Abstract
The mitogen-activated protein kinase (MAPK) pathway is a highly conserved signaling cascade that converts extracellular signals into various outputs. In Caenorhabditis elegans, asymmetric expression of the candidate odorant receptor STR-2 in either the left or the right of two bilaterally symmetrical olfactory AWC neurons is regulated by axon contact and Ca2+ signaling. We show that the MAPK kinase (MAPKK) SEK-1 is required for asymmetric expression in AWC neurons. Genetic and biochemical analyses reveal that SEK-1 functions in a pathway downstream of UNC-43 and NSY-1, Ca2+/calmodulin-dependent protein kinase II (CaMKII) and MAPK kinase kinase (MAPKKK), respectively. Thus, the NSY-1–SEK-1–MAPK cascade is activated by Ca2+ signaling through CaMKII and establishes asymmetric cell fate decision during neuronal development.
INTRODUCTION
Mitogen-activated protein kinase (MAPK) signal transduction pathways are evolutionarily conserved in eukaryotic cells and transduce signals in response to a variety of extracellular stimuli. Each pathway is composed of three classes of protein kinase: MAPK, MAPK kinase (MAPKK) and MAPK kinase kinase (MAPKKK) (Kyriakis and Avruch, 1996; Robinson and Cobb, 1997; Ip and Davis, 1998). MAPK is activated by phosphorylation of specific tyrosine and threonine residues by a family of dual-specificity protein kinase MAPKKs. MAPKK is in turn activated by phosphorylation of serine and serine/threonine residues by a family of upstream MAPKKKs.
Three subgroups of the MAPK superfamily have been identified (Kyriakis and Avruch, 1996; Robinson and Cobb, 1997; Ip and Davis, 1998): extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK) and p38. Further, several subgroups of the MAPKK superfamily have been identified, such as MEK1, MEK2, MKK3, MKK4, MKK6 and MKK7. The ERK group is activated by MEK1 and MEK2. Whereas MKK4 can activate both the JNK and p38 subgroups, MKK7 is specific to the JNK subgroup. On the other hand, MKK3 and MKK6 act solely as activators of the p38 subgroup. These members of the MAPKK superfamily are activated by members of the MAPKKK superfamily, such as Raf, MEKK, TAK1, MLK, Tpl2 and ASK1.
The JNK and p38 pathways have been implicated in a variety of biological functions in mammalian cells, including apoptosis and the responses to stress. However, the physiological role of these pathways in the normal development and function of the organism has not been fully elucidated. Recent studies using model genetic organisms have revealed some of the physiological roles of the JNK signaling pathway. In Drosophila, the JNK pathway is required for the normal morphogenetic process of dorsal closure (Noselli, 1998). In contrast, genetic studies of Caenorhabditis elegans demonstrate that the JNK pathway, consisting of JKK-1 (MAPKK) and JNK-1 (MAPK), regulates coordinated movement via type D GABAergic (GABA, γ-aminobutyric acid) motor neurons, but it does not appear to be essential for embryonic morphogenesis (Kawasaki et al., 1999).
Drosophila contains two p38 MAPKs, D-p38a and D-p38b. Phosphorylation and activation of D-p38a/b are mediated by the MAPKKs D-MKK3 and D-MKK4 (Han et al., 1998). A recent study has shown that D-MKK3 is encoded by the gene licorne (lic) (Suzanne et al., 1999), which plays an essential role in anterior–posterior and dorsal–ventral patterning during oogenesis by regulating the localization of cell fate determinants. Thus, the p38 pathway may play an intrinsic role in establishing the initial asymmetry in other organisms. Interestingly, p38 in zebrafish is asymmetrically activated during the cleavage period and plays a role in cleavage control on the prospective dorsal side (Fujii et al., 2000). Although there are two genes, pmk-1 and pmk-2, that encode p38 MAPKs in C. elegans (Kawasaki et al., 1999), pmk-1/2 mutants have yet to be isolated.
The C. elegans nsy-1 gene encodes a MAPKKK that is most similar to mammalian ASK1, an activator of JNK and p38 MAPKs (Ichijo et al., 1997; Sagasti et al., 2001). The nsy-1 locus was previously identified as mutations that affect asymmetric olfactory neuron fates (Troemel et al., 1999). Most neurons in C. elegans exist as bilaterally symmetric, morphologically similar pairs. However, the two AWC olfactory neurons interact with each other to generate distinct fates (White et al., 1986). The candidate odorant receptor STR-2 is expressed asymmetrically in either the left or right AWC neuron, but never in both. The asymmetric AWC cell fate is regulated by axon contact during development and by Ca2+ signaling (Troemel et al., 1999). nsy-1 mutants express STR-2 in both AWC neurons, disrupting AWC asymmetry. In this study, we show that the MAPKK SEK-1 determines asymmetric cell fates in the AWC neurons by functioning in a pathway downstream of Ca2+ signaling and NSY-1. Our results suggest that the NSY-1–SEK-1–MAPK cascade is a central component of the AWC cell fate decision.
RESULTS AND DISCUSSION
SEK-1 functions as a MAPKK
The yeast Hog1 MAPK pathway mediates cellular responses to an increase in external osmolarity (Posas and Saito, 1997). This signaling cascade consists of the Ssk2/22 (MAPKKK), Pbs2 (MAPKK) and Hog1 (MAPK) kinases (Figure 1A). The C. elegans JNK homolog, JNK-1, can complement the high osmolarity-sensitive (Osms) growth phenotype of a hog1Δ mutant (Kawasaki et al., 1999). However, expression of JNK-1 did not suppress the pbs2Δ Osms phenotype (Kawasaki et al., 1999), suggesting that Pbs2 is required for the activation of the C. elegans JNK-1. To identify the C. elegans MAPKKs activating JNK-1, we have previously isolated C. elegans cDNAs, which can suppress the pbs2Δ mutation in a JNK-1-dependent manner (Kawasaki et al., 1999). One of them is JKK-1-homologous to the MAPKK MKK7, an activator specific to JNK. The second, SEK-1, is also related to the MAPKK family (Figure 1B), having similarity to MKK3/6 (50% identity) and MKK4 (43% identity). MKK3 and MKK6 are specific activators of p38, whereas MKK4 can activate both the JNK and p38 subgroups of the MAPK superfamily (Derijard et al., 1995; Moriguchi et al., 1996; Stein et al., 1996). Suppression of the pbs2Δ Osms phenotype was also observed when SEK-1 was coexpressed with the C. elegans p38 MAPK homolog, PMK-1 (Figure 1A). This indicates that SEK-1 can activate both JNK-1 and PMK-1 in the yeast Hog pathway.
Fig. 1. Caenorhabditis elegans SEK-1 functions as a MAPKK. (A) Suppression of the pbs2Δ mutants by C. elegans SEK-1, JNK-1 and PMK-1. pbs2Δ mutants were transformed with the indicated C. elegans genes. Three independent transformants were streaked onto plates containing 1.2 M sorbitol and incubated at 30°C. The pbs2Δ mutants do not grow in the presence of sorbitol. Model for the yeast osmotic stress-activated Hog1 MAPK pathway is shown in the right panel. (B) Sequence alignment of SEK-1 with mammalian MAPKKs, MKK3, MKK6 and MKK4. Identical amino acids are shaded. The sites of activating phosphorylation in MAPKKs are indicated by asterisks and the kinase subdomains are marked with roman numbers below the sequences. The DDBJ/EMBL/GenBank accession number for the SEK-1 sequence is AB060731. (C) MAPKK activity of SEK-1. HEK 293 cells were transfected with Flag-SEK-1 and Flag-SEK-1(K79R). Anti-Flag antibody immunoprecipitates (IP) were used for in vitro kinase reactions with p38-KI as a substrate (top panel). The immunoprecipitates were immunoblotted (IB) with anti-Flag antibody (second panel). Whole-cell extracts were immunoblotted with anti-phospho-p38 MAPK antibody (third panel) and anti-p38 antibody (bottom panel).
Sequence information from the C. elegans Genome Consortium showed that sek-1 corresponds to R03G5.2. Comparison of the sequences between the database genomic DNA and the cloned cDNA revealed that the sek-1 gene has seven exons (Figure 2A). To determine the expression pattern of sek-1, we constructed a translational fusion between the promoter and coding sequences of sek-1 and green fluorescent protein (GFP) to generate sek-1::gfp. This fusion protein has functional MAPKK activity in C. elegans (see below). Transgenic C. elegans bearing the sek-1::gfp fusion exhibited fluorescence in excretory canal, rectal epithelial cells, uterine-vulval cells and several neurons (data not shown).
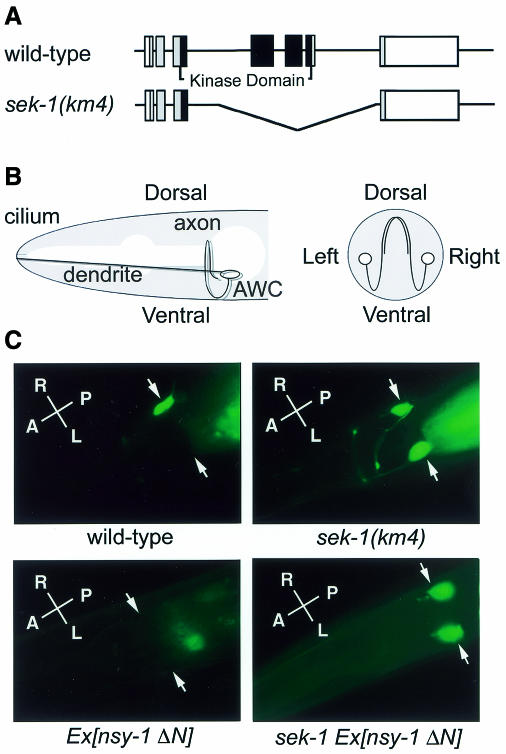
Fig. 2. Effect of the sek-1 mutation on str-2 asymmetry in AWC olfactory neurons. (A) Structure of the sek-1 gene. Exons are indicated by boxes. The shaded and open boxes are the translated and untranslated regions, respectively. The black boxes indicate kinase domains. sek-1(km4) is a 2083-bp deletion mutation in which three exons are missing. (B) The morphology of AWC neurons: a lateral diagram (left panel); a cross-sectional diagram (right panel). (C) Patterns of str-2::gfp expression in wild-type N2 animals, sek-1(km4) mutants, N2 carrying odr3p::nsy-1ΔN (Ex[nsy-1ΔN]) and sek-1(km4) carrying odr-3p::nsy-1ΔN (sek-1 Ex[nsy-1ΔN]). Arrows indicate AWC neurons. Fluorescence posterior to the head is gut auto-fluorescence. The photographs show dorsal views. A, anterior; P, posterior; L, left; R, right.
To determine whether SEK-1 has MAPKK activity, SEK-1 was cloned into a mammalian expression vector to generate a Flag-tagged protein (Flag-SEK-1). Human 293 cells were transiently transfected with the vector, and cell lysates were subjected to immunoprecipitation with anti-Flag antibody, followed by a kinase assay with kinase-inactive p38 MAPK (p38-KI) as a substrate (Figure 1C). SEK-1 phosphorylated p38-KI in vitro, but a catalytically inactive mutant, SEK-1(K79R), in which Lys-79 in the ATP-binding domain was replaced by Arg, did not phosphorylate p38-KI. We next examined whether SEK-1 is able to activate mammalian p38 in vivo. Since MAPKs are activated by dual phosphorylation, we performed western-blot analysis using an anti-phospho-p38 antibody that specifically recognizes the dually phosphorylated active form of p38 (Figure 1C). Activation of p38 MAPK in vivo was detected in cells expressing Flag-SEK-1 but not Flag-SEK-1(K79R). These results indicate that SEK-1 is a MAPKK that phosphorylates and activates p38 MAPK.
Isolation of a sek-1 deletion mutant
To examine the biological function of sek-1, we generated a sek-1 deletion mutant, sek-1(km4), by a transposon-based PCR sib-selection method (Zwaal et al., 1993). The sek-1(km4) mutant allele is missing an ∼2.1-kb region containing three exons, including kinase domains II–XI (Figure 2A). Therefore, sek-1(km4) is expected to be a null mutation. sek-1(km4) homozygous mutant animals were found to be viable but showed a variety of behavioral defects in egg laying and male turning during mating (data not shown).
SEK-1 regulates asymmetric AWC cell fate
The C. elegans nsy-1 gene encodes a homolog of the human MAPKKK ASK1 (Ichijo et al., 1997; Sagasti et al., 2001). The nsy-1 gene is involved in determining asymmetric cell fates in the AWC olfactory neurons (Troemel et al., 1999). AWC neurons exist as bilaterally symmetric pairs in the head (Figure 2B). Asymmetry between the two AWC neurons can be monitored by following expression of the candidate odorant receptor STR-2 (Troemel et al., 1999). A strain carrying a str-2::gfp fusion integrated into chromosome I expresses GFP in just one AWC neuron, with about half of the animals in a population expressing GFP in AWC-left and the other half expressing GFP in AWC-right. No animals express GFP in both AWC neurons (Figure 2C, Table I). Similar to nsy-1, we found that sek-1 also affected the asymmetric AWC cell fate. In sek-1(km4) mutants, str-2 was expressed in both AWC neurons (Figure 2C, Table I), indicating that SEK-1 is required for str-2 asymmetry. With the str-2::gfp fusion gene, AWC neuroanatomy was normal in sek-1(km4) mutants (data not shown).
Table I. Patterns of str-2 expression in mutants.
| Cells expressing str-2::gfp (%) | ||||
|---|---|---|---|---|
| 2AWCoff | 1AWCoff/1AWCon | 2AWCon | n | |
| N2 wild-type | 0 | 100 | 0 | 139 |
| nsy-1(ky400) | 0 | 2 | 98 | 117 |
| sek-1(km4) | 0 | 2 | 98 | 172 |
| sek-1(km4) Ex[odr-3p::sek-1] | 5 | 79 | 16 | 56 |
| N2 Ex[odr-3p::nsy-1ΔN] | 79 | 21 | 0 | 110 |
| sek-1(km4) Ex[ odr-3p::nsy-1ΔN] | 0 | 5 | 95 | 61 |
| N2 Ex[odr-3p::sek-1(STDD)] | 91 | 9 | 0 | 105 |
| nsy-1(ky400) Ex[odr-3p::sek-1(STDD)] | 82 | 1 | 17 | 110 |
| unc-43(n498) | 94 | 6 | 0 | 139 |
| unc-43(n498); sek-1(km4) | 0 | 10 | 90 | 124 |
Animals were scored as adults for str-2::gfp expression in AWC neurons. The AWC cells can be defined as AWCon and AWCoff based on their pattern of str-2::gfp.
To determine whether sek-1 expression in AWC neurons, or in an another cell type, is required for asymmetrical str-2 expression, sek-1 cDNA was expressed under the control of the odr-3 promoter (Roayaie et al., 1998), which drives expression in the AWC neurons. For transgenic sek-1(km4) mutants harboring the odr-3p::sek-1 transgene, most animals showed str-2 expression only in one AWC neuron, but not in both (Table I). Thus, SEK-1 acts within the AWC neurons to determine asymmetric expression of str-2.
MAPKKs are activated by phosphorylation within the activation loop between subdomains VII and VIII (Kyriakis and Avruch, 1996; Robinson and Cobb, 1997; Ip and Davis, 1998). These phosphorylation sites are conserved in SEK-1 (Ser 204 and Thr208) (Figure 1B). We generated sek-1(STDD) mutants, in which both Ser 204 and Thr 208 were replaced with Asp. The sek-1(STDD) mutants are predicted to have constitutively active kinase activity (Pages et al., 1994). When an odr-3p::sek-1(STDD) transgene was introduced into N2 wild-type animals, most animals had no str-2 expression in either of the AWC neurons, the opposite phenotype of sek-1(km4) (Table I). Taken together, the level of SEK-1 kinase activity can determine whether a cell adopts the fate that expresses str-2 or not.
SEK-1 acts downstream of NSY-1
The above results raised the possibility that NSY-1 and SEK-1 constitute a MAPK cascade that regulates str-2 asymmetry. To test this possibility, we examined genetic interactions between nsy-1 and sek-1. When a constitutively active form of NSY-1, NSY-1ΔN, lacking the N-terminal 640 amino acids was expressed by the odr-3 promoter, str-2 expression was abolished in both AWC neurons (Figure 2C, Table I) (Sagasti et al., 2001). In contrast, when this same odr-3p::nsy-1ΔN transgene was expressed in a transgenic sek-1 null strain, str-2 expression appeared in both AWC neurons, similar to the phenotype of sek-1 (km4) mutant (Figure 2C, Table I). Conversely, a gain-of-function odr-3p::sek-1(STDD) transgene increased the numbers of animals with no expression of str-2 in either of the AWC neurons in loss-of function nsy-1(ky400) mutants (Table I). These results indicate that the sek-1 mutations are epistatic to the nsy-1 mutations, suggesting that SEK-1 functions in a pathway downstream of NSY-1.
To obtain further support for the possibility that NSY-1 acts as a MAPKKK of SEK-1 MAPKK, we examined the biochemical interaction between NSY-1 and SEK-1. We transiently expressed T7-tagged NSY-1 (T7-NSY-1) together with a kinase-inactive Flag-SEK-1(K79R) in 293 cells. Cell extracts were subjected to immunoprecipitation with anti-Flag antibody, followed by both immunoblotting and in vitro kinase assay. NSY-1 was detected in SEK-1(K79R) immunoprecipitates (Figure 3A), indicating that NSY-1 is in a complex with SEK-1. When the immunoprecipitates were incubated with [γ-32P]ATP, SEK-1(K79R) became phosphorylated (Figure 3B). We generated a kinase-inactive form of NSY-1, NSY-1(K703M), in which Lys-703 in the ATP-binding domain has been mutated to Met. This mutant did not phosphorylate SEK-1(K79R) (Figure 3B), indicating that the phosphorylation of SEK-1(K79R) is dependent on NSY-1 kinase activity. These results demonstrate that NSY-1 acts as a MAPKKK that phosphorylates SEK-1 MAPKK.
Fig. 3. SEK-1 MAPKK functions downstream of UNC-43 CaMKII and NSY-1 MAPKKK. (A) Association between SEK-1 and NSY-1. HEK 293 cells were transfected with control vector (–), T7-NSY-1 and Flag-SEK-1(K79R) as indicated. Cell lysates were immunoprecipitated (IP) with anti-Flag antibody. Immunoprecipitates were immunoblotted (IB) with anti-T7 antibody (top panel) and anti-Flag antibody (middle panel). Whole-cell extracts were immunoblotted with anti-T7 antibody (bottom panel). (B) Phosphorylation of SEK-1 by NSY-1. HEK 293 cells were transfected with control vector (–), T7-NSY-1, T7-NSY-1(K703M) and Flag-SEK-1(K79R) as indicated. Immunoprecipitated complexes with anti-Flag antibody were incubated with [γ-32P]ATP and analyzed by autoradiography (upper panel). The immunoprecipitates were immunoblotted with anti-Flag antibody (lower panel). (C and D) Effects of nsy-1 (C) and unc-43 (D) mutations on SEK-1 activity in C. elegans. The sek-1::gfp (WT) and sek-1(K79R)::gfp (KN) transgenes were injected into wild-type N2 animals, nsy-1(ky400) and unc-43(n1186) mutants as indicated. Cell lysates from each animal were immunoprecipitated with anti-GFP antibody. The immunoprecipitates were used for in vitro kinase reactions with p38-KI (upper panel). The immunoprecipitates were immunoblotted with anti-GFP antibody (lower panel).
To confirm that NSY-1 activates SEK-1 in C. elegans, SEK-1 kinase activity was tested with the sek-1::gfp transgene. If NSY-1 functions as MAPKKK for SEK-1 in C. elegans, SEK-1::GFP should not show kinase activity in loss-of-function nsy-1(ky400) mutant animals. The sek-1::gfp transgene was introduced as an extrachromosomal array into both wild-type N2 and nsy-1(ky400) mutant animals. SEK-1::GFP was then immunoprecipitated with anti-GFP antibody and tested in a protein kinase assay using p38-KI as a substrate. SEK-1::GFP expressed in wild-type animals was found to have clearly detectable kinase activity, while a catalytically inactive SEK-1(K79R)::GFP negative control protein did not (Figure 3C). When SEK-1::GFP was immunoprecipitated from cell extracts of nsy-1(ky400) mutant animals, it had little kinase activity (Figure 3C), indicating that NSY-1 was required for the activation of SEK-1 in C. elegans. Taken together, these results indicate that NSY-1 MAPKKK acts in the same pathway upstream of SEK-1 MAPKK and functions to activate SEK-1 kinase activity.
SEK-1 acts downstream of the CaMKII homolog UNC-43
Previous studies have revealed that UNC-43 Ca2+/calmodulin-dependent protein kinase II (CaMKII) acts as a switch in determining str-2 expression in AWC neurons (Reiner et al., 1999; Troemel et al., 1999). While str-2 is expressed in both AWC neurons in loss-of-function unc-43(n1186) mutants, it is expressed in neither neuron in gain-of-function unc-43 (n498) mutants (Table I). To characterize the role of the sek-1 gene in AWC asymmetry, we analyzed the epistasis relationship between sek-1 and unc-43. Double mutant analysis with sek-1(km4) and unc-43(n498) indicated that the sek-1 mutation was epistatic to the unc-43 mutation (Table I). This suggests that sek-1 acts downstream of or in parallel to unc-43.
We next tested whether the unc-43(n1186) mutation affects SEK-1 kinase activity in C. elegans. The sek-1::gfp transgene was introduced into unc-43(n1186) mutant animals, and SEK-1::GFP immunoprecipitates from animals were assayed for kinase activity. The unc-43(n1186) mutation was found to cause a decrease in SEK-1 kinase activity (Figure 3D). These results are consistent with sek-1 acting downstream of unc-43 in a linear pathway. Thus, UNC-43 CaMKII functions as a positive mediator of the NSY-1–SEK-1–MAPK cascade regulating AWC asymmetry.
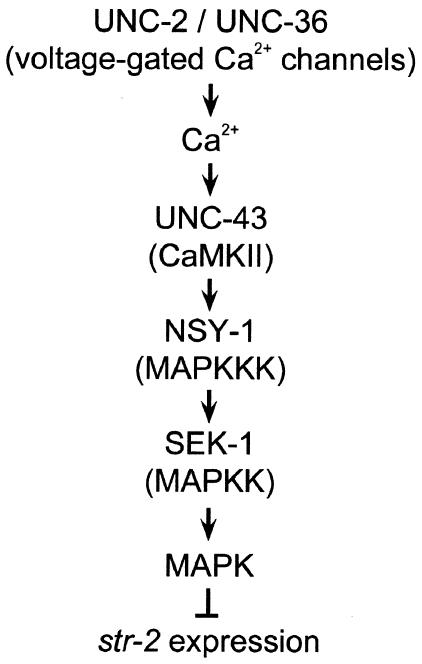
Asymmetric expression of str-2 creates two independent AWC neurons that each have a distinct olfactory specificity. This organization enables the animal to detect more odors and discriminate between them in complex environments (Wes and Bargmann, 2001). Asymmetrical str-2 expression in AWC neurons is initiated by regulating Ca2+ influx and it is maintained by cGMP signaling (Troemel et al., 1999). Ca2+ enters through the UNC-2 and UNC-36 voltage-gated Ca2+ channels (Schafer and Kenyon, 1995; Lee et al., 1997) and stimulates UNC-43 CaMKII activity, leading to the inhibition of str-2 expression in one AWC neuron. Furthermore, we recently showed that NSY-1, a homolog of the human MAPKKK ASK1, is involved in determining AWC asymmetry (Sagasti et al., 2001). In this study we show that SEK-1 MAPKK functions in a pathway downstream of UNC-43 and NSY-1 (Figure 4). Thus, CaMKII is a signal converter, transducing an increase in Ca2+ concentration into activation of the NSY-1–SEK-1–MAPK pathway that determines the fate of str-2 expression in AWC neurons. Mammalian ASK1 activates the JNK and p38 MAPKs (Ichijo et al., 1997). We have not yet identified a C. elegans MAPK that functions downstream of the NSY-1–SEK-1 pathway. Since the C. elegans JNK homolog jnk-1 did not affect AWC asymmetry (data not shown), p38 MAPK is a candidate that participates in the AWC cell fate determination. In the absence of lateral signaling between the AWC neurons, both AWC cells do not express str-2, and this str-2-negative state is probably maintained by the CaMKII–MAPK pathway. During normal development, AWC cells communicate with each other, probably through their axons, which contact each other in the nerve ring (White et al., 1986). This communication inhibits the activity of the CaMKII–MAPK signaling pathway in one of the neurons. Further understanding of this lateral signaling mechanism may be provided by identification of the receptors or ligands that allow the AWC neurons to recognize and signal to each other. The subsequent effects of MAPK on str-2 expression could be through either transcriptional regulation or the direct phosphorylation of target molecules. Given the power of genetic analysis in C. elegans, suppressor screens may lead to the identification of downstream genes encoding targets or downstream effectors of the NSY-1–SEK-1–MAPK pathway that play important roles in the regulation of the asymmetric AWC cell fate decision.
Fig. 4. Model for str-2 regulation by the CaMKII–MAPK pathway. See text for details.
METHODS
Assays of yeast Hog1 MAPK pathway. Yeast strain TM334 (MATa pbs2Δ::HIS3 ura3 leu2 trp1 his3) was transformed with yeast expression plasmids encoding PMK-1 and SEK-1. Transformants were scored for osmo-sensitivity on plates containing 1.2 M sorbitol.
Analysis of str-2 expression in C. elegans mutants. A str-2::gfp fusion gene and mutants of unc-43 and nsy-1 have been described previously (Reiner et al., 1999; Troemel et al., 1999). Germline transformation was carried out as described previously (Mello et al., 1992). Cell identification was based on characteristic morphology and position of GFP-positive cell nuclei as viewed by simultaneous fluorescence and Nomarski differential interference microscopy.
Isolation of the sek-1 null mutant. A Tc1 insertion mutant mut-2 (r459) I; sek-1::Tc1 X was isolated using a sib-selection protocol (Zwaal et al., 1993). This insertion was located in intron VI of sek-1. From mut-2(r459) I; sek-1::Tc1 X animals, a 2.1-kb deletion derivative (km4) was isolated. The PCR products derived from the deletion were directly sequenced. sek-1(km4) was isolated using a sib-selection protocol. The km4 allele was backcrossed twice to animals of a lon-2 background and then nine times to animals of wild-type N2 background.
Preparation of GFP immunoprecipitates from C. elegans. Sonicated worm lysates were incubated with anti-GFP polyclonal antibody (Clontech) in a buffer containing 50 mM Tris–HCl pH 8.0, 100 mM NaCl, 10% glycerol, 1% Triton X-100, 5 mM PMSF, 1 µg/ml leupeptin and 1 µg/ml pepstatin at 4°C for 3 h. Samples were precipitated with protein A–Sepharose beads (Pharmacia). Immunoprecipitates were subjected to protein kinase analysis and immunoblotting with anti-GFP monoclonal antibody (Clontech).
Immunoblotting. Immunoprecipitation from mammalian cells was carried out as described previously (Kawasaki et al., 1999). The immunoprecipitates and aliquots of total lysates were resolved on SDS–PAGE and transferred to PVDF membranes (Hybond-P, Amersham). The membranes were immunoblotted with antibodies, and bound antibodies were visualized with horseradish peroxidase-conjugated antibodies to mouse IgG using the Enhanced Chemiluminescence Western Blotting System (Amersham). Rabbit polyclonal antibodies to p38 and phospho-p38 MAPK (New England BioLabs) and mouse monoclonal antibodies to Flag (Sigma) and T7 (Novagen) were used.
In vitro kinase assays. Aliquots of immunoprecipitates were incubated in 10 µl kinase buffer containing 1 µg of bacterially expressed histidine-tagged p38-KI, 10 mM HEPES pH 7.4, 1 mM DTT, 5 mM MgCl2 and 5 µCi of [γ-32P]ATP at 25°C for 2 min. Samples were resolved on SDS–PAGE, and phosphorylated proteins were visualized by autoradiography.
Acknowledgments
ACKNOWLEDGEMENTS
We thank A. Coulson, A. Fire, J. Kaplan, J. MacGee and Caenorhabditis Genetics Center for materials and I. Mori for critical reading of the manuscript. This study was supported by Advanced Research on Cancer from the Ministry of Education, Culture and Science of Japan; the Uehara Foundation; and the Asahi Glass Foundation (to K.M.).
REFERENCES
- Derijard B., Raingeaud, J., Barrett, T., Wu, I.H., Han, J., Ulevitch, R.J. and Davis, R.J. (1995) Independent human MAP-kinase signal transduction pathways defined by MEK and MKK isoforms. Science, 267, 682–685. [DOI] [PubMed] [Google Scholar]
- Fujii R., Yamashita, S., Hibi, M. and Hirano, T. (2000) Asymmetric p38 activation in zebrafish: its possible role in symmetric and synchronous cleavage. J. Cell Biol., 150, 1335–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Z.S., Enslen, H., Hu, X., Meng, X., Wu, I.-H., Barrett, T., Davis, R.J. and Ip, Y.T. (1998) A conserved p38 mitogen-activated protein kinase pathway regulates Drosophila immunity gene expression. Mol. Cell. Biol., 18, 3527–3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichijo H. et al. (1997) Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science, 275, 90–94. [DOI] [PubMed] [Google Scholar]
- Ip Y.T. and Davis, R.J. (1998). Signal transduction by the c-Jun N-terminal kinase (JNK) from inflammation to development. Curr. Opin. Cell Biol., 10, 205–219. [DOI] [PubMed] [Google Scholar]
- Kawasaki M., Hisamoto, N., Iino, Y., Yamamoto, M., Ninomiya-Tsuji, J. and Matsumoto, K. (1999). A Caenorhabditis elegans JNK signal transduction pathway regulates coordinated movement via type-D GABAergic motor neurons. EMBO J., 18, 3604–3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakis J.M. and Avruch, J. (1996) Sounding the alarm: protein kinase cascades activated by stress and inflammation. J. Biol. Chem., 271, 24313–24316. [DOI] [PubMed] [Google Scholar]
- Lee R.Y., Lobel, L., Hengartner, M., Horvitz, H.R. and Avery, L. (1997) Mutations in the a subunit of an L-type voltage-activated Ca2+ channel cause myotonia in Caenorhabditis elegans. EMBO J., 16, 6066–6076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello C.C., Kramer, J.M., Stinchconb, D. and Ambros, V. (1992) Efficient gene transfer in C. elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J., 10, 3959–3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi T. et al. (1996) Purification and identification of a major activator for p38 from osmotically shocked cells. J. Biol. Chem., 271, 26981–26988. [DOI] [PubMed] [Google Scholar]
- Noselli S. (1998) JNK signaling and morphogenesis in Drosophila. Trends Genet., 14, 33–38. [DOI] [PubMed] [Google Scholar]
- Pages G., Brunet, A., L’Allemain, G. and Pouyssegur, J. (1994) Constitutive mutant and putative regulatory serine phosphorylation site of mammalian MAP kinase kinase (MEK1). EMBO J., 13, 3003–3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posas F. and Saito, H. (1997) Osmotic activation of the HOG MAPK pathway via Ste11p MAPKKK: scaffold role of Pbs2p MAPKK. Science, 276, 1702–1705. [DOI] [PubMed] [Google Scholar]
- Reiner D.J., Newton, E.M., Tian, H. and Thomas, J.H. (1999) Diverse behavioural defects caused by mutations in Caenorhabditis elegans unc-43 CaM kinase II. Nature, 402, 199–203. [DOI] [PubMed] [Google Scholar]
- Roayaie K., Crump, J.G., Sagasti, A. and Bargmann, C.I. (1998) The Gα protein ODR-3 mediates olfactory and nociceptive function and controls cilium morphogenesis in C. elegans olfactory neurons. Neuron, 20, 55–67. [DOI] [PubMed] [Google Scholar]
- Robinson M.J. and Cobb, M.H. (1997) Mitogen-activated protein kinase pathways. Curr. Opin. Cell Biol., 9, 180–186. [DOI] [PubMed] [Google Scholar]
- Sagasti A., Hisamoto, N., Hyodo, J., Tanaka-Hino, M., Matsumoto, K. and Bargmann, C.I. (2001) The CaMKII UNC-43 activates the MAPKKK NSY-1 to execute a lateral signaling decision required for asymmetric olfactory neuron fates. Cell, 105, 221–232. [DOI] [PubMed] [Google Scholar]
- Schafer W.R. and Kenyon, C.J. (1995) A calcium-channel homologue required for adaptation to dopamine and serotonin in Caenorhabditis elegans. Nature, 375, 73–78. [DOI] [PubMed] [Google Scholar]
- Stein B., Brady, H., Young, D.B. and Barbosa, M.S. (1996) Cloning and characterization of MEK6, a novel member of the mitogen-activated protein kinase kinase cascade. J. Biol. Chem., 271, 11427–11433. [DOI] [PubMed] [Google Scholar]
- Suzanne M., Irie, K., Glise, B., Agnes, F., Mori, E., Matsumoto, K. and Noselli, S. (1999) The Drosophila p38 MAPK pathway is required during oogenesis for egg asymmetric development. Genes Dev., 13, 1464–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troemel E.R., Sagasti, A. and Bargmann, C.I. (1999) Lateral signaling mediated by axon contact and calcium entry regulates asymmetric odorant receptor expression in C. elegans. Cell, 99, 387–398. [DOI] [PubMed] [Google Scholar]
- Wes P.D. and Bargmann, C.I. (2001) C. elegans odour discrimination requires asymmetric diversity in olfactory neurons. Nature, 410, 698–701. [DOI] [PubMed] [Google Scholar]
- White J.G., Southgate, E., Thomson, J.N. and Brenner, S. (1986) The structure of the nervous system of the nematode Caenorhabditis elegans. Phil. Trans. Roy. Soc. Lond. B, 314, 1–340. [DOI] [PubMed] [Google Scholar]
- Zwaal R.R., Broeks, A., van Meurs, J., Groenen, J.T. and Plasterk, R.H. (1993) Target-selected gene inactivation in Caenorhabditis elegans by using a frozen transposon insertion mutant bank. Proc. Natl Acad. Sci. USA, 90, 7431–7435. [DOI] [PMC free article] [PubMed] [Google Scholar]