Abstract
Background
Pulmonary trichomoniasis is considered a neglected disease due to failures in recognizing it, stemming from insensitive microbial methods and a lack of specific clinical features. This study aims to analyze the clinical implications of trichomonads detected in bronchoalveolar lavage fluid (BALF) by metagenomic next-generation sequencing (mNGS).
Methods
This multicenter retrospective study included patients diagnosed with pneumonia, admitted to three tertiary hospitals in China from July 2018 to September 2022, with trichomonads detected in BALF through mNGS. The analysis covered demographics, comorbidities, symptoms, laboratory findings, mNGS results, clinical treatment, and outcomes of these patients.
Results
A total of 17 patients were enrolled, comprising 14 males and 3 females. Trichomonas tenax and Trichomonas vaginalis were detected by mNGS in BALF samples of 15 and 2 patients, respectively. Patients were categorized into two groups based on the presence of risk factors for trichomonad infection, including immunocompromised conditions, uncontrolled diabetes mellitus, oral/periodontal diseases, and aspiration. Among 11 patients with risk factors (Case 1-11), 4 received nitromidazoles as part of comprehensive treatment, achieving a 100% treatment success rate. The remaining 7 patients, who did not receive nitromidazoles, had only one achieving relief after broad-spectrum antimicrobial therapy, resulting in a 14.3% treatment success rate. For the 6 patients without any risk factors for trichomonad infection (Case 12-17), none received nitromidazoles during hospitalization. However, 4 out of these 6 patients (66.7%) eventually recovered.
Conclusion
mNGS proves to be an efficient tool for detecting trichomonads in BALF samples. Comprehensive analysis of clinical features and laboratory indicators is essential to distinguish between infection and colonization of trichomonads. Pulmonary trichomoniasis should not be overlooked when trichomonads are detected in BALF from patients with risk factors.
Keywords: pulmonary trichomoniasis, trichomonads, metagenomic next-generation sequencing (mNGS), Trichomonas tenax, trichomonas vaginalis
Background
Trichomonads are flagellated protozoan parasites often found in the digestive or reproductive systems of many invertebrates and vertebrates. There are four trichomonad species classically recognized as human parasites, including Trichomonas tenax (T. tenax) in the oral cavity, Dientamoeba fragilis and Pentatrichomonas hominis in the intestinal tract, and Trichomonas vaginalis (T. vaginalis) in the genitourinary tract (Maritz et al., 2014). Among them, T. vaginalis and T. tenax are considered human-specific. T. vaginalis is the causative agent of trichomoniasis, one of the most common sexually transmitted diseases worldwide (Mielczarek and Blaszkowska, 2016). T. tenax, an obligate symbiont in the human oral cavity which could be pathogenic in some contexts (Hirt, 2019), especially in patients with poor oral hygiene and advanced periodontal diseases. There is a clear connection between T. tenax protozoa and periodontitis disease. Previous studies and meta-analyses support that T. tenax is involved in the pathophysiology of periodontal diseases (Marty et al., 2017; Eslahi et al., 2021; Martin-Garcia et al., 2022), even though its individual contribution requires further exploration by more studies. Specifically, T. tenax has been identified by microscopic and molecular methods in the lower respiratory tract specimens (Mallat et al., 2004).
Pulmonary trichomoniasis, which is caused by infections with trichomonads in the lungs, has been previously reported in only a few cases with risk factors, including immunocompromised conditions, uncontrolled diabetes mellitus, oral/periodontal diseases and aspiration. Most of these cases were infected with either T. tenax (Hersh, 1985; Lewis et al., 2003; Mallat et al., 2004) or T. vaginalis (Temesvari et al., 2002; Duboucher et al., 2003; Carter and Whithaus, 2008). Wet mount microscopy is widely used and highly specific, but its sensitivity is low in vaginal specimens (Bandea et al., 2013; Leli et al., 2016). Conceivably, it is even more difficult to sensitively detect trichomonads in BALF, which is considered as a dilute form of the composite epithelial lining fluid. While polymerase chain reaction (PCR) assay has been proved to detect trichomonads in both vaginal and BALF specimens in a sensitive and specific manner (Heine et al., 1997; Shipitsyna et al., 2013; Lin et al., 2019), it is not routinely applied for patients with pneumonia, due to the poor accessibility to the PCR assay of trichomonads and a lack of knowledge and vigilance on pulmonary trichomoniasis at present. Therefore, it remains challenging to achieve sensitive and specific detection of trichomonads in BALF, further aggravating the unawareness of trichomonads in the lungs.
Metagenomic next-generation sequencing (mNGS) is a promising microbial detection technology in infectious diseases (Jiang et al., 2021; Chen et al., 2022). Based on high-throughput sequencing, mNGS detects microbial nucleic acids in a variety of specimens and identifies a wide range of pathogens by a single test. Compared with conventional methods, mNGS is unbiased, rapid and highly sensitive, making it a popular microbial test in pulmonary infectious diseases, especially for detecting rare or novel pathogens. However, the utility of mNGS for the detection of trichomonads has not been reported so far.
In this study, we conducted a retrospective summary of the clinical characteristics, treatment processes, and outcomes of patients in whom trichomonads were detected in bronchoalveolar lavage fluid (BALF) using metagenomic next-generation sequencing (mNGS). Our objective was to analyze the potential clinical implications of trichomonad presence. The primary aim of this study is to propose feasible strategies for clinical management when trichomonads are identified in BALF. Additionally, we aim to raise awareness among clinical physicians regarding the potential occurrence of trichomonads, either alone or as coinfecting pathogens, in the context of pulmonary diseases.
Methods
Study design and subjects
In this retrospective multicenter study, we sequentially screened patients admitted to three tertiary hospitals in China—Xiangya Hospital, Zhuzhou Second Hospital, and Second Xiangya Hospital—between July 1, 2018, and September 30, 2022. A total of 1,680 patients underwent screening, among whom 17 individuals with trichomonads detected in BALF via mNGS were included. Patients were eligible for enrollment if they met the following criteria: (1) age ≥18 years old; (2) radiological evidence indicative of newly emerged pneumonia on chest computed tomography; (3) detection of trichomonads in BALF samples by mNGS. The study was approved by the Institutional Review Board and Ethics Committee of Xiangya Hospital, Central South University (202305381). The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because this is a retrospective study and all research data were de-identified and anonymously analyzed.
Data regarding demographics, comorbidities, symptoms, laboratory and radiological findings, mNGS results, clinical treatment, and outcomes were extracted from electronic medical records. Lymphopenia was defined as lymphocyte count less than 1.1 × 109/L in peripheral blood (Warny et al., 2018). According to the manufacturers’ instructions, the normal ranges of C-reactive protein, procalcitonin and lactate dehydrogenase were defined as 0-8 mg/L, 0-0.1 ng/mL and 120-250 U/L, respectively.
Given that trichomonads are opportunistic pathogens, risk factors for trichomonad infection at baseline were defined based on published literature on pulmonary trichomoniasis (Leterrier et al., 2012; Lopez-Escamilla et al., 2013; Dybicz et al., 2018; Dong et al., 2019): (1) Primary or secondary immunocompromised conditions (Ramirez et al., 2020), including but not limited to hematologic malignancies, solid tumors, rheumatic diseases, long-term systemic use of corticosteroids, use of immunosuppressive agents, solid organ transplantation and hematopoietic stem cell transplantation. (2) Uncontrolled diabetes mellitus. (3) Presence of oral/periodontal diseases and risk factors for aspiration, including sedative use, drug abuse, alcohol consumption, dementia, traumatic brain injury, stroke, seizures, and any therapeutic or diagnostic procedures involving the upper thorax or esophagus. The third risk factor specifically pertains to T. tenax infection in the lungs, given T. tenax’s residence in the human oral cavity.
Sample processing and DNA extraction for mNGS
BALF samples were processed as described in a previous study of our group (Jiang et al., 2021). Briefly, BALF was mixed with lysozyme and 1 g of 0.5-mm glass beads, and then the mixture was attached to a horizontal platform on a vortex mixer and agitated vigorously at 2800–3200 rpm for 30 min. For nucleic acid extraction, 300 mL of supernatant was transferred to a 1.5-mL centrifuge tube. Subsequently, DNA was extracted using the TIANamp Micro DNA kit (Tiangen Biotech) according to standard procedures.
DNA library preparation and sequencing for mNGS
The DNA library was constructed by DNA fragmentation, end repair and PCR amplification using MGIEasy Cell-free DNA Library Prep Set (MGI Tech). Agilent 2100 (Agilent Technologies) and Qubit 2.0 (Invitrogen) were used as library quality control. The double-stranded DNA library was converted into single-stranded circular DNA using DNA degradation and circularization. The DNA Nanoballs were generated by rolling circle amplification technology. Qualified DNA Nanoballs were loaded on the chip and then performed 20 M 50-bp single-end sequencing on the MGISEQ-2000 sequencing platform (BGI Genomics) (Jeon et al., 2014).
Bioinformatic analysis for mNGS
High-quality sequencing data were generated by removing low-quality reads, followed by computational subtraction of human host sequences mapped to the human reference genome (hg19) using Burrows-Wheeler Alignment (Li and Durbin, 2010; Schmieder and Edwards, 2011). The remaining high-quality data were classified by simultaneously aligning to Pathogens metagenomics Database (PMDB) to obtain the specific number of reads for a particular microbial species, including bacteria, fungi, viruses and parasites. The classification reference databases were downloaded from NCBI (ftp://ftp.ncbi.nlm.nih.gov/genomes/). RefSeq contains the whole genome sequence of 4,945 viruses, 6,350 bacteria, 1064 fungi related to human infections, and 234 parasites associated with human diseases. The coverage ratio and depth of each microorganism were calculated using BEDTools (Quinlan and Hall, 2010). Clinically significant microbes detected by mNGS were determined based on a comprehensive analysis of the read number, coverage ratio, relative abundance, as well as the clinical, laboratory, and radiological characteristics. The sequencing data presented in the study are deposited in the CNGB Sequence Archive (CNSA) of China National GeneBank DataBase (CNGBdb) repository(https://db.cngb.org/cnsa/), accession number CNP0005055.
PCR assay for the detection of trichomonads
PCR assay was performed to confirm the existence of trichomonads in BALF according to the standardized protocol. Briefly, the DNA lysates were extracted and purified from BALF samples that were positive for trichomonads identified by mNGS by using QIAamp DNA Mini Kit (QIAGEN, Hilden, Germany) according to manufacturer’s instructions. Two pairs of primers targeting a conserved region of either T. vaginalis or T. tenax with good sensitivity and specificity were synthesized and utilized according to published literatures (Kikuta et al., 1997; Bandea et al., 2013). The sequences were as follows: T. vaginalis Former primer 5’-CGAATGGTATAACGAATGCGAC-3’, and T. vaginalis Reverse primer 5’-CAACCTTTCTTGTCAGACAACTTG-3’, with product size of 237 bases (NW_001820796.1:258918-259154 Trichomonas vaginalis G3 1047229024264 genomic scaffold); T. tenax Former primer 5’-AGTTCCATCGATGCCATTC-3’, and T. tenax Reverse primer 5’-GCATCTAAGGACTTAGACG-3’, with product size of 776 bases in 18S ribosomal RNA region. All primer pairs generated fragments of correct size and specificity as verified by DNA sequencing. For PCR amplification, one nanogram of DNA template was mixed with 22 μL of Super PCR Mix (BGI, Beijing, China) and 1 μL of each primer. The steps of PCR were described as follows: initial denaturation for 5 minutes at 96°C, 35 cycles of 20 seconds at 96°C for denaturation, 30 seconds at 52°C for annealing, 30 seconds at 72°C for elongation, a final elongation step for 10 minutes at 72°C. Agarose gel electrophoresis was then performed to assess PCR product yield. Subsequently, PCR products were purified by magnetic beads-based system (BGI, Beijing, China) according to the manufacturer’s instruction. Purified DNA fragments were further confirmed by DNA sequencing.
Results
Baseline clinical characteristics
A total of 17 patients were enrolled in this study, comprising 14 males and 3 females. As depicted in Table 1 , their ages ranged from 29 to 81 years, with 7 patients having a history of current or former smoking. Five patients reported prior oral or periodontal diseases. The most prevalent comorbidities were high blood pressure and diabetes mellitus. Four patients had a history of using immunosuppressive agents before the disease onset. The commonly reported symptoms included fever, cough, and dyspnea. Patients exhibited an oxygenation index ranging from 73 to 250, indicating severe hypoxemia. Elevated white blood cell counts were observed in 6 out of 17 patients. Lymphopenia was frequently noted, whereas increased eosinophils in peripheral blood were seen in only one patient. Elevated levels of C-reactive protein, procalcitonin, and lactate dehydrogenase were found in 100%, 88.2%, and 76.5% of patients, respectively. All patients displayed radiological abnormalities in bilateral lungs with multifocal lesions. In summary, these patients did not exhibit distinctive symptoms, laboratory, or radiological findings. This suggests difficulty in distinguishing pulmonary trichomoniasis from pneumonia caused by other pathogens based solely on clinical features.
Table 1.
Baseline clinical characteristics of patients.
| Case No. | Gender | Age (years) | Current or former smoker | Oral or periodontal diseases | Comorbidities | Use of immunosuppressive agents | Main symptoms | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 63 | No | Yes | Follicular lymphoma | Chemotherapy | Fever, cough and dyspnea | |||||
| 2 | F | 29 | No | Yes | Kidney transplantation | Corticosteroids and Tacrolimus | Fever and dyspnea | |||||
| 3 | F | 50 | No | No | AML | Chemotherapy | Fever, dyspnea and fatigue | |||||
| 4 | M | 64 | Yes | Yes | DM, HBP | No | Fever, cough and dyspnea | |||||
| 5 | M | 65 | No | No | DM, HBP, CAD | No | Fever, cough and dyspnea | |||||
| 6 | M | 72 | Yes | No | DM, COPD, HBP | No | Cough and dyspnea | |||||
| 7 | M | 69 | No | No | DM, HBP | No | Fever and dyspnea | |||||
| 8 | F | 68 | No | No | DM, HBP, CAD | No | Fever, cough and dyspnea | |||||
| 9 | M | 74 | Yes | Yes | Lung cancer, COPD | Anti-PD-1 monoclonal antibody | Fever and dyspnea | |||||
| 10 | M | 45 | No | Yes | Cerebral hemorrhage | No | Fever and dyspnea | |||||
| 11 | M | 57 | No | No | DM, HBP, CAD | No | Cough and dyspnea | |||||
| 12 | M | 81 | No | No | HBP | No | Fever | |||||
| 13 | M | 70 | Yes | No | Chronic bronchitis | No | Cough and dyspnea | |||||
| 14 | M | 49 | No | No | HBP, CVD | No | Fever and cough | |||||
| 15 | M | 53 | Yes | No | HBP, CVD | No | Fever | |||||
| 16 | M | 75 | Yes | No | CAD | No | Cough and dyspnea | |||||
| 17 | M | 74 | Yes | No | COPD | No | Fever, cough and dyspnea | |||||
F, female; M, male; AML, acute myeloid leukemia; DM, diabetes mellitus; HBP, high blood pressure; CVD, cerebrovascular disease; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; PD-1, programmed cell death protein 1.
Table 1.
(continued).
| Case No. | Oxyge-nation index | WBC (×109/L) |
Neutrophils (×109/L) |
Lymphocytes (×109/L) |
Eosinophils (×109/L) |
CRP (mg/L) |
Procalcitonin (ng/mL) |
LDH (U/L) |
Radio-logical abnormalities | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bilateral lungs | Multifocal lesions | |||||||||||||||
| 1 | 250 | 4.2 | 3.24 | 0.2 | <0.01 | 145 | 4.30 | 410 | Yes | Yes | ||||||
| 2 | 188 | 8.89 | 7.83 | 0.25 | 0.08 | 114 | 8.83 | 358 | Yes | Yes | ||||||
| 3 | 150 | 0.44 | 0.28 | 0.1 | 0.05 | 226 | 1.25 | 580 | Yes | Yes | ||||||
| 4 | 138 | 9.7 | 9.0 | 0.4 | <0.01 | 337 | 1.52 | 194 | Yes | Yes | ||||||
| 5 | 78 | 16.34 | 15.62 | 0.39 | <0.01 | 347 | 24.37 | 373 | Yes | Yes | ||||||
| 6 | 167 | 18.56 | 17.37 | 0.87 | 0.02 | 55 | 0.14 | 280 | Yes | Yes | ||||||
| 7 | 316 | 5.64 | 4.9 | 0.46 | <0.01 | 28 | 0.17 | 382 | Yes | Yes | ||||||
| 8 | 120 | 5.93 | 4.23 | 0.7 | 0.80 | 30 | 0.51 | 178 | Yes | Yes | ||||||
| 9 | 73 | 8 | 6.4 | 0.6 | 0.31 | 177 | 0.21 | 308 | Yes | Yes | ||||||
| 10 | 210 | 11 | 8.61 | 1.28 | <0.01 | 79 | 0.51 | 454 | Yes | Yes | ||||||
| 11 | 90 | 16.19 | 14.4 | 1.3 | <0.01 | 20 | 1.12 | 2504 | Yes | Yes | ||||||
| 12 | 147 | 7.97 | 6.79 | 0.33 | <0.01 | 35 | 1.97 | 238 | Yes | Yes | ||||||
| 13 | 248 | 2.9 | 2.2 | 0.4 | 0.01 | 115 | 2.17 | 412 | Yes | Yes | ||||||
| 14 | 208 | 11.9 | 9.7 | 1.6 | 0.09 | 89 | 0.11 | 192 | Yes | Yes | ||||||
| 15 | 350 | 8.47 | 6.91 | 1.11 | 0.06 | 14 | 0.09 | 336 | Yes | Yes | ||||||
| 16 | 90 | 4.21 | 2.82 | 0.74 | 0.27 | 43 | <0.05 | 293 | Yes | Yes | ||||||
| 17 | 120 | 19.2 | 17.28 | 0.86 | <0.01 | 118 | 3.12 | 760 | Yes | Yes | ||||||
WBC, white blood cell; CRP, C-reactive protein; LDH, lactate dehydrogenase.
Microbiological findings and clinical implications
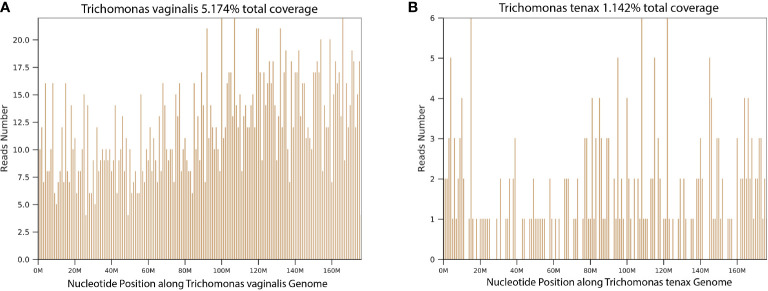
In this cohort, T. tenax and T. vaginalis were detected in BALF samples of 15 and 2 patients, respectively. The coverage of T. vaginalis and T. tenax in the metagenomic next-generation sequencing (mNGS) results of two representative cases is illustrated in Figure 1 , indicating the reliable detection of trichomonads. Co-pathogens identified in BALF included bacteria (10 patients), fungi (4 patients), and viruses (6 patients). Notably, two out of 17 patients (Case 4 and 17) had trichomonads as the sole microbes detected in BALF by mNGS. PCR confirmation using the same BALF samples was conducted, and all showed positive detections of T. tenax or T. vaginalis. The total number of reads, number of reads mapped on reference genomes, number of reads mapped on the human genomes and specific number of reads at the species level are all listed in Supplementary Table 1 .
Figure 1.
The coverage of Trichomonads vaginalis and Trichomonads tenax in the mNGS results. (A) Trichomonads vaginalis (Case 1); (B) Trichomonads tenax (Case 4).
As indicated in Table 2 , patients were categorized into two groups based on the presence of risk factors for trichomonad infection, as described in the methods. Among the 11 patients with risk factors (Case 1-11), 4 had immunocompromised conditions, 6 had uncontrolled diabetes mellitus, and 1 had aspiration due to cerebral hemorrhage. Four out of the 11 patients received nitromidazoles as either the first or second-line treatment, all experiencing relief, resulting in a 100% treatment success rate. Strikingly, among the 7 patients with risk factors who did not receive nitromidazoles, only one achieved relief despite receiving broad-spectrum and targeted antibiotic therapy based on mNGS and other microbial test results, leading to a low treatment success rate of 14.3%. Among patients without risk factors for trichomonad infection (Case 12-17), none received nitromidazoles during hospitalization, and 4 out of 6 (66.7%) eventually experienced relief.
Table 2.
Microbiological findings and clinical outcomes of patients.
| Case No. | Trichomonads species | Other microbes identified by mNGS | PCR confirmation | With risk factors | Efficacy of initial antibiotic treatment | Use of nitromidazoles | Clinical outcomes |
|---|---|---|---|---|---|---|---|
| 1 | T. vaginalis | P. jirovecii, HSV1 | Positive | Yes | Failed | Second line | Relieved |
| 2 | T. tenax | S. pneumoniae, H. influenzae, S.aureus | Positive | Yes | Failed | Second line | Relieved |
| 3 | T. vaginalis | H. parainfluenzae, C. albicans, HSV1 | Positive | Yes | Failed | Second line | Relieved |
| 4 | T. tenax | None | Positive | Yes | Succeeded | First line | Relieved |
| 5 | T. tenax | L. pneumophila, Tropheryma whipplei | Positive | Yes | Succeeded | No | Relieved |
| 6 | T. tenax | A. fumigatus | Positive | Yes | Failed | No | Death |
| 7 | T. tenax | Stenotrophomonas maltophilia | Positive | Yes | Failed | No | Death |
| 8 | T. tenax | Burkholderia cepacia | Positive | Yes | Failed | No | Death |
| 9 | T. tenax | A. baumanii, Tropheryma whipplei | Positive | Yes | Failed | No | Death |
| 10 | T. tenax | A. baumanii, K.pneumoniae | Positive | Yes | Failed | No | Death |
| 11 | T. tenax | Candida albicans, HSV1 | Positive | Yes | Failed | No | Death |
| 12 | T. tenax | K.pneumoniae | Positive | No | Failed | No | Relieved |
| 13 | T. tenax | K.pneumoniae, EBV | Positive | No | Failed | No | Relieved |
| 14 | T. tenax | A. baumanii, S.aureus | Positive | No | Succeeded | No | Relieved |
| 15 | T. tenax | Human herpesvirus 7 | Positive | No | Succeeded | No | Relieved |
| 16 | T. tenax | P. jirovecii, HSV1 | Positive | No | Failed | No | Death |
| 17 | T. tenax | None | Positive | No | Failed | No | Death |
T. tenax, Trichomonas tenax; T. vaginalis, Trichomonas vaginalis; mNGS, metagenomic next-generation sequencing; PCR, polymerase chain reaction; IMV, invasive mechanical ventilation; H. parainfluenzae, Haemophilus parainfluenzae; C. albicans, Candida albicans; HSV1, Herpes simplex virus 1; S. pneumoniae, Streptococcus pneumoniae; H. influenzae, Haemophilus influenza; S.aureus, Staphylococcus aureus; K.pneumoniae, Klebsiella pneumoniae; EBV, Epstein-Barr Virus; A. baumanii, Acinetobacter baumannii; L. pneumophila, Legionella pneumophila; A. fumigatus, Aspergillus fumigatus.
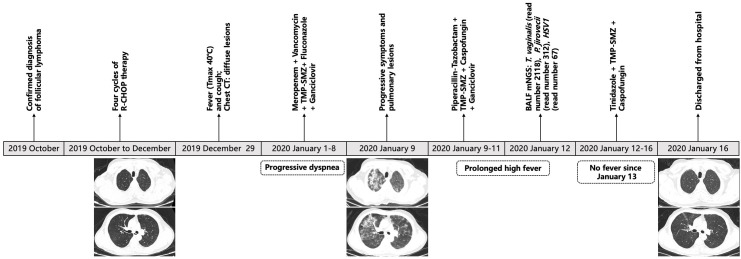
Description of case 1
The course of illness of Case 1 was shown in Figure 2 . This 63-year-old man was diagnosed with follicular lymphoma in October 2019 and received 4 cycles of R-CHOP therapy, a standard combination chemotherapy for non-Hodgkin lymphoma, from October to December 2019. A chest tomography on December 20, 2019, showed no apparent abnormality in the bilateral lungs. On January 1, 2020, the patient began to present with fever, cough, and dyspnea, and his chest tomography showed diffuse pulmonary lesions. After comprehensive and strong antimicrobial therapy with a combination of meropenem, vancomycin, trimethoprim-sulfamethoxazole, fluconazole, and ganciclovir, his symptoms still deteriorated. Therefore, the patient was admitted to our hospital on January 9, 2020. He had a periodontal disease but did not report any history of oral sex. Piperacillin-tazobactam, trimethoprim-sulfamethoxazole, caspofungin, and ganciclovir were empirically used for 72 hours. However, his symptoms of high fever and dyspnea did not relieve. A bronchoscopic examination was performed, and the mNGS result revealed T. vaginalis (specific read number 2118), Pneumocystis jirovecii (specific read number 312), Herpes simplex virus 1 (specific read number 67) in the BALF sample on January 12. Subsequently, tinidazole was given against the infection of T. vaginalis, and trimethoprim-sulfamethoxazole combined with caspofungin were continued against Pneumocystis jirovecii. His body temperature fell to the normal range within 48 hours. On January 16, chest tomography showed that pulmonary infiltrates were completely absorbed, and then the patient was discharged.
Figure 2.
The disease course of a patient (Case 1) who was diagnosed with pulmonary trichomoniasis. R-CHOP, a combination chemotherapy including rituximab, cyclophosphamide, doxorubicin hydrochloride (hydroxydaunorubicin), vincristine sulfate (Oncovin), and prednisone; Tmax, maximum body temperature; TMP-SMZ, trimethoprim-sulfamethoxazole; mNGS, metagenomic next-generation sequencing; HSV1, Herpes simplex virus 1.
Discussion
The prevalence of pulmonary trichomoniasis remains uncertain, primarily attributed to the challenge of recognizing this disease. This difficulty arises from insensitive microbial methods and the absence of specific clinical features. Recently, the identification of T. tenax and T. vaginalis in BALF by mNGS has garnered attention, yet its clinical implications remain unclear. In this retrospective multicenter study, our data suggests that trichomonad infection should be considered in patients with identified risk factors. Consequently, we provide practical suggestions for cases where trichomonads are detected in BALF by mNGS. These findings contribute to enhancing awareness and understanding of trichomoniasis in the context of pulmonary diseases.
Over the last two decades, various trichomonad species have been identified in human lungs through immunological and molecular methods, including T. tenax (Duboucher et al., 2003), T. vaginalis (Duboucher et al., 2003; Mallat et al., 2004), and Pentatrichomonas hominis (Jongwutiwes et al., 2000), which suggests that human trichomonad infections may not be restricted to a specific anatomic site (Maritz et al., 2014). Trichomonads can also invade the pleura and cause empyema (Lewis et al., 2003; Mantini et al., 2009). However, the epidemiology, pathophysiology, clinical features, and management options of pulmonary trichomoniasis remain largely unclear (Duboucher et al., 2008; Martínez-Girón et al., 2008). Due to a lack of recognition of this disease, the possibility of trichomonad infection in human lungs has often been ignored among patients with suspected pneumonia. Exclusive reliance on empirical antibiotic treatment does not cover trichomonads, leading to a worse prognosis in patients with pulmonary trichomoniasis. Therefore, an efficient microbial method for detecting trichomonads is urgently needed in clinical practice.
Currently, the most common approach for detecting trichomonads in hospitals is through microscopic examination of staining smears. However, its low sensitivity is attributed to numerous factors (Dos Santos et al., 2017). The immobility of trichomonads due to low temperature or extended periods in vitro, the similarity in movement between many epithelial cells of lung alveoli and trichomonads, and the ability of trichomonads to develop into an unrecognizable amoeboid form contribute to this low sensitivity (Duboucher et al., 2007a; Li and Gao, 2016). BALF samples are considered diluted, further reducing the sensitivity of trichomonad detection. PCR emerges as an alternative effective tool due to its high sensitivity and specificity (Lin et al., 2019). Although the advantages of PCR have been confirmed through direct comparisons in both vaginal and BALF samples (Heine et al., 1997; Lawing et al., 2000; Lin et al., 2019), routine use of PCR assays targeting trichomonads in pneumonia patients is impractical for physicians due to a lack of awareness and limited knowledge related to pulmonary trichomoniasis. Moreover, PCR assays for trichomonads are unavailable at most hospitals. In our present study, the data indicate that mNGS enables efficient detection of trichomonads in BALF samples. Its advantages, including being untargeted and high-throughput, make it a valuable tool, and the results can be validated by PCR assays. The application of mNGS provides crucial guidance for antimicrobial treatment. Recently, metatranscriptomics emerges as a valuable and complementary approach to metagenomics in studying infectious diseases. It analyzes the total RNA content of a sample, revealing which genes are actively being transcribed by the microbial community. Therefore, metatranscriptomics offers insights into the gene expression profiles of microorganisms within a sample, providing information about actively transcribing genes. This can be particularly relevant in understanding the dynamics of microbial activity, such as during active infections (Adekoya et al., 2023; Wang et al., 2023).
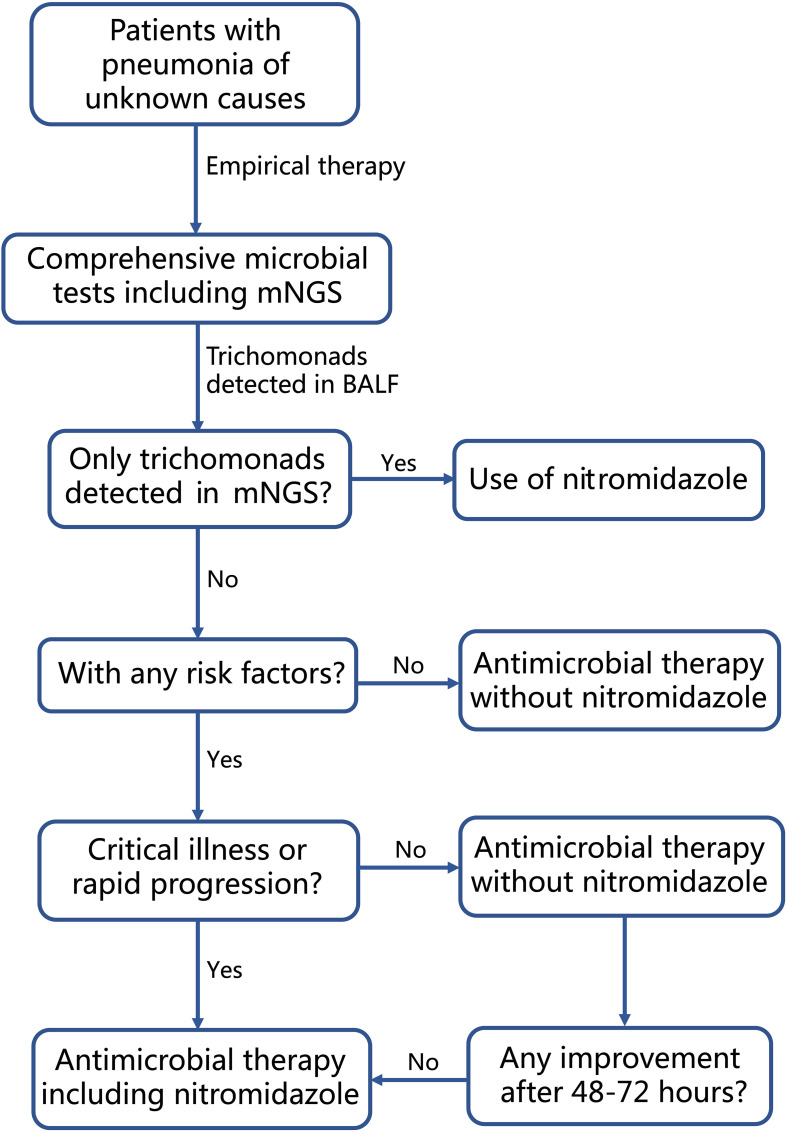
When advanced molecular methods, such as mNGS and PCR, are employed to detect trichomonads in respiratory tract specimens, a crucial question arises: how to distinguish infection from contamination and colonization? In this study, T. tenax and T. vaginalis were detected in BALF samples of 15 and 2 patients, respectively. As a common obligate symbiont in the human mouth, especially in patients with poor oral hygiene, the presence of T. tenax in the human respiratory tract may be attributed to the aspiration of the parasite from the oral cavity, potentially not leading to pulmonary infections. Hence, oral or periodontal diseases and aspiration could be considered risk factors for T. tenax infection. Previous literature has indicated that various degrees of immunosuppression, uncontrolled diabetes mellitus, dysphagia and alcohol abuse are common comorbidities in patients with trichomonad infections, including T. tenax and T. vaginalis (Duboucher et al., 2007b; Leterrier et al., 2012; Lopez-Escamilla et al., 2013; Dybicz et al., 2018). Consequently, we categorized the 17 patients into two groups based on the presence or absence of these risk factors. Among the 11 patients with risk factors (4 with immunocompromised conditions, 6 with uncontrolled diabetes mellitus, 5 with oral or periodontal diseases, and 1 with aspiration due to cerebral hemorrhage), the administration of nitromidazoles correlated with better outcomes, resulting in a higher treatment success rate (4/4 vs. 1/7). In contrast, among the 6 patients without risk factors, none received nitromidazoles, yet 4 showed relief. This suggests that T. tenax detected in these patients could be either colonization or contamination during bronchoscopy. This observation reinforces the identification of risk factors for trichomonad infection and supports the use of nitromidazoles as salvage therapy when the first-line treatment fails in patients with these risk factors and trichomonads detected in BALF. For critically ill patients with rapidly progressive pneumonia, we propose the inclusion of nitromidazoles as a component of the first-line therapy to prevent delayed treatment against trichomonads. In cases where trichomonads are the sole microbes detected in BALF by mNGS, as seen in Case 4 and Case 17 in our cohort, the use of nitromidazoles is recommended. Based on our data and clinical experience, we developed a clear guiding strategy flowchart for the clinical management of patients with trichomonads detected in BALF, as shown in Figure 3 .
Figure 3.
Flow-chart for clinical management of patients diagnosed with pneumonia and having trichomonads detected in BALF by mNGS.
It is noteworthy that two patients (Case 1 and 3) had T. vaginalis detected in BALF samples in this study. According to existing knowledge, T. vaginalis should not exist in healthy human lungs, except for its identification in the pharynx and lower respiratory tract of neonates born to mothers with T. vaginalis infection and adults with a history of urogenital contact (Salvador-Membreve et al., 2014). Both Case 1 and 3 were immunocompromised patients with hematological malignancies, received nitromidazoles, and eventually experienced relief. Although the invasion pathway and pathogenicity of T. vaginalis remain unclear, our data suggest that close attention should be paid when T. vaginalis is detected in BALF samples.
The co-occurrence of trichomonads and Pneumocystis jirovecii in the lungs is a compelling area of research, especially in immunocompromised individuals. While both pathogens can independently affect the respiratory system, their potential interaction within the lung environment has attracted attention in clinical settings. Research has indicated that trichomonads are frequently found in the course of human Pneumocystis jiroveci pneumonia (Duboucher et al., 2007b). The hypothesis is that local alveolar conditions, rather than immunodepression, are the main factors favoring the development of trichomonads (Duboucher et al., 2008). However, in our present study, only two cases (Case 1 and 16) showed trichomonads and Pneumocystis jirovecii simultaneously detected in BALF samples. Furthermore, a previous study conducted by our team showed that trichomonads were barely detected in BALF from 60 patients with Pneumocystis jirovecii pneumonia (Jiang et al., 2021). Therefore, the precise relationship between trichomonads infections in the lungs and co-infection with Pneumocystis jirovecii remains relatively understudied and not well understood. Further epidemiological studies are needed to understand the prevalence, risk factors, and clinical outcomes associated with the co-infection of trichomonads and Pneumocystis jirovecii in immunocompromised individuals.
The present study has limitations. Firstly, it is a retrospective study, and only a small number of patients are included due to the low detection rate of trichomonads in BALF. Because of its retrospective nature, microscopic examination of staining smears was not performed to further confirm the presence of trichomonads. Multicenter studies with a larger sample size are required to confirm our findings and provide physicians with more comprehensive guidance when dealing with trichomonads in BALF. Secondly, there are no widely accepted diagnostic criteria for pulmonary trichomoniasis so far, and physicians must recognize that trichomonads detected in BALF by mNGS cannot definitively indicate infection. A comprehensive analysis of clinical, laboratory, and radiological characteristics, as well as the response to empirical antimicrobial treatment, is necessary for the diagnosis of pulmonary trichomoniasis. In this study, several samples have very low number of reads mapping onto Trichomonas genomes (such as Case 12 and 14), which suggests the possibility of contamination either in the sample or on the sequencing platform. Therefore, accurate interpretation of mNGS results is crucial when clinical physicians use this technology.
Conclusion
The prevalence, characteristics, and impact of trichomonads in the lungs deserve much more attention. While mNGS is an effective tool for detecting trichomonads in BALF of patients with pneumonia, it cannot distinguish between infection, colonization, and contamination. Trichomonad infection should be considered if detected in BALF samples of patients with risk factors. When broad-spectrum antibiotics targeting other coexisting pathogens fail, nitroimidazoles are recommended against trichomonads, which could potentially improve prognosis. Further investigations are required to understand the significance of the pulmonary settlement of trichomonads, their real incidence, invasion pathway, and pathogenicity.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://db.cngb.org/cnsa/, CNP0005055, CNR1065172, CNR1065173, CNR1065174, CNR1065187, CNR1065188, CNR1065189, CNR1065190, CNR1065191, CNR1065192, CNR1065193, CNR1065194, CNR1065195, CNR1065196, CNR1065197, CNR1065198, CNR1065199, CNR1065200.
Ethics statement
The studies involving humans were approved by the Institutional Review Board and Ethics Committee of Central South University. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because this is a retrospective study and all research data were de-identified and anonymously analyzed.
Author contributions
JJ: Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Writing – original draft. YL: Conceptualization, Data curation, Investigation, Validation, Writing – review & editing. QW: Investigation, Resources, Writing – review & editing. HZ: Investigation, Resources, Writing – review & editing. WY: Investigation, Writing – review & editing. YW: Investigation, Writing – review & editing. WP: Investigation, Writing – review & editing. PP: Funding acquisition, Supervision, Writing – review & editing. CH: Funding acquisition, Supervision, Writing – review & editing. PD: Conceptualization, Data curation, Formal Analysis, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft.
Acknowledgments
The authors acknowledge the professionalism and compassion demonstrated by all the healthcare workers involved in patient care. The authors also appreciate BGI Genomics and Genskey Medicine for their technical assistance in the current study.
Funding Statement
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by grants from Respiratory and Critical Care Medicine Department - National Key Clinical Specialty Construction Project (Z047-02), National Multidisciplinary Cooperative Diagnosis and Treatment Capacity Building Project for Major Diseases (Lung Cancer, z027002), National Natural Science Foundation of China (82100099), and Natural Science Foundation of Hunan Province (2022JJ40792).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2024.1289231/full#supplementary-material
References
- Adekoya A. E., Kargbo H. A., Ibberson C. B. (2023). Defining microbial community functions in chronic human infection with metatranscriptomics. mSystems 8 (6), e0059323. doi: 10.1128/msystems.00593-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandea C. I., Joseph K., Secor E. W., Jones L. A., Igietseme J. U., Sautter R. L., et al. (2013). Development of PCR assays for detection of Trichomonas vaginalis in urine specimens. J. Clin. Microbiol. 51, 1298–1300. doi: 10.1128/JCM.03101-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter J. E., Whithaus K. C. (2008). Neonatal respiratory tract involvement by Trichomonas vaginalis: a case report and review of the literature. Am. J. Trop. Med. Hygiene. 78, 17–19. doi: 10.4269/ajtmh.2008.78.17 [DOI] [PubMed] [Google Scholar]
- Chen S., Kang Y., Li D., Li Z. (2022). Diagnostic performance of metagenomic next-generation sequencing for the detection of pathogens in bronchoalveolar lavage fluid in patients with pulmonary infections: Systematic review and meta-analysis. Int. J. Infect. Dis. 122, 867–873. doi: 10.1016/j.ijid.2022.07.054 [DOI] [PubMed] [Google Scholar]
- Dong N., Jin X., Huang J., Chen K., Li Y., Chen C., et al. (2019). Tetratrichomonas in pyopneumothorax. Am. J. Emergency Med. 37, 1215. e1211–1215. e1214. doi: 10.1016/j.ajem.2019.03.029 [DOI] [PubMed] [Google Scholar]
- Dos Santos C. S., De Jesus V. L. T., Mcintosh D., Carreiro C. C., Batista L. C. O., Do Bomfim Lopes B., et al. (2017). Morphological, ultrastructural, and molecular characterization of intestinal tetratrichomonads isolated from non-human primates in southeastern Brazil. Parasitol. Res. 116, 2479–2488. doi: 10.1007/s00436-017-5552-5 [DOI] [PubMed] [Google Scholar]
- Duboucher C., Barbier C., Beltramini A., Rona M., Ricome J.-L., Morel G., et al. (2007. a). Pulmonary superinfection by trichomonads in the course of acute respiratory distress syndrome. Lung 185, 295–301. doi: 10.1007/s00408-007-9022-1 [DOI] [PubMed] [Google Scholar]
- Duboucher C., Boggia R., Morel G., Capron M., Pierce R. J., Dei-Cas E., et al. (2007. b). Pneumocystis pneumonia: immunosuppression, Pneumocystis jirovecii … and the third man. Nat. Rev. Microbiol. 5, 967–967. doi: 10.1038/nrmicro1621-c1 [DOI] [PubMed] [Google Scholar]
- Duboucher C., Noël C., Durand-Joly I., Gerbod D., Delgado-Viscogliosi P., Jouveshomme S., et al. (2003). Pulmonary coinfection by Trichomonas vaginalis and Pneumocystis sp. as a novel manifestation of AIDS. Hum. Pathol. 34, 508–511. doi: 10.1016/S0046-8177(03)00088-1 [DOI] [PubMed] [Google Scholar]
- Duboucher C., Pierce R. J., Capron M., Dei-Cas E., Viscogliosi E. (2008). Recent advances in pulmonary trichomonosis. Trends Parasitol. 24, 201–202. doi: 10.1016/j.pt.2008.02.005 [DOI] [PubMed] [Google Scholar]
- Dybicz M., Perkowski K., Sedzikowska A., Baltaza W., Chomicz L. (2018). Studies on prevalence of infection with Trichomonas tenax identified by molecular techniques-in respect to oral health of patients with various systemic disease requiring immunosuppressive therapy. Ann. Parasitol. 64, 193–197. doi: 10.17420/ap6403.150 [DOI] [PubMed] [Google Scholar]
- Eslahi A. V., Olfatifar M., Abdoli A., Houshmand E., Johkool M. G., Zarabadipour M., et al. (2021). The neglected role of trichomonas tenax in oral diseases: A systematic review and meta-analysis. Acta Parasitol. 66, 715–732. doi: 10.1007/s11686-021-00340-4 [DOI] [PubMed] [Google Scholar]
- Heine R. P., Wiesenfeld H. C., Sweet R. L., Witkin S. S. (1997). Polymerase chain reaction analysis of distal vaginal specimens: a less invasive strategy for detection of Trichomonas vaginalis. Clin. Infect. Dis. 24, 985–987. doi: 10.1093/clinids/24.5.985 [DOI] [PubMed] [Google Scholar]
- Hersh S. (1985). Pulmonary trichomoniasis and Trichomonas tenax. J. Med. Microbiol. 20, 1–10. doi: 10.1099/00222615-20-1-1 [DOI] [PubMed] [Google Scholar]
- Hirt R. P. (2019). Mucosal microbial parasites/symbionts in health and disease: an integrative overview. Parasitology 146, 1109–1115. doi: 10.1017/S0031182019000647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon Y. J., Zhou Y., Li Y., Guo Q., Chen J., Quan S., et al. (2014). The feasibility study of non-invasive fetal trisomy 18 and 21 detection with semiconductor sequencing platform. PloS One 9, e110240. doi: 10.1371/journal.pone.0110240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J., Bai L., Yang W., Peng W., An J., Wu Y., et al. (2021). Metagenomic next-generation sequencing for the diagnosis of pneumocystis jirovecii pneumonia in non-HIV-infected patients: A retrospective study. Infect. Dis. Ther. 10, 1733–1745. doi: 10.1007/s40121-021-00482-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongwutiwes S., Silachamroon U., Putaporntip C. (2000). Pentatrichomonas hominis in empyema thoracis. Trans. R. Soc. Trop. Med. Hygiene. 94, 185–186. doi: 10.1016/s0035-9203(00)90270-0 [DOI] [PubMed] [Google Scholar]
- Kikuta N., Yamamoto A., Fukura K., Goto N. (1997). Specific and sensitive detection of Trichomonas tenax by the polymerase chain reaction. Lett Appl Microbiol 24, 193–197. doi: 10.1046/j.1472-765x.1997.00379.x [DOI] [PubMed] [Google Scholar]
- Lawing L. F., Hedges S. R., Schwebke J. R. (2000). Detection of trichomonosis in vaginal and urine specimens from women by culture and PCR. J. Clin. Microbiol. 38, 3585–3588. doi: 10.1128/JCM.38.10.3585-3588.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leli C., Castronari R., Levorato L., Luciano E., Pistoni E., Perito S., et al. (2016). Molecular sensitivity threshold of wet mount and an immunochromatographic assay evaluated by quantitative real-time PCR for diagnosis of Trichomonas vaginalis infection in a low-risk population of childbearing women. Le Infezioni. Med. 24, 112–116. [PubMed] [Google Scholar]
- Leterrier M., Morio F., Renard B., Poirier A.-S., Miegeville M., Chambreuil G. (2012). Trichomonads in pleural effusion: case report, literature review and utility of PCR for species identification. Microbiologica-Quarterly. J. Microbiol. Sci. 35, 83. [PubMed] [Google Scholar]
- Lewis K. L., Doherty D. E., Ribes J., Seabolt J. P., Bensadoun E. S. (2003). Empyema caused by trichomonas. Chest 123, 291–292. doi: 10.1378/chest.123.1.291 [DOI] [PubMed] [Google Scholar]
- Li H., Durbin R. (2010). Fast and accurate long-read alignment with burrows-wheeler transform. Bioinformatics 26, 589–595. doi: 10.1093/bioinformatics/btp698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Gao Z. C. (2016). Lophomonas blattarum infection or just the movement of ciliated epithelial cells? Chin. Med. J. (Engl) 129, 739–742. doi: 10.4103/0366-6999.178025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C., Ying F., Lai Y., Li X., Xue X., Zhou T., et al. (2019). Use of nested PCR for the detection of trichomonads in bronchoalveolar lavage fluid. BMC Infect. Dis. 19, 512. doi: 10.1186/s12879-019-4118-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Escamilla E., Sanchez-Aguillon F., Alatorre-Fernandez C. P., Aguilar-Zapata D., Arroyo-Escalante S., Arellano T., et al. (2013). New Tetratrichomonas species in two patients with pleural empyema. J. Clin. Microbiol. 51, 3143–3146. doi: 10.1128/JCM.01056-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallat H., Podglajen I., Lavarde V., Mainardi J. L., Frappier J., Cornet M. (2004). Molecular characterization of Trichomonas tenax causing pulmonary infection. J. Clin. Microbiol. 42, 3886–3887. doi: 10.1128/JCM.42.8.3886-3887.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantini C., Souppart L., Noël C., Duong T. H., Mornet M., Carroger G., et al. (2009). Molecular characterization of a new Tetratrichomonas species in a patient with empyema. J. Clin. Microbiol. 47, 2336–2339. doi: 10.1128/JCM.00353-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maritz J. M., Land K. M., Carlton J. M., Hirt R. P. (2014). What is the importance of zoonotic trichomonads for human health? Trends Parasitol. 30, 333–341. doi: 10.1016/j.pt.2014.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Garcia D. F., Sallam M., Garcia G., Santi-Rocca J. (2022). Parasites in periodontal health and disease: A systematic review and meta-analysis. Adv. Exp. Med. Biol. 1373, 95–111. doi: 10.1007/978-3-030-96881-6_5 [DOI] [PubMed] [Google Scholar]
- Martínez-Girón R., Esteban J., Ribas A., Doganci L. (2008). Protozoa in respiratory pathology: a review. Eur. Respir. J. 32, 1354–1370. doi: 10.1183/09031936.00022008 [DOI] [PubMed] [Google Scholar]
- Marty M., Lemaitre M., Kémoun P., Morrier J. J., Monsarrat P. (2017). Trichomonas tenax and periodontal diseases: a concise review. Parasitology 144, 1417–1425. doi: 10.1017/S0031182017000701 [DOI] [PubMed] [Google Scholar]
- Mielczarek E., Blaszkowska J. (2016). Trichomonas vaginalis: pathogenicity and potential role in human reproductive failure. Infection 44, 447–458. doi: 10.1007/s15010-015-0860-0 [DOI] [PubMed] [Google Scholar]
- Quinlan A. R., Hall I. M. (2010). BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26, 841–842. doi: 10.1093/bioinformatics/btq033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez J. A., Musher D. M., Evans S. E., Cruz C. D., Crothers K. A., Hage C. A., et al. (2020). Treatment of community-acquired pneumonia in immunocompromised adults: a consensus statement regarding initial strategies. Chest 158, 1896–1911. doi: 10.1016/j.chest.2020.05.598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvador-Membreve D. M. C., Jacinto S. D., Rivera W. L. (2014). Trichomonas vaginalis induces cytopathic effect on human lung alveolar basal carcinoma epithelial cell line A549. Exp. Parasitol. 147, 33–40. doi: 10.1016/j.exppara.2014.10.003 [DOI] [PubMed] [Google Scholar]
- Schmieder R., Edwards R. (2011). Quality control and preprocessing of metagenomic datasets. Bioinformatics 27, 863–864. doi: 10.1093/bioinformatics/btr026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipitsyna E., Zolotoverkhaya E., Chen C., Chi K., Grigoryev A., Savicheva A., et al. (2013). Evaluation of polymerase chain reaction assays for the diagnosis of Trichomonas vaginalis infection in Russia. J. Eur. Acad. Dermatol. Venereol. 27, e217–e223. doi: 10.1111/j.1468-3083.2012.04593.x [DOI] [PubMed] [Google Scholar]
- Temesvari P., Kerekes A., Tege A., Szarka K. (2002). Demonstration of Trichomonas vaginalis in tracheal aspirates in infants with early respiratory failure. J. Maternal-Fetal. Neonatal. Med. 11, 347–349. doi: 10.1080/jmf.11.5.347.349 [DOI] [PubMed] [Google Scholar]
- Wang L., Cao J. B., Xia B. B., Li Y. J., Zhang X., Mo G. X., et al. (2023). Metatranscriptome of human lung microbial communities in a cohort of mechanically ventilated COVID-19 Omicron patients. Signal Transduct. Target. Ther. 8, 432. doi: 10.1038/s41392-023-01684-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warny M., Helby J., Nordestgaard B. G., Birgens H., Bojesen S. E. (2018). Lymphopenia and risk of infection and infection-related death in 98,344 individuals from a prospective Danish population-based study. PloS Med. 15, e1002685. doi: 10.1371/journal.pmed.1002685 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://db.cngb.org/cnsa/, CNP0005055, CNR1065172, CNR1065173, CNR1065174, CNR1065187, CNR1065188, CNR1065189, CNR1065190, CNR1065191, CNR1065192, CNR1065193, CNR1065194, CNR1065195, CNR1065196, CNR1065197, CNR1065198, CNR1065199, CNR1065200.