Abstract
Background
The anti-programmed death 1 (PD-1) antibody has led to durable clinical responses in a wide variety of human tumors. We have previously developed the caninized anti-canine PD-1 antibody (ca-4F12-E6) and evaluated its therapeutic properties in dogs with advance-staged oral malignant melanoma (OMM), however, their therapeutic effects on other types of canine tumors remain unclear.
Objective
The present clinical study was carried out to evaluate the safety profile and clinical efficacy of ca-4F12-E6 in dogs with advanced solid tumors except for OMM.
Methods
Thirty-eight dogs with non-OMM solid tumors were enrolled prospectively and treated with ca-4F12-E6 at 3 mg/kg every 2 weeks of each 10-week treatment cycle. Adverse events (AEs) and treatment efficacy were graded based on the criteria established by the Veterinary Cooperative Oncology Group.
Results
One dog was withdrawn, and thirty-seven dogs were evaluated for the safety and efficacy of ca-4F12-E6. Treatment-related AEs of any grade occurred in 13 out of 37 cases (35.1%). Two dogs with sterile nodular panniculitis and one with myasthenia gravis and hypothyroidism were suspected of immune-related AEs. In 30 out of 37 dogs that had target tumor lesions, the overall response and clinical benefit rates were 6.9% and 27.6%, respectively. The median progression-free survival and overall survival time were 70 days and 215 days, respectively.
Conclusions
The present study demonstrated that ca-4F12-E6 was well-tolerated in non-OMM dogs, with a small number of cases showing objective responses. This provides evidence supporting large-scale clinical trials of anti-PD-1 antibody therapy in dogs.
Keywords: Dog, immunotherapy, immune checkpoint inhibitor, monoclonal antibody, tumor
INTRODUCTION
Immune checkpoint inhibitor (ICI) development has led to numerous clinical benefits, including long-term survival in a wide range of advanced tumors in humans [1]. Notably, the inhibition of programmed death 1 (PD-1), PD-1 ligand (PD-L1), and cytotoxic T-lymphocyte-associated protein 4 by each target-specific monoclonal antibody has strongly induced anti-tumor immunity via T cell re-activation and achieved the approval by the Food and Drug Administration as the standard care for various tumor types [2]. In 2017, one of the great predictive biomarkers for tumor response to anti-PD-1 antibody pembrolizumab was approved, which is high microsatellite instability (MSI-H) or mismatch repair deficient (dMMR) status in any solid tumor [3]. This was the first of its kind that used common indications for anti-tumor agents across tumor types, regardless of the tumor origin. Further clinical studies using ICIs revealed other cleared diagnostics and predictive biomarkers, including staining scores of PD-L1 and tumor mutation burden in various tumor types [2].
Recent veterinary studies have determined the PD-L1 expression in various canine tumor tissues, including oral malignant melanoma (OMM), nasal adenocarcinoma, squamous cell carcinoma (SCC), urothelial carcinoma (UC), and soft tissue sarcoma [4,5,6,7]. Additionally, mitogen-stimulated canine lymphocytes expressed PD-1 molecule on the cell surface [8,9,10]. Thus, ICIs targeting PD-1/PD-L1 molecules can be clinically utilized in canine tumors as a novel tumor therapy.
Clinical pilot studies used chimeric or caninized anti-canine PD-1 antibodies and the chimeric anti-canine PD-L1 antibody in dogs with advanced tumors [4,11,12]; most of those were OMM cases. Our previous studies produced rat-canine chimeric anti-canine PD-1 antibody (ch-4F12-E6) and caninized anti-canine PD-1 therapeutic antibody (ca-4F12-E6) and confirmed that these antibodies can block the binding between canine PD-1 and canine PD-L1 molecules followed by T cell re-activation [11,13]. We investigated the safety profile and efficacy in 30 dogs with advanced tumors, including OMM (21 cases), mammary gland tumor (MGT, 1 case), SCC (2 cases), renal cell carcinoma (1 case), lymphoma (1 case), sebaceous carcinoma (1 case), lung adenocarcinoma (1 case), and skin melanoma (2 cases), after the safety examination of those antibodies against healthy beagles. This pilot study revealed that ca-4F12-E6 had clinical benefits for OMM cases while unknown for other tumor types due to the limited number of cases. Therefore, the present study evaluated the safety profile and clinical efficacy of ca-4F12-E6 in dogs with advanced solid tumors except for OMM. Additionally, we assessed the tumor-infiltrating lymphocytes (TILs) using immunohistochemistry (IHC) in cases with available pre- and post-treatment tumor tissues.
MATERIALS AND METHODS
Ethics statement
The present clinical study for clients’ dogs including the study design was approved by the Ethics Review Board of the Joint Faculty of Veterinary Medicine of Yamaguchi University (Approval No. 17). The owners all agreed and gave written informed consent.
Study design and treatment regimen
The present open-label, single-arm, investigator-initiated clinical study was conducted at Yamaguchi University Animal Medical Center from January 7, 2020, to April 15, 2022. Inclusion and exclusion criteria based on our previous report [11] are shown in Supplementary Table 1. Inclusion criteria is as follows; the case with a confirmed tumor diagnosis by cytology or histopathology except for a heart base tumor, a tumor whose size that can be measured by one of either caliper, X-ray, computed tomography (CT) scan, or ultrasonography and a life expectancy of at least 1 month. Cases with previous treatments such as radiation or chemotherapy were allowable if they had stable disease (SD) or progressive disease (PD) at the start of the trial. We excluded dogs with initial concurrent treatment such as systemic chemotherapies at the start of the trial. However, the use of concurrent continuous treatments with non-steroidal drugs and without apparent therapeutic effects before study inclusion was included. If cases had received radiation or toceranib prior to the start of the trial and the tumor had progressed, the concomitant treatment was permitted.
The present study primarily aimed to evaluate the safety profile of caninized anti-canine PD-1 monoclonal antibody (ca-4F12-E6) in dogs with advanced solid tumors except for OMM. The secondary objective is to evaluate the treatment response in enrolled dogs.
The detail of the caninized anti-canine PD-1 monoclonal antibody, ca-4F12-E6, was described in our previous report [11]. Eligible cases received 1 hour of ca-4F12-E6 intravenous constant rate infusion at 3 mg/kg every 2 weeks of each 10-week treatment cycle until disease progression, an unacceptable adverse event (AE), and a withdrawal of consent. Continuous ca-4F12-E6 administration was permitted over the first assessment of PD if only acceptable AEs were observed, the dogs remained in better condition as compared to prior to enrollment as assessed by investigators, and the owner made the decision to continue treatment. Dose modification of ca-4F12-E6 was not implemented throughout the study except for Case 6.
Clinical assessments
All enrolled dogs were evaluated for AEs. The assessment duration is from initial treatment to trial termination. Complete blood count and blood biochemistry were performed every two weeks. AEs were graded based on the Veterinary Cooperative Oncology Group-Common Terminology Criteria for Adverse Events v2 scale [14].
Tumor response was assessed according to the response evaluation criteria for solid tumors in dogs cRECIST v1.0 [15]. All enrolled dogs were assessed for tumor response either by caliper, X-ray, or CT scan, followed by comparing tumor diameter between baseline and termination of each treatment cycle. A target lesion was defined as a > 10-mm tumor diameter while a non-target lesion as a < 10-mm tumor diameter. Dogs with measurable disease were defined as having tumor mass as a target lesion, and dogs with non-measurable disease were defined as having tumor mass as a non-target lesion only. Tumor response was separately evaluated for a target and non-target lesion based on cRESIST v1.0 every 10 weeks, i.e., two weeks after the end of one cycle of five doses every two weeks, except the case that was evaluated as PD in the middle of the cycle due to clinical progression. A new metastatic tumor lesion, non-target lesions progression, or clinical progression was determined as PD, even if the target lesion had shrunk. Cases with changes in tumor diameter of < 20% increase or 30% decrease are considered SD after the completion of each treatment cycle (every 10 weeks). The overall response rate (ORR) was calculated as the number of dogs whose target lesion had a complete response (CR) and partial response (PR) at the first treatment cycle termination. The clinical benefit rate (CBR) was defined as the objective status, including CR, PR, and SD. The overall survival (OS) was defined as the duration from initiation of the treatment to death. The progression-free survival (PFS) was defined as the length of time from the initial treatment until PD or death at the end of the study.
IHC
Formalin-fixed, paraffin-embedded tumor tissues obtained from case 6 were used for IHC of infiltrating lymphocytes. No tumor tissue samples post-treatment were obtained from other cases. According to our previous reports [16,17], tissue sections (5-μm) were subjected to antigen retrieval by incubation in a citric acid buffer (pH 6.0) using a pressure cooker (Dako Japan, Tokyo, Japan). The sections were incubated with 3% of hydrogen peroxide in phosphate-buffered saline (PBS) to block endogenous peroxidase activity. The sections were incubated with a rat anti-canine CD8 monoclonal antibody (F3-B2, produced by our laboratory; 5 μg/mL dilution), a rat anti-mouse Foxp3 monoclonal antibody (FJK-16s, Thermo Fisher Scientific, Waltham, MA, USA; 1:200 dilution), or a rat IgG2a isotype control (RTK2578, BioLegend; 1:400 dilution) as a primary antibody after the incubation with 5% of skimmed milk in PBS. Histofine Simple Stain Rat MAX PO (Nichirei Bioscience Inc., Tokyo, Japan) was used as a secondary antibody. Peroxidase Stain DAB Kit (Nacalai Tesque, Kyoto, Japan) was used to visualize the immunolabelling. The sections were counter-stained with Mayer’s hematoxylin.
The immunohistochemical staining using the melanoma diagnostics markers PNL2 and Melan-A was performed at IDEXX laboratories (Tokyo, Japan) for histopathologically undistinguished OMM and undifferentiated sarcoma. Cases with oral mass having negative markers were diagnosed as undifferentiated sarcoma.
Statistical analysis of clinical assessment
All enrolled dogs that received ca-4F12-E6 administrations were considered evaluable for safety endpoints. Safety evaluation only included data until the date of withdrawal when enrolled dogs were found ineligible. Tumor response evaluation included both cases with measurable disease and non-measurable diseases. However, the calculation of ORR and CBR after the first treatment cycle was limited to dogs with measurable diseases. Cases 22 and 26 in which toceranib or radiation was used in combination at start to the trial were excluded from the calculation of tumor response rate. The mean or median value of age, the number of treatments, PFS, and OS were calculated using JMP pro software (SAS Institute Japan, Tokyo, Japan).
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
RESULTS
Case characteristics
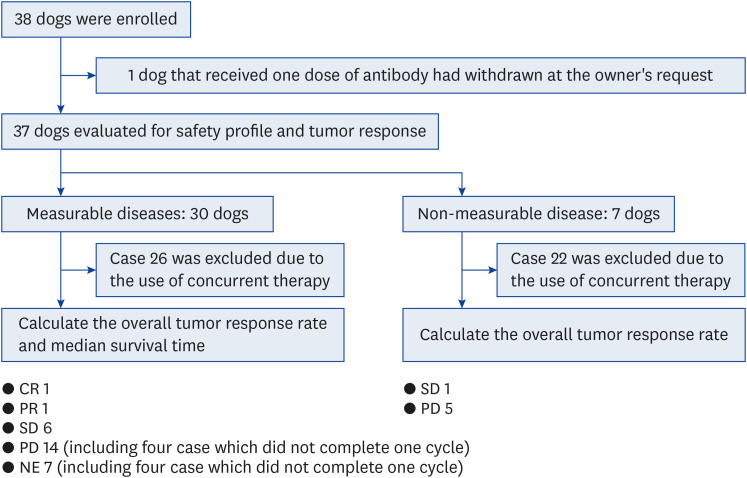
This study included 38 dogs with advanced non-OMM solid tumors from January 7, 2020, to April 15, 2022. However, one dog was withdrawn from the trial at the owner’s request, for unknown reason. Then, we assessed 37 enrolled dogs for further analysis (Fig. 1). The characteristics of 37 dogs were summarized in Table 1. The median age for entry to this study was 11 years (range, 1–15). There were 6 intact males, 12 castrated males, 3 intact females, and 16 spayed females. The most common breed was Miniature Dachshund (n = 9). The tumor types included UC (n = 5), SCC (n = 6), undifferentiated sarcoma (n = 5), nasal adenocarcinoma (n = 3), MGT (n = 3), lung carcinoma (n = 2), heart base tumor (n = 2), adenocarcinoma of unknown origin (n = 1), hepatocellular carcinoma (n = 2), colorectal adenocarcinoma (n = 1), osteosarcoma (n = 2), soft tissue sarcoma (n = 3), and skin melanoma (n = 2). Primary tumor lesion, tumor, node, and metastasis classification, and the target lesion in each case were shown in Table 1. Of 37 dogs, 34 received prior therapies, including surgery, radiation (orthovoltage [kV] and megavoltage [MV]), and chemotherapeutic drugs such as toceranib and nonsteroidal anti-inflammatory agents (NSAIDs). The median time from the completion of prior therapy to the start of this trial was 35 days (range, 0–1019 days). Along with ca-4F12-E6 treatment, 4 UC cases (Cases no. 1, 2, 3, and 5), 1 undifferentiated sarcoma (case 16), and 1 nasal adenocarcinoma (case 19) were treated with NSAIDs, and cases 22 and 26 were allowed concurrently treatment with orthovoltage radiation (kV) and toceranib phosphate, respectively, because we confirmed tumor progression at the start of ca-4F12-E6. The ca-4F12-E6 treatment regimen was referred to in our previous study [11]. The median numbers of ca-4F12-E6 administrations were 6 (range, 1–43). Exceptionally, the ca-4F12-E6 dosage was increased up to 6 mg/kg from day 221 because of tumor progression (Fig. 2A) and the owner’s request for case 6. Case 27 was started on toceranib phosphate along with ca-4F12-E6 administration from day 385 because of the new lesions of tumor metastasis (Fig. 2A).
Fig. 1. The flow chart of the enrollment of eligible dogs in the clinical study.
CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; NE, not evaluation.
Table 1. Case demographics and tumor responses.
| Case No. | Age | Sex | Breed | Tumor type | Primary tumor lesion | TNM classification | Prior therapy | Time from completion of prior therapy to start of trial (days) | Measurable or non-measurable cases | Non-target lesions/target lesionsa | Concomitant medications | Numbers of injection of ca-4F12-E6 | Overall tumor response at first evaluation | Tumor response in non-target lesions at first evaluation | Overall best response at subsequent time point | Progression-free survival (days) | Overall Survival (days) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 10Y | MC | Miniature Dachshund | UC | Bladder | T0N1M1 | Surgery, NSAIDs | 0 | Non-measurable | Lymph node and lung | NSAIDs | 6 | - | PDb | - | 72 | 139 |
| 4 | 12Y | F | Welsh Corgi | UC | Bladder | T0N1M0 | Surgery, NSAIDs | 197 | Lymph node | - | 6 | - | PDb | - | 112 | 245 | |
| 12 | 6Y | FS | French Bulldog | Undifferentiated sarcoma | Oral | T0N0M1 | Radiation (MV) | 56 | Lung | - | 43 | - | PD | CR | 70 | > 917 (alive) | |
| 13 | 14Y | M | Miniature Dachshund | Undifferentiated sarcoma | Oral | T0N0M1 | Radiation (MV) | 63 | Lung | - | 7 | - | PDb,c | - | 63 | 95 | |
| 20 | 15Y | FS | Italian Greyhound | MGT | Mammary gland | T0N0M1 | Surgery, chemotherapy | 5 | Lung | - | 11 | - | SD | PDb | 145 | > 145 (ongoing) | |
| 22 | 9Y | FS | French Bulldog | MGT | Mammary gland | T0N0M1 | Radiation (kV) | 0 | Lung and skin | Radiation (kV) | 5 | - | - | - | 25 | 57 | |
| 23 | 14Y | MC | Chihuahua | LC | Lung | T0N0M1 | Surgery | 352 | Lung | - | 31 | - | PD | PDb | 72 | > 513 (alive) | |
| 2 | 14Y | FS | Miniature Dachshund | UC | Bladder | T0N0M1 | Surgery, NSAIDs, radiation (kV) | 0 | Measurable | M (skin) | NSAIDs | 5 | PDb,c | - | - | 63 | 93 |
| 3 | 9Y | F | Shetland Sheepdog | UC | Bladder | T0N2M0 | Surgery, NSAIDs | 0 | M (lymph node) | NSAIDs | 6 | PD | PD | - | 70 | > 70 | |
| 5 | 10Y | MC | Miniature Dachshund | UC | Prostatic urethra | T0N1M1 | Surgery, NSAIDs | 0 | M (lung) | NSAIDs | 6 | PD | PD | - | 72 | > 72 (alive) | |
| 6 | 9Y | M | Golden Retriever | SCC | Nasal | T2N0M0 | Surgery, radiation (kV) | 34 | P | - | 20 | PR | - | CR | 277 | 472 | |
| 7 | 12Y | FS | Toy Poodle | SCC | Oral | T2aN0M0 | - | 0 | P | - | 5 | PD | - | - | 67 | > 97 | |
| 8 | 4Y | FS | French Bulldog | SCC | Nasal | T3N0M0 | Radiation (MV) | 35 | P | - | 7 | PD | - | - | 70 | 101 | |
| 9 | 9Y | FS | Maltese | SCC | Nasal | T3N0M0 | Radiation (MV) | 469 | P | - | 6 | NE | - | - | 70 | 73 | |
| 10 | 14Y | M | Shiba | SCC | Skin | T2N1M0 | Surgery | 122 | P | - | 5 | NE | - | - | 46 | > 66 | |
| 11 | 7Y | MC | Weimaraner | SCC | Oral | T3bN1M0 | Surgery | 30 | P | - | 2 | NE | - | - | 14 | 31 | |
| 14 | 13Y | MC | Labrador Retriever | Undifferentiated sarcoma | Oral | T0N0M1 | Radiation (MV) | 161 | M (lung) | - | 5 | CR | - | - | > 63 | > 63 (alive) | |
| 15 | 13Y | FS | Miniature Dachshund | Undifferentiated sarcoma | Oral | T3bN1M1 | Radiation (kV), toceranib | 0 | P | - | 2 | NE | - | - | 14 | 15 | |
| 16 | 1Y | M | Great dane | Undifferentiated sarcoma | Oral | T3bN1M1 | Surgery | 41 | P | NSAIDs | 5 | NE | - | - | 54 | 56 | |
| 17 | 8Y | MC | Chihuahua | Nasal adenocarcinoma | Nasal | T3N0M0(Stage 4d) | Radiation (MV) | 404 | P | - | 37 | SD | - | PD | 205 | > 597 (ongoing) | |
| 18 | 10Y | MC | Toy Poodle | Nasal adenocarcinoma | Nasal | T2N0M0(Stage 2d) | Radiation (MV), NSAIDs | 28 | P | - | 16 | SD | - | SD | > 350 | > 350 (alive) | |
| 19 | 6Y | M | Shiba | Nasal adenocarcinoma | Nasal | T3N0M0(Stage 3d) | Radiation (MV) | 72 | P | NSAIDs | 5 | PD | - | - | 70 | > 70 | |
| 21 | 12Y | FS | Toy Poodle | MGT | Mammary gland | T0N0M1 | Surgery, chemotherapy, toceranib | 0 | M (lymph node) | - | 5 | PD | SD | - | 70 | 215 | |
| 24 | 14Y | MC | Miniature Dachshund | LC | Lung | T2N0M0 | - | 0 | P | - | 15 | PDc | PD | PDb,c | 55 | 300 | |
| 25 | 10Y | FS | French Bulldog | HBT | Heart | - | Radiation (MV), toceranib | 0 | P | - | 10 | SD | - | SD | > 479 | > 479 (alive) | |
| 26 | 14Y | MC | Miniature Dachshund | HBT | Heart | - | Toceranib | 0 | P | Toceranib | 6 | - | - | 77 | 96 | ||
| 27 | 11Y | FS | Miniature Dachshund | Adenocarcinoma of unknown origin | Intraperitoneal | T2N0M1 | Surgery, toceranib | 35 | P | Toceranib (day385-) | 32 | SD | SD | PDb | 140 | 583 | |
| 28 | 13Y | MC | Standard Poodle | HCC | Liver | T2N0M0 | - | 0 | P | - | 20 | SD | - | SD | > 217 | 506 | |
| 29 | 10Y | F | Chihuahua | HCC | Liver | T2N1M0 | Surgery | 1,019 | P | - | 1 | NE | - | - | 10 | 10 | |
| 30 | 8Y | MC | Shetland Sheepdog | Colorectal adenocarcinoma | Colon | T2N0M0 | Surgery | 100 | P | - | 6 | PD | - | - | 70 | 423 | |
| 31 | 6Y | M | Rottweiler | OSA | Maxillofacial | T2N-M0 | Radiation (MV), chemotherapy | 3 | P | - | 23 | SD | - | PR | > 497 | > 497 (ongoing) | |
| 32 | 12Y | FS | Miniature Dachshund | OSA | Spleen | T0N0M1 | Surgery | 347 | M (liver) | - | 2 | PDe | - | - | 21 | 33 | |
| 33 | 14Y | MC | Toy Poodle | STS | Skin | T0N0M1 | Surgery, radiation (kV) | 10 | M (skin) | - | 4 | NE | - | - | 46 | 63 | |
| 34 | 12Y | FS | Toy Poodle | STS | Spleen | T0N0M1 | Chemotherapy | 93 | M (liver) | - | 3 | PDe | - | - | 41 | 51 | |
| 35 | 10Y | FS | Mixed | STS | Skin | T4N0M1 | Radiation (MV), chemotherapy | 519 | P | - | 3 | PDe | - | - | 34 | 36 | |
| 36 | 13Y | FS | Miniature Schnauzer | Skin melanoma | Skin | T0N0M1 | Surgery | 110 | M (lung) | - | 11 | PDb,c | - | PD | 74 | > 144 (ongoing) | |
| 37 | 14Y | FS | Mixed | Skin melanoma | Skin | T3N1M0 | Surgery | 55 | P | - | 2 | PDe | - | - | 14 | 27 |
TNM, tumor, node, metastasis; MC, castrated male; UC, urothelial carcinoma; NSAID, nonsteroidal anti-inflammatory agent; PD, progressive disease; F, female; FS, spayed female; CR, complete response; M, male; MGT, mammary gland tumor; SD, stable disease; LC, lung carcinoma; SCC, squamous cell carcinoma; PR, partial response; NE, not evaluation; HBT, heart base tumor; HCC, hepatocellular carcinoma; OSA, osteosarcoma; STS, soft tissue sarcoma; -, negative or none.
aTarget lesions were primary (P) or metastatic (M) sites. In dogs with non-measurable disease, the location of non-target lesions was indicated.
bNew tumor lesions were found.
cThis showed mixed tumor response.
dAdams Modified Staging System.
eClinical progression was observed before the end of the trial.
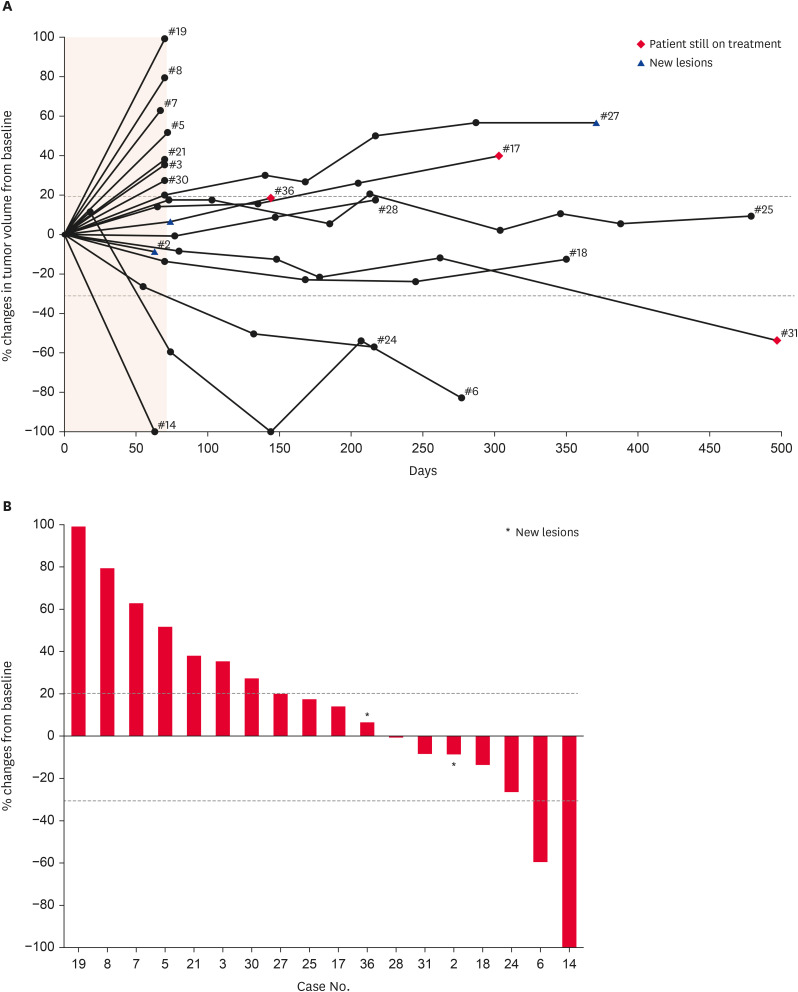
Fig. 2. Tumor response of ca-4F12-E6 in dogs with target tumor lesions (n = 19). (A) Percent changes of individual tumor volume of target lesions over time from baseline. The shaded lesion indicates the endpoint of the first treatment cycle. Dashed lines correspond to a 30% decrease and a 20% increase in tumor volume. The end of the line indicates the termination of this trial. (B) Percentage changes in the size of the target lesion from baseline at the endpoint of the first treatment cycle are indicated by a waterfall plot.
Asterisks indicate new lesions.
AEs
Treatment-related, treatment-unlikely, or unrelated AEs were summarized in Table 2. Treatment-related AEs with any grade occurred in 13 out of 37 cases (35.1%). The most frequent AEs were diarrhea and elevated liver enzymes (alanine transaminase, aspartate transaminase, and alkaline phosphatase). AEs of grade 4 were only seen in elevated alanine transaminase (cases 4 and 25). Two cases (cases 7 and 14) of aspiration pneumonia (grade 3) and a case (case 24) of a seizure (grade 2) were observed as treatment-unlikely or unrelated AEs. Additionally, the secondary tumor occurred in two cases (cases 6 and 27).
Table 2. Adverse events in dogs received ca-4F12-E6 (n = 37).
| Variables | All grade | Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
|---|---|---|---|---|---|---|
| Treatment-related | ||||||
| Appetite, altered | 2 | 1 | 1 | 0 | 0 | |
| Fatigue | 1 | 1 | 0 | 0 | 0 | |
| Diarrhea | 5 | 4 | 1 | 0 | 0 | |
| Vomiting | 1 | 1 | 0 | 0 | 0 | |
| Colitis | 1 | 0 | 1 | 0 | 0 | |
| ALT | 3 | 0 | 1 | 0 | 2 | |
| AST | 2 | 0 | 0 | 2 | 0 | |
| ALP | 3 | 0 | 1 | 2 | 0 | |
| Bil | 1 | 1 | 0 | 0 | 0 | |
| Megaesophagusa | 1 | 0 | 1 | 0 | 0 | |
| Myasthenia gravisa | 1 | 0 | 1 | 0 | 0 | |
| Hypothyroidisma | 1 | 0 | 0 | 1 | 0 | |
| Sterile nodular panniculitis | 2 | - | ||||
| Treatment-unlikely or unrelated | ||||||
| Aspiration pneumonia | 2 | 0 | 0 | 2 | 0 | |
| BUN | 1 | 0 | 0 | 1 | 0 | |
| ALT | 3 | 0 | 0 | 2 | 1 | |
| ALP | 3 | 0 | 1 | 1 | 1 | |
| Seizure | 1 | 0 | 1 | 0 | 0 | |
| Occurring the other tumor | 2 | - | ||||
ALT, alanine transaminase; AST, aspartate transaminase; ALP, alkaline phosphatase; Bil, bilirubin; BUN, blood urine nitrogen.
aCase 27 received ca-4F12-E6 combined with toceranib.
The suspected immune-related adverse events (irAEs)
Sterile nodular panniculitis, known as an immune-mediated disease, occurred in two cases (cases 27 and 28) in this study, however, was improved after 70 days and 128 days, respectively, without anti-inflammatory therapy. Moreover, in case 27, megaesophagus, myasthenia gravis, and hypothyroidism simultaneously occurred on day 455, after starting concurrent use of toceranib phosphate on day 385 in addition to ca-4F12-E6 treatment (Table 2). The concentrations of thyroid-stimulating hormone (TSH), total T4, and free T4 were 3.21 ng/mL (reference range, 0.9–2.6 ng/mL), < 0.5 μg/dL (reference range, 0.5–0.32 μg/dL), and 0.731 ng/dL (reference range, 1.1–3.6 ng/dL), respectively. Furthermore, the serum concentration of the anti-acetylcholine receptor antibody was increased (12.71 nM, reference range, 0–0.6 nM), although anti-thyroglobulin antibody was not detected. Hypoadrenocorticism had not been observed in case 27. Therefore, case 27 was diagnosed with hypothyroidism and acquired megaesophagus, which has been associated with myasthenia gravis, regardless of not having a proven detailed mechanism.
Efficacy
Initially, based on the tumor mass diameter for the cRECIST criteria, we divided 37 enrolled dogs into two groups: those with and without target lesions (i.e. measurable or non-measurable diseases) (Fig. 1 and Table 1). Then, 30 cases with target lesions were eligible for tumor response evaluation (Table 1). The remaining 7 cases have not been included in the tumor response rate calculation; however, their treatment response was similarly evaluated in cases with target lesions (Table 1).
Fig. 2A depicted changes in the tumor burden of the target lesions over time for each case. The waterfall plot indicated the percent change in tumor burden from baseline when cases achieved the evaluation of tumor size at the end of the first treatment cycle (Fig. 2B). Of 30 cases, case 26 was excluded from the calculation of the tumor response rate due to concomitant treatment. Of remaining 29 cases, one dog has CR, one dog has PR and 6 have SD while 14 have PD (Fig. 1), and the remaining 7 dogs could not be evaluated for tumor size at the end of the initial treatment cycle, as they either died or their owner’s consent for examinations could not be obtained. Notably, case 14 with undifferentiated sarcoma whose primary oral mass had been controlled after radiation therapy for 161 days prior to this trial showed complete tumor regression for lung metastasis by ca-4F12-E6 treatment at the first cycle (Fig. 3A). Hence, ORR and CBR were 6.9% and 27.6%, respectively. Median PFS and OS were 70 days (95% confidential interval, 46–72) and 215 days (95% confidential interval, 63–506), respectively. The tumor burden of case 6 was completely reduced on day 144 with subsequent follow-up, and case 31 revealed a PR after four treatment cycles. The treatment for three cases (cases 17, 31, and 36) remained ongoing.
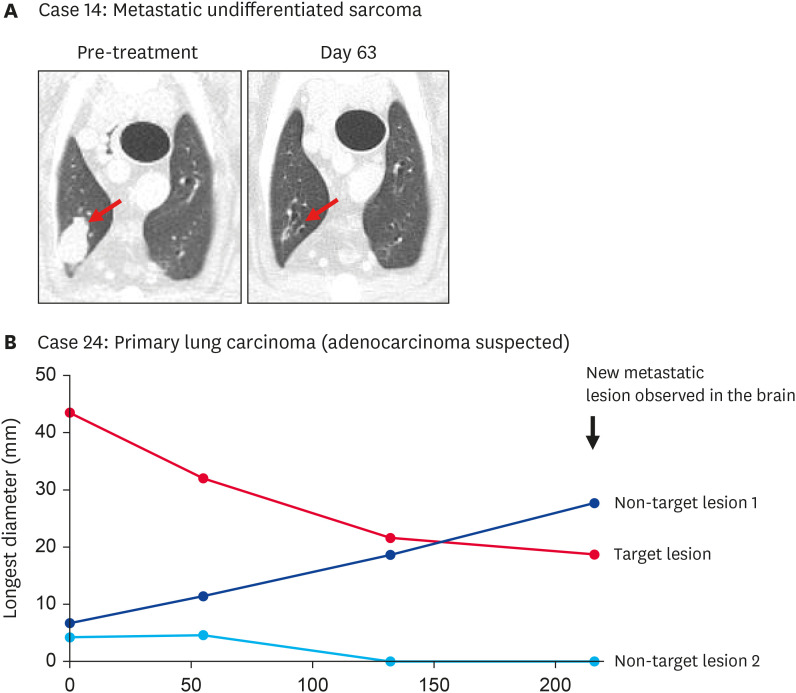
Fig. 3. The clinical course of two dogs with metastatic undifferentiated sarcoma and primary lung epithelial tumor. (A) Case 14: Representative CT scan images of lung metastasis of an undifferentiated sarcoma dog treated with ca-4F12-E6 for the first cycle. The red arrow indicates the target lesion. (B) Case 24: Measurement of the longest diameter of each lung tumor, including one target lesion and two non-target lesions. Case 24 received three cycles of ca-4F12-E6.
Tumor responses including mixed response and pseudoprogression/slow onset
Case 24 with treatment-naïve primary lung carcinoma with multiple lesions showed unique treatment responsiveness different from conventional therapies (Fig. 3B). The diameter of the target lesion and non-target lesion 2 was gradually decreased over time by ca-4F12-E6 treatment while non-target lesion 1 was apparently increased. A suspected metastatic brain lesion was detected using magnetic resonance imaging on day 216. Based on cRESIST criteria, this case categorized tumor response as PD, however, this clinical response indicated the mixed tumor response pattern, which means discordant reactivity of lesions evaluated, as sometimes seen in human patients treated with ICIs. Additionally, there was similarly mixed tumor responsiveness in cases 2, 13, and 36. Briefly, the tumor response of target lesions showed SD but new tumor lesions were observed in a lung and an iliac lymph node of case 2, and an axillary lymph node of case 36 (Table 1). In case 13, the metastatic lesions in the lung showed a mixture of shrinking and enlarging.
The result of case 12 with lung metastatic undifferentiated sarcoma revealed another unique responsiveness, such as pseudoprogression or slow onset (Supplementary Fig. 1A). The non-target lesions were enlarged at the end of the first treatment cycle. Unfortunately, the CT scan at the second time point could not be performed due to a problem with anesthesia, and the owner decided to withdraw from this trial. A CT scan revealed the first registered non-target lesions that disappeared completely but new lung metastatic lesions were observed on day 294 (Supplementary Fig. 1B). We re-started the ca-4F12-E6 administration for those new lesions. Hence, further ca-4F12-E6 treatment induced complete tumor regression on day 364. These data suggested that anti-PD-1 therapy using ca-4F12-E6 was effective in canine advanced solid tumors in addition to OMM, but variable response patterns, such as mixed responses, were identified in human studies.
IHC of TILs in a dog with SCC
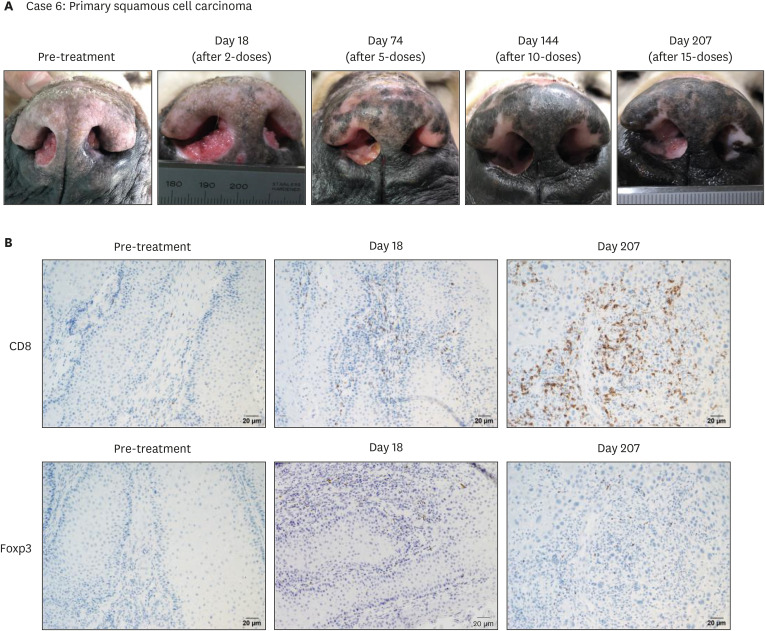
We obtained tumor tissue samples during the pre- and post-treatment (day 18 and day 207) in case 6 who was a ca-4F12-E6 treatment responder. Case 6 had a PR at the first time point of evaluation (day 74), as shown in Fig. 4A. A CR was achieved on day 144 with a continuous ca-4F12-E6 treatment. However, a relapse was observed on day 207. TILs, including cytotoxic T cells and regulatory T cells, stained with anti-CD8 and anti-Foxp3 antibodies, respectively, and were evaluated. Tumor tissues on pre-treatment indicated few T cell infiltrations (Fig. 4B). Interestingly, the IHC result showed the number of CD8-positive T cells increased after ca-4F12-E6 treatment in contrast to that of Foxp3-positive T cells slightly increased (Fig. 4B). We determined to continue ca-4F12-E6 treatment with increment dosage up to 6 mg/kg because cytotoxic T cells were accumulated in the relapsed tumor. Then, the tumor burden in case 6 was mildly decreased (Fig. 2A). This suggested that cytotoxic T cell accumulation might be induced by ca-4F12-E6 treatment even if the anti-tumor effect of those T cells had not emerged upon recurrence.
Fig. 4. Tumor response and immunohistochemistry for tumor-infiltrating lymphocytes in a case with nasal squamous cell carcinoma. (A) Case 6 received a total of four ca-4F12-E6 cycles. The pictures show the target lesion at the indicated time point. (B) Tumor-biopsy specimens with immunohistochemistry using anti-CD8 antibody or anti-Foxp3 antibody for lymphocytic markers reveal that cytotoxic T cells (indicated as CD8-positive cells) were accumulated in post-treatment tumor tissues. The peroxidase and DAB staining method was used to visualize the immunolabelling. Scale bars: 20 μm.
DISCUSSION
The present study revealed that caninized anti-canine PD-1 monoclonal antibody (ca-4F12-E6) demonstrated relatively safe and anti-tumor activity against some of the advanced canine solid tumors as observed in OMM. Some cases received > 20 ca-4F12-E6 doses, but the overall AEs rate was 35.1%. This result was relatively lower than previous canine studies (51.7%–63.3%) [4,11]. Moreover, no AEs with grade 5 were observed in the present study.
irAEs are frequently observed AEs in patients treated with ICI, and sometimes are one of the reasons for discontinuing ICI treatment in humans. Additionally, irAEs were distinct from conventional chemotherapy-related toxicities [18]. The occurrence of irAEs involved various organs, including the skin, digestive system, endocrine system, respiratory system, and urinary system. Previous studies in dogs revealed suspected irAEs in liver injury and pneumonitis [4,11]. Elevation of grade 4 alanine transaminase was observed in two cases; however, as we did not perform liver biopsies, these cases could not be assessed whether irAEs or not. Both of these cases did not show any clinical symptoms, and their liver enzymes decreased immediately with the intervention of hepatoprotective therapies such as S-adenosylmethionine and ursodeoxycholic acid. However, the present study revealed two cases representing sterile nodular panniculitis, which is the first report of irAEs involved in dog skin. The anti-CTLA4 antibody in humans is more frequently associated with dermatological irAEs than the anti-PD-1 antibody, but the anti-PD-1 antibody seems to have a wider range of symptoms. Granulomatous reactions of the skin after ICI treatments are rare in human [19,20], but the incidence of sterile nodular panniculitis is needed to investigate further clinical studies in dogs.
Moreover, case 27 showed megaesophagus, myasthenia gravis, and hypothyroidism simultaneously, maybe due to irAEs. Megaesophagus was considered to occur secondary to myasthenia gravis and/or hypothyroidism because clinical symptoms, including regurgitation, were improved using the thyroid replacement hormone and steroid therapy along with anti-PD-1 antibody discontinuation. However, those irAEs occurred after initiating the combination with toceranib phosphate, and whether the symptoms were caused either alone or in combination was unclear. Serum TSH levels are elevated although toceranib phosphate was not concluded to cause hypothyroidism [21]. There were no reports of myasthenia gravis induced by treatment with toceranib phosphate. We speculated that the combination of the anti-PD-1 antibody caused these irAEs because case 27 had received toceranib phosphate before inclusion in the present study without any AEs. The tyrosine kinase inhibitor axitinib combined with anti-PD-1 antibody pembrolizumab caused the acute onset of myasthenia gravis in addition to hypothyroidism and adrenal insufficiency in a patient with human renal cell carcinoma [22]. Therefore, further clinical studies are required, considering AEs, although the combination of toceranib phosphate and ICIs are promising for canine tumors.
ORR in non-OMM cases (6.9%) was relatively similar to that in previously reported OMM cases (7.7%–16.7%) [4,11,12]. The anti-PD-1 antibody may be considered as therapy for advanced solid tumors except for OMM despite a remarkable clinical efficacy exhibited in a significant minority of dogs. The number of infiltrating lymphocytes in tumor tissue post-treatment was increased from that of pre-treatment, as shown in Fig. 4B. This suggests that anti-PD-1 antibodies may affect not only pre-existing exhausted T cells but also newly recruited cytotoxic T cells from lymph nodes or peripheral blood [23]. Therefore, further investigation using TILs will be required to confirm the suitability of this phenomenon as a treatment-response biomarker. Recent veterinary studies established the evaluation methods of MSI-H/dMMR and IHC of PD-L1 expression [4,16]. Hence, clinical trials incorporating more dogs with each tumor type are needed using those methods to develop a predictive biomarker for treatment-response, such as human studies [24].
Atypical tumor responses, including mixed response and pseudoprogression/slow onset, were observed in five cases (cases 2, 12, 13, 24, and 36). Among these cases, some may have the potential for clinical efficacy with the anti-PD-1 antibody when observed over a more extended evaluation period. However, according to cREISCT criteria, they were all categorized as PD. Our data suggested anti-PD-1 antibodies may take time to develop efficacy, i.e., unlike conventional chemotherapies, tumor response differs from PD to CR depending on the timing of evaluation. In particular, early diagnosis of PD may lead to discontinuation of treatment. Novel immunotherapies, such as ICIs, have been observed in distinct immune-related tumor response patterns in human patients, which trigger changes in the new criteria irRECIST or iRECIST from traditional RECIST [25,26]. We suggest that a similar evaluation method to replace cRECIST is needed in dogs, following humans.
In conclusion, the present study described the safety profile and clinical activity of the caninized anti-canine PD-1 antibody, ca-4F12-E6, in various types of canine tumors. The administration of ca-4F12-E6 was well-tolerated and had objective responses in dogs with SCC and undifferentiated sarcoma. However, study limitations included the small number of cases with each tumor type, the absence of optimal disease controls, unconfirmed PD-1 and PD-L1 expression on tumor tissues due to lack of commercially available antibodies, and inconsistency with the prior and concurrent treatment. However, the present study influences the development of a novel immunotherapeutic approach in veterinary medicine. It is necessary to plan further clinical trials based on this pilot study to validate the treatment efficacy more accurately. It is also crucial to investigate whether the treatment responsiveness of PD-1 antibodies changes due to prior therapy.
ACKNOWLEDGMENTS
The authors would like to acknowledge the all YUAMEC clinical staff and the laboratory members for helping us succeed in the present study. Finally, we thank the dogs and their owners for making this study possible.
Footnotes
Funding: This work was supported by JSPS KAKENHI Grants Number 21H04754.
Conflict of Interest: Takuya Mizuno received research funding from Nippon Zenyaku Kogyo Co., Ltd. The remaining authors declare no conflicts of interest.
- Conceptualization: Igase M, Mizuno T.
- Data curation: Igase M, Itamoto K, Sunahara H, Nemoto Y, Tani K, Horikirizono H, Nakaichi M, Baba K.
- Formal analysis: Igase M, Itamoto K, Sunahara H, Nemoto Y, Tani K, Horikirizono H, Nakaichi M, Baba K.
- Funding acquisition: Mizuno T.
- Investigation: Igase M, Inanaga S, Nishibori S, Sakai Y, Sakurai M.
- Methodology: Igase M, Mizuno T.
- Resources: Kato M, Tsukui T.
- Supervision: Kambayashi S, Okuda M.
- Validation: Kambayashi S, Okuda M.
- Writing - original draft: Igase M.
- Writing - review & editing: Mizuno T.
SUPPLEMENTARY MATERIALS
Inclusion and exclusion criteria in the present study
A detailed description of tumor response in case 12 with metastatic undifferentiated sarcoma. (A) Measurement of the longest diameter of each lung tumor as non-target lesions. Case 12 received seven ca-4F12-E6 cycles. (B) Representative CT scan images of lung metastasis. Red arrows indicate tumor lesions.
References
- 1.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Twomey JD, Zhang B. Cancer immunotherapy update: FDA-approved checkpoint inhibitors and companion diagnostics. AAPS J. 2021;23(2):39. doi: 10.1208/s12248-021-00574-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marcus L, Lemery SJ, Keegan P, Pazdur R. FDA approval summary: pembrolizumab for the treatment of microsatellite instability-high solid tumors. Clin Cancer Res. 2019;25(13):3753–3758. doi: 10.1158/1078-0432.CCR-18-4070. [DOI] [PubMed] [Google Scholar]
- 4.Maekawa N, Konnai S, Nishimura M, Kagawa Y, Takagi S, Hosoya K, et al. PD-L1 immunohistochemistry for canine cancers and clinical benefit of anti-PD-L1 antibody in dogs with pulmonary metastatic oral malignant melanoma. NPJ Precis Oncol. 2021;5(1):10. doi: 10.1038/s41698-021-00147-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maekawa N, Konnai S, Okagawa T, Nishimori A, Ikebuchi R, Izumi Y, et al. Immunohistochemical analysis of PD-L1 expression in canine malignant cancers and PD-1 expression on lymphocytes in canine oral melanoma. PLoS One. 2016;11(6):e0157176. doi: 10.1371/journal.pone.0157176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shosu K, Sakurai M, Inoue K, Nakagawa T, Sakai H, Morimoto M, et al. Programmed cell death ligand 1 expression in canine cancer. In Vivo. 2016;30(3):195–204. [PubMed] [Google Scholar]
- 7.Pinard CJ, Hocker SE, Poon AC, Inkol JM, Matsuyama A, Wood RD, et al. Evaluation of PD-1 and PD-L1 expression in canine urothelial carcinoma cell lines. Vet Immunol Immunopathol. 2022;243:110367. doi: 10.1016/j.vetimm.2021.110367. [DOI] [PubMed] [Google Scholar]
- 8.Nemoto Y, Shosu K, Okuda M, Noguchi S, Mizuno T. Development and characterization of monoclonal antibodies against canine PD-1 and PD-L1. Vet Immunol Immunopathol. 2018;198:19–25. doi: 10.1016/j.vetimm.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 9.Choi JW, Withers SS, Chang H, Spanier JA, De La Trinidad VL, Panesar H, et al. Development of canine PD-1/PD-L1 specific monoclonal antibodies and amplification of canine T cell function. PLoS One. 2020;15(7):e0235518. doi: 10.1371/journal.pone.0235518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pinard CJ, Stegelmeier AA, Bridle BW, Mutsaers AJ, Wood RD, Wood GA, et al. Evaluation of lymphocyte-specific programmed cell death protein 1 receptor expression and cytokines in blood and urine in canine urothelial carcinoma patients. Vet Comp Oncol. 2022;20(2):427–436. doi: 10.1111/vco.12788. [DOI] [PubMed] [Google Scholar]
- 11.Igase M, Nemoto Y, Itamoto K, Tani K, Nakaichi M, Sakurai M, et al. A pilot clinical study of the therapeutic antibody against canine PD-1 for advanced spontaneous cancers in dogs. Sci Rep. 2020;10(1):18311. doi: 10.1038/s41598-020-75533-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maekawa N, Konnai S, Takagi S, Kagawa Y, Okagawa T, Nishimori A, et al. A canine chimeric monoclonal antibody targeting PD-L1 and its clinical efficacy in canine oral malignant melanoma or undifferentiated sarcoma. Sci Rep. 2017;7(1):8951. doi: 10.1038/s41598-017-09444-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Igase M, Inanaga S, Tani K, Nakaichi M, Sakai Y, Sakurai M, et al. Long-term survival of dogs with stage 4 oral malignant melanoma treated with anti-canine PD-1 therapeutic antibody: a follow-up case report. Vet Comp Oncol. 2022;20(4):901–905. doi: 10.1111/vco.12829. [DOI] [PubMed] [Google Scholar]
- 14.LeBlanc AK, Atherton M, Bentley RT, Boudreau CE, Burton JH, Curran KM, et al. Veterinary Cooperative Oncology Group-Common Terminology Criteria for Adverse Events (VCOG-CTCAE v2) following investigational therapy in dogs and cats. Vet Comp Oncol. 2021;19(2):311–352. doi: 10.1111/vco.12677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nguyen SM, Thamm DH, Vail DM, London CA. Response evaluation criteria for solid tumours in dogs (v1.0): a Veterinary Cooperative Oncology Group (VCOG) consensus document. Vet Comp Oncol. 2015;13(3):176–183. doi: 10.1111/vco.12032. [DOI] [PubMed] [Google Scholar]
- 16.Inanaga S, Igase M, Sakai Y, Hagimori K, Sunahara H, Horikirizono H, et al. Relationship of microsatellite instability to mismatch repair deficiency in malignant tumors of dogs. J Vet Intern Med. 2022;36(5):1760–1769. doi: 10.1111/jvim.16454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakai O, Ii T, Uchida K, Igase M, Mizuno T. Establishment and characterization of monoclonal antibody against canine CD8 alpha. Monoclon Antib Immunodiagn Immunother. 2020;39(4):129–134. doi: 10.1089/mab.2020.0002. [DOI] [PubMed] [Google Scholar]
- 18.Martins F, Sofiya L, Sykiotis GP, Lamine F, Maillard M, Fraga M, et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. 2019;16(9):563–580. doi: 10.1038/s41571-019-0218-0. [DOI] [PubMed] [Google Scholar]
- 19.Singh P, Wolfe SP, Alloo A, Gottesman SP. Interstitial granulomatous dermatitis and granulomatous arteritis in the setting of PD-1 inhibitor therapy for metastatic melanoma. J Cutan Pathol. 2020;47(1):65–69. doi: 10.1111/cup.13562. [DOI] [PubMed] [Google Scholar]
- 20.Trinidad C, Nelson KC, Glitza Oliva IC, Torres-Cabala CA, Nagarajan P, Tetzlaff MT, et al. Dermatologic toxicity from immune checkpoint blockade therapy with an interstitial granulomatous pattern. J Cutan Pathol. 2018;45(7):504–507. doi: 10.1111/cup.13150. [DOI] [PubMed] [Google Scholar]
- 21.Harper A, Blackwood L, Mason S. Investigation of thyroid function in dogs treated with the tyrosine kinase inhibitor toceranib. Vet Comp Oncol. 2020;18(3):433–437. doi: 10.1111/vco.12538. [DOI] [PubMed] [Google Scholar]
- 22.Figueroa-Perez N, Kashyap R, Bal D, Anjum Khan S, Pattan V. Autoimmune myasthenia, primary adrenal insufficiency, and progressive hypothyroidism due to pembrolizumab and axitinib combination regimen. Cureus. 2021;13(8):e16933. doi: 10.7759/cureus.16933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Callahan MK, Wolchok JD. Recruit or reboot? How does anti-PD-1 therapy change tumor-infiltrating lymphocytes? Cancer Cell. 2019;36(3):215–217. doi: 10.1016/j.ccell.2019.08.009. [DOI] [PubMed] [Google Scholar]
- 24.Havel JJ, Chowell D, Chan TA. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat Rev Cancer. 2019;19(3):133–150. doi: 10.1038/s41568-019-0116-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seymour L, Bogaerts J, Perrone A, Ford R, Schwartz LH, Mandrekar S, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18(3):e143–e152. doi: 10.1016/S1470-2045(17)30074-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tazdait M, Mezquita L, Lahmar J, Ferrara R, Bidault F, Ammari S, et al. Patterns of responses in metastatic NSCLC during PD-1 or PDL-1 inhibitor therapy: Comparison of RECIST 1.1, irRECIST and iRECIST criteria. Eur J Cancer. 2018;88:38–47. doi: 10.1016/j.ejca.2017.10.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Inclusion and exclusion criteria in the present study
A detailed description of tumor response in case 12 with metastatic undifferentiated sarcoma. (A) Measurement of the longest diameter of each lung tumor as non-target lesions. Case 12 received seven ca-4F12-E6 cycles. (B) Representative CT scan images of lung metastasis. Red arrows indicate tumor lesions.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.