sequential interaction of the FtsQ TM with SecY and YidC during membrane insertion in vitro
Previously, we have used bifunctional- and photo-crosslinking to study the molecular interactions of membrane-targeted nascent FtsQ of 108 amino acids (108FtsQ) (Valent et al., 1998; Scotti et al., 2000). At this nascent chain length the cytoplasmic domain and TM are fully exposed outside the ribosome (Figure 1B). In addition, assuming that the ribosome covers ∼35 residues, ∼19 residues of the periplasmic domain are exposed. The TM of 108FtsQ appeared in close proximity to both YidC and lipids, whereas the flanking hydrophilic regions were close to both SecA and SecY.
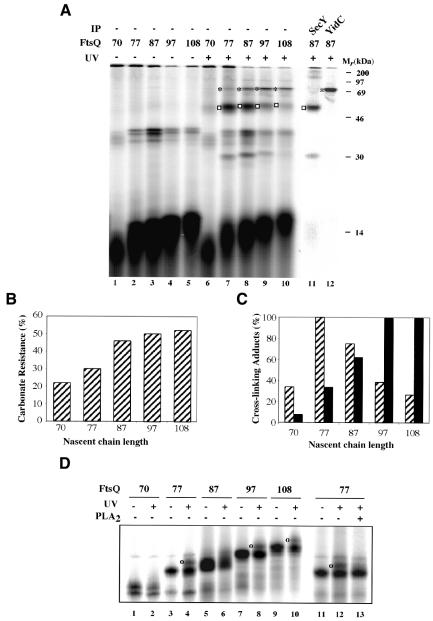
Obviously, these crosslinking experiments provide a static impression of the molecular environment of 108FtsQ. No conclusions could be drawn as to the role of the ribosome and the dynamics of the insertion reaction. To obtain insight into the order of the interactions of nascent FtsQ, we analyzed the earliest stages of membrane insertion, focusing on interactions of the TM domain probed by photo-crosslinking. Nascent chains of different length (70–108 amino acids) were prepared by translating truncated mRNA in the presence of [35S]methionine in a cell- and membrane-free E. coli lysate. Purified inverted inner membrane vesicles (IMVs) were added from the start of the translation reaction to allow co-translational membrane targeting and insertion of the translation intermediates. To specifically probe the molecular environment of the TM, a stop codon (TAG) was introduced at position 40 in the TM region of all constructs, and suppressed during in vitro synthesis by the addition of (Tmd)Phe-tRNASup, which carries a photoreactive probe. After the translation–insertion reaction, one half of each sample was irradiated with UV light to induce crosslinking while the other half was kept in the dark to serve as a control (Figure 3A).
Fig. 3. The TM sequence of FtsQ RNCs interacts sequentially with SecY and YidC. (A) In vitro synthesis of nascent FtsQ 70mer, 77mer, 87mer, 97mer and 108mer (all with a TAG codon at position 40) was carried out in the presence of IMVs and (Tmd)Phe-tRNASup. Aliquots were TCA-precipitated directly to evaluate translation and suppression efficiencies (not shown). After translation, samples were kept in the dark or UV-irradiated and subsequently extracted with carbonate. UV-irradiated pellet fractions were immunoprecipitated using antiserum against SecY or YidC. Immunoprecipitated adducts obtained with 87FtsQTAG40 are shown (lanes 11 and 12). The translation product at 35–40 kDa, present in lanes 1–10, is the peptidyl-tRNA form of the nascent FtsQ. SecY adducts are indicated by an open square; YidC adducts are indicated by an asterisk. (B) Nascent chains present in carbonate pellets from non-irradiated samples (A, lanes 1–5) were quantified. Carbonate resistance was quantified and expressed relative to the total amount of suppressed nascent chains present in TCA-precipitated translation totals (not shown). (C) SecY and YidC crosslinking adducts from (A) (lanes 6–10) were quantified and each signal was corrected for the total amount of carbonate-resistant material. Highest values for crosslinking efficiency were taken as 100%. The quantification is the average of two independent experiments. Hatched boxes, SecY adduct; black boxes, YidC adduct. For details on the quantification procedure see Supplementary data. (D) FtsQ nascent chains were produced and crosslinked as described in (A) and analyzed by tricine SDS–PAGE. To identify lipid crosslinking adducts (indicated by an ‘o’), photo-crosslinked 77FtsQTAG40 nascent chains were incubated with bee venom phospholipase (PLA2).
In all constructs, the TAG40 mutation was efficiently suppressed by (Tmd)Phe-tRNASup (not shown; see also Scotti et al., 2000), resulting in nascent FtsQ of the expected molecular weight. The shortest construct, 70FtsQ, was less efficiently integrated into the membrane, as judged by the criterion of carbonate resistance (Figure 3B), and generated only weak crosslinking products. In this construct the photo-crosslinking probe is expected to be in the ribosome interior (Figure 1B). In marked contrast, the 77FtsQ, while integrating only slightly more efficient (Figure 3B), gave rise to a major crosslinking product of ∼50 kDa. Immunoprecipitation identified SecY as the crosslinking partner (not shown; see also Figure 3A, lane 11). In addition, a weak crosslinking product of ∼70 kDa was detected that represents crosslinking to YidC (identified by immunoprecipitation; see also Figure 3A, lane 12). At this nascent chain length, the TM is still not fully exposed outside the ribosome, but the photo-crosslinking probe nevertheless can still reach out and interact with translocon proteins (Figure 1B).
Nascent chains of 87-, 97- and 108FtsQ have a fully exposed TM (Figure 1B) and integrated more efficiently (Figure 3B). 87FtsQ, which exposes the photo-crosslinking probe ∼12 residues outside the ribosome, showed crosslinking to SecY and, to a lesser extent, to YidC. In addition, a ∼30 kDa crosslinking adduct was detected that could be immunoprecipitated with anti-SecY (Figure 3A, lane 12) and probably represents a degradation fragment of the SecY adduct (Joly et al., 1994). 97- and 108FtsQ showed strong crosslinking to YidC and relatively weak crosslinking to SecY.
The crosslinking efficiencies were quantified and the results of this calculation, shown in Figure 3C, confirm the visual inspection of the gel image and show that crosslinking of the TM to SecY is optimal for short (77- and 87-) FtsQ, whereas crosslinking to YidC is optimal for longer (97- and 108-) FtsQ. Crosslinking efficiencies are relatively high for both components, indicating close contacts considering the short spacer arm (7 Å) of the photoprobe.
Previously, we showed photo-crosslinking data to suggest that the TM in membrane-targeted 108FtsQ is not only close to YidC but also to lipids (Scotti et al., 2000). Crosslinking adducts were identified that migrated slightly slower than the nascent chains in tricine SDS–PAGE. Analysis of the shorter nascent chains showed similar adducts for the 77- and 97FtsQ (and perhaps very weakly for 87-FtsQ) but not for the 70-FtsQ (Figure 3D). The adducts were not detected upon phospholipase treatment of the crosslinked samples, confirming that they represent crosslinking to lipids (shown for 77FtsQ in Figure 3D, lanes 11–13).
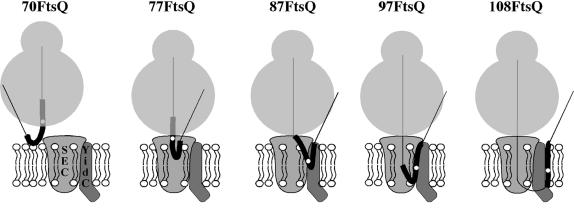
Taken together, the results indicate that the FtsQ TM encounters distinct protein–lipid environments early during biogenesis. Initial insertion of the TM of FtsQ takes place at a very early stage in biogenesis, in the vicinity of both SecY and lipids, perhaps at an interface between both compounds. Upon elongation of the nascent chain to 87 and 97 amino acids, the TM moves to a combined SecY/YidC/lipid environment, whereas in the longest nascent chains tested (108 amino acids) the TM is closest to YidC and lipids.
Role of the ribosome in interactions of 108FtsQ with translocase components
A recent study indicates that E. coli ribosomes bind with high affinity to the SecYEG complex (Prinz et al., 2000). To study the role of the ribosome in the membrane insertion of 108FtsQ in more detail, nascent 108FtsQ that carries (Tmd)Phe at position 40 in the TM domain was prepared in the presence of IMVs. After the translation–insertion reaction, one third of each sample was irradiated with UV light to induce crosslinking from position 40, whereas the rest of the sample was incubated with disuccinimidyl suberate (DSS) to induce crosslinking from the endogenous lysine at position 59 and possibly from the N-terminal amino-group (Figure 4).
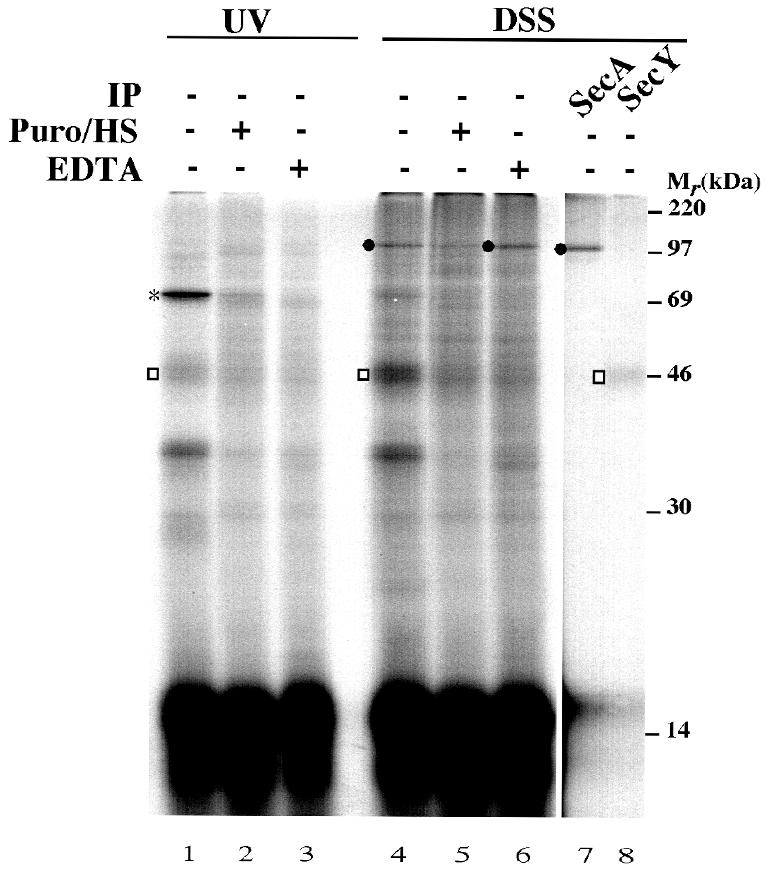
Fig. 4. The interaction between the TM domain of 108FtsQ and SecY/YidC is ribosome dependent. 108FtsQTAG40 nascent chains were synthesized in the presence of IMVs. The samples were split into equal aliquots and treated either with 0.2 mg/ml puromycin and 0.4 M KOAc (Puro/HS), with 25 mM EDTA, or mock-treated with incubation buffer. After 10 min of incubation at 37°C, one third of each aliquot was photo-crosslinked, whereas two thirds were subjected to DSS crosslinking. Carbonate-insoluble fractions are shown. To characterize the DSS crosslinking adducts, immunoprecipitations were carried out on mock-treated samples using antisera against SecA and SecY. SecA adduct is indicated by a black circle; other adducts are indicated as described in Figure 3.
Upon UV irradiation, the ∼69 kDa YidC adduct was observed (Figure 4, lane 1; see also Scotti et al., 2000). Upon treatment with DSS, ∼46 and ∼120 kDa products were detected, which could be immunoprecipitated using antibodies against SecY and SecA, respectively (Figure 4, lanes 4, 7 and 8). Apparently, the modification at position 40 does not alter these interactions, which have also been observed in wt 108FtsQ (Valent et al., 1998). Release of the nascent chain from the ribosome by a puromycin/high salt treatment after the membrane insertion reaction diminished the interaction with YidC and SecY, but had a less pronounced effect on the interaction with SecA. Membrane association of the nascent chain per se was not affected, as judged by its resistance to carbonate extraction (data not shown). Complete disassembly of the ribosome nascent chain complex by EDTA strongly affected the interaction with YidC and SecY, but not the association of the nascent chains with the membrane. Interestingly, a slightly stronger crosslinking to SecA was observed under these conditions (Figure 4, lane 6). Possibly, SecA has better access to the nascent chain upon disassembly of the ribosome. Alternatively, EDTA influences the insertion state of SecA and thereby the interaction with nascent FtsQ (Nishiyama et al., 1999).
Taken together, the results demonstrate that different domains of membrane-inserted nascent 108FtsQ interact with YidC, SecA and SecY. Premature release of the nascent chain from the ribosome appears to alter its conformation in the translocase.
Concluding remarks
Our results suggest sequential interactions of nascent FtsQ with membrane components starting very early in biosynthesis when the TM is not even fully exposed outside the ribosome (Figure 5). Insertion of the TM takes place adjacent to SecY and lipids, implying close contact between translating ribosome and translocase, consistent with the recently described affinity of ribosomes for the SecYEG complex (Prinz et al., 2000). It remains to be demonstrated whether insertion takes place at an outer interface between the translocase and lipids or whether lipids have access to the translocase interior. In fact, it is unclear whether or not the translocase is fully assembled at this stage, and whether the ribosome plays any role in this process. Upon elongation, the TM moves to a YidC/lipid environment, whereas the hydrophilic flanking region is close to SecA and SecY, presumably in the translocation channel as has been suggested for Lep (Nout–Cout topology) (Houben et al., 2000). The observed order of interactions of nascent FtsQ is consistent with the recent in vitro reconstitution of the insertion reaction using proteoliposomes that contain SecYEG, YidC, or both SecYEG and YidC (van der Laan et al., 2001). This latter study shows that nascent FtsQ engages YidC only when SecYEG is present as well. Together, the data are in agreement with the proposed role of YidC in the lateral diffusion of TM domains from the translocase into the lipids (Samuelson et al., 2000; Scotti et al., 2000). It should be noted, however, that a large part of FtsQ (and other Sec-dependent IMPs; Samuelson et al., 2000) still assembles in the membrane in vivo upon depletion of YidC (Figure 2), indicating that YidC may not be absolutely required for this process.
Fig. 5. Model for the membrane insertion of short nascent FtsQ. The TM region and photo-crosslinking probe are indicated as in Figure 1.
To some extent, the results are reminiscent of the early interactions of nascent Lep in the ER, which suggest insertion of the first TM at a Sec61α/lipid interface when it is only partly exposed outside the ribosome (Heinrich et al., 2000). Later interactions include the TRAM protein, depending on the hydrophobicity of the TM domain, suggesting a critical role for TRAM in the lipid partitioning of TM domains. While the analogy in timing of interactions is suggestive, the precise roles of TRAM and YidC remain elusive.
METHODS
Strains, plasmids and growth conditions.
Strain Top10F′ (Stratagene) was used for routine cloning of plasmid constructs. Strain MRE600 (xyl rna lpcA lam) was used to prepare a lysate for translation of in vitro synthesized mRNA and suppression of UAG stop codons in the presence of (Tmd)Phe-tRNASup. Strain MC4100 (F– ΔlacU169 araD136 rpsL thi relA) grown in Luria–Bertani medium was used to isolate IMVs.
The temperature-sensitive SecA strain BA13 and the control strain DO251 were cultured and depleted for SecA essentially as described (Qi and Bernstein, 1999). SecE and YidC depletion was carried out using strains CM124 and JS7131, respectively, as described (Traxler and Murphy, 1996; de Gier et al., 1998; Samuelson et al., 2000).
FtsQ was expressed by l-arabinose induction from the pBAD24 vector (Guzman et al., 1997) in strains BA13 and DO251, and by isopropyl-β-d-thiogalactopyranoside (IPTG) induction from the pEH1 vector (Hashemzadeh-Bonehi et al., 1998) in strains CM124 and JS7131.
Plasmids pC4Meth70FtsQTAG40, pC4Meth77FtsQTAG40, pC4Meth87FtsQTAG40 and pC4Meth97FtsQTAG40 were obtained by PCR using pC4Meth108FtsQTAG40 as a template (Scotti et al., 2000).
In vivo assay for membrane assembly.
Cells were grown to mid-log phase. Expression of FtsQ was induced with either IPTG (1 mM) for 3 min in strain CM124 and 10 min in strain JS7131, or with l-arabinose (0.2%) for 3 min in strains BA13 and DO251. BA13, DO251 and CM124 cells were labeled for 30 sec, and JS7131 cells were labeled for 2 min with [35S]methionine (Amersham). After labeling, cells were converted to spheroplasts and incubated in the presence or absence of proteinase K as described previously (de Gier et al., 1998). Finally, the samples were TCA-precipitated and immunoprecipitated with antisera to FtsQ, OmpA (an outer membrane protein with a large periplasmic domain) and AraB/bandX (a cytoplasmic control) as described previously (de Gier et al., 1998). Depletion of SecA and SecE was checked by monitoring the accumulation of pro-OmpA, whereas depletion of YidC was checked by western blotting.
In vitro transcription, translation, targeting and crosslinking.
Truncated mRNA was prepared as previously described from HindIII-linearized pC4MethFtsQ derivative plasmids (Scotti et al., 2000). For photo-crosslinking, (Tmd)Phe was site-specifically incorporated into FtsQ nascent chains by suppression of UAG stop codons using (Tmd)Phe-tRNASup in an E. coli in vitro translation system containing [35S]methionine to label the nascent chains. This procedure has been described previously (Scotti et al., 2000) but has been improved by small modifications that are described in the Supplementary data. Targeting to IMVs, photo-crosslinking and phospholipid analysis were carried out as described previously (Scotti et al., 2000). Bifunctional crosslinking was induced with 0.5 mM DSS (Pierce) for 10 min at 37°C and quenched at 4°C by adding 1/10 volume of quench buffer (1 M glycine, 100 mM NaHCO3 pH 8.5). Carbonate extraction to separate soluble and peripheral membrane proteins from integral membrane proteins was as described (Scotti et al., 2000). Carbonate-insoluble fractions were either TCA-precipitated or immunoprecipitated. The material used for immunoprecipitation was 4-fold the amount used for TCA precipitation. Sample analysis (SDS–PAGE and phosphorImaging) was as described previously (Scotti et al., 2000). The quantification of labeled bands is described in detail in the Supplementary data.
Supplementary data.
Supplementary data are available at EMBO reports Online.
Supplementary Material
Acknowledgments
ACKNOWLEDGEMENTS
We thank C.M. ten Hagen-Jongman for technical support. This work was supported by grants from the EC (to J.L.), from the Swedish Natural Sciences Research Council, the Swedish Foundation for Strategic Research and the Carl Trygger Foundation (to J.W.de G.) and from the Swiss National Science Foundation (to J.B.).
REFERENCES
- Beck K., Wu, L.F., Brunner, J. and Müller, M. (2000) Discrimination between SRP- and SecA/SecB-dependent substrates involves selective recognition of nascent chains by SRP and trigger factor. EMBO J., 19, 134–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein H.D. (2000) The biogenesis and assembly of bacterial membrane proteins. Curr. Opin. Microbiol., 3, 203–209. [DOI] [PubMed] [Google Scholar]
- de Gier J.W.L., Scotti, P.A., Sääf, A., Valent, Q.A., Kuhn, A., Luirink, J. and von Heijne, G. (1998) Differential use of the signal recognition particle translocase targeting pathway for inner membrane protein assembly in Escherichia coli. Proc. Natl Acad. Sci. USA, 95, 14646–14651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekkes P. and Driessen, A.J.M. (1999) Protein targeting to the bacterial cytoplasmic membrane. Microbiol. Mol. Biol. Rev., 63, 161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman L.M., Weiss, D.S. and Beckwith, J. (1997) Domain-swapping analysis of FtsI, FtsL, and FtsQ, bitopic membrane proteins essential for cell division in Escherichia coli. J. Bacteriol., 179, 5094–5103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashemzadeh-Bonehi L., Mehraein-Ghomi, F., Mitsopoulos, C., Jacob, J.P., Hennessey, E.S. and Broome-Smith, J.K. (1998) Importance of using lac rather than ara promoter vectors for modulating the levels of toxic gene products in Escherichia coli. Mol. Microbiol., 30, 676–678. [DOI] [PubMed] [Google Scholar]
- Heinrich S.U., Mothes, W., Brunner, J. and Rapoport, T.A. (2000) The Sec61p complex mediates the integration of a membrane protein by allowing lipid partitioning of the transmembrane domain. Cell, 102, 233–244. [DOI] [PubMed] [Google Scholar]
- Herskovits A.A., Bochkareva, E.S. and Bibi, E. (2000) New prospects in studying the bacterial signal recognition particle pathway. Mol. Microbiol., 38, 927–939. [DOI] [PubMed] [Google Scholar]
- Houben E.N.G., Scotti, P.A., Valent, Q.A., Brunner, J., de Gier, J.W.L., Oudega, B. and Luirink, J. (2000) Nascent Lep inserts into the Escherichia coli inner membrane in the vicinity of YidC, SecY and SecA. FEBS Lett., 476, 229–233. [DOI] [PubMed] [Google Scholar]
- Joly J.C., Leonard, M.R. and Wickner, W.T. (1994) Subunit dynamics in Escherichia coli preprotein translocase. Proc. Natl Acad. Sci. USA, 91, 4703–4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manting E.H. and Driessen, A.J.M. (2000) Escherichia coli translocase: the unravelling of a molecular machine. Mol. Microbiol., 37, 226–238. [DOI] [PubMed] [Google Scholar]
- Nishiyama K., Fukuda, A., Morita, K. and Tokuda, H. (1999) Membrane deinsertion of SecA underlying proton motive force-dependent stimulation of protein translocation. EMBO J., 18, 1049–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz A., Behrens, C., Rapoport, T.A., Hartmann, E. and Kalies, K.U. (2000) Evolutionarily conserved binding of ribosomes to the translocation channel via the large ribosomal RNA. EMBO J., 19, 1900–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi H.Y. and Bernstein, H.D. (1999) SecA is required for the insertion of inner membrane proteins targeted by the Escherichia coli signal recognition particle. J. Biol. Chem., 274, 8993–8997. [DOI] [PubMed] [Google Scholar]
- Samuelson J.C., Chen, M.Y., Jiang, F.L., Moller, I., Wiedmann, M., Kuhn, A., Phillips, G.J. and Dalbey, R.E. (2000) YidC mediates membrane protein insertion in bacteria. Nature, 406, 637–641. [DOI] [PubMed] [Google Scholar]
- Scotti P.A., Urbanus, M.L., Brunner, J., de Gier, J.W., von Heijne, G., van der Does, C., Driessen, A.J., Oudega, B. and Luirink, J. (2000) YidC, the Escherichia coli homologue of mitochondrial Oxa1p, is a component of the Sec translocase. EMBO J., 19, 542–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian H.P., Boyd, D. and Beckwith, J. (2000) A mutant hunt for defects in membrane protein assembly yields mutations affecting the bacterial signal recognition particle and Sec machinery. Proc. Natl Acad. Sci. USA, 97, 4730–4735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traxler B. and Murphy, C. (1996) Insertion of the polytopic membrane protein MalF is dependent on the bacterial secretion machinery. J. Biol. Chem., 271, 12394–12400. [DOI] [PubMed] [Google Scholar]
- Valent Q.A., Scotti, P.A., High, S., de Gier, J.-W.L., von Heijne, G., Lentzen, G., Wintermeyer, W., Oudega, B. and Luirink, J. (1998) The E.coli SRP and SecB targeting pathways converge at the translocon. EMBO J., 17, 2504–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Laan M., Houben, E.N.G., Nouwen, N., Luirink, J. and Driessen, A.J.M. (2001) Reconstruction of Sec-dependent membrane protein secretion: nascent FtsQ interacts with YidC in a SecYEG-dependent manner. EMBO Rep., 2, 519–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.