Abstract
Background
External parasites, particularly ticks and fleas, are among the most common problems affecting dogs. Chemical medicines are commonly used to prevent and eliminate such external parasites, but their improper use can cause adverse reactions, and the toxins they contain may remain in the environment.
Objectives
The objective of this study was to investigate the in vitro efficacy of Zanthoxylum limonella, citronella, clove, peppermint, and ginger essential oils against dog ticks and fleas and to test the sensitivity of dogs’ skin to these essential oils.
Methods
The five essential oils were tested for in vitro efficacy against ticks and fleas, and the two most effective essential oils were then tested on the dogs’ skin.
Results
The results revealed that these five essential oils at 16% concentrations effectively inhibited the spawning of female engorged ticks. In addition, all five essential oils had a strong ability to kill tick larvae at concentrations of 2% upward. Furthermore, 4% concentrations of the five essential oils quickly eliminated fleas, especially clove oil, which killed 100% of fleas within 1 h. A 50%, 90%, and 99% lethal concentration (LC50, LC90, and LC99) for the essential oils on tick larvae in 24 h were found to be low values. LC50, LC90, and LC99 for the essential oils on flea in 1 h was lowest values. Clove oil at 16% concentration was the most satisfactory essential oil for application on dogs’ skin, with a low percentage of adverse effects.
Conclusions
This study confirmed the effectiveness of essential oils for practical use as tick and flea repellents and eliminators. Essential-oil-based pharmaceutical can replace chemical pesticides and provide benefits for both consumers and the environment.
Keywords: Essential oil, pharmaceutics, ticks, fleas, ectoparasites infestations, dogs
INTRODUCTION
Pet companions especially dogs are important family members, but external parasites, particularly ticks and fleas, are among the most common problems affecting dogs. They can cause allergic skin problems, septicaemia, and even death [1]. Furthermore, ticks and fleas are vectors of zoonotic diseases that can be transmitted to humans, such as Query fever, Rocky Mountain spotted fever, Ehrlichosis, and Lyme disease [2]. There are several ticks genera that can be found in pets such as genera Amblyomma, Dermacentor, Ixodes and Rhipicephalus [3]. Furthermore, there are several flea species that can be found in pets such as Ctenocephalides felis, Ctenocephalides canis, Archaeopsylla erinacei, Pulex irritans, Ceratophyllus gallinae [4]. However, tick species Rhipicephalus sanguineus tick species and the Ctenocephalides felis flea species (cat flea) are the most common pests affecting dogs and cats [4,5,6]. The entire life cycle of ticks (Rhipicephalus sanguineus) occurs on dog hosts [7] and be completed in 70-126 days under normal environmental conditions and host availability [8], whereas the life cycle of fleas mostly occurs outside dogs [9]. The female fleas can produce 40-50 eggs per day and average of 1,350 eggs over a 50 day period [9]. Flea eggs are laid on the host and then drop from host and can spread into the environment. The entire flea life cycle takes 20–30 days [10]. Chemical pesticides, administered topically, orally, by injection, or through bathing/soaking, are usually used to eliminate ticks and fleas [11]. However, the misuse or excessive administration of these pesticides can poison dogs, and the toxins they contain may remain in the environment.
Herb extracts are alternative treatments for preventing and eradicating many external parasites in animals. For example, the citronella species Cymbopogon nardus (L.) Rendle proved effective for controlling bovine ticks [12]. Additionally, a study conducted by [13] found that Cymbopogon nardus (L.) Rendle was effective against Amblyomma cajennense and Anocentor niten larvae. Study of [14] found that the most abundant constituents of citronella were citronellal, citronellol, and geraniol. Likewise, Mentha piperita (peppermint) effectively controlled internal parasites in rabbits and chickens in British Columbia, Canada [15]. The chemically characterised peppermint was rich in pulegone, menthol, carvone 1, 8-cineole, limonene, and β-caryophyllene [16]. In addition, the Zanthoxylum genus, including Z. caribaeum Lamarck, at a concentration of 5% effectively eliminated the cattle ticks species Rhipicephalus microplus [17]. The active ingredient of the essential oil of the clove species Syzygium aromaticum (eugenol) proved to be effective against Rhipicephalus microplus larvae and adult ticks [18]. In addition, the essential oil of the basil species Hyptis suaveolens was effective against Ixodes ricinus larvae [19].
The Zanthoxylum limonella, citronella, clove, peppermint, and ginger essential oils, which are extracted from locally grown herbs, may provide an alternative means of eliminating external parasites on dogs and be safe for animals, people, and the environment. The objective of this study was to investigate the in vitro efficacy of these five essential oils against dog ticks and fleas and to test the sensitivity of dogs’ skin.
MATERIALS AND METHODS
Essential oils
Z. limonella, citronella, clove, peppermint, and ginger are grown in northern Thailand. These Thai herbs were grown naturally for this study and collected in 2020. These were screened for contaminants, cut into roughly 1–3 inch pieces, and then weighed and the weights recorded. All herbs were transferred to extract the essential oil. The herbs were distilled with water until the first drop of essential oil appeared, and distillation continued thereafter for 5 h. The essential oil layer was separated from the water layer by adding anhydrous sodium sulphate to the water. The percentage yields (% yields) of the essential oils were calculated by weight and stored in a sealed container protected from light. They were kept in a refrigerator at 2–8°C.
In vitro insecticide test of essential oils against ticks
Adult immersion test
Seven-hundred-and-twenty engorged female ticks were collected from untreated dogs, placed in Petri dishes, washed immediately, and kept dry. Twelve engorged female ticks were used for each replication of the adult immersion test, and the total tick weight was 0.50–0.59 g per replication. Thereafter, each batch of engorged ticks was immersed in one of the Z. limonella, citronella, clove, peppermint, and ginger essential oils at 2%, 4%, 8%, and 16% concentrations, respectively, and a negative control solution (essential oil solvent-ethyl alcohol 95%) for 5 min. After 5 min, removed the ticks from the essential oils that had been soaked and placed in a petri dish lined with filter paper. Each exposure was repeated three times with batches of essential oils. Survival rates were counted at 30 min; 1, 2, 4, 6, 12, and 24 h; and 2–15 days after exposure to the essential oils. On day 15, eggs were weighed to calculate the reproductive index (RI) and inhibit the ovulation percentage (IO%) [20]:
Larvae immersion test
Eighty engorged female ticks were collected from untreated dogs and incubated under laboratory conditions at 27 ± 1.5°C and 70–80% relative humidity (RH) to encourage egg laying. Tick eggs were weighed at 0.01 g (200 eggs) after laying within 12 h and placed in a 6 × 6 cm2 envelope made of filter paper. Larva immersion tests were conducted after ticks were hatched from the eggs. Each packet of tick larvae was impregnated with one of the five essential oils (Z. limonella, citronella, clove, peppermint, and ginger) at concentrations of 2%, 4%, 8%, and 16%, respectively, and with a negative control solution for 5 min. Each exposure was repeated three times for each essential oil. The mortality rate of larvae was then calculated at 24 h [20].
In vitro insecticide test of essential oils against fleas
Seven-hundred and twenty fleas (Ctenocephalides spp.) were collected gently by hand from untreated dogs or cats within 12 h. This test was modified following the study of [21]. Twelve fleas per replication were placed in clear plastic tubes with lids containing small holes. Strips of filter paper (1 × 10 cm2) were impregnated with each essential oil at concentrations of 0.5%, 1%, 2%, and 4%, respectively, and with control solution 1 (essential oil solvent-ethyl alcohol 95%) and control solution 2 (filter paper only). Then, each strip was transferred to a clear plastic tube containing 12 fleas. The motility rates were investigated at 10 and 30 min and at 1, 2, 6, 12, 24, and 48 h after exposure to the essential oils and the control solution and filter paper.
Dogs’ skin allergy test
Sixty dogs were randomly selected to test their skin for sensitivity to essential oils, calculated using the G*power programme at an effect size of 0.6, α err prob of 0.05, and a power of (1 − β err prob). The following criteria were used to select the dogs: 1) ticks found on the body, especially in the armpit area; 2) not treated with flea and tick prevention or elimination products for at least 1 month; 3) not treated with steroids or antihistamines for at least 2 weeks; 4) in good health with no skin disease; 5) aged between 2 months and 10 years; 6) weight at least 1 kg; 7) the owner’s or caretaker’s consent for the experiment; and 8) from the Chiang Mai area. The dogs were divided into 15 dogs per treatment group for 4 treatments: Treatment 1, 16% Z. limonella oil; Treatment 2, 16% clove oil; Treatment 3, negative control group (essential oil solvent-ethyl alcohol 95%); Treatment 4, positive control (Bayticol; Bayer, Germany). Dogs were sprayed 10 times on a 10 × 20 cm2 armpit area for each treatment. Approval for the study was granted by the Maejo University Animal Care and Use Committee (MACUC; approval No. MACUC023A/2561).
Statistical analysis
Differences in the means for 1) the inhibition of ovulation of engorged female ticks, 2) the mortality rate of tick larva within 24 h, and 3) the mortality rate of fleas within 1 h of exposure to one of the five essential oils at each concentration were analysed with a one-way analysis of variance followed by Tukey’s test using R Studio. A value of p < 0.05 was considered significant. Survivor analysis was used to compare insect mortality over time using the Kaplan–Meier method. A 50%, 90%, or 99% lethal concentration (LC) of tick and flea was done by probit analysis. To calculate LC50, LC90, and LC99 by probit analysis, the concentration obtained from definitive test were converted into log concentration and corrected % [22]. The 0 and 100% of mortality were corrected before the determination of probit as followed:
| For 0% mortality = 100(0.25/n) |
| For 100% mortality = 100(n − 0.25/n) |
n is number of ticks or flea used in the experiment. The probit value of correct % mortality were obtained from Finney’s table. Furthermore, descriptive statistics were used to summarise the data on dogs’ allergic reactions and behaviour after exposing them to the products.
RESULTS
In vitro insecticide test of essential oils against ticks
Adult immersion test
There were no significant differences in the ability of the Z. limonella, citronella, clove, and peppermint essential oils (p > 0.05) to inhibit spawning in female engorged ticks. However, the spawning of female engorged ticks was more effectively inhibited by the Z. limonella, clove, and peppermint essential oils than by the ginger oil (p > 0.05). Additionally, there were no significant differences between citronella and ginger oil in inhibiting spawning in female engorged ticks (Table 1).
Table 1. IO% for female engorged ticks when tested with five essential oils at different concentrations.
| Essential oil | IO% | |||
|---|---|---|---|---|
| 16% | 8% | 4% | 2% | |
| Zanthoxylum limonella | 98.77 ± 1.41a,A | 51.81 ± 32.13A | 40.03 ± 2.51A | 5.09 ± 61.22B |
| Citronella | 90.71 ± 12.84a,b | 31.97 ± 43.35 | 28.79 ± 19.41 | −117.07 ± 264.92 |
| Clove | 98.17 ± 3.17a,A | 40.61 ± 15.59A | 32.38 ± 32.19A | 3.57 ± 44.06B |
| Peppermint | 91.43 ± 3.85a,A | 27.14 ± 22.06A,C | 11.98 ± 20.34B,C | 1.69 ± 241.13B,C |
| Ginger | 66.53 ± 23.93b,A | 8.87 ± 7.91A,C | 12.73 ± 29.37A,C | 2.33 ± 17.68B,C |
IO%, inhibit the ovulation percentage.
a,bThese letters within columns indicate statistical differences (p < 0.05) in the ability of the essential oils to inhibit ovulation.
A,B,CThese letters within rows indicate a statistical difference (p < 0.05).
Larvae immersion test
Furthermore, all five essential oils had a strong ability to kill tick larvae at concentrations of 2% upward within 24 h. There were no significant differences among the essential oils in eliminating ticks at the larval stage (p > 0.05). Additionally, all essential oils were significantly more effective against ticks in the larval stage than the controls (p > 0.05; Table 2).
Table 2. Mortality rates of tick larvae within 24 h in the larvae immersion test for the essential oils.
| Essential oil | Mortality (%) | |||
|---|---|---|---|---|
| 16% | 8% | 4% | 2% | |
| Zanthoxylum limonella | 100 ± 0a | 100 ± 0a | 99.65 ± 0.13a | 99.50 ± 0.53a |
| Citronella | 100 ± 0a | 100 ± 0a | 99.56 ± 0.04a | 99.58 ± 0.41a |
| Clove | 99.73 ± 0.32a | 99.84 ± 0.14a | 99.53 ± 0.28a | 98.99 ± 0.34a |
| Peppermint | 100 ± 0a | 100 ± 0a | 100 ± 0a | 100 ± 0a |
| Ginger | 100 ± 0a | 100 ± 0a | 99.76 ± 0.25a | 99.86 ± 0.24a |
| Control | 10.71 ± 0.25b | 10.23 ± 0.78b | 8.29 ± 0.73b | 11.20 ± 1.84b |
a,bThese letters within columns indicate statistical differences (p < 0.05) in the mortality rates for the essential oils.
Lethal concentrations (LC50, LC90, and LC99) of 5 essential oils on ticks
The 50%, 90%, and 99% lethal concentrations (LC50, LC90, and LC99) of peppermint oil at 24 h had no data because there were 100% of mortality of all concentrations. Additionally, LC50 and LC90 of ginger oil could not be calculated for tick larvae because their mortality was too high. The LC50 of clove oil at 24 h was the lowest, followed by the citronella and Z. limonella oil. Furthermore, the LC90 of clove oil at 24 h was the lowest, followed by the citronella, Z. limonella oils, respectively. The LC99 of citronella at 24 h was the lowest, followed by the clove and Z. limonella oils, respectively (Table 3).
Table 3. Lethal concentrations (LC50, LC90, and LC99) for the essential oils on tick larvae in 24 hours (ppm).
| Essential oil | LC50 | LC90 | LC99 |
|---|---|---|---|
| Zanthoxylum limonella | 0.45 | 95.06 | 9,826.52 |
| Citronella | 0.17 | 55.98 | 6,456.54 |
| Clove | 0.04 | 32.06 | 8,298.51 |
| Peppermint | ND | ND | ND |
| Ginger | NaN | NaN | 0.56 |
LC50, 50% lethal concentration; LC90, 90% lethal concentration; LC99, 99% lethal concentration; ND, no data because 100% of mortality of all concentrations; NaN, could not be calculated because the tick mortality rate was too high.
In vitro insecticide test of essential oils against fleas
For the in vitro test of essential oils as insecticides again fleas, fleas exposed to 4% concentrations of clove oil for 1 h exhibited 100% mortality rates, but the mortality rates did not significantly different (p > 0.05). However, fleas exposed to the Z. limonella, clove, and peppermint essential oils had a significantly higher mortality rate than fleas exposed to the solvent and filter paper controls (p > 0.05). Fleas exposed to citronella oil tended to have different mortality rates than fleas exposed to the solvent (p = 0.06), but statistically significantly higher mortality rates than fleas exposed to filter paper only (p < 0.05). In addition, the mortality rates did not differ significantly (p > 0.05; Table 4). Fleas could start to die within 10 min.
Table 4. Mean mortality rates of fleas within 1 h when exposed to essential oils at concentrations of 0.5%, 1%, 2%, and 0.5% and to solvent and filter paper.
| Essential oil | Mortality rate at 1 h | |||
|---|---|---|---|---|
| 4% | 2% | 1% | 0.50% | |
| Zanthoxylum limonella | 94.44 ± 4.81a | 35.42 ± 31.46 | 50 ± 38.17 | 44.44 ± 26.79 |
| Citronella | 77.78 ± 38.49a,b | 66.67 ± 16.67 | 38.89 ± 53.58 | 63.89 ± 24.06 |
| Clove | 100 ± 0a | 69.44 ± 20.97 | 44.44 ± 26.79 | 41.67 ± 36.32 |
| Peppermint | 94.44 ± 9.62a | 47.22 ± 39.38 | 58.33 ± 38.19 | 41.67 ± 28.87 |
| Ginger | 55.55 ± 38.49a,b,c | 66.67 ± 22.05 | 38.87 ± 19.25 | 44.44 ± 33.68 |
| Essential oil solvent | 22.22 ± 12.73b,c | 6.25 ± 12.50 | 25 ± 14.43 | 25 ± 14.43 |
| Filter paper | 8.33 ± 6.80c | 0 | 5.56 ± 4.81 | 0 |
a,b,cThese letters within columns indicate statistical differences (p < 0.05) in the mortality rates for the essential oils.
Lethal concentrations (LC50, LC90, and LC99) of 5 essential oils on ticks
The LC50 for fleas at 1 h were lowest for citronella oil, followed by the clove, peppermint, Z. limonella, and ginger oils, respectively. LC90 for fleas at 1 h were lowest for clove oil, followed by the peppermint, Z. limonella, citronella oil and ginger oils, respectively. LC99 for fleas at 1 h were lowest for clove oil, followed by the peppermint, Z. limonella, ginger and citronella oil, respectively (Table 5).
Table 5. Lethal concentration (LC50, LC90, and LC99) at 1 h for the essential oils on fleas (ppm).
| Essential oil | LC50 | LC90 | LC99 |
|---|---|---|---|
| Zanthoxylum limonella | 9,632.01 | 56,599.03 | 241,938.43 |
| Citronella | 4,492.79 | 425,044.79 | 17,754,373.06 |
| Clove | 8,292.88 | 19,274.11 | 38,497.22 |
| Peppermint | 8,470.76 | 50,490.56 | 218,367.69 |
| Ginger | 11,907.16 | 2,845,157.29 | 254,128,798.59 |
LC50, 50% lethal concentration; LC90, 90% lethal concentration; LC99, 99% lethal concentration.
Survival test of fleas
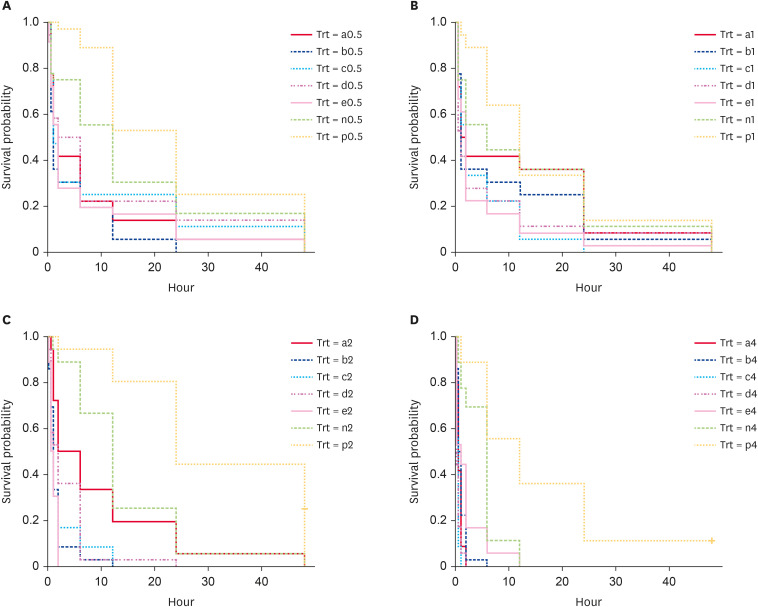
The effectiveness of the five essential oils against fleas was analysed using the Kaplan-Meier survival test. All the essential oils were effective in eliminating fleas at a 4% concentration compared with the solvent and filter paper controls, and the flea survival rates of control 1 were significantly lower than those of control 2 (p < 0.01; Fig. 1). Clove oil at a concentration of 4% most effectively eliminated fleas, with the lowest flea survival rate (p < 0.01). However, all five essential oils were effective against fleas at a concentration of 0.5%, and the flea survival rates were significantly less than those of the control groups (p < 0.05).
Fig. 1. Survival curves showing the survival rates of fleas exposed to the five essential oils at concentrations of 0.5%, 1%, 2%, 4% and to the solvent and filter paper controls, separated by concentration: a = Zanthoxylum limonella oil, b = citronella oil, c = clove oil, d = peppermint oil, e = ginger oil, n = control group 1 (essential oil solvent-ethyl alcohol 95%), p = control group 2 (filter paper), 4 = 4% concentration, 2 = 2% concentration, 1 = 1% concentration, 0.5 = 0.5% concentration; (A) = survival rates of fleas exposed to the five essential oils at concentrations of 0.5%, (B) = survival rates of fleas exposed to the five essential oils at concentrations of 1%, (C) = survival rates of fleas exposed to the five essential oils at concentrations of 2%, (D) = survival rates of fleas exposed to the five essential oils at concentrations of 4%.
Dogs’ skin allergy test
Due to their greater effectiveness, the Z. limonella and clove oils were selected to test for adverse effects on dogs’ skin compared to the negative control (solvent) and the positive control (Bayticol; Bayer). The results revealed low percentages of adverse effects on dogs’ skin for all groups (Table 6).
Table 6. Allergic reactions within 14 days of applying the oils to dogs’ skin.
| Parameters | 16% Clove oil | 16% Zanthoxylum limonella oil | Positive control (Bayticol) | Negative control (essential oil solvent-ethyl alcohol 95 %) | |
|---|---|---|---|---|---|
| Allergic reactions occurred within 15 min of applying the oils to the dogs’ skin | |||||
| - Abnormalities (scratching, itching, rubbing the body against furniture or objects) | 29.41% | 41.18% | 46.67% | 16.67% | |
| - Abnormalities (red bumps) | 0.00% | 0.00% | 0.00% | 0.00% | |
| - Abnormalities (dermatitis) | 0.00% | 0.00% | 0.00% | 0.00% | |
| - No abnormalities | 70.59% | 58.82% | 53.33% | 83.33% | |
| Allergic reactions occurred 2 days after applying the oils to the dogs’ skin | |||||
| - Abnormalities (scratching, itching, rubbing the body against furniture or objects) | 5.88% | 17.65% | 20.00% | 0.00% | |
| - Abnormalities (red bumps) | 0.00% | 0.00% | 0.00% | 5.56% | |
| - Abnormalities (dermatitis) | 0.00% | 0.00% | 0.00% | 5.56% | |
| - No abnormalities | 94.12% | 82.35% | 80.00% | 88.89% | |
| Allergic reactions occurred 3 days after applying the oils to the dogs’ skin | |||||
| - Abnormalities (scratching, itching, rubbing the body against furniture or objects) | 5.88% | 17.65% | 13.33% | 5.56% | |
| - Abnormalities (red bumps) | 0.00% | 0.00% | 0.00% | 0.00% | |
| - Abnormalities (dermatitis) | 0.00% | 0.00% | 0.00% | 5.56% | |
| - No abnormalities | 94.12% | 82.35% | 86.67% | 88.89% | |
| Allergic reactions occurred 7 days after applying the oils to the dogs’ skin | |||||
| - Abnormalities (scratching, itching, rubbing the body against furniture or objects) | 5.88% | 17.65% | 13.33% | 11.11% | |
| - Abnormalities (red bumps) | 0.00% | 0.00% | 0.00% | 0.00% | |
| - Abnormalities (dermatitis) | 0.00% | 0.00% | 0.00% | 0.00% | |
| - No abnormalities | 94.12% | 82.35% | 86.67% | 88.89% | |
| Allergic reactions occurred 14 days after applying the oils to the dogs’ skin | |||||
| - Abnormalities (scratching, itching, rubbing the body against furniture or objects) | 0.00% | 6.25% | 26.67% | 11.11% | |
| - Abnormalities (red bumps) | 0.00% | 0.00% | 0.00% | 5.56% | |
| - Abnormalities (dermatitis) | 0.00% | 0.00% | 0.00% | 0.00% | |
| - No abnormalities | 100.00% | 93.75% | 73.33% | 83.33% | |
DISCUSSION
There were no statistically significant differences in the ability of the Z. limonella, citronella, clove, and peppermint essential oils to inhibit the spawning of female engorged ticks at concentrations of 16% (p > 0.05). Studies of [23,24] revealed that Zanthoxylum armatum from Nepal effectively killed insects, which is relevant to this study. Another study [25] showed a repellent effect of 3% clove oil against Dermacentor reticulatus, with 83% effectiveness. Interestingly, no studies have shown that clove oil can kill ticks. However, citronella oil at 16% concentration was highly tick repellent and reduced the reproductive efficiency of ticks. At concentrations of 16%, the Z. limonella, clove, peppermint, and ginger essential oils were more effective in inhibiting egg laying than the same oils at 2% concentrations, suggesting that higher concentrations of essential oils may have a stronger inhibitory effect on tick egg laying than lower concentrations. This supports the general principle that the concentration of an active ingredient in a substance can influence its efficacy as a repellent or insecticidal agent. In addition, the high standard deviations for the lower concentrations of essential oils (2%, 4%, and 8%) were more widely distributed and varied than those for the higher 16% concentration. This suggests that the higher standard deviations indicated relatively unstable effects at the lower concentrations. In contrast, the smaller standard deviation for the 16% concentration indicated that the effects on egg laying inhibition were more consistent, and the more stable and reliable response to the higher concentration of essential oil had a stronger ability to prevent or reduce the reproductive capabilities of female engorged ticks.
The mortality rate of tick larvae was as high as 99.58% when exposed to 2% citronella oil, whereas [26] observed a mortality rate of 66%. Interestingly, all five essential oils tested in this study were highly effective in treating tick larvae when used at concentrations of 2% or higher. The 50%, 90%, and 99% lethal concentrations (LC50, LC90, and LC99) for the essential oils on tick larvae in 24 h were found to be low values. This represented that all essential oils were highly toxic to tick larva. This suggests that these essential oils have potential as tick control agents during the larval stage and can potentially disrupt the tick life cycle.
The testing of five essential oils against fleas indicated that a concentration of 4% was the most effective in terms of flea control. Specifically, clove oil at a 4% concentration demonstrated the highest efficacy, achieving 100% flea control. However, although the 0.5% concentration of essential oils was effective in killing but it required a longer exposure to achieve the same level of flea control as the 1%, 2%, and 4% concentrations, respectively. In this study, clove oil at the minimal concentration achieved relatively low LC90, and LC99 values, indicating its potency in killing fleas within a short exposure time and highly toxic to fleas. Clove oil was effective in eliminating Ctenocephalides spp. fleas, similar to a study by [26]. However, no study of the effectiveness of the Z. limonella, citronella, peppermint, and ginger essential oils against Ctenocephalides spp. fleas has been conducted.
Essential oils have insecticidal effects by acting on the insects’ nervous systems to inhibit the activity of the acetylcholinesterase enzyme. This leads to an accumulation of acetylcholine, disrupting the normal functioning of the nervous system and affecting the movement and coordination of insects, ultimately leading to their death [27,28,29,30]. Additionally, essential oils may disrupt various metabolic processes in insects, including nutrient absorption, utilisation, and storage. By reducing the availability of essential energy sources, such as protein, fat, and glycogen, insects can be deprived of energy, thus affecting larval growth and development and the reproductive capabilities of adult insects [28,31].
Clove oil at a concentration of 16% could potentially be developed as a pharmaceutical agent to effectively kill fleas and ticks based on the observation of few side effects 15 min after applying the oil to dogs’ skin. This is important for ensuring the safety and welfare of treated dogs. This study confirmed the effectiveness of essential oils for practical use as tick and flea repellents and eliminators. However, [32] retrospective study from 2006 to 2008 showed that plant-derived flea products containing mixtures of essential oils such as peppermint oil (0.25%–3%), thyme oil (1.7%–5%), 2-phenethypropionate (1.7%–5%), cinnamon oil (1.5%–4.5%), lemongrass oil (1.5%–4.5%), clove oil (1.7%–5%), and isopropyl myristate had potentially adverse effects on dogs. The reported abnormalities, such as lethargy and vomiting, highlighted the need for caution when using essential oils on dogs, which should only be done under veterinary supervision.
This study’s findings will help to understand the problem of killing ticks and fleas on dogs. All essential oil tested as pharmaceutical agent can replace chemical pesticides and be highly beneficial for both consumers and the environment, as well as for increasing the value of local herbal products.
ACKNOWLEDGMENTS
We gratefully appreciate the students who helped with sample collection and processing. The authors gratefully thank the dogs’ owner for their cooperation and help with the study. Finally, we would like to express our thanks to Faculty of Animal Science and Technology, Maejo University for their significant contributions.
Footnotes
Funding: This research was supported by Maejo university research fund, Fundamental Fund (MJU1-63-07-003).
Conflict of Interest: The authors declare no conflicts of interest.
- Conceptualization: Tadee P, Chukiatsiri K.
- Data curation: Tadee P, Chukiatsiri K.
- Formal analysis: Tadee P, Tadee PP.
- Funding acquisition: Tadee P.
- Investigation: Tadee P, Khaodang P.
- Methodology: Tadee P, Khaodang P, Chukiatsiri K, Chansakaow S, Tipduangta P.
- Project administration: Tadee P.
- Resources: Chukiatsiri K, Chansakaow S, Tipduangta P.
- Writing - review & editing: Tadee P.
References
- 1.Otranto D, Dantas-Torres F. Canine and feline vector-borne diseases in Italy: current situation and perspectives. Parasit Vectors. 2010;3(1):2. doi: 10.1186/1756-3305-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moriello KA. Zoonotic skin diseases of dogs and cats. Anim Health Res Rev. 2003;4(2):157–168. doi: 10.1079/ahr200355. [DOI] [PubMed] [Google Scholar]
- 3.Saleh MN, Allen KE, Lineberry MW, Little SE, Reichard MV. Ticks infesting dogs and cats in North America: biology, geographic distribution, and pathogen transmission. Vet Parasitol. 2021;294:109392. doi: 10.1016/j.vetpar.2021.109392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beck W, Boch K, Mackensen H, Wiegand B, Pfister K. Qualitative and quantitative observations on the flea population dynamics of dogs and cats in several areas of Germany. Vet Parasitol. 2006;137(1-2):130–136. doi: 10.1016/j.vetpar.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 5.Beugnet F, Marié JL. Emerging arthropod-borne diseases of companion animals in Europe. Vet Parasitol. 2009;163(4):298–305. doi: 10.1016/j.vetpar.2009.03.028. [DOI] [PubMed] [Google Scholar]
- 6.Rust MK, Dryden MW. The biology, ecology, and management of the cat flea. Annu Rev Entomol. 1997;42(1):451–473. doi: 10.1146/annurev.ento.42.1.451. [DOI] [PubMed] [Google Scholar]
- 7.Dantas-Torres F. The brown dog tick, Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae): from taxonomy to control. Vet Parasitol. 2008;152(3-4):173–185. doi: 10.1016/j.vetpar.2007.12.030. [DOI] [PubMed] [Google Scholar]
- 8.Bandaranayaka KO, Dissanayake UI, Rajakaruna RS. Diversity and geographic distribution of dog tick species in Sri Lanka and the life cycle of brown dog tick, Rhipicephalus sanguineus under laboratory conditions. Acta Parasitol. 2022;67(4):1708–1718. doi: 10.1007/s11686-022-00622-5. [DOI] [PubMed] [Google Scholar]
- 9.Dryden MW. Host association, on-host longevity and egg production of Ctenocephalides felis felis . Vet Parasitol. 1989;34(1-2):117–122. doi: 10.1016/0304-4017(89)90171-4. [DOI] [PubMed] [Google Scholar]
- 10.Dryden MW, Blakemore JC. A review of flea allergy dermatitis in the dog and cat. Companion Anim Pract. 1989;19:10–17. [Google Scholar]
- 11.Garris GI. Control of ticks. Vet Clin North Am Small Anim Pract. 1991;21(1):173–183. doi: 10.1016/s0195-5616(91)50017-6. [DOI] [PubMed] [Google Scholar]
- 12.Agnolin C, Olivo C, Leal M, Beck R, Meinerz G, Parra C, et al. Eficácia do óleo de citronela [Cymbopogon nardus (L.) Rendle] no controle de ectoparasitas de bovinos. Rev Bras Plantas Med. 2010;12(4):482–487. [Google Scholar]
- 13.Veeraphant C, Mahakittikun V, Soonthornchareonnon N. Acaricidal effects of Thai herbal essential oils against Dermatophagoides pteronyssinus . Mahidol Univ J Pharm Sci. 2011;38:1–12. [Google Scholar]
- 14.Hamzah MH, Man HC, Abidin ZZ, Jamaludin H. Comparison of citronella oil extraction methods from Cymbopogon nardus grass by ohmic-heated hydro-distillation, hydro-distillation, and steam distillation. Bioresour. 2014;9(1):256–272. [Google Scholar]
- 15.Lans C, Turner N. Organic parasite control for poultry and rabbits in British Columbia, Canada. J Ethnobiol Ethnomed. 2011;7(1):21. doi: 10.1186/1746-4269-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh P, Pandey AK. Prospective of essential oils of the genus Mentha as biopesticides: a review. Front Plant Sci (Lausanne) 2018;9:1295. doi: 10.3389/fpls.2018.01295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nogueira J, Vinturelle R, Mattos C, Tietbohl LA, Santos MG, Junior IS, et al. Acaricidal properties of the essential oil from Zanthoxylum caribaeum against Rhipicephalus microplus . J Med Entomol. 2014;51(5):971–975. doi: 10.1603/me13236. [DOI] [PubMed] [Google Scholar]
- 18.Ferreira FM, Delmonte CC, Novato TL, Monteiro CM, Daemon E, Vilela FM, et al. Acaricidal activity of essential oil of Syzygium aromaticum, hydrolate and eugenol formulated or free on larvae and engorged females of Rhipicephalus microplus . Med Vet Entomol. 2018;32(1):41–47. doi: 10.1111/mve.12259. [DOI] [PubMed] [Google Scholar]
- 19.Ashitani T, Garboui SS, Schubert F, Vongsombath C, Liblikas I, Pålsson K, et al. Activity studies of sesquiterpene oxides and sulfides from the plant Hyptis suaveolens (Lamiaceae) and its repellency on Ixodes ricinus (Acari: Ixodidae) Exp Appl Acarol. 2015;67(4):595–606. doi: 10.1007/s10493-015-9965-5. [DOI] [PubMed] [Google Scholar]
- 20.Ribeiro VL, Avancini C, Gonçalves K, Toigo E, von Poser G. Acaricidal activity of Calea serrata (Asteraceae) on Boophilus microplus and Rhipicephalus sanguineus . Vet Parasitol. 2008;151(2-4):351–354. doi: 10.1016/j.vetpar.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 21.Batista LC, Cid YP, De Almeida AP, Prudêncio ER, Riger CJ, De Souza MA, et al. In vitro efficacy of essential oils and extracts of Schinus molle L. against Ctenocephalides felis felis . Parasitology. 2016;143(5):627–638. doi: 10.1017/S0031182016000081. [DOI] [PubMed] [Google Scholar]
- 22.Ghosh M. Fundamentals of experimental pharmacology. Indian J Pharmacol. 2007;39(4):216–216. [Google Scholar]
- 23.Phuyal N, Jha PK, Prasad Raturi P, Rajbhandary S. Zanthoxylum armatum DC.: current knowledge, gaps and opportunities in Nepal. J Ethnopharmacol. 2019;229:326–341. doi: 10.1016/j.jep.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 24.Subedi R. Ethnobotanical study of panchase protected forest, Kaski District, Central Nepal. Kathmandu, Nepal: Central Department of Botany Tribhuvan University Kirtipur; 2017. [Google Scholar]
- 25.Štefanidesová K, Škultéty Ľ, Sparagano OA, Špitalská E. The repellent efficacy of eleven essential oils against adult Dermacentor reticulatus ticks. Ticks Tick Borne Dis. 2017;8(5):780–786. doi: 10.1016/j.ttbdis.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 26.da Silva LC, de Souza Perinotto WM, Sá FA, de Souza MA, de Oliveira Barbosa Bitencourt R, Sanavria A, et al. In vitro acaricidal activity of Cymbopogon citratus, Cymbopogon nardus and Mentha arvensis against Rhipicephalus microplus (Acari: Ixodidae) Exp Parasitol. 2020;216:107937. doi: 10.1016/j.exppara.2020.107937. [DOI] [PubMed] [Google Scholar]
- 27.Abdelgaleil SA, Mohamed MI, Shawir MS, Abou-Taleb HK. Chemical composition, insecticidal and biochemical effects of essential oils of different plant species from Northern Egypt on the rice weevil, Sitophilus oryzae L. J Pest Sci. 2016;89(1):219–229. [Google Scholar]
- 28.Campolo O, Giunti G, Russo A, Palmeri V, Zappalà L. Essential oils in stored product insect pest control. J Food Qual. 2018;2018:1–18. [Google Scholar]
- 29.Fang F, Candy K, Melloul E, Bernigaud C, Chai L, Darmon C, et al. In vitro activity of ten essential oils against Sarcoptes scabiei . Parasit Vectors. 2016;9(1):594. doi: 10.1186/s13071-016-1889-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jankowska M, Rogalska J, Wyszkowska J, Stankiewicz M. Molecular targets for components of essential oils in the insect nervous system—a review. Molecules. 2017;23(1):34. doi: 10.3390/molecules23010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Borzoui E, Naseri B, Abedi Z, Karimi-Pormehr MS. Lethal and sublethal effects of essential oils from Artemisia khorassanica and Vitex pseudo-negundo against Plodia interpunctella (Lepidoptera: Pyralidae) Environ Entomol. 2016;45(5):1220–1226. doi: 10.1093/ee/nvw100. [DOI] [PubMed] [Google Scholar]
- 32.Genovese AG, McLean MK, Khan SA. Adverse reactions from essential oil-containing natural flea products exempted from Environmental Protection Agency regulations in dogs and cats. J Vet Emerg Crit Care. 2012;22(4):470–475. doi: 10.1111/j.1476-4431.2012.00780.x. [DOI] [PubMed] [Google Scholar]