Abstract
The tuberculin skin test currently used to diagnose infection with Mycobacterium tuberculosis has poor diagnostic value, especially in geographic areas where the prevalence of tuberculosis is low or where the environmental burden of saprophytic, nontuberculous mycobacteria is high. Inaccuracy of the tuberculin skin test often reflects a low diagnostic specificity due to the presence in tuberculin of antigens shared by many mycobacterial species. Thus, a skin test specific for tuberculosis requires the development of new tuberculins consisting of antigens specific to M. tuberculosis. We have formulated cocktails of two to eight antigens of M. tuberculosis purified from recombinant Escherichia coli. Multiantigen cocktails were evaluated by skin testing guinea pigs sensitized with M. bovis BCG. Reactivity of multiantigen cocktails was greater than that of any single antigen. Cocktail activity increased with the number of antigens in the cocktail even when the same amount of total protein was used for cocktails and for each single antigen. A cocktail of four purified antigens specific for the M. tuberculosis complex elicited skin test responses only in BCG-immunized guinea pigs, not in control animals immunized with M. avium. These findings open the way to designing a multiantigen formulation for a skin test specific for tuberculosis.
Identification of individuals infected with Mycobacterium tuberculosis, who account for approximately one-third of the world’s population (36), is of paramount importance for the control of tuberculosis (TB). TB control programs are usually established on the basis of the proportion of infected individuals in a given community (10). Moreover, infection with M. tuberculosis often constitutes an indication for prophylactic chemotherapy against TB, especially in individuals at risk of rapid progression to disease (6). The method currently used to detect infection with M. tuberculosis, the tuberculin skin test, is based on measuring delayed-type hypersensitivity (DTH) responses (local skin induration and erythema) to the intradermal injection of purified protein derivative (PPD) of tuberculin (reviewed in references 5 and 6). Unfortunately, the tuberculin skin test has low diagnostic specificity, because PPD contains antigens that are shared by many mycobacterial species (8, 11). Thus, a positive test result is not necessarily associated with M. tuberculosis infection but may also be caused by immune cross-reactions in individuals vaccinated with the bacille Calmette-Guérin (BCG) attenuated strain of M. bovis or sensitized with nontuberculous mycobacteria (5, 6). Cross-reactions greatly complicate interpretation of skin test results in subjects living in or originating from geographic areas with a high environmental load of nontuberculous mycobacteria (4–6). Thus, there is a need to develop new reagents that are specific for TB to overcome the limitations of the current tuberculin skin test.
Development of new TB-specific tuberculins requires identification of M. tuberculosis-specific antigens that elicit DTH responses in TB. Since measurement of DTH activity is usually part of the characterization of M. tuberculosis antigens, many antigens active in DTH-based assays have been described (for a partial list, see references 15, 23, 26 to 28, and 38). However, efforts to identify a potent, species-specific antigen that could replace PPD for skin testing have been disappointing. For example, in a study performed with human volunteers (35), only few PPD-positive subjects responded to MPT64, an M. tuberculosis complex-specific antigen that elicits strong DTH responses in tuberculous guinea pigs (28). We argue that a single antigen, however potent, is bound to be inadequate for skin testing because (i) one antigen may contain too few epitopes to recruit to the site of antigen injection the number of DTH effector T cells necessary to obtain a response measurable by skin testing and (ii) antigen recognition in TB is broad and highly variable from individual to individual (21, 32). Thus, multiple antigens should be required to detect infection with M. tuberculosis by skin testing.
To evaluate cocktails of multiple antigens for skin testing, we chose antigens found in the filtrate of M. tuberculosis cell cultures because culture filtrate antigens are usually potent in DTH-based immunoassays (15, 23, 26, 27). Culture filtrate antigens were purified as recombinant proteins from Escherichia coli cells and tested in combination for DTH responses in guinea pigs sensitized with M. bovis BCG, an avirulent member of the M. tuberculosis complex. To assess specificity for the M. tuberculosis complex in skin test, multiantigen cocktails were also tested in control guinea pigs immunized with M. avium, a nontuberculous mycobacterial species commonly found in the environment. We report that (i) skin test activity of a cocktail is greater than that of any single antigen and increases with the number of antigens in the cocktail, even when the same amount of total protein is used for the cocktail and for each single antigen, and (ii) a cocktail of M. tuberculosis complex-specific antigens elicits DTH responses in BCG-immunized guinea pigs but not in M. avium-immunized animals. These findings indicate that the use of multiantigen cocktails should yield a new, specific skin test for TB.
MATERIALS AND METHODS
Bacterial strains and products.
E. coli strains were grown in standard liquid and solid media (31). M. bovis BCG Japanese ATCC 35737 and M. avium ATCC 25291 were obtained from the American Type Culture Collection. The Japanese substrain of BCG was chosen because it produces at high levels the MPB64 antigen (19), whose M. tuberculosis homolog, MPT64, was used in this study. Mycobacteria were grown at 37°C in rotating bottles in 7H9 medium enriched with 0.05% Tween 80 and standard albumin-dextrose additive. PPD produced from M. tuberculosis (PPD-CT-68) was purchased from Connaught Laboratories Inc. (Swiftwater, Pa.). PPDs from M. bovis and from M. avium were purchased from Kursk Biofactory (Kursk, Russia).
Gene cloning and protein purification.
Ten genes encoding M. tuberculosis culture filtrate proteins (Table 1) were cloned in the pQE30 (Qiagen) plasmid vector of E. coli as described previously (23, 24). Recombinant proteins were expressed as NH2-terminally polyhistidine-tagged fusion proteins and purified to near homogeneity from E. coli cells by using a three-step protocol consisting of sequential chromatography with metal chelate affinity, size exclusion, and anion-exchange columns, as reported elsewhere (7).
TABLE 1.
Recombinant protein antigens of M. tuberculosis used in this study
Guinea pig sensitization.
Groups of six female guinea pigs of the outbred strain Hsd:DH (Harlan Sprague Dawley) weighing 300 to 350 g were sensitized by intradermal injection in the abdomen with 107 live M. bovis BCG Japanese or M. avium cells in 0.2 ml of phosphate-buffered saline (PBS), pH 7.2.
Skin tests.
Five to eight weeks after sensitization, animals were shaved on the back and injected intradermally with 2 μg of each purified antigen in 0.1 ml of PBS or with 0.5 to 8 μg of multiantigen cocktails in 0.1 ml of PBS, as indicated. Each animal was also injected with 10 tuberculin units (TU) of PPD as a control for sensitization. Skin reactions (diameters of erythema, in millimeters) were independently measured 24 h after antigen injection by two investigators. Single purified antigens and antigen cocktails were tested in three or four separate experiments. Results were expressed as means of diameters of erythema ± standard deviations.
RESULTS
Skin test reactivity of recombinant antigens of M. tuberculosis.
Ten purified recombinant proteins of M. tuberculosis (Table 1) were tested for tuberculin-like activity and specificity to the M. tuberculosis complex, using two groups of guinea pigs, one sensitized with M. bovis BCG and the other sensitized with the nontuberculous species M. avium. Measurement of skin reactions to PPDs from M. bovis and M. avium indicated that similar degrees of sensitization were obtained in the two groups of animals (Table 2), a prerequisite for interpretation of results obtained with purified antigens. All 10 purified antigens elicited DTH responses of similar intensities in the BCG-immunized group (Table 2). In contrast, DTH responses in the M. avium-immunized guinea pigs differed from antigen to antigen. Some were equally active in the BCG- and M. avium-immunized animals, while others displayed little, if any, reactivity in the M. avium-immunized group. The specificity index (SpI) (Table 2), which was obtained by dividing sizes of skin reactions in the BCG-immunized group by those obtained in the M. avium-immunized group, measured specificity of antigen for the M. tuberculosis complex. Six antigens were cross-reactive (SpI ∼ 1 [Table 2]). The presence of homologous proteins in M. avium or the demonstration of shared T-cell epitopes has been reported for some of these antigens by us (23) and others (14, 17, 20, 30). Four antigens (MPT63, MPT64, MTC28, and MPT70) elicited DTH responses 8 to 15 times stronger in BCG- than in M. avium-immunized guinea pigs (Table 2). Specificity for tubercle bacilli in guinea pig skin tests has already been reported for three of these four antigens by us (MTC28) (23) and others (MPT64 and MPT70) (2, 12, 26). The quantitative assessment of skin test reactivity and specificity for the M. tuberculosis complex obtained for each antigen in this set of experiments provided baseline values to evaluate multiantigen cocktails.
TABLE 2.
Guinea pig DTH responses to purified proteins of M. tuberculosis
| Antigen | Diam (mm) of skin test reactions in guinea pigs immunized witha:
|
SpIb | |
|---|---|---|---|
| M. bovis BCG | M. avium | ||
| Tuberculins | |||
| PPD-t | 8.9 ± 1.3 | 8.0 ± 1.4 | 1.11 |
| PPD-b | 12.1 ± 1.3 | 9.5 ± 1.5 | 1.27 |
| PPD-a | 8.0 ± 1.5 | 12.6 ± 1.4 | 0.63 |
| Cross-reactive antigens | |||
| 19 kDa | 7.0 ± 2.0 | 9.3 ± 1.6 | 0.75 |
| MPT51 | 6.2 ± 0.5 | 5.0 ± 0.7 | 1.24 |
| Ag85B | 7.9 ± 2.1 | 8.9 ± 0.8 | 0.89 |
| 38 kDa | 6.4 ± 0.9 | 5.0 ± 0.8 | 1.28 |
| MPT32 | 7.2 ± 1.3 | 7.8 ± 1.7 | 0.92 |
| KatG | 7.4 ± 1.2 | 4.1 ± 0.9 | 1.80 |
| Specific antigens | |||
| MPT63 | 7.5 ± 1.6 | 0.5 ± 1.0 | 15.00 |
| MPT70 | 7.6 ± 1.2 | 1.0 ± 1.2 | 7.60 |
| MPT64 | 7.6 ± 1.0 | 0.5 ± 1.0 | 15.20 |
| MTC28 | 6.6 ± 1.4 | 0.8 ± 1.5 | 8.25 |
Means ± standard deviations of diameters of erythema after intradermal injection of 2 μg of each purified antigen in six guinea pigs immunized with M. bovis BCG and six guinea pigs immunized with M. avium, as described in Materials and Methods; 10-TU doses of PPD from M. tuberculosis (PPD-t), M. bovis (PPD-b), and M. avium (PPD-a) were used as controls for sensitization.
Determined by dividing mean diameters of skin reactions measured in BCG-immunized guinea pigs by those in M. avium-immunized animals.
Activity of multiantigen cocktails.
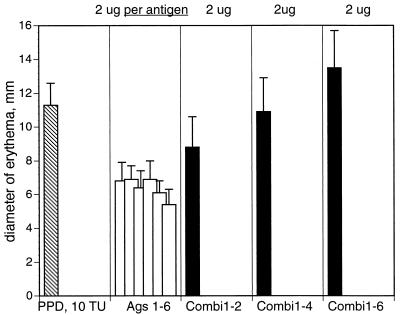
We next formulated multiantigen cocktails and compared the activity of cocktails with that of each antigen in the cocktails by skin testing BCG-immunized guinea pigs. In this set of experiments, we used six antigens (MPT63, MPT64, MTC28, MPT32, MPT51, and 38 kDa) to formulate cocktails containing two, four, and six antigens. Single antigens and antigen cocktails were all tested at the same amount (2 μg) of total protein. Skin test reactivity of the cocktails was greater than that of any single antigen and increased with the number of antigens in the cocktail (Fig. 1), even though the amount of each antigen in the cocktail decreased. This finding suggests an additive, perhaps even cooperative, effect of multiple T-cell epitopes on the development of DTH responses elicited by antigen cocktails.
FIG. 1.
Skin test reactivity of purified recombinant antigens of M. tuberculosis tested singly and in combinations in guinea pigs immunized with M. bovis BCG. Six guinea pigs were sensitized as described in Materials and Methods. Five weeks after sensitization, animals were intradermally injected with 10 TU of PPD and 2 μg of purified recombinant antigens, singly or in combination. Results are expressed as the means (plus standard deviations) of the diameters of erythema measured 24 h after antigen injection. Ags 1–6, six antigens injected singly (1, MPT63; 2, MPT64; 3, MTC28; 4, MPT32; 5, MPT51; 6, 38 kDa); Combi1-2, two-antigen cocktail (antigens 1 and 2); Combi1-4, four-antigen cocktail (antigens 1 through 4); Combi1-6, six-antigen cocktail (antigens 1 through 6).
Specificity of multiantigen cocktails.
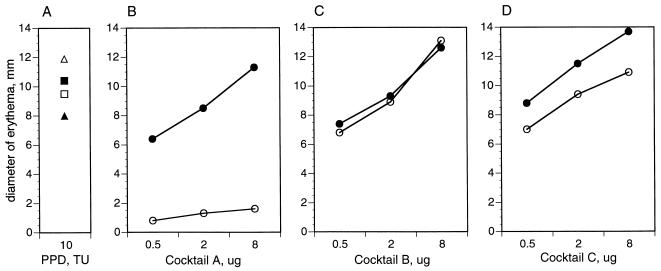
We next set out to assess skin test specificity of multiantigen cocktails for tuberculous mycobacteria. We formulated three cocktails. Cocktail A contained four M. tuberculosis complex-specific antigens (MPT63, MPT64, MTC28, and MPT70) (Table 2), cocktail B contained four cross-reactive antigens (MPT51, MPT32, Ag85B, and KatG) (Table 2), and cocktail C contained all eight antigens present in cocktails A and B. Each of the three multiantigen cocktails was evaluated at different concentrations (from 0.5 to 8 μg) by skin testing guinea pigs sensitized with BCG and with M. avium. Both animal groups responded to PPD from M. bovis and PPD from M. avium with similar-size skin reactions (Fig. 2A). In sharp contrast, BCG-immunized, but not M. avium-immunized, animals mounted DTH responses to the specific cocktail A (Fig. 2B). Both groups of animals gave DTH responses similar in strength to that of the cross-reactive cocktail B (Fig. 2C). Cocktail C, a mixture of specific plus cross-reactive antigens, was of intermediate specificity, as it elicited slightly stronger responses in the BCG-immunized animals than in the group immunized with M. avium (Fig. 2D). Thus, the specificity of the antigen cocktail is that of its components.
FIG. 2.
Skin test reactivity of multiantigen cocktails in guinea pigs immunized with M. bovis BCG (solid symbols) and with M. avium (open symbols). Six guinea pigs were sensitized and skin tested as described in Materials and Methods. In this set of experiments, animals were skin tested with 10 TU of PPD and increasing amounts (0.5 to 8 μg) of multiantigen cocktails. Results are expressed as means of the diameters of erythema measured 24 h after antigen injection. Cocktail A, four M. tuberculosis complex-specific antigens (MPT63, MPT64, MTC28, and MPT70); cocktail B, four cross-reactive antigens (MPT51, Ag85B, MPT32, and KatG); cocktail C, eight-antigen cocktail (antigens in cocktails A plus B). ■, □, M. bovis PPD; ▴, ▵, M. avium PPD; •, ○, cocktails of purified antigens.
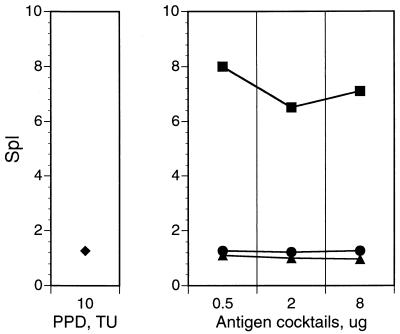
Results shown in Fig. 2 also indicated that skin reactivity of cocktails was dose dependent, with cocktails used in the range of 2 to 8 μg of total protein eliciting skin reactions similar in size to those elicited by 10 TU of PPD (compare Fig. 2B to D to Fig. 2A). In contrast, cocktail specificity for the M. tuberculosis complex was dose independent (Fig. 3). This property is important, because high doses of the immunoreagent may be required in skin tests for accurate discrimination between M. tuberculosis-infected and noninfected individuals.
FIG. 3.
Specificity for tuberculous mycobacteria of multiantigen cocktails tested at different doses. Protocols of guinea pig sensitization and skin testing with PPD and multiantigen cocktails were as described in the legend to Fig. 2. SpI was calculated as described in Table 2, footnote b. ⧫, PPD; ■, cocktail A (M. tuberculosis complex specific); ▴, cocktail B (cross-reactive); •, cocktail C (antigens in cocktails A plus B).
DISCUSSION
The findings described in this report establish that (i) cocktails of purified antigens of M. tuberculosis are significantly more active in skin tests than any of the single antigens in the cocktail and (ii) a cocktail retains the specificity to tubercle bacilli of the antigens in the cocktail. Involvement of many antigens in DTH responses to infection with tubercle bacilli (all 10 antigens tested in the present study [Table 2]), together with the above-described properties in skin tests of multiantigen cocktails, provide a basis for the rational design of a skin test specific for TB that uses cocktails of purified, M. tuberculosis complex-specific antigens.
A requirement for multiple, rather than single, purified antigens as skin test reagents can be due to several factors. First, multiantigen formulations can presumably recruit many antigen-specific T cells to the site of antigen injection to afford a skin reaction of the appropriate size. Second, numerous antigens may be required to overcome problems related to genetic restriction in antigen recognition (9) that causes some individuals to react to certain antigens and not to others.
Formulations of purified antigens offer several advantages over PPD. First, PPD is a highly cross-reactive antigen that does not always allow distinction between tuberculous infection, infection with nonpathogenic mycobacteria, and vaccination with BCG. The present study indicates that use of M. tuberculosis complex-specific antigens can yield a skin test that discriminates between tuberculous infection and infection with M. avium, a nonpathogenic mycobacterial species commonly found in the environment. Further experimentation may be needed to evaluate skin test specificity of cocktails containing M. tuberculosis complex-specific antigens vis-à-vis sensitization with additional nontuberculous mycobacteria. Similar principles can also be applied to the design of multiantigen cocktails that may possibly discriminate between BCG vaccination and infection with virulent tuberculous mycobacteria by selecting antigens, such as ESAT-6 (33) and MPT64, that are produced by virulent mycobacteria but are absent in all (ESAT-6) or some (MPB64) BCG substrains (13, 19, 22). A second advantage is that the use of purified recombinant antigens should facilitate manufacturing and quality control of skin test reagents.
Antigens of M. tuberculosis should be purified as recombinant proteins, since the large-scale requirement for skin test reagents (many million doses are used each year worldwide) is incompatible with purification of native protein from M. tuberculosis cells. Recombinant proteins purified from E. coli should be suitable reagents for diagnostic skin testing, since several recombinant antigens were found indistinguishable in guinea pig skin tests vis-à-vis the corresponding native proteins by us (MPB70, MPT63, Ag85B, and MPT51) (our unpublished observations) and others (MPT64) (28). Should optimal activity of certain antigens require posttranslational modification of protein, which might occur in M. tuberculosis but not in E. coli, alternative recombinant DNA techniques in fast-growing, nonpathogenic mycobacteria could be adopted.
The present study strongly suggests that a multiantigen cocktail should be more effective than PPD or single antigens as a reagent for TB skin testing. The choice of antigens to formulate optimal immunodiagnostic cocktails for human use will have to be guided by ex vivo and in vivo human studies.
ACKNOWLEDGMENTS
We thank Maarten Bosland and staff of the Nelson Institute Environmental Medicine, New York University Medical Center (Tuxedo, N.Y.) for excellent animal care and assistance in handling of guinea pigs; Harald Wiker for proteins purified from culture filtrates of M. tuberculosis and M. bovis BCG; and Karl Drlica and Jeannie Dubnau for comments on the manuscript.
This work was supported by NIH grant AI-36896 (M.L.G). C.M. was a Fellow in the Graduate Program of Microbial Technologies, Department of Agricultural Sciences, University of Sassari, Sassari, Italy. R.C. was the recipient of an AIDS training fellowship from the Istituto Superiore di Sanità, Rome, Italy.
REFERENCES
- 1.Andersen Å B, Hansen E B. Structure and mapping of antigenic domains of protein antigen b, a 38,000-molecular-weight protein of Mycobacterium tuberculosis. Infect Immun. 1989;57:2481–2488. doi: 10.1128/iai.57.8.2481-2488.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen Å B, Kjungqvist L, Hasløv K, Bentzon M W. MPT64 possesses “tuberculosis-complex”-specific B- and T-cell epitopes. Scand J Immunol. 1991;34:365–372. doi: 10.1111/j.1365-3083.1991.tb01558.x. [DOI] [PubMed] [Google Scholar]
- 3.Ashbridge K R A, Booth R J, Watson J D, Lathigra R B. Nucleotide sequence of the 19kDa antigen gene from Mycobacterium tuberculosis. Nucleic Acids Res. 1989;17:1249. doi: 10.1093/nar/17.3.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bass J B, Sanders R V, Kirkpatrick M B. Choosing an appropriate cutting point for conversion in annual tuberculin skin testing. Am Rev Respir Dis. 1985;132:379–381. doi: 10.1164/arrd.1985.132.2.379. [DOI] [PubMed] [Google Scholar]
- 5.Bass J B., Jr . The tuberculin test. In: Reichmann L B, Hershfield E S, editors. Tuberculosis. A comprehensive international approach. New York, N.Y: Marcel Dekker, Inc.; 1993. pp. 139–148. [Google Scholar]
- 6.Bates J H. The tuberculin skin test and preventive treatment for tuberculosis. In: Rom W N, Garay S, editors. Tuberculosis. Boston, Mass: Little, Brown and Co.; 1996. pp. 865–872. [Google Scholar]
- 7.Colangeli, R., A. Heijbel, A. Williams, C. Manca, J. Chan, K. Lyashchenko, and M. L. Gennaro. Three-step purification of lipopolysaccharide-free, polyhistidine-tagged recombinant antigens of Mycobacterium tuberculosis. J. Chromatogr., in press. [DOI] [PubMed]
- 8.Daniel T M, Janicki B W. Mycobacterial antigens: a review of their isolation, chemistry, and immunological properties. Microbiol Rev. 1978;42:84–113. doi: 10.1128/mr.42.1.84-113.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.deVries R R P. Regulation of T cell responsiveness against mycobacterial antigens by HLA class 2 immune response genes. Rev Infect Dis. 1989;11:S400–S403. doi: 10.1093/clinids/11.supplement_2.s400. [DOI] [PubMed] [Google Scholar]
- 10.Enarson D A, Murray J F. Global epidemiology of tuberculosis. In: Rom W N, Garay S, editors. Tuberculosis. Boston, Mass: Little, Brown and Co.; 1996. pp. 57–76. [Google Scholar]
- 11.Harboe M. Antigens of PPD, old tuberculin, and autoclaved Mycobacterium bovis BCG studied by crossed immunoelectrophoresis. Am Rev Respir Dis. 1981;124:80–87. doi: 10.1164/arrd.1981.124.1.80. [DOI] [PubMed] [Google Scholar]
- 12.Harboe M, Nagai S, Patarroyo M E, Torres M, Ramirez C, Cruz N. Properties of MPB64, MPB70, and MPB80 of Mycobacterium bovis BCG. Infect Immun. 1986;52:293–302. doi: 10.1128/iai.52.1.293-302.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harboe M, Oettinger T, Wiker H G, Rosenkrands L, Andersen P. Evidence for occurrence of the ESAT-6 protein in Mycobacterium tuberculosis and virulent Mycobacterium bovis and for its absence in Mycobacterium bovis BCG. Infect Immun. 1996;64:16–22. doi: 10.1128/iai.64.1.16-22.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris D P, Vordermeier H M, Roman E, Lathigra R, Brett S J, Moreno C, Ivanyi J. Murine T cell-stimulatory peptides from the 19-kDa antigen of Mycobacterium tuberculosis. J Immunol. 1991;147:2706–2712. [PubMed] [Google Scholar]
- 15.Hasløv K, Andersen Å, Nagai S, Gottschau A, Sørensen T, Andersen P. Guinea pig cellular immune responses to proteins secreted by Mycobacterium tuberculosis. Infect Immun. 1995;63:804–810. doi: 10.1128/iai.63.3.804-810.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heym B, Zhang Y, Pulet S, Young D, Cole S T. Characterization of the katG gene encoding a catalase-peroxidase required for the isoniazid susceptibility of Mycobacterium tuberculosis. J Bacteriol. 1993;175:4255–4259. doi: 10.1128/jb.175.13.4255-4259.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kadival G V, Chaparas S D, Hussong D. Characterization of serologic and cell-mediated reactivity of a 38 kDa antigen isolated from Mycobacterium tuberculosis. J Immunol. 1987;139:2447–2451. [PubMed] [Google Scholar]
- 18.Laqueyrerie A, Militzer P, Romain F, Eiglmeier K, Cole S, Marchal G. Cloning, sequencing, and expression of the apa gene coding for the Mycobacterium tuberculosis 45/47-kilodalton secreted antigen complex. Infect Immun. 1995;63:4003–4010. doi: 10.1128/iai.63.10.4003-4010.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li H, Ulstrup J C, Jonassen T Ø, Melby K, Nagai S, Harboe M. Evidence for the absence of the MPB64 gene in some substrains of Mycobacterium bovis BCG. Infect Immun. 1993;61:1730–1734. doi: 10.1128/iai.61.5.1730-1734.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lozes E, Denis O, Drowart A, Jurion F, Palfliet K, Vanonckelen A, Bruyn J D, Cock M D, Vooren J-P V, Huygen K. Cross-reactive immune responses against Mycobacterium bovis BCG in mice infected with non-tuberculous mycobacteria belonging to the MAIS group. Scand J Immunol. 1997;46:16–26. doi: 10.1046/j.1365-3083.1997.d01-99.x. [DOI] [PubMed] [Google Scholar]
- 21.Lyashchenko K, Colangeli R, Houde M, Al Jahdali H, Menzies D, Gennaro M L. Heterogeneous antibody responses in tuberculosis. Infect Immun. 1998;66:3936–3940. doi: 10.1128/iai.66.8.3936-3940.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahairas G G, Sabo P J, Hickey M J, Singh D C, Stover C K. Molecular analysis of genetic differences between Mycobacterium bovis BCG and virulent M. bovis. J Bacteriol. 1996;178:1274–1282. doi: 10.1128/jb.178.5.1274-1282.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manca C, Lyashchenko K, Colangeli R, Gennaro M L. MTC28, a novel 28-kilodalton proline-rich secreted antigen specific for the Mycobacterium tuberculosis complex. Infect Immun. 1997;65:4951–4957. doi: 10.1128/iai.65.12.4951-4957.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manca C, Lyashchenko K, Wiker H G, Usai D, Colangeli R, Gennaro M L. Molecular cloning, purification, and serological characterization of MPT63, a novel secreted antigen of Mycobacterium tuberculosis. Infect Immun. 1997;65:16–23. doi: 10.1128/iai.65.1.16-23.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsuo K, Yamaguchi R, Yamazaki A, Tasaka H, Yamada T. Cloning and expression of the Mycobacterium bovis BCG gene for extracellular α-antigen. J Bacteriol. 1988;170:3847–3854. doi: 10.1128/jb.170.9.3847-3854.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagai S, Matsumoto J, Nagasuga T. Specific skin test-reactive protein from culture filtrate of Mycobacterium bovis BCG. Infect Immun. 1981;31:1152–1160. doi: 10.1128/iai.31.3.1152-1160.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagai S, Wiker H G, Harboe M, Kinomoto M. Isolation and partial characterization of major protein antigens in the culture fluid of Mycobacterium tuberculosis. Infect Immun. 1991;59:372–382. doi: 10.1128/iai.59.1.372-382.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oettinger T, Holm A, Mtoni I M, Andersen Å B, Hasløv K. Mapping of the delayed-type hypersensitivity-inducing epitope of secreted protein MPT64 from Mycobacterium tuberculosis. Infect Immun. 1995;63:4613–4618. doi: 10.1128/iai.63.12.4613-4618.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohara N, Kitaura H, Hotokezaka H, Nishiyama T, Wada N, Matsumoto S, Matsuo T, Naito M, Yamada T. Characterization of the gene encoding the MPB51, one of the major secreted protein antigens of Mycobacterium bovis BCG, and identification of the secreted protein closely related to the fibronectin binding 85 complex. Scand J Immunol. 1995;41:433–442. doi: 10.1111/j.1365-3083.1995.tb03589.x. [DOI] [PubMed] [Google Scholar]
- 30.Ohara N, Ohara-Wada N, Kitaura H, Nishiyama T, Matsumoto S, Yamada T. Analysis of genes encoding the antigen 85 complex and MPT51 from Mycobacterium avium. Infect Immun. 1997;65:3680–3685. doi: 10.1128/iai.65.9.3680-3685.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 32.Schoel B, Gulle H, Kaufmann S H E. Heterogeneity of the repertoire of T cells of tuberculosis patients and healthy contacts to Mycobacterium tuberculosis antigens separated by high-resolution techniques. Infect Immun. 1992;60:1717–1720. doi: 10.1128/iai.60.4.1717-1720.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sørensen A L, Nagai S, Houen G, Andersen P, Andersen Å B. Purification and characterization of a low-molecular-mass T-cell antigen secreted by Mycobacterium tuberculosis. Infect Immun. 1995;63:1710–1717. doi: 10.1128/iai.63.5.1710-1717.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Terasaka K, Yamaguchi R, Matsuo K, Yamasaki A, Nagai S, Yamada T. Complete nucleotide sequence of immunogenic protein MPB70 from Mycobacterium bovis BCG. FEMS Microbiol Lett. 1989;58:273–276. doi: 10.1016/0378-1097(89)90052-9. [DOI] [PubMed] [Google Scholar]
- 35.Wilcke J T R, Jensen B N, Ravn P, Andersen Å B, Hasløv K. Clinical evaluation of MPT-64 and MPT-59, two proteins secreted from Mycobacterium tuberculosis, for skin test reagents. Tubercle Lung Dis. 1996;77:250–256. doi: 10.1016/s0962-8479(96)90009-x. [DOI] [PubMed] [Google Scholar]
- 36.World Health Organization. Report on the tuberculosis epidemic. Geneva, Switzerland: World Health Organization; 1996. [Google Scholar]
- 37.Yamaguchi R, Matsuo K, Yamazaki A, Abe C, Nagai S, Teresaka K, Yamada T. Cloning and characterization of the gene for immunogenic protein MPB64 of Mycobacterium bovis BCG. Infect Immun. 1989;57:283–288. doi: 10.1128/iai.57.1.283-288.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Young D B, Kaufmann S H E, Hermans P W M, Thole J E R. Mycobacterial protein antigens: a compilation. Mol Microbiol. 1992;6:133–145. doi: 10.1111/j.1365-2958.1992.tb01994.x. [DOI] [PubMed] [Google Scholar]