Abstract
Depression, a global health problem with growing prevalence, brings serious impacts on the daily life of patients. However, the antidepressants currently used in clinical are not perfectly effective, which greatly reduces the compliance of patients. Berberine is a natural quaternary alkaloid which has been shown to have a variety of pharmacological effects, such as hypoglycemic, lipid-regulation, anti-cancer, antibacterial, anti-oxidation, anti-inflammatory, and antidepressant. This review summarizes the evidence of pharmacological applications of berberine in treating depression and elucidates the mechanisms of berberine regulating neurotransmitter levels, promoting the regeneration of hippocampal neurons, improving hypothalamic-pituitary-adrenal axis dysfunction, anti-oxidative stress, and suppressing inflammatory status in order to provide a reference for further research and clinical application of berberine.
Keywords: berberine, depression, neurotransmitter, cell regeneration, HPA axis, oxidative stress, inflammation
1 Introduction
Depression is a common mood disorder characterized by low mood, anxiety, insomnia, loss of appetite, and poor concentration (Malhi and Mann, 2018; McCarron et al., 2021). In 2021, approximately 280 million people suffered from depression with a global prevalence rate of 3.8%, and severe depression can even lead to suicide, with more than 700,000 deaths due to suicide each year (World Health Organization, 2022). Meanwhile, depression is the sixth leading cause of disability-adjusted life-years (DALYs) among 20- to 50-year-olds (GBD, 2019 Diseases and Injuries Collaborators, 2020). As a result, depression significantly diminishes the quality of life and places a huge burden on the global economy (Chisholm et al., 2016). However, depression is frequently comorbid with other mental and chronic medical conditions (Berk et al., 2023). Clinical diagnosis of depression relies on the identification of several key symptoms mentioned above, while it is difficult to diagnose due to none of the symptoms is pathognomonic for depression (Malhi and Mann, 2018). Therefore, it is critical to improve early detection and management for people suffering from depression due to the complexity and seriousness of the pathophysiology of depression.
Selective serotonin reuptake inhibitors (SSRIs), serotonin and norepinephrine reuptake inhibitors (SNRIs), monoamine oxidase inhibitors (MAOIs), and tricyclic and tetracyclic antidepressants are the most commonly used antidepressants in clinical, with small differences between them were found (Cipriani et al., 2018). Most of these drugs are slow-acting, one-third of patients with major depressive disorder (MDD) did not improve significantly after taking multiple consecutive courses of antidepressants (Jha and Mathew, 2023). A significant proportion of patients develop treatment-resistant depression, requiring medication changes, additional treatment cycles, or adjunctive therapies (Lundberg et al., 2023). Besides, most of these medications have side effects including but not limited to gastrointestinal reactions, hepatotoxicity and hypersensitivity reactions, weight gain and metabolic disturbances, sexual dysfunction, and sleep disturbances (Gill et al., 2020; Rothmore, 2020; Oliva et al., 2021). Carvalho et al. demonstrated that the long-term treatment with these novel-generation antidepressant drugs should be avoided if alternative treatments are available (Carvalho et al., 2016). It is evident that the current treatment of depression remains difficult and there is an urgent need to find safer and more effective antidepressant drugs.
Traditional Chinese medicine (TCM) profits from the flexible utilization of the concepts of holistic view and diagnosis and treatment in clinical, plays a role in the treatment of multiple diseases, especially in the fight against COVID-19, which has shown the world the great potential of TCM (Lyu et al., 2021; Chen et al., 2022). In recent years, the antidepressant effects of herbal formulas and individual components have been gradually confirmed (Li et al., 2020; He et al., 2022). For instance, Lu et al. found that the methanol extract, ethanol extract, aqueous extract, and its volatile oil of Rhizoma Cyperi have antidepressant activity (Lu et al., 2022); muscone may alleviate lipopolysaccharide (LPS)-induced depression-like behaviors through TLR/MyD88 and TLR4/NLRP3 pathways (He et al., 2020); Lily Bulb and Rehmannia decoction can improve depression by reducing MAO activity, increasing monoamine neurotransmitter levels and regulating the hypothalamic-pituitary-adrenal (HPA) axis dysfunction (Chi et al., 2019; Zhang et al., 2021), a meta-analysis showed that chaihu-jia-longgu-muli-tang can ameliorate the depressive manifestations of the patients via suppressing inflammation (Zhao et al., 2023).
Berberine (Ber, Figure 1), a quaternary ammonium alkaloid, is one of the important constituents of Chinese herbal medicines such as Rhizoma Coptidis, Rhizoma Cyperus, and Rhizoma Rhei. Studies have shown that Ber possesses a variety of biological activities (Song et al., 2020), including hypoglycemic (Xie et al., 2022), hypolipidemic (Wang et al., 2022), antimicrobial (Jamshaid et al., 2020), anti-inflammatory (Li et al., 2020), and antitumor (Liu et al., 2020), etc. Therefore, the exploration of the pharmacological effects of Ber and its derivatives has a broad prospect. In recent years, the role of Ber in the neuropsychiatric field has greatly attracted the attention of researchers, and a large number of studies have been conducted to explore the effects of Ber on neuropsychiatric diseases including anxiety disorders and Alzheimer’s disease (Akbar et al., 2021; Fang et al., 2021; Raju et al., 2021; YU et al., 2021; Nguyen et al., 2022).
FIGURE 1.
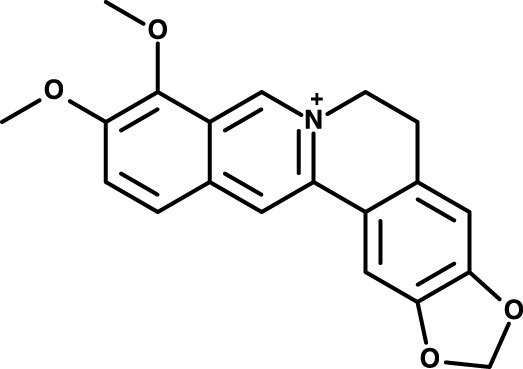
Chemical structure berberine.
Studies have shown that multiple factors are involved in the pathogenesis of depression, such as low functioning of the monoamine nervous system (Borroto-Escuela et al., 2021), inflammation (Beurel et al., 2020; Novakovic et al., 2023), nerve damage and regeneration disorders (Tartt et al., 2022; Thompson, 2023), dysfunction of the HPA axis (Herzog et al., 2023), oxidative stress (Bhatt et al., 2020), and genetic and psychosocial factors (Zhao et al., 2022; Ross et al., 2023), etc. The pathogenesis fits right in with the broad pharmacologic effects of Ber as mentioned above, and an increasing number of in vitro and in vivo experiments have focused on the validation of the antidepressant effects of Ber (Zhu et al., 2017; LU et al., 2021; YU et al., 2021; Wang et al., 2022b). Therefore, in this paper, we searched for related studies in databases such as PubMed, ScienceDirect, Web of Science, Chinese National Knowledge Infrastructure (CNKI), and Wanfang Data Resource System Chinese Science databases from inception till October 2023 to review the potential mechanisms of Ber in the treatment of depression, in order to provide a scientific basis for its in-depth study and clinical application. Table 1 presents relevant information on the studies focused on the antidepressant effects of Ber.
TABLE 1.
The in vivo studies on the antidepressant effects of berberine.
| Ref. | Animals | Modeling methods | Drugs | Usage | Dose and duration | Evaluation methods | Functions |
|---|---|---|---|---|---|---|---|
| Zhang et al. (2021) | SD rats | CUMS | Ber plus GRb1 | i.g | 150 mg/kg Ber and 20 mg/kg GRb1, for 4 weeks | FST, SPT, EPM, OFT | Ber + GRb1 reduced the immobility time in FST, upregulated the percentages of sugar water preference in SPT and the activity times in EPM, and increased the maximum travel distance, total travel distance, and time spent at the area center in the OFT. |
| Xu et al. (2021) | SD rats | reserpine intervention | Ber plus EVO | i.n | 0.05, 0.10, 0.15 mg/kg Ber and 0.008, 0.017, 0.025 mg/kg EVO, not mentioned | eyelid ptosis score | Ber plus EVO increased eyelid ptosis score |
| Zhu et al. (2017) | SD rats | CUMS | Ber | not mentioned | 40, 200 mg/kg, not mentioned | OFT, FST, SPT | Ber (200 mg/kg) increased the traversing times, vertical movement, and grooming times in the OFT, reduced the motionless time in the FST, and improved the sucrose preference in the SPT. |
| Lee et al. (2012) | SD rats | morphine withdrawal | Ber | i.p | 10, 20, and 50 mg/kg, for 10 days | FST, EPM | Ber (50 mg/kg) decreased the immobility time and restored climbing behavior in the FST, increased the time in the open arms, and the number of entries into the open arms of the maze in the EPM. |
| Huang et al. (2023) | Wistar rats | CUMS | Ber | i.g | 50, 100 mg/kg, for 14 days | OFT, SPT | Ber increased the rearing numbers and total distances in the OFT and prevented CUMS-induced abnormal SPT. |
| Wang et al. (2020) | Wistar rats | CUMS | BBH/HP-β-CD inclusion complex | i.n | 0.05, 0.10, and 0.15 mg/kg, for 14 days | OFT, SPT | BBH/HP-β-CD inclusion complex increased the number of rearing and total distance in the OFT, and improved the sucrose intake in the SPT. |
| Wang et al. (2022) | ICR mice | CUMS | Berberine | p.o | 25, 50, and 100 mg/kg, for 21 days | SPT, FST, TST, OFT, NSFT | Ber increased the sucrose preference in the SPT, improved the immobility time in the FST and TST, increased the number of crossings, rearing, and total moving distance in the OFT, and decreased the latency to feed in the NSFT. |
| Fan et al. (2017) | ICR mice | ovariectomize | Ber | i.p | 5, 10 mg/kg, for 7 days | OFT, FST | Ber (10 mg/kg) reduced the immobility time in the FST, and did not affect locomotor activity or rearing in the OFT. |
| Liu et al. (2017) | ICR mice | CUMS | Ber | p.o | 50, 100 mg/kg, for 4 weeks | SPT, NSFT | Ber increased the sucrose preference in the SPT, 100 mg/kg Ber decreased the latency to feed in the NSFT. |
| Shen et al. (2016) | ICR mice | CORT intervention | Ber | i.g | 50, 100 mg/kg, for 21 days | SPT, FST, OFT | Ber increased the sucrose preference in the SPT and the immobility time in the FST, while having no significant effects on the number of crossings and rearing in the OFT. |
| Qin et al. (2023) | C57BL/6N mice | CORT intervention | Ber | i.g | 100, 200 mg/kg, for 28 days | OFT, TST, FST, SPT | Ber (200 mg/kg increased the center zone time duration, total distance as well as zone transition number in OFT, reduced immobile duration in the FST and TST, and improved the consumption of sucrose solution in SPT. |
| Yang et al. (2023) | C57BL/6J mice | CUMS | Ber | i.g | 5, 10 mg/kg, for 3 weeks | SPT, FST, TST, OFT | Ber increased the proportion of sucrose preference in SPT and the immobility in the TST and FST, the difference in the distance traveled in the OFT was little |
| Zhan et al. (2021) | C57BL/6J mice | CUMS | Ber | i.g | 20 mg/kg, for 14 days | FST, SPT | Ber improved the sucrose preference in the SPT and increased the swimming time in the FST. |
| Yi et al. (2021) | C57BL/6J mice | CUMS | Ber | i.g | 100 mg/kg, for 7 days | SPT, NSFT | Ber increased the sucrose preference in the SPT and reduced the latency to feed in the NSFT. |
| Ge et al. (2023) | C57BL/6 mice | CUMS | Ber | i.g | 2.5, 5, and 10 mg/kg, for 1 week | OFT, FST, NSFT | Ber reduced the immobility in the FST, increased the proportion of distance in the center, the total walking distance, total walking time, and central activity time in the OFT, and improved the latency period in the NSFT. |
| Gong et al. (2019) | C57BL/6 mice | CORT intervention | Ber | i.g | 150 mg/kg, for 14 days | SPT | Ber increased the sucrose intake in the SPT. |
Ber, berberine; GRb1, ginsenoside Rb1; BBH, berberine hydrochloride; HP-β-CD, hydroxylpropyl-β-cyclodextrin; EVO, evodiamine; CUMS, chronic unpredictable mild stress; CRS, chronic restraint stress; CORT, corticosterone; OFT, open-field test; FST, forced swimming test; TST, tail suspension test; EPM, elevated plus maze; SPT, sucrose preference test; NSFT, novelty-suppressed feeding test; ICR, institute of cancer research; SD, sprague dawley.
2 Potential mechanisms underlying the antidepressant effects of berberine
2.1 Berberine modulates neurotransmitter levels
The discovery of tricyclic antidepressants (TCAs) represented by promethazine, led to the formation of the monoamine theory of depression (Barsa and Kline, 1957) which proposed that depression may be caused by a decrease in the availability of monoamine neurotransmitters such as serotonin (5-HT) and noradrenaline (NE) in the central nervous system (CNS), and was one of the early hypotheses aiming to explain the pathophysiology of depression (Krishnan and Nestler, 2008). In the 1950s, the antitubercular drug, iproniazid, was shown to have antidepressant effects in tuberculosis patients, followed by the discovery that iproniazid inhibits the monoamine oxidase (MAO), which is involved in the catabolism of 5-HT, NE, and dopamine (DA) (Shulman et al., 2013). Meanwhile, two other pieces of evidence provide support for this theory, one of which is reserpine is thought to provoke depression essentially through catecholamine depletion (Strawbridge et al., 2023), and the other is that serotonin transporter knockout mice showed depression-like behaviors (Haenisch and Bönisch, 2011). What’s more, the first-line antidepressants increase acute delivery of monoamine neurotransmitters through inhibition of neuronal reuptake (e.g., SSRIs) or inhibition of degradation (e.g., MAOIs), which indicates the crucial role of monoamine neurotransmitters in the onset of depression. Although these hypotheses are constantly being updated, the strong link between monoamine neurotransmitters and the development of depression has never been questioned.
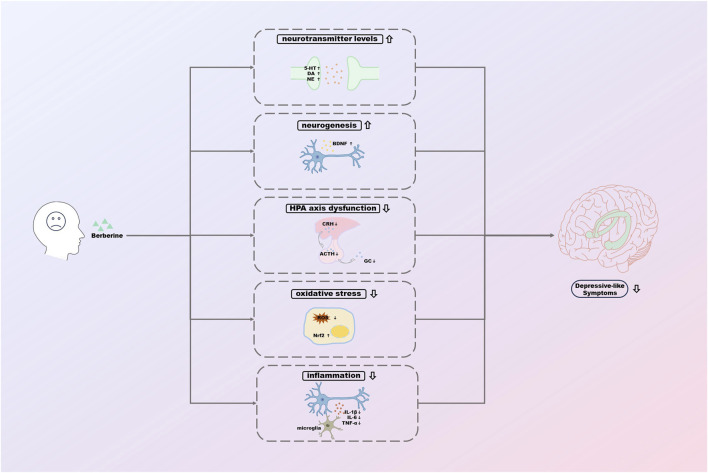
Ber can alleviate depression-like symptoms by modulating the levels of these neurotransmitters. Studies have shown that Ber could improve depressive-like behavior in mice by increasing the levels of NE, 5-HT, and DA in the hippocampus and frontal cortex as detected by high-performance liquid chromatography (HPLC) (Peng et al., 2007; Xu et al., 2021) and enzyme-linked immunosorbent assay (ELISA) (Huang et al., 2023). The 5-HT transporter (5-HTT) is an integral membrane protein that functions as a transporter protein and mediates the reuptake of 5-HT from inter-synaptic space, ensuring its recirculation into new cytoplasmic vesicles, and thus the duration and intensity of the biological action of 5-HT is largely dependent on 5-HTT (Iurescia et al., 2016). A study in the immortalized rat raphe-derived neuronal cell line RN46A cells showed that Ber (100 μM) can increase the mRNA and protein expression of 5-HTT, thereby enhancing the reuptake of 5-HT, which mechanism similar to that of SSRIs (Hu et al., 2012). Furthermore, Ber can also increase 5-HT levels in the hippocampus by regulating enzymes such as tryptophan 5-hydroxylase-1 (TPH1) and indoleamine 2,3-dioxygenase-1 (IDO1), thus shifting the kynurenine (KYN) pathway in tryptophan metabolism more towards the 5-HT pathway for the treatment of depression (Wang et al., 2022c; Ge et al., 2023). In addition, Ber may be an agonist of tyrosine hydroxylase (TH) in Enterococcus, which could lead to the production of L-dopa by the gut microbiota and finally transform into DA in the brain through a vitamin-like effect, thereby improving the brain function (Wang et al., 2021). We hypothesize that berberine may treat depression by modulating gut microbiota, which are likely important players in the diagnosis and treatment of depression due to their involvement in the bidirectional communication system of the gastrointestinal tract with the brain (Cryan et al., 2019).
2.2 Berberine enhances hippocampal neurogenesis
The cause of depression is far from being a simple deficiency of central monoamines. Subsets of depressed patients have been observed to exhibit volumetric reductions within the hippocampus and other forebrain regions, providing support for another prevalent hypothesis regarding depression, which posits a crucial role for neurodevelopmentally expressed growth factors in regulating plasticity within the adult brain (Monteggia et al., 2004). Several antidepressant treatments such as SSRIs, MAOIs, and SNRIs exhibit a notable cellular effect in the induction of adult hippocampal neurogenesis, a process characterized by the mitotic division of neural progenitors residing in the subgranular zone (SGZ) of the hippocampal region, leading to the formation of new neurons that subsequently undergo differentiation and integration within the dentate gyrus (DG) (Pittenger and Duman, 2008). Also, antidepressants could elevate the levels of various growth factors within the hippocampus, potentially through the modulation of cyclic adenosine monophosphate (CREB) or other transcription regulators, which exerts a significant influence on the process of neurogenesis (Krishnan and Nestler, 2008), further indicating the importance of neurogenesis in combating depression.
Ber could protect hippocampal nerves directly. MicroRNA (miR), a non-coding RNA with a size of approximately 22 nucleotides, frequently modulates gene expression at the post-transcriptional level. Mounting evidence indicates numerous miRNAs are specifically expressed or enriched in the brain, with aberrant miRNA expressions accompanying various neurological disorders in depression sufferers as well as depressive-like animals (Allen and Dwivedi, 2020; Fan et al., 2022). Studies showed that miR-34a overexpression in depressed mice impaired neurogenesis, and targeted inhibition of miR-34a expression by Ber could reverse this process and play an antidepressant role (Yi et al., 2021). Previous research suggested disrupting Jun N-terminal kinase (JNK)-Akt signaling could prevent hippocampal neuron apoptosis during ischemic brain damage (Gong et al., 2016). Zhang et al. demonstrated that the insulin-like growth factor receptor (IGFR) inhibitor remarkably enhances JNK and Akt expression, thereby inhibiting the Ber-augmented proliferation in hippocampal pyramidal neurons, which indicated a potential neuroprotective role for Ber (2 mg/kg/d) in the facial nerve axotomy damage mice model (Zhang et al., 2018).
In addition, Ber can also indirectly promote neurogenesis by modulating the levels of brain-derived neurotrophic factor (BDNF). BDNF, a neurotrophic factor that increases the proportion of neural stem cells that differentiate into neurons, and promotes the survival, proliferation, and maturation of neurons in the adult olfactory bulb and DG (Eliwa et al., 2017), has been validated as a key factor in promoting synaptic plasticity for antidepressant effects (Erickson et al., 2012; Zhang et al., 2016). Wang et al. demonstrated that exogenous BDNF administration and genetically engineered deletion of the DG resulted in the induction and attenuation of antidepressant response, respectively (Wang et al., 2022). Ber (100 mg/kg) can attenuate the depressive-like behavior (detected by SPT, FST, and open-field test (OFT)) by increasing BDNF expression in the hippocampal CA1 region (MA et al., 2012; Shen et al., 2016), and overexpressing BDNF can reverse the effects of miR-34b-5p and miR-470-5p on depressive-like behavior in CUMS mice (Zhan et al., 2021). Moreover, in de-ovulated model mice, the detection of CREB and eukaryotic translation elongation factor 2 (eEF2) suggested that Ber (10 mg/kg) can improve depression through the BDNF/CREB/eEF2 pathway, and the onset of action is 2–4 weeks faster than SSRI (Fan et al., 2017).
2.3 Berberine improves HPA axis function
The HPA axis is an important component of the neuroendocrine system, which has a close relationship with depression, with up to 40%–60% of depressed patients having hypercortisolemia or other HPA axis abnormalities (Keller et al., 2017). The activation of the HPA axis is characterized by an increase in hypothalamic production of corticotrophin-releasing factor (CRF), followed by increased pituitary release of adrenocorticotrophin (ACTH). There is strong evidence that stressful situations activate the HPA axis and increase circulating levels of glucocorticoid (GC) (Haleem and Gul, 2020), while overactivity of the HPA axis and increased circulating GC can affect brain serotonin and dependent responses to stress, precipitating depression (Haleem, 2022). Moreover, the observed correlation between heightened cortisol levels and the onset of depression may be attributed to the deleterious impact of excessive adrenal activity on the hippocampus (Mikulska et al., 2021). Chronic stress or the dysregulation of GC negative feedback receptors can result in elevated GC levels that can lead to significant damage to the hippocampus and hypothalamus (Hu et al., 2016). Ultimately, this neurogenic damage can lead to the proliferation of oligodendrocytes and exacerbation of depressive symptoms (Komoltsev and Gulyaeva, 2022). Chronic antidepressant treatment can restore the negative feedback function of the HPA axis, which either precedes or coincides with the relief of depression symptoms (Gobinath et al., 2014).
At present, many studies have demonstrated that Ber inhibits the abnormal activity of the HPA axis. In chronic unpredictable mild stress (CUMS)-induced mice, Ber (150 mg/kg) combined with ginsenosides could upregulate the expression levels of BDNF and downregulate the levels of corticosterone (CORT) and ACTH in plasma. Thereby attenuating depressive-like behaviors, including reducing the immobility time in FST, upregulating the percentages of sugar water preference in SPT and the activity times in EPM, increasing the maximum travel distance, total travel distance, and time spent at the area center in the OFT (Zhang et al., 2021). For the upstream hormones of CORT and ACTH, Ber (50 mg/kg) significantly reduced the expression of hypothalamic CRH and TH and showed greater improvement in depression and anxiety-like behavior (detected FST, EPM) in chronic morphine withdrawal rats (Lee et al., 2012). In addition, since excessive CORT is one of the important triggers for the onset of depression, quantitative proteomics of depressed mice revealed the inhibitory effects of CORT on the expression of mitochondrial oxidative phosphorylation-related proteins, and Ber could antagonize this effect and protect the neuronal physiological functions, which might be one of the mechanisms of the antidepressant effects of Ber (Gong et al., 2019).
2.4 Berberine reduces oxidative stress
Oxidative stress is another important factor that impairs neuroplasticity and contributes to the development of depression, it serves as a primary catalyst for neurodegeneration because reactive oxygen species (ROS) possess a profound relationship with a diverse array of pathophysiological processes (Bitanihirwe and Woo, 2011). When cells fail to maintain redox homeostasis and consequently generate proinflammatory mediators, cell necrosis ensues. The brain is particularly vulnerable to oxidative stress due to its elevated oxygen consumption, substantial lipid content, and relatively weak antioxidant defense system (Fesharaki-Zadeh, 2022). A clinical investigation demonstrated an elevated level of serum malondialdehyde (MDA) among individuals suffering from MDD, in comparison to a control population (Sarandol et al., 2007). In CUMS-induced depressed mice, the synthesis of peroxides such as MDA increased and the activity of antioxidant enzymes such as superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) decreased (Cheng et al., 2018), and antidepressant drugs could increase the levels of antioxidant enzymes, including catalase (CAT), SOD, and GSH-Px, in depressed patients or animals (Sherkawy et al., 2018; Meejuru et al., 2021; Mishra et al., 2021), the above suggests that improving the oxidative stress state might be an important direction for the treatment of depression.
In the study for type 2 diabetes mellitus (T2DM) model mice, Ber could increase the mRNA expression of SOD in the liver and the activities of SOD and CAT in the kidney tissue (Chatuphonprasert et al., 2013), which showed that Ber has a role in combating oxidative stress. What’s more, Ber was involved in the regulation of the GSH/GSH-Px antioxidant system in diabetic patients (Ma et al., 2018), indicating that Ber plays a role in ameliorating oxidative stress, and studies have shown that many signaling pathways may be involved. Sirtuin1 (SIRT1) is a deacetylase with excellent antioxidant properties whose expression level is significantly increased by Ber and triggers the transcription of forkhead box protein O (FoxO) target genes, including SOD, that affects the oxidative stress state (Hill et al., 2000; Chen and Yang, 2017). Ber mediates the inhibition of oxidative stress through the nuclear factor erythroid 2-related factor 2 (Nrf2) pathway (Yang et al., 2011; Mo et al., 2014) and the antioxidant activity of Ber can be eliminated by pharmacological blockade of Nrf2 in neurons and macrophages, verifying that the effects of Ber may be related to Nrf2 (Wen et al., 2020). However, fewer studies are validating the antidepressant effects of Ber from the perspective of oxidative stress, and the association between the two needs to be further explored in the future.
2.5 Berberine can inhibit inflammatory responses
Oxidative stress is always linked to inflammation. Inflammatory cells produce ROS, which can activate intracellular signaling and lead to the activation of proinflammatory genes. Peripheral cytokines undoubtedly have a role in behavioral effects, as evidenced by data indicating that blocking peripheral cytokines tightens the blood-brain barrier (BBB), and stopping BBB breakdown demonstrates antidepressant effects (Cheng et al., 2018). Beurel et al. demonstrated that peripheral cytokines can reach the brain maybe through “leaky” regions of the BBB, through a neural route via afferent nerve fiber cytokine receptors that relay the signal to the brain parenchyma, and through infiltration of immune cells (Beurel et al., 2020). At the molecular level, proinflammatory cytokines can reduce the supply of 5-HT, DA, and NE by increasing the expression and function of presynaptic 5-HT reuptake transporter proteins and activating the IDO to reduce related monoamine precursors (Maes et al., 2011). Moreover, inflammation affects growth factors, such as BDNF in the DG of the hippocampus, resulting in the damage of neuronal integrity, including neurogenesis, long-duration potentiation, and dendritic germination (Miller and Raison, 2016), which is important in the onset of depression. A meta-analysis found that depression is associated with concurrent and future inflammation in children and adolescents (Colasanto et al., 2020), Similarly, depressed patients were confirmed to have greater levels of proinflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), IL-1, IL-4, IL-5, IL-12, interferon-γ (IFN-γ), and C-reactive protein (CRP) in their blood (Hussain et al., 2016), while antidepressant treatment significantly could reduce peripheral levels of IL-6, TNF, IL-10 (Köhler et al., 2018). Furthermore, anti-inflammatory drug supplementary use of antidepressants appears to boost antidepressant efficacy, and treatment-resistant depressive patients may benefit from anti-inflammatory drugs as well (Raison et al., 2013). The above supports that inflammation is closely associated with the pathogenesis of depression.
The anti-inflammatory effects of Ber have long been well documented (Zhang et al., 2019; Wang et al., 2020; Naz et al., 2022), and many studies have shown that the effects of Ber in reducing neuropsychiatric symptoms are related to its anti-inflammatory effects. Ber (100 mg/kg) reduced the levels of IL-1β, IL-6, and TNF-α in the hippocampus and inhibited the activation of microglia in mice, thus alleviating their depressive symptoms (detected by SPT, novelty-suppressed feeding test (NSFT)) induced by CUMS (Liu et al., 2017). In another animal model, depressive symptoms complicated by inflammatory pain were significantly improved after Ber (50 mg/kg) intervention, which may be related to the reduction of IL-1β, IL-6, and TNF-α levels (Xu et al., 2018). Meanwhile, proteomics analysis of reserpine-induced depressed mice revealed that retinoic acid-inducible gene I (RIG-I) was highly expressed in the model group while negative in the Ber group, whereas RIG-I-mediated neuroinflammation may be involved in the pathogenesis of depression (Yang et al., 2022). In addition, activation of neuronal nitric oxide synthases (NOS) raises the concentration of NO, which ultimately leads to the development of depression (Adell, 2020; Kang et al., 2020), while the inhibitory effects of Ber on inducible NOS has been recognized (Zhu et al., 2018; Zhu et al., 2019). The tripartite motif (TRIM) family is a subfamily of E3 ubiquitin ligases that regulate the ubiquitination of target proteins in biological processes such as proliferation, apoptosis, development, differentiation, inflammation, and immunology. Yang et al., 2023 showed that Ber inhibits NLRP3 inflammasome activity by increasing Trim65 conjugation to NLRP3 and NLRP3 ubiquitination, effectively alleviates depressive symptoms (detected by SPT, FST, OFT, and tail suspension test (TST)), and reduces hippocampal neuronal functional damage in CUMS mice.
3 The effect of Chinese medicine prescription containing berberine on depression
Traditional herbal formulas tend to target more than a single herb and therefore have a more multifaceted therapeutic effect. Ber is one of the most important components in Rhizoma Coptidis (Huang Lian, HL), and current research has found that many formulas containing HL have antidepressant effects. Table 2 presents relevant information on the related studies.
TABLE 2.
The in vivo studies on the antidepressant effects of traditional herbal formulas which containing berberine.
| Formulas | Components | Ref. | Animals | Modeling methods | Usage | Dose and duration | Evaluation methods | Functions |
|---|---|---|---|---|---|---|---|---|
| Jiao-tai-wan (JTW) | Rhizoma Coptidis and Cinnamon | Bai et al. (2022) | Kunming mice | CORT intervention | i.g | 2.1 and 4.2 g/kg, for 2 weeks | OFT, EPM, FST, TST | JTW increased the travel distance and time spent in the center in the OFT, improved the numbers into open arms and time on open arms in the EPM, and reduced the swing immobility time and floating immobility time in the TST and FST. |
| Tang et al. (2022) | C57BL/6 mice | CRS | i.g | 1.6 and 3.2 g/kg, for 21 days | OFT, TST, FST | JTW (3.2 g/kg) decreased the immobility time in the TST and FST and increased the total distance traveled in the OFT. | ||
| Jiao et al. (2021) | SD rats | CUMS | i.g | 0.75, 1.5, and 3 g/kg, for 14 days | OFT, SPT | JTW improved the sucrose preference in the SPT, and 3 g/kg JTW increased the upright numbers and crossing numbers in the OFT. | ||
| Zhe et al. (2017) | ICR mice | LPS intervention | i.g | 4.2 and 8.4 g/kg, for 7 days | FST, SPT, OFT, TST | JTW increased the sucrose preference in the SPT, decreased the immobility time in the TST and FST, and improved the crossings, rearing, and grooming numbers in the OFT. | ||
| Zuojin pill (ZJP) | Rhizoma Coptidis and Evodia rutaecarpa | Tao et al. (2023) | C57BL/6 mice | CUMS | i.g | 450 and 910 mg/kg, for 3 weeks | SPT, FST, TST, OFT | ZJP increased the percent sucrose preference in the SPT and decreased the immobility time of the TST and FST, while having no significant effect on central movement distance in the OFT. |
| Wang et al. (2023) | C57BL/6 mice | CUMS | i.g | 225, 450, and 910 mg/kg, for 3 weeks | TST, SPT, FST | ZJP increased the sucrose preference level in the SPT and decreased the duration of immobility in the TST and FST. | ||
| Wang et al. (2020) | SD rats | CUMS | i.g | 0.6 and 1.2 g/kg, for 5 weeks | OFT, SPT | ZJP improved the sucrose preference in the SPT, ZJP (1.2 g/kg) increased the crossing, grooming, and rearing, and decreased time spent in the central area in the OFT. | ||
| Wang et al. (2013) | ICR mice | reserpine intervention | p.o | 5, 10, and 20 mg/kg, for 10 days | TST, FST, OFT | The ethanol extract of ZJP decreased the immobility time in the FST at 5 and 10 mg/kg, reduced the immobility time in the TST at 5 and 20 mg/kg, and did not affect the crossings and rearings in the OFT. | ||
| HuangLian JieDu Decoction (HLJDD) | Rhizoma Coptidis, Radix Scutellariae, Cortex Phellodendri, and Fructus Gardeniae | Qu et al. (2021) | C57BL/6 mice | CUMS | p.o | 590 mg/kg, for 2 weeks | FST, OFT, NSFT | HLJDD decreased the immobility time in the FST, reduced the latency to feed in the NSFT, and increased total traveling time and distance, distance traveled at the center, and travel duration in the OFT. |
| Zheng et al. (2023) | C57BL/6 mice | DSS intervention | p.o | 2 and 4 g/kg, for 7 weeks | OFT, EPM, NORT | HLJDD increased the central travel distance and time ratio in the OFT, increased the time spent exploring the novel object in the NORT, and improved the time and distance on exploring the open arms in the EPM. | ||
| Banxia Xiexin Decoction (BXXXD) | Pinelliae Rhizoma, Scutellariae Radix, Zingiberis Rhizoma, Ginseng Radix, Glycyrrhizae Radix, Coptidis Rhizoma, and Jujubae Fructus | Liao et al. (2023) | C57BL/6 mice | High fat combined with bind stimulation | i.g | 0.45, 1.35, and 4.05 g/kg, for 16 weeks | SPT, OFT, TST | BXXXD (1.35 and 4.05 g/kg) improved the rate of sugar-water consumption in the SPT, decreased the total motor distance and the central residence time in the OFT and the immobility time in the TST. |
CUMS, chronic unpredictable mild stress; CRS, chronic restraint stress; CORT, corticosterone; LPS, lipopolysaccharide; DSS, dextran sulfate sodium; OFT, open-field test; FST, forced swimming test; TST, tail suspension test; EPM, elevated plus maze; SPT, sucrose preference test; NSFT, novelty-suppressed feeding test; NORT, novel object recognition test; ICR, institute of cancer research; SD, sprague dawley.
3.1 Jiao-tai-wan
JTW, composed of Rhizoma Coptidis and Cinnamon (Figure 2A), has been applied for insomnia since ancient times, and its antidepressant effects are been explored. JTW can ameliorate depression-like symptoms in depression mice induced by chronic restraint stress (CRS), and has a protective effect on the damage to hippocampal neurons (Tang et al., 2022). Bai et al., 2022 found that JTW could ameliorate CORT-induced depressive-like behaviors and neuronal damage and enhance the levels of monoamine neurotransmitters in the serum of mice, which were also seen in the LPS-induced mice (Zhe et al., 2017). The therapeutic effects of JTW in the above experiments all involved an anti-inflammatory response. In addition, the metabolomics of serum from CUMS-induced rats showed that the antidepressant effects of JTW may be attributed to the regulation of amino acid metabolism, glycerophospholipid metabolism, and energy metabolism (Jiao et al., 2021).
FIGURE 2.
The components of the traditional herbal formulas in this review. (A) Chinese herbal medicine contained in Jiao-tai-wan. (B) Chinese herbal medicine contained in Zuojin Pill. (C) Chinese herbal medicine contained in HuangLian JieDu Decoction. (D) Chinese herbal medicine contained in Banxia Xiexin Decoction.
3.2 Zuojin pill
ZJP, a classic herbal formula consisting of Rhizoma Coptidis and Evodia Rutaecarpa (Figure 2B) is widely used clinically to treat gastrointestinal diseases, and there have been confirmed that ZJP may have a role in alleviating depressive-like behavior. Wang et al. found that ZJP can improve CUMS-induced depression-like behavior via the TPH2/5-HT pathway (Wang et al., 2023). And the anti-inflammatory and antidepressant effects of ZJP are primarily attributed to the promotion of the ubiquitination of MyD88 and the inhibition of the activation of downstream inflammatory signals (Wang et al., 2020; Tao et al., 2023). Except for ZJP itself, the ethanol extract of ZJP also showed antidepressant-like effects in reserpine-induced depressed mice with a mechanism involving the central monoaminergic neurotransmitter system (Wang et al., 2013).
3.3 Huanglian Jiedu decoction
HLJDD includes Rhizoma Coptidis, Scutellariae Radix, Cortex Phellodendri, and Fructus Gardeniae (Figure 2C), and has been implicated as effective in treating inflammation-related diseases. HLJDD was able to alleviate depressive-like behaviors in colitis mice by inhibiting the Trem2/Dap12 signaling pathway in the microglia of the lateral habenula (Zheng et al., 2023). Additionally, network pharmacology analysis and metabolomics examination revealed that tryptophan metabolism serves as the primary target for HLJDD in CUMS mice, and SLC6A4 and MAOA within the tryptophan metabolic pathway were effectively modulated by Ber, baicalein, tetrahydro berberine, candicine, could be classified as the primary antidepressant targets for HLJDD (Qu et al., 2021), highlighting the key role of Ber in HLJDD.
3.4 Banxia Xiexin decoction
BXXXD is a formula consisting of seven herbs including Pinelliae Rhizoma, Scutellariae Radix, Zingiberis Rhizoma, Ginseng Radix, Glycyrrhizae Radix, Coptidis Rhizoma, and Jujubae Fructus (Figure 2D), which can lower lipids and alleviate depressive disorders, but fewer studies have been conducted to date. Liao et al. showed that BXXXD may exert a therapeutic effect by modulating the abundance of gut microbiota and thus intervening lipid metabolism in the peripheral and hippocampus (Liao et al., 2023). Another network pharmacology suggested the antidepressant effects of BXXXD are related to drug response, steroid metabolism, lipid metabolism, inflammatory response, oxidative stress response, and other biological functions (Yu et al., 2020), which need further validation.
4 Discussion
This paper reviews the potential mechanisms of antidepressant effects of Ber based on the existing studies (Figure 3). However, there is still a long way to go before Ber can truly be used as an antidepressant in the clinic.
FIGURE 3.
The potential mechanisms of antidepressant effects of berberine.
One problem that cannot be avoided by applying Ber in the clinic is its low bioavailability. As an alkaloid with poor aqueous solubility (Shen et al., 2016), the bioavailability of Ber is less than 1% of the dose in rats, mostly owing to high intestinal first-pass metabolism (more than 98% of the absorbed amount), insufficient intestinal absorption (about 50% of the dose), and hepatic first-pass metabolism (20%–30% of that reached portal vein) (Murakami et al., 2023). The intrinsic mechanism may be related to the fact that Ber is P-glycoprotein (P-gp) (Zhang Y. et al., 2019), and cytochrome P450s (CYPs) (Johnson et al., 2002) expressed abundantly in the small intestine and the liver. Given the above, it has been shown that the bioavailability can be effectively improved by changing the route of administration (Murakami et al., 2023), co-administration with absorption enhancers (Xu et al., 2021; Wang et al., 2022), formulations containing solubilizer exhibiting P-gp and/or CYPs inhibitors (Kwon et al., 2020), and development of Ber analogs or derivatives (Wang et al., 2020; Kohli et al., 2021).
In addition, there is a need to focus on the safety of Ber. Ber may be cardiotoxic (Zhi et al., 2015) and hepatotoxic (Moreira et al., 2022), and the LD50 (median lethal dosage) of intravenous and intraperitoneal berberine was 9.0386 mg/kg and 57.6103 mg/kg, respectively. Ber has a high oral safety dose, because the absorption of Ber by the animal’s intestine system has its limit, and no matter how much the orally administered dosage is raised, the absorption rate would not increase at this internal limit (Kheir et al., 2010). Table 3 compares the toxicity of Ber with some clinically used antidepressants. Of these antidepressants, ketamine seems particularly dangerous as a street drug. Intravenous ketamine and intranasal esketamine (in combination with antidepressants) have proven efficacy in the management of treatment-resistant depression and are thus of high interest (Reif et al., 2023), and they seem to exert effects within 1 day whereas antidepressants generally take weeks (Popova et al., 2019). However, after acute dosing, psychiatric, psychotomimetic, cardiovascular, neurological, and other side-effects were more commonly observed after ketamine treatment than after placebo in patients with depression (Short et al., 2018). Interestingly, one study showed Ber can inhibit avoidance memory impairment of Toxoplasma gondii-infected rat model of ketamine-induced schizophrenia (Gholizadeh et al., 2023). Meanwhile, levo-tetrahydropalmatine, one of tetrahydroprotoberberines, could increase the bioavailability of ketamine and promote the metabolism of ketamine (Du et al., 2020), so the combination of ketamine and Ber might be a valuable new idea.
TABLE 3.
Comparison of toxicity between berberine and commonly used antidepressants.
| Drugs | Toxicity | Drugs | Toxicity |
|---|---|---|---|
| Berberine | Cardiotoxicity | MAOIs | Serotonin syndrome |
| Hepatotoxicity | Hypertensive crisis | ||
| NMDA antagonist (ketamine) | Sedation | Vilazodone | Drowsiness |
| Dissociation | Vomiting | ||
| Ulcerative or Interstitial Cystitis | Tachycardia | ||
| Embryo-fetal Toxicity | Serotonin syndrome (altered mental status, autonomic instability, and neuromuscular abnormalities) | ||
| Trazodone | Arrhythmias | Mirtazapine | Disorientation |
| Respiratory arrest | Drowsiness | ||
| Coma | Impaired memory | ||
| Priapism | Bradyarrhythmias | ||
| Bupropion | Tachycardia | Dextromethorphan/bupropion | Seizures |
| Hypertension | Psychosis | ||
| Seizure | Serotonin Syndrome | ||
| SSRIs | Drowsiness | TCAs | Dilated pupils |
| Tremor | Absent bowel sounds | ||
| QRS and QTc interval prolongation (especially with citalopram and escitalopram) | Constipation | ||
| Potential serotonin syndrome (hyperthermia, hypertonia, hyperreflexia, clonus) | Urinary retention | ||
| Electrocardiogram changes (tachycardia, hypotension, conduction abnormalities, QRS duration >100 msec) | |||
| Sedation | |||
| Seizures | |||
| SNRIs | Tachycardia | ||
| Hypertension | |||
| Electrocardiogram changes (e.g., prolongation of QT interval, bundle branch block, QRS prolongation), ventricular tachycardia | |||
| Changes in the level of consciousness (ranging from somnolence to coma) | |||
| Mydriasis | |||
| Serotonin syndrome | |||
| Rhabdomyolysis | |||
| Liver necrosis | |||
| Death |
MAOI, monoamine oxidase inhibitor; NMDA, n-methyl-d-aspartic acid; SSRI, selective serotonin reuptake inhibitor; TCAs, tricyclic antidepressants; SNRI, serotonin and noradrenalin reuptake inhibitors.
Clinical trials of Ber in the treatment of depression are still lacking. A clinical randomized controlled trial that included 164 patients showed that Ber hydrochloride improved performance on a depression scale in patients with irritable bowel syndrome (Chen et al., 2015). However, such a change might be related to the improvement of the patient’s intestinal symptoms and does not directly reflect the role of berberine in the treatment of depression. Another clinical study that included 52 opioid addicts demonstrated that there were no significant differences in depression, anxiety, stress, and sleep quality scores in the treatment group given capsules of Berberis vulgaris extract (Dabaghzadeh et al., 2023). Therefore, more rigorously designed and targeted clinical trials are needed to guide the clinical application of Ber in the future.
Due to the late discovery of the antidepressant effect of berberine, there are still some problems in the experimental research on this area: 1) Currently, the in vitro experiments of the antidepressant effects of Ber mainly used the HT22 cell line, and due to the less frequent use of primary cells and the lack of a recognized modeling method, the progress in the exploration of the mechanisms is slow. 2) The anti-oxidative stress effect of Ber is clear and there is a strong correlation between oxidative stress and depression, but there has not been a study directly focused on the relationship between the anti-oxidative stress and antidepressant effects of Ber, which needs to be explored in the future. 3) Doses (from 2 to 200 mg/kg) and duration (from 1 to 4 weeks) of Ber have varied considerably from study to study, and it has not yet been possible to determine an appropriate dose range for the treatment of depression. It is necessary to compare different doses and duration of Ber in different depression models to investigate the most reasonable dose, with the use of consistent behavioral tests.
In summary, the relevant studies suggest that the mechanisms of the antidepressant effects of Ber may be related to the regulation of neurotransmitter levels, enhancement of hippocampal neurogenesis, improvement of HPA axis function, reduction of oxidative stress, and inhibition of inflammatory responses. These pathways are essential in the pathogenesis of depression and are also crucial for the efficient treatment of depression. Ber, as a monomer of TCM with rich pharmacological effects, the exploration of its relevant mechanisms is still in its infancy, although there have been several studies on its antidepressant effects. We believe that the exploration of the efficacy of Ber will be deepened gradually, and the potential mechanisms of the antidepressant effects of Ber will be clarified, which will have a broader application prospect in the future.
Funding Statement
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Natural Science Foundation of China (No. 82174159).
Author contributions
YG: Writing–original draft. KN: Data curation, Writing–original draft. HW: Data curation, Writing–original draft. HD: Funding acquisition, Writing–review and editing. YT: Writing–original draft, Writing–review and editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
| DALYs | disability-adjusted life-years |
| SSRIs | selective serotonin reuptake inhibitors |
| SNRIs | serotonin and norepinephrine reuptake inhibitors |
| MAOIs | monoamine oxidase inhibitors |
| MDD | major depressive disorder |
| TCM | traditional Chinese medicine |
| LPS | lipopolysaccharide |
| Ber | berberine |
| HPA | hypothalamic-pituitary-adrenal |
| CNKI | Chinese National Knowledge Infrastructure |
| TCAs | tricyclic antidepressants |
| 5-HT | serotonin |
| NE | noradrenaline |
| CNS | central nervous system |
| MAO | monoamine oxidase |
| DA | dopamine |
| HPLC | high-performance liquid chromatography |
| ELISA | enzyme-linked immunosorbent assay |
| 5-HTT | 5-HT transporter |
| TH | tyrosine hydroxylase |
| TPH1 | tryptophan 5-hydroxylase-1 |
| IDO1 | indoleamine 2,3-dioxygenase-1 |
| KYN | kynurenine |
| SGZ | subgranular zone |
| DG | dentate gyrus |
| miR | microRNA |
| JNK | Jun N-terminal kinase |
| CREB | cyclic adenosine monophosphate |
| IGFR | insulin-like growth factor receptor |
| BDNF | brain-derived neurotrophic factor |
| OFT | open-field test |
| eEF2 | elongation factor 2 |
| CRF | corticotrophin-releasing factor |
| ACTH | adrenocorticotrophin |
| GC | glucocorticoid |
| CUMS | chronic unpredictable mild stress |
| CORT | corticosterone |
| FST | forced swimming test |
| SPT | sucrose preference test |
| EPM | elevated plus maze |
| ROS | reactive oxygen species |
| MDA | malondialdehyde |
| SOD | superoxide dismutase |
| GSH-Px | glutathione peroxidase |
| CAT | catalase |
| T2DM | type 2 diabetes mellitus |
| SIRT1 | sirtuin1 |
| FoxO | forkhead box protein O |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| BBB | blood-brain barrier |
| TNF-α | tumor necrosis factor-α |
| IL-6 | interleukin-6 |
| IFN-γ | interferon-γ |
| CRP | C-reactive protein |
| RIG-I | retinoic acid-inducible gene I |
| NSFT | novelty-suppressed feeding test |
| NOS | nitric oxide synthases |
| TRIM | tripartite motif |
| TST | tail suspension test |
| HL | Huang Lian |
| JTW | jiao-tai-wan |
| CRS | chronic restraint stress |
| HLJDD | HuangLian JieDu Decoction |
| ZJP | Zuojin pill |
| BXXXD | Banxia Xiexin Decoction |
| P-gp | P-glycoprotein |
| CYPs | cytochrome P450s |
| LD 50 | median lethal dosage |
References
- Adell A. (2020). Brain NMDA receptors in schizophrenia and depression. Biomolecules 10 (6), 947. 10.3390/biom10060947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbar M., Shabbir A., Rehman K., Akash M. S. H., Shah M. A. (2021). Neuroprotective potential of berberine in modulating Alzheimer's disease via multiple signaling pathways. J. Food Biochem. 45 (10), e13936. 10.1111/jfbc.13936 [DOI] [PubMed] [Google Scholar]
- Allen L., Dwivedi Y. (2020). MicroRNA mediators of early life stress vulnerability to depression and suicidal behavior. Mol. Psychiatry 25 (2), 308–320. 10.1038/s41380-019-0597-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai G., Qiao Y., Lo P. C., Song L., Yang Y., Duan L., et al. (2022). Anti-depressive effects of Jiao-Tai-Wan on CORT-induced depression in mice by inhibiting inflammation and microglia activation. J. Ethnopharmacol. 283, 114717. 10.1016/j.jep.2021.114717 [DOI] [PubMed] [Google Scholar]
- Barsa J. A., Kline N. S. (1957). Depression treated with chlorpromazine and promethazine. Am. J. Psychiatry 113 (8), 744–745. 10.1176/ajp.113.8.744 [DOI] [PubMed] [Google Scholar]
- Berk M., Köhler-Forsberg O., Turner M., Penninx B., Wrobel A., Firth J., et al. (2023). Comorbidity between major depressive disorder and physical diseases: a comprehensive review of epidemiology, mechanisms and management. World Psychiatry 22 (3), 366–387. 10.1002/wps.21110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurel E., Toups M., Nemeroff C. B. (2020). The bidirectional relationship of depression and inflammation: double trouble. Neuron 107 (2), 234–256. 10.1016/j.neuron.2020.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt S., Nagappa A. N., Patil C. R. (2020). Role of oxidative stress in depression. Drug Discov. Today 25 (7), 1270–1276. 10.1016/j.drudis.2020.05.001 [DOI] [PubMed] [Google Scholar]
- Bitanihirwe B. K., Woo T. U. (2011). Oxidative stress in schizophrenia: an integrated approach. Neurosci. Biobehav Rev. 35 (3), 878–893. 10.1016/j.neubiorev.2010.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borroto-Escuela D. O., Ambrogini P., Chruścicka B., Lindskog M., Crespo-Ramirez M., Hernández-Mondragón J. C., et al. (2021). The role of central serotonin neurons and 5-HT heteroreceptor complexes in the pathophysiology of depression: a historical perspective and future prospects. Int. J. Mol. Sci. 22 (4), 1927. 10.3390/ijms22041927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho A. F., Sharma M. S., Brunoni A. R., Vieta E., Fava G. A. (2016). The safety, tolerability and risks associated with the use of newer generation antidepressant drugs: a critical review of the literature. Psychother. Psychosom. 85 (5), 270–288. 10.1159/000447034 [DOI] [PubMed] [Google Scholar]
- Chatuphonprasert W., Lao-Ong T., Jarukamjorn K. (2013). Improvement of superoxide dismutase and catalase in streptozotocin-nicotinamide-induced type 2-diabetes in mice by berberine and glibenclamide. Pharm. Biol. 52, 419–427. 10.3109/13880209.2013.839714 [DOI] [PubMed] [Google Scholar]
- Chen C., Tao C., Liu Z., Lu M., Pan Q., Zheng L., et al. (2015). A randomized clinical trial of berberine hydrochloride in patients with diarrhea-predominant irritable bowel syndrome. Phytother. Res. 29 (11), 1822–1827. 10.1002/ptr.5475 [DOI] [PubMed] [Google Scholar]
- Chen D. L., Yang K. Y. (2017). Berberine alleviates oxidative stress in islets of diabetic mice by inhibiting miR-106b expression and up-regulating SIRT1. J. Cell Biochem. 118 (12), 4349–4357. 10.1002/jcb.26089 [DOI] [PubMed] [Google Scholar]
- Chen S., Tang Y., Gao Y., Nie K., Wang H., Su H., et al. (2022). Antidepressant potential of quercetin and its glycoside derivatives: a comprehensive review and update. Front. Pharmacol. 13, 865376. 10.3389/fphar.2022.865376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J., Dong S., Yi L., Geng D., Liu Q. (2018a). Magnolol abrogates chronic mild stress-induced depressive-like behaviors by inhibiting neuroinflammation and oxidative stress in the prefrontal cortex of mice. Int. Immunopharmacol. 59, 61–67. 10.1016/j.intimp.2018.03.031 [DOI] [PubMed] [Google Scholar]
- Cheng Y., Desse S., Martinez A., Worthen R. J., Jope R. S., Beurel E. (2018b). TNFα disrupts blood brain barrier integrity to maintain prolonged depressive-like behavior in mice. Brain Behav. Immun. 69, 556–567. 10.1016/j.bbi.2018.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi X., Wang S., Baloch Z., Zhang H., Li X., Zhang Z., et al. (2019). Research progress on classical traditional Chinese medicine formula Lily Bulb and Rehmannia Decoction in the treatment of depression. Biomed. Pharmacother. 112, 108616. 10.1016/j.biopha.2019.108616 [DOI] [PubMed] [Google Scholar]
- Chisholm D., Sweeny K., Sheehan P., Rasmussen B., Smit F., Cuijpers P., et al. (2016). Scaling-up treatment of depression and anxiety: a global return on investment analysis. Lancet Psychiatry 3 (5), 415–424. 10.1016/s2215-0366(16)30024-4 [DOI] [PubMed] [Google Scholar]
- Cipriani A., Furukawa T. A., Salanti G., Chaimani A., Atkinson L. Z., Ogawa Y., et al. (2018). Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet 391 (10128), 1357–1366. 10.1016/s0140-6736(17)32802-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colasanto M., Madigan S., Korczak D. J. (2020). Depression and inflammation among children and adolescents: a meta-analysis. J. Affect Disord. 277, 940–948. 10.1016/j.jad.2020.09.025 [DOI] [PubMed] [Google Scholar]
- Cryan J. F., O'Riordan K. J., Cowan C. S. M., Sandhu K. V., Bastiaanssen T. F. S., Boehme M., et al. (2019). The microbiota-gut-brain Axis. Physiol. Rev. 99 (4), 1877–2013. 10.1152/physrev.00018.2018 [DOI] [PubMed] [Google Scholar]
- Dabaghzadeh F., Sharififar F., Ahmadzadeh A. M., Karami-Mohajeri S. (2023). The effects of Berberis vulgaris L. root extract on the opiate withdrawal syndrome and psychological factors: a randomized double-blind clinical trial. J. Basic Clin. Physiol. Pharmacol. 34 (4), 465–472. 10.1515/jbcpp-2020-0327 [DOI] [PubMed] [Google Scholar]
- Du Y., Su H., Cao J., Wei Z., Wang Y., Yun K. (2020). Pharmacokinetic effects of l-tetrahydropalmatine on ketamine in rat plasma by ultraperformance liquid chromatography tandem mass spectrometry. Biomed. Res. Int. 2020, 9259683. 10.1155/2020/9259683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliwa H., Belzung C., Surget A. (2017). Adult hippocampal neurogenesis: is it the alpha and omega of antidepressant action? Biochem. Pharmacol. 141, 86–99. 10.1016/j.bcp.2017.08.005 [DOI] [PubMed] [Google Scholar]
- Erickson K. I., Miller D. L., Roecklein K. A. (2012). The aging hippocampus: interactions between exercise, depression, and BDNF. Neuroscientist 18 (1), 82–97. 10.1177/1073858410397054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan C., Li Y., Lan T., Wang W., Long Y., Yu S. Y. (2022). Microglia secrete miR-146a-5p-containing exosomes to regulate neurogenesis in depression. Mol. Ther. 30 (3), 1300–1314. 10.1016/j.ymthe.2021.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J., Li B., Ge T., Zhang Z., Lv J., Zhao J., et al. (2017). Berberine produces antidepressant-like effects in ovariectomized mice. Sci. Rep. 7 (1), 1310. 10.1038/s41598-017-01035-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y., Zhang J., Zhu S., He M., Ma S., Jia Q., et al. (2021). Berberine ameliorates ovariectomy-induced anxiety-like behaviors by enrichment in equol generating gut microbiota. Pharmacol. Res. 165, 105439. 10.1016/j.phrs.2021.105439 [DOI] [PubMed] [Google Scholar]
- Fesharaki-Zadeh A. (2022). Oxidative stress in traumatic brain injury. Int. J. Mol. Sci. 23 (21), 13000. 10.3390/ijms232113000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD2019 Diseases and Injuries Collaborators (2020). Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 396(10258), 1204–1222. 10.1016/s0140-6736(20)30925-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge P. Y., Qu S. Y., Ni S. J., Yao Z. Y., Qi Y. Y., Zhao X., et al. (2023). Berberine ameliorates depression-like behavior in CUMS mice by activating TPH1 and inhibiting Ido1-associated with tryptophan metabolism. Phytother. Res. 37 (1), 342–357. 10.1002/ptr.7616 [DOI] [PubMed] [Google Scholar]
- Gholizadeh N., Dalimi A., Ghaffarifar F., Nader-Mohammadi M., Molavi P., Dadkhah M., et al. (2023). Berberine improves inhibitory avoidance memory impairment of Toxoplasma gondii-infected rat model of ketamine-induced schizophrenia. BMC Complement. Med. Ther. 23 (1), 303. 10.1186/s12906-023-04107-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill H., Gill B., El-Halabi S., Chen-Li D., Lipsitz O., Rosenblat J. D., et al. (2020). Antidepressant medications and weight change: a narrative review. Obes. (Silver Spring) 28 (11), 2064–2072. 10.1002/oby.22969 [DOI] [PubMed] [Google Scholar]
- Gobinath A. R., Mahmoud R., Galea L. A. (2014). Influence of sex and stress exposure across the lifespan on endophenotypes of depression: focus on behavior, glucocorticoids, and hippocampus. Front. Neurosci. 8, 420. 10.3389/fnins.2014.00420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong H. Y., Zheng F., Zhang C., Chen X. Y., Liu J. J., Yue X. Q. (2016). Propofol protects hippocampal neurons from apoptosis in ischemic brain injury by increasing GLT-1 expression and inhibiting the activation of NMDAR via the JNK/Akt signaling pathway. Int. J. Mol. Med. 38 (3), 943–950. 10.3892/ijmm.2016.2663 [DOI] [PubMed] [Google Scholar]
- Gong Q., Yan X. J., Lei F., Wang M. L., He L. L., Luo Y. Y., et al. (2019). Proteomic profiling of the neurons in mice with depressive-like behavior induced by corticosterone and the regulation of berberine: pivotal sites of oxidative phosphorylation. Mol. Brain 12 (1), 118. 10.1186/s13041-019-0518-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haenisch B., Bönisch H. (2011). Depression and antidepressants: insights from knockout of dopamine, serotonin or noradrenaline re-uptake transporters. Pharmacol. Ther. 129 (3), 352–368. 10.1016/j.pharmthera.2010.12.002 [DOI] [PubMed] [Google Scholar]
- Haleem D. J. (2022). Glucocorticoids in the physiological and transcriptional regulation of 5-ht1a receptor and the pathogenesis of depression. Neuroscientist 28 (1), 59–68. 10.1177/1073858420975711 [DOI] [PubMed] [Google Scholar]
- Haleem D. J., Gul S. (2020). Circulating leptin, cortisol and gender differences associated with anorexia or obesity in depression. World J. Biol. Psychiatry 21 (3), 195–202. 10.1080/15622975.2019.1648870 [DOI] [PubMed] [Google Scholar]
- He M. C., Feng R., Wang J., Xia S. H., Wang Y. J., Zhang Y. (2022). Prevention and treatment of natural products from Traditional Chinese Medicine in depression: potential targets and mechanisms of action. Front. Aging Neurosci. 14, 950143. 10.3389/fnagi.2022.950143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M. C., Shi Z., Qin M., Sha N. N., Li Y., Liao D. F., et al. (2020). Muscone ameliorates LPS-induced depressive-like behaviors and inhibits neuroinflammation in prefrontal cortex of mice. Am. J. Chin. Med. 48 (3), 559–577. 10.1142/s0192415x20500287 [DOI] [PubMed] [Google Scholar]
- Herzog S., Galfalvy H., Keilp J. G., Mann J. J., Sublette M. E., Burke A., et al. (2023). Relationship of stress-reactive cortisol to suicidal intent of prior attempts in major depression. Psychiatry Res. 327, 115315. 10.1016/j.psychres.2023.115315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill C., Flyvbjerg A., Grønbaek H., Petrik J., Hill D. J., Thomas C. R., et al. (2000). The renal expression of transforming growth factor-beta isoforms and their receptors in acute and chronic experimental diabetes in rats. Endocrinology 141 (3), 1196–1208. 10.1210/endo.141.3.7359 [DOI] [PubMed] [Google Scholar]
- Hu W., Zhang Y., Wu W., Yin Y., Huang D., Wang Y., et al. (2016). Chronic glucocorticoids exposure enhances neurodegeneration in the frontal cortex and hippocampus via NLRP-1 inflammasome activation in male mice. Brain Behav. Immun. 52, 58–70. 10.1016/j.bbi.2015.09.019 [DOI] [PubMed] [Google Scholar]
- Hu Y., Ehli E. A., Hudziak J. J., Davies G. E. (2012). Berberine and evodiamine influence serotonin transporter (5-HTT) expression via the 5-HTT-linked polymorphic region. Pharmacogenomics J. 12 (5), 372–378. 10.1038/tpj.2011.24 [DOI] [PubMed] [Google Scholar]
- Huang M., He Y., Tian L., Yu L., Cheng Q., Li Z., et al. (2023). Gut microbiota-SCFAs-brain axis associated with the antidepressant activity of berberine in CUMS rats. J. Affect Disord. 325, 141–150. 10.1016/j.jad.2022.12.166 [DOI] [PubMed] [Google Scholar]
- Hussain T., Tan B., Yin Y., Blachier F., Tossou M. C., Rahu N. (2016). Oxidative stress and inflammation: what polyphenols can do for us? Oxid. Med. Cell Longev. 2016, 7432797. 10.1155/2016/7432797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iurescia S., Seripa D., Rinaldi M. (2016). Role of the 5-HTTLPR and SNP promoter polymorphisms on serotonin transporter gene expression: a closer look at genetic architecture and in vitro functional studies of common and uncommon allelic variants. Mol. Neurobiol. 53 (8), 5510–5526. 10.1007/s12035-015-9409-6 [DOI] [PubMed] [Google Scholar]
- Jamshaid F., Dai J., Yang L. X. (2020). New development of novel berberine derivatives against bacteria. Mini Rev. Med. Chem. 20 (8), 716–724. 10.2174/1389557520666200103115124 [DOI] [PubMed] [Google Scholar]
- Jha M. K., Mathew S. J. (2023). Pharmacotherapies for treatment-resistant depression: how antipsychotics fit in the rapidly evolving therapeutic landscape. Am. J. Psychiatry 180 (3), 190–199. 10.1176/appi.ajp.20230025 [DOI] [PubMed] [Google Scholar]
- Jiao Z., Zhao H., Huang W., Liang R., Liu Y., Li Z., et al. (2021). An investigation of the antidepressant-like effect of Jiaotaiwan in rats by nontargeted metabolomics based on ultra-high-performance liquid chromatography quadrupole time-of-flight mass spectrometry. J. Sep. Sci. 44 (2), 645–655. 10.1002/jssc.202000576 [DOI] [PubMed] [Google Scholar]
- Johnson B. M., Charman W. N., Porter C. J. (2002). An in vitro examination of the impact of polyethylene glycol 400, Pluronic P85, and vitamin E d-alpha-tocopheryl polyethylene glycol 1000 succinate on P-glycoprotein efflux and enterocyte-based metabolism in excised rat intestine. AAPS PharmSci 4 (4), E40. 10.1208/ps040440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang M., Noh J., Chung J. M. (2020). NMDA receptor-dependent long-term depression in the lateral habenula: implications in physiology and depression. Sci. Rep. 10 (1), 17921. 10.1038/s41598-020-74496-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller J., Gomez R., Williams G., Lembke A., Lazzeroni L., Murphy G. M., Jr., et al. (2017). HPA axis in major depression: cortisol, clinical symptomatology and genetic variation predict cognition. Mol. Psychiatry 22 (4), 527–536. 10.1038/mp.2016.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheir M. M., Wang Y., Hua L., Hu J., Li L., Lei F., et al. (2010). Acute toxicity of berberine and its correlation with the blood concentration in mice. Food Chem. Toxicol. 48 (4), 1105–1110. 10.1016/j.fct.2010.01.033 [DOI] [PubMed] [Google Scholar]
- Köhler C. A., Freitas T. H., Stubbs B., Maes M., Solmi M., Veronese N., et al. (2018). Peripheral alterations in cytokine and chemokine levels after antidepressant drug treatment for major depressive disorder: systematic review and meta-analysis. Mol. Neurobiol. 55 (5), 4195–4206. 10.1007/s12035-017-0632-1 [DOI] [PubMed] [Google Scholar]
- Kohli K., Mujtaba A., Malik R., Amin S., Alam M. S., Ali A., et al. (2021). Development of natural polysaccharide-based nanoparticles of berberine to enhance oral bioavailability: formulation, optimization, ex vivo, and in vivo assessment. Polym. (Basel) 13 (21), 3833. 10.3390/polym13213833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komoltsev I. G., Gulyaeva N. V. (2022). Brain trauma, glucocorticoids and neuroinflammation: dangerous liaisons for the Hippocampus. Biomedicines 10 (5), 1139. 10.3390/biomedicines10051139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V., Nestler E. J. (2008). The molecular neurobiology of depression. Nature 455 (7215), 894–902. 10.1038/nature07455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon M., Lim D. Y., Lee C. H., Jeon J. H., Choi M. K., Song I. S. (2020). Enhanced intestinal absorption and pharmacokinetic modulation of berberine and its metabolites through the inhibition of P-glycoprotein and intestinal metabolism in rats using a berberine mixed micelle formulation. Pharmaceutics 12 (9), 882. 10.3390/pharmaceutics12090882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B., Sur B., Yeom M., Shim I., Lee H., Hahm D. H. (2012). Effect of berberine on depression- and anxiety-like behaviors and activation of the noradrenergic system induced by development of morphine dependence in rats. Korean J. Physiol. Pharmacol. 16 (6), 379–386. 10.4196/kjpp.2012.16.6.379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Huang J., Cheng Y. C., Zhang Y. W. (2020a). Traditional Chinese medicine in depression treatment: from molecules to systems. Front. Pharmacol. 11, 586. 10.3389/fphar.2020.00586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Feng C., Fan C., Yang Y., Yang X., Lu H., et al. (2020b). Intervention of oncostatin M-driven mucosal inflammation by berberine exerts therapeutic property in chronic ulcerative colitis. Cell Death Dis. 11 (4), 271. 10.1038/s41419-020-2470-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao X. X., Hu K., Xie X. H., Wen Y. L., Wang R., Hu Z. W., et al. (2023). Banxia Xiexin decoction alleviates AS co-depression disease by regulating the gut microbiome-lipid metabolic axis. J. Ethnopharmacol. 313, 116468. 10.1016/j.jep.2023.116468 [DOI] [PubMed] [Google Scholar]
- Liu Y., Liu X., Zhang N., Yin M., Dong J., Zeng Q., et al. (2020). Berberine diminishes cancer cell PD-L1 expression and facilitates antitumor immunity via inhibiting the deubiquitination activity of CSN5. Acta Pharm. Sin. B 10 (12), 2299–2312. 10.1016/j.apsb.2020.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. M., Niu L., Wang L. L., Bai L., Fang X. Y., Li Y. C., et al. (2017). Berberine attenuates depressive-like behaviors by suppressing neuro-inflammation in stressed mice. Brain Res. Bull. 134, 220–227. 10.1016/j.brainresbull.2017.08.008 [DOI] [PubMed] [Google Scholar]
- Lu J., Li W., Gao T., Wang S., Fu C., Wang S. (2022). The association study of chemical compositions and their pharmacological effects of Cyperi Rhizoma (Xiangfu), a potential traditional Chinese medicine for treating depression. J. Ethnopharmacol. 287, 114962. 10.1016/j.jep.2021.114962 [DOI] [PubMed] [Google Scholar]
- Lu S.-f., Bao-ying W., Ming B., Er-ping X., Yu-cheng L. (2021). Effects of berberine on energy metabolism of hippocampus in depression mice. China J. Traditional Chin. Med. Pharm. 36 (06), 3580–3584. [Google Scholar]
- Lundberg J., Cars T., Lööv S., Söderling J., Sundström J., Tiihonen J., et al. (2023). Association of treatment-resistant depression with patient outcomes and health care Resource utilization in a population-wide study. JAMA Psychiatry 80 (2), 167–175. 10.1001/jamapsychiatry.2022.3860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu M., Fan G., Xiao G., Wang T., Xu D., Gao J., et al. (2021). Traditional Chinese medicine in COVID-19. Acta Pharm. Sin. B 11 (11), 3337–3363. 10.1016/j.apsb.2021.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X., Chen Z., Wang L., Wang G., Wang Z., Dong X., et al. (2018). The pathogenesis of diabetes mellitus by oxidative stress and inflammation: its inhibition by berberine. Front. Pharmacol. 9, 782. 10.3389/fphar.2018.00782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D., Liu J., Weiwei J., Qiang F., Shiping M. (2012). Effect of berberine on hippocampal tissue oxidative stress levels and BDNF expression in diabetic encephalopathy rats. Pharmacol. Clin. Chin. Materia Medica 28 (05), 39–41. 10.13412/j.cnki.zyyl.2012.05.018 [DOI] [Google Scholar]
- Maes M., Leonard B. E., Myint A. M., Kubera M., Verkerk R. (2011). The new '5-HT' hypothesis of depression: cell-mediated immune activation induces indoleamine 2,3-dioxygenase, which leads to lower plasma tryptophan and an increased synthesis of detrimental tryptophan catabolites (TRYCATs), both of which contribute to the onset of depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 35 (3), 702–721. 10.1016/j.pnpbp.2010.12.017 [DOI] [PubMed] [Google Scholar]
- Malhi G. S., Mann J. J. (2018). Depression. Lancet 392 (10161), 2299–2312. 10.1016/s0140-6736(18)31948-2 [DOI] [PubMed] [Google Scholar]
- McCarron R. M., Shapiro B., Rawles J., Luo J. (2021). Depression. Ann. Intern Med. 174 (5), Itc65–itc80. 10.7326/aitc202105180 [DOI] [PubMed] [Google Scholar]
- Meejuru G. F., Somavarapu A., Danduga R., Nissankara Roa L. S., Kola P. K. (2021). Protective effects of duloxetine against chronic immobilisation stress-induced anxiety, depression, cognitive impairment and neurodegeneration in mice. J. Pharm. Pharmacol. 73 (4), 522–534. 10.1093/jpp/rgaa003 [DOI] [PubMed] [Google Scholar]
- Mikulska J., Juszczyk G., Gawrońska-Grzywacz M., Herbet M. (2021). HPA Axis in the pathomechanism of depression and schizophrenia: new therapeutic strategies based on its participation. Brain Sci. 11 (10), 1298. 10.3390/brainsci11101298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. H., Raison C. L. (2016). The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 16 (1), 22–34. 10.1038/nri.2015.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra S. K., Hidau M. K., Rai S. (2021). Memantine treatment exerts an antidepressant-like effect by preventing hippocampal mitochondrial dysfunction and memory impairment via upregulation of CREB/BDNF signaling in the rat model of chronic unpredictable stress-induced depression. Neurochem. Int. 142, 104932. 10.1016/j.neuint.2020.104932 [DOI] [PubMed] [Google Scholar]
- Mo C., Wang L., Zhang J., Numazawa S., Tang H., Tang X., et al. (2014). The crosstalk between Nrf2 and AMPK signal pathways is important for the anti-inflammatory effect of berberine in LPS-stimulated macrophages and endotoxin-shocked mice. Antioxid. Redox Signal 20 (4), 574–588. 10.1089/ars.2012.5116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteggia L. M., Barrot M., Powell C. M., Berton O., Galanis V., Gemelli T., et al. (2004). Essential role of brain-derived neurotrophic factor in adult hippocampal function. Proc. Natl. Acad. Sci. U. S. A. 101 (29), 10827–10832. 10.1073/pnas.0402141101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira E. S., Ames-Sibin A. P., Bonetti C. I., Leal L. E., Peralta R. M., de Sá-Nakanishi A. B., et al. (2022). The short-term effects of berberine in the liver: narrow margins between benefits and toxicity. Toxicol. Lett. 368, 56–65. 10.1016/j.toxlet.2022.08.005 [DOI] [PubMed] [Google Scholar]
- Murakami T., Bodor E., Bodor N. (2023). Approaching strategy to increase the oral bioavailability of berberine, a quaternary ammonium isoquinoline alkaloid: Part 1. Physicochemical and pharmacokinetic properties. Expert Opin. Drug Metab. Toxicol. 19 (3), 129–137. 10.1080/17425255.2023.2203857 [DOI] [PubMed] [Google Scholar]
- Naz I., Masoud M. S., Chauhdary Z., Shah M. A., Panichayupakaranant P. (2022). Anti-inflammatory potential of berberine-rich extract via modulation of inflammation biomarkers. J. Food Biochem. 46 (12), e14389. 10.1111/jfbc.14389 [DOI] [PubMed] [Google Scholar]
- Nguyen L. T. H., Choi M. J., Shin H. M., Yang I. J. (2022). Coptisine alleviates imiquimod-induced psoriasis-like skin lesions and anxiety-like behavior in mice. Molecules 27 (4), 1412. 10.3390/molecules27041412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novakovic M. M., Korshunov K. S., Grant R. A., Martin M. E., Valencia H. A., Budinger G. R. S., et al. (2023). Astrocyte reactivity and inflammation-induced depression-like behaviors are regulated by Orai1 calcium channels. Nat. Commun. 14 (1), 5500. 10.1038/s41467-023-40968-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva V., Lippi M., Paci R., Del Fabro L., Delvecchio G., Brambilla P., et al. (2021). Gastrointestinal side effects associated with antidepressant treatments in patients with major depressive disorder: a systematic review and meta-analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry 109, 110266. 10.1016/j.pnpbp.2021.110266 [DOI] [PubMed] [Google Scholar]
- Peng W. H., Lo K. L., Lee Y. H., Hung T. H., Lin Y. C. (2007). Berberine produces antidepressant-like effects in the forced swim test and in the tail suspension test in mice. Life Sci. 81 (11), 933–938. 10.1016/j.lfs.2007.08.003 [DOI] [PubMed] [Google Scholar]
- Pittenger C., Duman R. S. (2008). Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology 33 (1), 88–109. 10.1038/sj.npp.1301574 [DOI] [PubMed] [Google Scholar]
- Popova V., Daly E. J., Trivedi M., Cooper K., Lane R., Lim P., et al. (2019). Efficacy and safety of flexibly dosed esketamine nasal spray combined with a newly initiated oral antidepressant in treatment-resistant depression: a randomized double-blind active-controlled study. Am. J. Psychiatry 176 (6), 428–438. 10.1176/appi.ajp.2019.19020172 [DOI] [PubMed] [Google Scholar]
- Qin Z., Shi D. D., Li W., Cheng D., Zhang Y. D., Zhang S., et al. (2023). Berberine ameliorates depression-like behaviors in mice via inhibiting NLRP3 inflammasome-mediated neuroinflammation and preventing neuroplasticity disruption. J. Neuroinflammation 20 (1), 54. 10.1186/s12974-023-02744-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu S. Y., Li X. Y., Heng X., Qi Y. Y., Ge P. Y., Ni S. J., et al. (2021). Analysis of antidepressant activity of huang-lian jie-du decoction through network pharmacology and metabolomics. Front. Pharmacol. 12, 619288. 10.3389/fphar.2021.619288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison C. L., Rutherford R. E., Woolwine B. J., Shuo C., Schettler P., Drake D. F., et al. (2013). A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry 70 (1), 31–41. 10.1001/2013.jamapsychiatry.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju M., Kunde S. S., Auti S. T., Kulkarni Y. A., Wairkar S. (2021). Berberine loaded nanostructured lipid carrier for Alzheimer's disease: design, statistical optimization and enhanced in vivo performance. Life Sci. 285, 119990. 10.1016/j.lfs.2021.119990 [DOI] [PubMed] [Google Scholar]
- Reif A., Bitter I., Buyze J., Cebulla K., Frey R., Fu D. J., et al. (2023). Esketamine nasal spray versus quetiapine for treatment-resistant depression. N. Engl. J. Med. 389 (14), 1298–1309. 10.1056/NEJMoa2304145 [DOI] [PubMed] [Google Scholar]
- Ross R. E., VanDerwerker C. J., Saladin M. E., Gregory C. M. (2023). The role of exercise in the treatment of depression: biological underpinnings and clinical outcomes. Mol. Psychiatry 28 (1), 298–328. 10.1038/s41380-022-01819-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothmore J. (2020). Antidepressant-induced sexual dysfunction. Med. J. Aust. 212 (7), 329–334. 10.5694/mja2.50522 [DOI] [PubMed] [Google Scholar]
- Sarandol A., Sarandol E., Eker S. S., Erdinc S., Vatansever E., Kirli S. (2007). Major depressive disorder is accompanied with oxidative stress: short-term antidepressant treatment does not alter oxidative-antioxidative systems. Hum. Psychopharmacol. 22 (2), 67–73. 10.1002/hup.829 [DOI] [PubMed] [Google Scholar]
- Shen J. D., Ma L. G., Hu C. Y., Pei Y. Y., Jin S. L., Fang X. Y., et al. (2016a). Berberine up-regulates the BDNF expression in hippocampus and attenuates corticosterone-induced depressive-like behavior in mice. Neurosci. Lett. 614, 77–82. 10.1016/j.neulet.2016.01.002 [DOI] [PubMed] [Google Scholar]
- Shen R., Kim J. J., Yao M., Elbayoumi T. A. (2016b). Development and evaluation of vitamin E d-α-tocopheryl polyethylene glycol 1000 succinate-mixed polymeric phospholipid micelles of berberine as an anticancer nanopharmaceutical. Int. J. Nanomedicine 11, 1687–1700. 10.2147/ijn.S103332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherkawy M. M., Abo-Youssef A. M., Salama A. A. A., Ismaiel I. E. (2018). Fluoxetine protects against OVA induced bronchial asthma and depression in rats. Eur. J. Pharmacol. 837, 25–32. 10.1016/j.ejphar.2018.08.026 [DOI] [PubMed] [Google Scholar]
- Short B., Fong J., Galvez V., Shelker W., Loo C. K. (2018). Side-effects associated with ketamine use in depression: a systematic review. Lancet Psychiatry 5 (1), 65–78. 10.1016/s2215-0366(17)30272-9 [DOI] [PubMed] [Google Scholar]
- Shulman K. I., Herrmann N., Walker S. E. (2013). Current place of monoamine oxidase inhibitors in the treatment of depression. CNS Drugs 27 (10), 789–797. 10.1007/s40263-013-0097-3 [DOI] [PubMed] [Google Scholar]
- Song D., Hao J., Fan D. (2020). Biological properties and clinical applications of berberine. Front. Med. 14 (5), 564–582. 10.1007/s11684-019-0724-6 [DOI] [PubMed] [Google Scholar]
- Strawbridge R., Javed R. R., Cave J., Jauhar S., Young A. H. (2023). The effects of reserpine on depression: a systematic review. J. Psychopharmacol. 37 (3), 248–260. 10.1177/02698811221115762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y., Wang H., Nie K., Gao Y., Su H., Wang Z., et al. (2022). Traditional herbal formula Jiao-tai-wan improves chronic restrain stress-induced depression-like behaviors in mice. Biomed. Pharmacother. 153, 113284. 10.1016/j.biopha.2022.113284 [DOI] [PubMed] [Google Scholar]
- Tao W., Su K., Huang Y., Lu Z., Wang Y., Yang L., et al. (2023). Zuojinwan ameliorates CUMS-induced depressive-like behavior through inducing ubiquitination of MyD88 via SPOP/MyD88/NF-κB pathway. J. Ethnopharmacol. 312, 116487. 10.1016/j.jep.2023.116487 [DOI] [PubMed] [Google Scholar]
- Tartt A. N., Mariani M. B., Hen R., Mann J. J., Boldrini M. (2022). Dysregulation of adult hippocampal neuroplasticity in major depression: pathogenesis and therapeutic implications. Mol. Psychiatry 27 (6), 2689–2699. 10.1038/s41380-022-01520-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson S. M. (2023). Plasticity of synapses and reward circuit function in the genesis and treatment of depression. Neuropsychopharmacology 48 (1), 90–103. 10.1038/s41386-022-01422-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A., Yang W., Yang X., Mei X., Hu T., Liang R., et al. (2020a). MgAl monolayer hydrotalcite increases the hypoglycemic effect of berberine by enhancing its oral bioavailability. Biomed. Pharmacother. 127, 110140. 10.1016/j.biopha.2020.110140 [DOI] [PubMed] [Google Scholar]
- Wang C. S., Kavalali E. T., Monteggia L. M. (2022a). BDNF signaling in context: from synaptic regulation to psychiatric disorders. Cell 185 (1), 62–76. 10.1016/j.cell.2021.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Jingyuan L., Xiaojuan Q. (2022b). Experimental study of the effect of berberine on behaviors of mice with reserpine-induced depression. China Med. Pharm. 12 (05), 23–26. [Google Scholar]
- Wang Q., Sun Y. N., Zou C. M., Zhang T. L., Li Z., Liu M., et al. (2022c). Regulation of the kynurenine/serotonin pathway by berberine and the underlying effect in the hippocampus of the chronic unpredictable mild stress mice. Behav. Brain Res. 422, 113764. 10.1016/j.bbr.2022.113764 [DOI] [PubMed] [Google Scholar]
- Wang Q. S., Ding S. L., Mao H. P., Cui Y. L., Qi X. J. (2013). Antidepressant-like effect of ethanol extract from Zuojin Pill, containing two herbal drugs of Rhizoma Coptidis and Fructus Evodiae, is explained by modulating the monoaminergic neurotransmitter system in mice. J. Ethnopharmacol. 148 (2), 603–609. 10.1016/j.jep.2013.05.011 [DOI] [PubMed] [Google Scholar]
- Wang S., Ren H., Zhong H., Zhao X., Li C., Ma J., et al. (2022d). Combined berberine and probiotic treatment as an effective regimen for improving postprandial hyperlipidemia in type 2 diabetes patients: a double blinded placebo controlled randomized study. Gut Microbes 14 (1), 2003176. 10.1080/19490976.2021.2003176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Yan Y. F., Yang L., Huang Y. Z., Duan X. H., Su K. H., et al. (2020b). Effects of Zuojin pill on depressive behavior and gastrointestinal function in rats with chronic unpredictable mild stress: role of the brain-gut axis. J. Ethnopharmacol. 254, 112713. 10.1016/j.jep.2020.112713 [DOI] [PubMed] [Google Scholar]
- Wang Y., Huang Y., Zhao M., Yang L., Su K., Wu H., et al. (2023). Zuojin pill improves chronic unpredictable stress-induced depression-like behavior and gastrointestinal dysfunction in mice via the theTPH2/5-HT pathway. Phytomedicine 120, 155067. 10.1016/j.phymed.2023.155067 [DOI] [PubMed] [Google Scholar]
- Wang Y., Tong Q., Ma S. R., Zhao Z. X., Pan L. B., Cong L., et al. (2021). Oral berberine improves brain dopa/dopamine levels to ameliorate Parkinson's disease by regulating gut microbiota. Signal Transduct. Target Ther. 6 (1), 77. 10.1038/s41392-020-00456-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Zhou X., Zhao D., Wang X., Gurley E. C., Liu R., et al. (2020c). Berberine inhibits free fatty acid and LPS-induced inflammation via modulating ER stress response in macrophages and hepatocytes. PLoS One 15 (5), e0232630. 10.1371/journal.pone.0232630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen C., Huang C., Yang M., Fan C., Li Q., Zhao J., et al. (2020). The secretion from bone marrow mesenchymal stem cells pretreated with berberine rescues neurons with oxidative damage through activation of the keap1-nrf2-HO-1 signaling pathway. Neurotox. Res. 38 (1), 59–73. 10.1007/s12640-020-00178-0 [DOI] [PubMed] [Google Scholar]
- World Health Organization (2022). Depression. [Google Scholar]
- Xie W., Su F., Wang G., Peng Z., Xu Y., Zhang Y., et al. (2022). Glucose-lowering effect of berberine on type 2 diabetes: a systematic review and meta-analysis. Front. Pharmacol. 13, 1015045. 10.3389/fphar.2022.1015045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D., Qiu C., Wang Y., Qiao T., Cui Y. L. (2021). Intranasal co-delivery of berberine and evodiamine by self-assembled thermosensitive in-situ hydrogels for improving depressive disorder. Int. J. Pharm. 603, 120667. 10.1016/j.ijpharm.2021.120667 [DOI] [PubMed] [Google Scholar]
- Xu F., Yang J., Meng B., Zheng J. W., Liao Q., Chen J. P., et al. (2018). The effect of berberine on ameliorating chronic inflammatory pain and depression. Zhonghua Yi Xue Za Zhi 98 (14), 1103–1108. 10.3760/cma.j.issn.0376-2491.2018.14.011 [DOI] [PubMed] [Google Scholar]
- Yang L., Huang Y., Chen F., Wang Y., Su K., Zhao M., et al. (2023). Berberine attenuates depression-like behavior by modulating the hippocampal NLRP3 ubiquitination signaling pathway through Trim65. Int. Immunopharmacol. 123, 110808. 10.1016/j.intimp.2023.110808 [DOI] [PubMed] [Google Scholar]
- Yang N., Zou L., Wang Y. (2022). Label-free quantitative proteomic analysis of reserpine-induced depression in mice intervened by berberine. Pak J. Pharm. Sci. 35 (1), 151–155. 10.36721/PJPS.2022.35.1.REG.151-155.1 [DOI] [PubMed] [Google Scholar]
- Yang Q. H., Hu S. P., Zhang Y. P., Xie W. N., Li N., Ji G. Y., et al. (2011). Effect of berberine on expressions of uncoupling protein-2 mRNA and protein in hepatic tissue of non-alcoholic fatty liver disease in rats. Chin. J. Integr. Med. 17 (3), 205–211. 10.1007/s11655-011-0668-4 [DOI] [PubMed] [Google Scholar]
- Yi L. T., Zhu J. X., Dong S. Q., Chen M., Li C. F. (2021). Berberine exerts antidepressant-like effects via regulating miR-34a-synaptotagmin1/Bcl-2 axis. Chin. Herb. Med. 13 (1), 116–123. 10.1016/j.chmed.2020.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y., Zhang G., Han T., Huang H. (2020). Analysis of the pharmacological mechanism of Banxia Xiexin decoction in treating depression and ulcerative colitis based on a biological network module. BMC Complement. Med. Ther. 20 (1), 199. 10.1186/s12906-020-02988-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W., Li D., Yu-qian C., Xiang-feng W., Jun C. (2021). Effect of berberine on depression and anxiety in 5XFAD mice. Neural Inj. Funct. Reconstr. 16 (09), 497–501. 10.16780/j.cnki.sjssgncj.20201283 [DOI] [Google Scholar]
- Zhan Y., Han J., Xia J., Wang X. (2021). Berberine suppresses mice depression behaviors and promotes hippocampal neurons growth through regulating the miR-34b-5p/miR-470-5p/BDNF Axis. Neuropsychiatr. Dis. Treat. 17, 613–626. 10.2147/ndt.S289444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Xue X., Pan J., Song X., Chang X., Mao Q., et al. (2021a). Integrated analysis of the chemical-material basis and molecular mechanisms for the classic herbal formula of Lily Bulb and Rehmannia Decoction in alleviating depression. Chin. Med. 16 (1), 107. 10.1186/s13020-021-00519-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H. N., Sun Y. J., He H. Q., Li H. Y., Xue Q. L., Liu Z. M., et al. (2018). Berberine promotes nerve regeneration through IGFR-mediated JNK-AKT signal pathway. Mol. Med. Rep. 18 (6), 5030–5036. 10.3892/mmr.2018.9508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. C., Yao W., Hashimoto K. (2016). Brain-derived neurotrophic factor (BDNF)-TrkB signaling in inflammation-related depression and potential therapeutic targets. Curr. Neuropharmacol. 14 (7), 721–731. 10.2174/1570159x14666160119094646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. H., Yang H. Z., Su H., Song J., Bai Y., Deng L., et al. (2021b). Berberine and ginsenoside Rb1 ameliorate depression-like behavior in diabetic rats. Am. J. Chin. Med. 49 (5), 1195–1213. 10.1142/s0192415x21500579 [DOI] [PubMed] [Google Scholar]
- Zhang W., Xu J. H., Yu T., Chen Q. K. (2019a). Effects of berberine and metformin on intestinal inflammation and gut microbiome composition in db/db mice. Biomed. Pharmacother. 118, 109131. 10.1016/j.biopha.2019.109131 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Guo L., Huang J., Sun Y., He F., Zloh M., et al. (2019b). Inhibitory effect of berberine on broiler P-glycoprotein expression and function: in situ and in vitro studies. Int. J. Mol. Sci. 20 (8), 1966. 10.3390/ijms20081966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Han L., Teopiz K. M., McIntyre R. S., Ma R., Cao B. (2022). The psychological factors mediating/moderating the association between childhood adversity and depression: a systematic review. Neurosci. Biobehav Rev. 137, 104663. 10.1016/j.neubiorev.2022.104663 [DOI] [PubMed] [Google Scholar]
- Zhao Y., Xu D., Wang J., Zhou D., Liu A., Sun Y., et al. (2023). The pharmacological mechanism of chaihu-jia-longgu-muli-tang for treating depression: integrated meta-analysis and network pharmacology analysis. Front. Pharmacol. 14, 1257617. 10.3389/fphar.2023.1257617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhe Q., Sulei W., Weiwei T., Hongyan L., Jianwei W. (2017). Effects of Jiaotaiwan on depressive-like behavior in mice after lipopolysaccharide administration. Metab. Brain Dis. 32 (2), 415–426. 10.1007/s11011-016-9925-8 [DOI] [PubMed] [Google Scholar]
- Zheng J. Y., Li X. X., Lin W. Y., Su S., Wu H. C., Hu R. D., et al. (2023). Huang-Lian-Jie-Du decoction alleviates depressive-like behaviors in dextran sulfate sodium-induced colitis mice via Trem2/Dap12 pathway. J. Ethnopharmacol. 315, 116658. 10.1016/j.jep.2023.116658 [DOI] [PubMed] [Google Scholar]
- Zhi D., Feng P. F., Sun J. L., Guo F., Zhang R., Zhao X., et al. (2015). The enhancement of cardiac toxicity by concomitant administration of Berberine and macrolides. Eur. J. Pharm. Sci. 76, 149–155. 10.1016/j.ejps.2015.05.009 [DOI] [PubMed] [Google Scholar]
- Zhu L., Gu P., Shen H. (2019). Protective effects of berberine hydrochloride on DSS-induced ulcerative colitis in rats. Int. Immunopharmacol. 68, 242–251. 10.1016/j.intimp.2018.12.036 [DOI] [PubMed] [Google Scholar]
- Zhu L., Han J., Yuan R., Xue L., Pang W. (2018). Berberine ameliorates diabetic nephropathy by inhibiting TLR4/NF-κB pathway. Biol. Res. 51 (1), 9. 10.1186/s40659-018-0157-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X., Sun Y., Zhang C., Liu H. (2017). Effects of berberine on a rat model of chronic stress and depression via gastrointestinal tract pathology and gastrointestinal flora profile assays. Mol. Med. Rep. 15 (5), 3161–3171. 10.3892/mmr.2017.6353 [DOI] [PubMed] [Google Scholar]