Abstract
β-catenin mediates Wnt signaling by acting as the essential co-activator for TCF transcription factors. Wnt signaling increases the half-life and therefore the absolute level of β-catenin in responding cells. The current model states that these changes in β-catenin stability set the threshold for Wnt signaling. However, we find that pharmacological inhibition of proteasome activity by ALLN leads to accumulation of cytosolic β-catenin but not to increased TCF-mediated transcription. In addition, in temperature-sensitive ubiquitylation mutant CHO cells inhibition of ubiquitylation increases β-catenin levels, but does not induce transcriptional activation of TCF reporter genes. Using an antibody specific for β-catenin dephosphorylated at residues Ser37 and Thr41, we show that Wnt signals specifically increase the levels of dephosphorylated β-catenin, whereas ALLN does not. We conclude that changes in the phosphorylation status of the N-terminus of β-catenin that occur upon Wnt signaling independently affect the signaling properties and half-life of β-catenin. Hence, Wnt signals are transduced via N-terminally dephosphorylated β-catenin.
INTRODUCTION
Wnt genes encode a large family of secreted glycoproteins that modulate developmental processes through interaction with Frizzled receptors and subsequent activation of intracellular signal transduction pathways. The Wnt1 ortholog in Drosophila is Wingless (Wg), a segment polarity gene product that constitutes the first component of the Wg signaling pathway (Nusse et al., 1991; Nusse and Varmus, 1992). A central effector of the Wg/Wnt pathway is β-catenin/Armadillo (Arm), a multifunctional 92-kD protein involved also in cell adhesion (Miller and Moon, 1996). Some years ago, studies provided evidence that members of the TCF/Lef family of DNA-binding factors bind with β-catenin/Arm to form the ultimate downstream effectors of this pathway (Behrens et al., 1996; Molenaar et al., 1996; Brunner et al., 1997; Riese et al., 1997; van de Wetering et al., 1997). The emerging theme from these examples is that β-catenin/Arm interacts with a member of the TCF family to form a bipartite transcription factor complex where TCFs mediate DNA binding and β-catenin the transactivation domain.
In the Wg/Wnt pathway, suppression of β-catenin degradation has been proposed as the key signaling event (Miller and Moon, 1996). In the absence of Wg/Wnt, the serine/threonine protein kinase zeste white 3 (Zw3), or its vertebrate homolog glycogen synthase kinase-3β (GSK-3β) (Yost et al., 1996; Young et al., 1998), is active and promotes degradation by N-terminal phosphorylation and subsequent ubiquitylation and proteasome targeting (Peifer et al., 1994a). In response to Wg/Wnt, a cytoplasmic protein called dishevelled (Dsh) inactivates Zw3/GSK-3β through largely unknown mechanisms, leading to accumulation of β-catenin by suppressing its breakdown (Miller and Moon, 1996). Indeed, oncogenic mutations in β-catenin in putative GSK-3β phosphorylation sites stabilize β-catenin (Morin et al., 1997).
In the cytoplasm, β-catenin participates in a large protein complex, containing GSK-3β, the tumor suppressor gene product adenomatous polyposis coli (APC) (Papkoff et al., 1996), and axin (Hart et al., 1998; Kishida et al., 1998; Sakanaka et al., 1998) or its analog conductin (Behrens et al., 1998), which co-ordinately regulate the modifications of β-catenin and which lead to ubiquitylation and proteasomal breakdown. The activity of the β-catenin degradation complex may be regulated by phosphorylation of the components APC, axin and GSK-3β (Hart et al., 1998; Kishida et al., 1998; Sakanaka et al., 1998). The importance of regulation via this complex is illustrated by the fact that mutations in APC or β-catenin that stabilize β-catenin in colon epithelial cells cause aberrant activation of TCF4 leading to colorectal tumorigenesis (Korinek et al., 1997; Morin et al., 1997), and that mutations in Zw3 that stabilize Arm mimic Wg signaling (Peifer et al., 1994b).
In the present study, we investigated whether increases in β-catenin levels are sufficient to activate the ultimate step in Wnt signaling, i.e. TCF-dependent transcription. We provide two lines of evidence suggesting that the increased half-life of β-catenin as a consequence of Wnt signaling is not directly related to transduction of Wnt signals. We also show that Wnt signals, in contrast to pharmacological agents that inhibit β-catenin breakdown, specifically increase the dephosphorylated form of β-catenin.
RESULTS
Current models of Wnt signaling state that Wnt signals inhibit the phosphorylation of β-catenin, which results in accumulation of β-catenin, and that this accumulation is the key signaling event. However, oncogenic forms of β-catenin all have mutations in putative GSK3-β phosphorylation sites.
Pharmacological inhibition of β-catenin breakdown
We first investigated whether increasing the β-catenin half-life by pharmacological reagents would influence TCF-dependent transcription. We made use of the peptide aldehyde ALLN (Calpain inhibitor I), which inhibits proteasome-mediated proteolysis and has been shown to dramatically increase cellular β-catenin levels (Aberle et al., 1997). Indeed, treatment of 293T cells with ALLN for up to 6 h strongly increased β-catenin levels (Figure 1A). Surprisingly, when 293T cells were transfected with TCF reporter constructs (termed TOP-Flash and the mutant control FOP-Flash) and, after overnight recovery, were treated with ALLN for 6 h, we did not observe any increase in TCF-mediated transcription (Figure 1B, left panels). Similar results were obtained with C57MG, Rat-1 and Jurkat cells (data not shown).
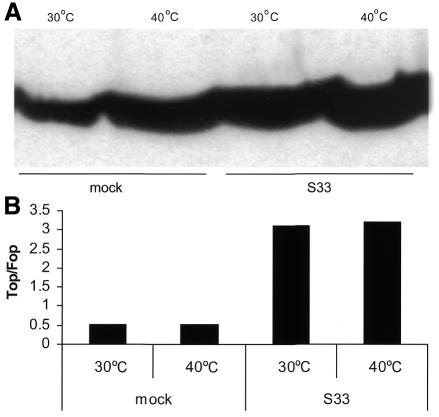
Fig. 1. Lack of transactivation by ALLN-induced increase in β-catenin levels. 293T cells were treated with the proteasome inhibitor ALLN for 6 h and analysed for β-catenin levels by western blotting (A). Equal amounts of protein were loaded in each lane. (B) In parallel, 293T were transfected with TOP and FOP reporter plasmids, Renilla-luciferase control vectors and S33 β-catenin. ALLN treatment does not induce activation of the reporter constructs, despite accumulation of β-catenin. Co-transfection of S33 β-catenin can still induce TCF-dependent transcription under ALLN treatment, indicating that the cells are signaling-competent.
It could be that the cells are not signaling-competent because of the non-specific toxic effects of ALLN treatment. Therefore, we investigated whether ALLN-treated cells are signaling-competent. Co-transfection of S33 β-catenin led to a strong increase in TCF-dependent transcription in both ALLN-treated and non-treated 293T cells (Figure 1B, right panel), showing that cells can activate TCF reporter genes under ALLN treatment. Similar results were obtained with Rat-1 fibroblasts and Jurkat T cells (data not shown).
Genetic experiments to increase β-catenin levels
β-catenin is phosphorylated, ubiquitylated and then degraded in the proteasome (Peifer et al., 1994a; Orford et al., 1997). Thus, inhibition of ubiquitylation should also lead to increases in the amounts of cellular β-catenin. The ts20 CHO-cell line harbors a specific temperature-sensitive mutation in the E1 ubiquitin conjugation enzyme and has been widely used in studies aimed at understanding the role of ubiquitylation in signaling pathways (Kulka et al., 1988; Strous et al., 1996, 1997). When shifted from the permissive temperature (30°C) to the restrictive temperature (40°C), these cells fail to ubiquitylate β-catenin. Indeed, upon growing the cells at 40°C, β-catenin levels increase (Figure 2A). The parental CHO cells (E36) did not show this increase in β-catenin levels (data not shown). We transfected these ts20 CHO cells with the TCF reporter constructs TOP-Flash and FOP-Flash, control plasmids and a TCF4 expression construct. Increasing β-catenin levels by inhibition of ubiquitylation at 40°C did not result in increased TCF-controled transcription (Figure 2B), similar to results obtained when β-catenin degradation was blocked by inhibition of proteasome activity. Importantly, the cells incubated for 6 h at the restrictive temperature were still capable of activating TCF-dependent transcription when co-transfected with S33 β-catenin (Figure 2B), indicating that the lack of reporter gene activity was not caused by toxic effects of blocking the ubiquitylation nor by shifting the temperature.
Fig. 2. Increased β-catenin half-life by inhibition of ubiquitylation does not lead to increased TCF-dependent transcription (A). ts20 cells were incubated at the restrictive temperature (40°C) for 6 h. Subsequently the cells were lysed and analyzed on western blot using the TL α-β-catenin antibody (B). ts20 cells were transfected with reporter constructs with or without S33 β-catenin and immediately shifted to restricted temperature (40°C) for 6 h. While β-catenin levels increased (Figure 2A), TOP/FOP ratios remained the same. In addition, under conditions where ubiquitylation is inhibited, the cells are still capable of activating TCF-dependent transcription when transfected with β-catenin.
Dephosphorylated β-catenin and Wnt signaling
The above data show that increased levels of β-catenin per se are not sufficient to activate TCF-mediated transcription. Therefore, we reasoned that dephosphorylation of β-catenin could constitute an essential activating modification induced by Wnt signals. We set out to generate an antibody specific for the non-phosphorylated form of β-catenin to test this hypothesis. This antibody, called αABC (anti-active β-catenin), was generated using the first 100 aa of the human β-catenin sequence. The specificity of the antibody was established using deletion constructs, the Pepscan method and α-phosphopeptide ELISAs. The epitope of αABC was found to be HSGATTTAP (residues 36–44), thus harbouring dephosphorylated S37 and T41 (M. van Noort, unpublished data).
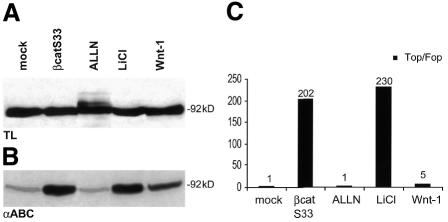
To investigate the role of dephosphorylated β-catenin in Wnt signaling, we used four different treatments to increase β-catenin levels and simultaneously detect its phosphorylation status and its transactivation potential (Figure 3). These treatments include transfection with S33 β-catenin, Wnt1 and incubations with LiCl or ALLN. LiCl is an inhibitor of GSK-3β activity and is widely used to mimic Wnt signaling. S33 β-catenin is a dominant-positive mutant of β-catenin where S33 is mutated into a tyrosine. This serine is not part of the epitope of αABC. The levels of β-catenin as detected with the commercially available C-terminal pan-β-catenin antibody were only slightly increased upon transfection of S33 β-catenin and Wnt1 as well as after treatment with LiCl (in some experiments, total β-catenin levels increased somewhat more; cf. Figure 5). Treatment of the cells with ALLN resulted in an increase in β-catenin levels with the typical pattern of multiple ubiquitylated bands (Figure 3A). In contrast, probing the same lysates with the antibody specific for dephosphorylated β-catenin showed dramatic differences. Transfection with Wnt1 and treatment with LiCl drastically increased the levels of dephosphorylated β-catenin. The S33-β-catenin transfection also showed a striking increase in the amounts of dephosphorylated β-catenin, whereas these levels did not alter upon ALLN treatment (Figure 3B). In the same experiment, these cells were used to investigate the induction of TCF-mediated transcription (Figure 3C). As expected, TOP/FOP activity was induced by transfection with S33 β-catenin (200-fold) and Wnt1 (5-fold), as well as by treating the cells with LiCl (230-fold). ALLN, however, failed to elevate the TOP/FOP ratio. Thus, treatments that resulted in increased amounts of dephosphorylated β-catenin all resulted in increased TCF-dependent transcription.
Fig. 3. Levels of non-phosphorylated β-catenin correlate with the transcriptional activity. (A) 293T cells were transfected with S33 β-catenin, Wnt1 or treated with ALLN and LiCl. Cells were analyzed by western blot using a pan-anti-β-catenin antibody directed against the C-terminus. Equal amounts of protein, as confirmed by Ponceau S staining, were loaded onto each lane. (B) The same samples were analyzed by western blot with an antibody directed against N-terminally dephosphorylated β-catenin. Protein amounts (intensity of specific bands) were measured by densitometry. Wnt, 2-fold increase over untreated cells; lithium and S33 β-catenin, 10-fold; ALLN, no change. (C) Parallel samples of the conditions under (A) and (B) were transfected with optimal (TOP) or mutated (FOP) luciferase reporter plasmids and analyzed for TCF-dependent transcription by measuring luciferase activity. The ratio of the specific versus control signals (corrected for transfection efficiencies), termed TOP/FOP, is shown.
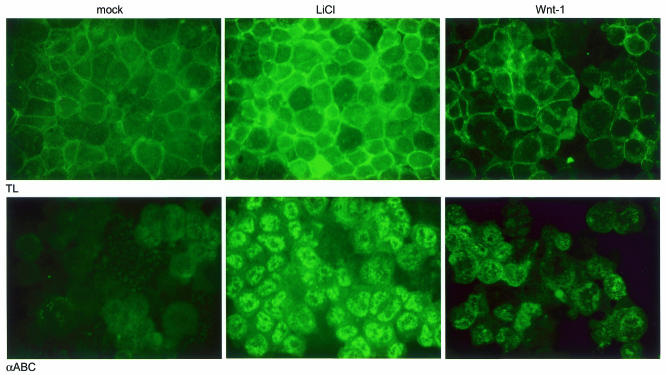
Fig. 5. Strongly increased levels of nuclear dephosphorylated β-catenin after Wnt signaling. 293T cells were treated with LiCl or transfected with Wnt, cytospins were prepared and slides were stained with an antibody specific for total β-catenin (top) or dephosphorylated β-catenin (bottom), followed by an FITC-labeled secondary antibody. The levels of β-catenin increase in all cases compared to mock-treated cells, but a dramatic increase in dephosphorylated β-catenin is seen with LiCl and to a lesser extend with Wnt1.
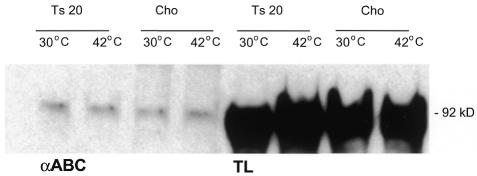
Subsequently, we confirmed these data in ts20 CHO cells (Figure 4). As shown in Figure 2A, extracts from cells incubated at the restrictive temperature show increased levels of β-catenin. However, the levels of dephosphorylated β-catenin, as visualized by αABC antibody, remained low, corroborating that an increase in dephosphorylated β-catenin, but not in phosphorylated/ubiquitylated β-catenin, is required for TCF-mediated signaling.
Fig. 4. ts20 CHO cells fail to show increased levels of dephosphorylated β-catenin. The ts20 CHO cells were kept at the restrictive temperature for 6 h, before they were analysed on a western blot for their levels of dephosphorylated β-catenin and for total β-catenin levels. Whereas total β-catenin levels increase, dephosphorylated levels remain low.
Surprisingly, the increase in TCF-mediated transcription for transfection with S33-β-catenin and LiCl treatment is far greater (>200-fold) than the increase in the active form of β-catenin (∼10-fold), as was shown in Figure 3. For Wnt1 transfection, which results in a modest increase in the amounts of active β-catenin (2-fold), the increase of TCF activity also is modest (5-fold). These discrepancies could be accounted for by potential differences in cellular localization of the activated form of β-catenin. Therefore, we investigated localization of the total and dephosphorylated β-catenin pools by immunofluorescence after LiCl treatment and Wnt transfection. Both treatments with LiCl and Wnt1 transfection lead to increases in the total pools of β-catenin, mostly in the cadherin-associated plasma membrane pool. Interestingly, most of the dephosphorylated β-catenin is found in the nucleus, where it can interact with TCF, and far more dephosphorylated β-catenin is found in the nucleus after LiCl treatment than with Wnt1 transfection. This increased pool of nuclear β-catenin available to interact with TCF-1 and induce transcription can explain the much higher reporter gene activity after LiCl treatment (Figure 3).
DISCUSSION
Several studies have shown that β-catenin levels increase upon Wg/Wnt signaling (Miller and Moon, 1996; Yost et al., 1996; Young et al., 1998). Here, we have investigated whether this increased half-life sets the threshold for signaling. Using ALLN and ts20 cells, we demonstrate that signaling by β-catenin/TCF is not a direct consequence of the increased β-catenin half-life that occurs upon Wnt signaling. Rather, the data suggest that changes in the phosphorylation status of the N-terminus of β-catenin that occur upon Wnt signaling independently affect the signaling properties and half-life of β-catenin. Using a monoclonal antibody (αABC) directed against dephosphorylated β-catenin, we show that only bona fide Wnt signals lead to an increase in unphosphorylated β-catenin.
We noticed that the increase in TCF-mediated transcription for LiCl treatment is far greater than the increase in the active form of β-catenin, whereas Wnt1 transfection results in a modest increase in both the amounts of active β-catenin and TCF reporter gene activity. Several reasons could account for these discrepancies between Wnt1 and LiCl. First, there might be a threshold in the amount of β-catenin required for transcription, and the amount of active β-catenin induced by Wnt1 may only just reach this threshold. Secondly, since Wnt1 operates more upstream in the pathway, its activity might be subjected to negative regulators that are bypassed by LiCl. Thirdly, and most importantly, the amounts of dephosphorylated β-catenin in the nucleus are different with these two treatments (Figure 5), and therefore the pool of signaling-competent β-catenin that interacts with TCF and induces transcription is different. It is likely that LiCl inhibits GSK-3β more than Wnt1 transfection, raising the possibility that the export of β-catenin from the nucleus could be impaired upon LiCl treatment. Indications for this notion come from studies demonstrating that GSK-3β is involved in the nuclear export of the transcription factor NF-AT (Devereux et al., 1999). In this context, it is of interest that the import of β-catenin in the nucleus has recently been shown to constitute a regulated event (Fagotto et al., 1998). Perhaps regulated nuclear import of β-catenin is dependent on its phosphorylation status and represents a key regulatory step in the pathway.
The lack of induction of TCF-dependent transcription by the proteasome inhibitor ALLN that we observed contrasts with reports by Easwaran et al. (1999). They used a different cell type with a mutation in the APC gene and much longer (24 h) incubations than we used (6 h), perhaps allowing for secondary events (e.g. de novo synthesis of β-catenin) to induce activation of the reporter gene. The reporter gene used was different from ours in that it contains a long ‘minimal promoter’, containing potential binding sites for other transcription factors, instead of just a TATA box, as in the reporter gene that we used. Collectively, this may explain the differences found between the two systems.
Support for our model of Wnt signaling comes from two studies by Gumbiner’s group done in a cell-free system based on early Xenopus embryos (Nelson and Gumbiner, 1999; Guger and Gumbiner, 2000). They showed that β-catenin derived from a pre-existing pool can be activated to signal and that accumulation of this active form does not require ongoing synthesis. This contrasts with the established model where β-catenin accumulates through the build-up of newly synthesized protein (Peifer et al., 1994b). We also find that accumulation of β-catenin is insufficient for transmission of Wnt signals and additionally show that the activation signal proposed by Nelson and Gumbiner (1999) is represented by dephosphorylated β-catenin. The other report by Guger and Gumbiner (2000) showed that mutations in the putative GSK-3β phosphorylation sites of β-catenin enhance its signaling activity, but that this cannot be accounted for by accumulation of either total or cadherin-free protein. Again, this corroborates our conclusion that accumulation of β-catenin is insufficient for signaling.
Several studies have reported that β-TrCP is a component of the ubiquitin ligase complex that specifically interacts with phosphorylated β-catenin (Hart et al., 1999; Liu et al., 1999). β-TrCP contains two important domains, the F-box and the WD40 repeat region. The F-box interacts with the ubiquitin complex, whereas the WD40 repeats mediate the interaction of the N-terminus of β-catenin. In apparent contradiction of our results, the ΔF-box β-TrCP deletion mutant (which still binds phosphorylated β-catenin) induces TCF-dependent transcription (Hart et al., 1999; Liu et al., 1999). An explanation to reconcile these apparently contradictory results may be found in the notion that the deletion mutant will directly bind to phosphorylated amino-acid stretches and may thus neutralize the presence of the charged phosphate groups. Only in this special case would phosphorylated β-catenin induce TCF-mediated transcription. This hypothesis is supported by the fact that β-catenin lacking the complete N-terminus is a potent transcriptional activator, because it can no longer bind to β-TrCP and cannot contain negatively charged phosphate groups.
Additionally, it is possible that ΔF-box β-TrCP affects not only β-catenin but also other proteins involved in TCF-controled transcription. Experimental evidence supporting this possibility stems from experiments in which mutants of β-TrCP2 are used. β-TrCP2 is incapable of interacting with β-catenin and is therefore not involved in its degradation. As reported by Hart et al. (1999), the F-box mutant of β-TrCP2 induces TCF-dependent transcription, apparently in a β-catenin-independent fashion. β-TrCP could interact with the same unidentified factor as β-TrCP2. Thus, the effect of ΔF-box β-TrCP on TCF-mediated transcription could be a composite of the direct effect on β-catenin and on the unidentified factor and of the increase in dephosphorylated β-catenin.
Loss of function of the Drosophila β-TrCP homolog Slmb leads, as expected, to high levels of Arm, the fly β-catenin homolog (Jiang and Struhl, 1998). If control mechanisms similar to those of β-catenin regulate Arm transactivation, we would predict that Wg target genes would not be induced in Slmb mutant cells. However, the Wg target gene Scute is induced in Slmb mutants, suggesting that simple accumulation of Arm is sufficient for Wg signal transduction in flies. This is in contrast to our findings and those of Gumbiner in vertebrate cells. Perhaps dephosphorylated Arm can accumulate under these conditions, or perhaps additional control mechanisms that modify TCF and β-catenin in vertebrates are absent in Drosophila. It would be of interest to investigate the phosphorylation status of Arm capable of signaling with a similar antibody as described here for β-catenin.
In summary, in contrast to widely held belief that accumulated β-catenin levels are sufficient to transduce Wnt signals, we demonstrate here that this is mediated by N-terminally dephosphorylated β-catenin. Thus, the accumulation of β-catenin and transduction of Wnt signals are separable events, in that effects on stability of β-catenin can be dissociated from its effects on signaling. In a variety of tumors the putative GSK-3β phosphorylation sites are mutated, which is regarded as a major oncogenic event in the pathogenesis of these malignancies. Our data suggest that it is not the accumulation of β-catenin per se but the lack of phosphorylation of this increased pool of β-catenin that is the key oncogenic step.
METHODS
Constructs, transfections and reporter gene assays. Expression constructs of β-catenin have been described previously (Molenaar et al., 1996). In some experiments, pCI-β-catenin or pCI-S33-β-catenin constructs were used instead of pCDNA constructs. The TK-TOP and TK-FOP optimal and mutated TCF luciferase-reporter constructs have been described previously (Staal et al., 1999). CHO cells were transfected using lipofectamine (Gibco BRL), 293T cells using FuGene-6 (Boehringer Mannheim). pSV40-CAT was used to normalize for transfection efficiency. CAT and luciferase values were determined as described by van de Wetering et al. (1997). For the 293T cells, TOP-Glow and FOP-Glow, which are similar to TK-TOP and TK-FOP except for a TATA box instead of the TK minimal promoter, were transfected. For 293T cells, 300 ng of the β-catenin constructs, 100 ng reporter, 20 ng CMV-Renilla and, where appropriate, pCi to equilibrate the amount of plasmid DNA transfected were used.
Cells and reagents. For ALLN treatment, cells were washed and treated for 6 h with 10 µM ALLN from a 1000× stock prepared in ethanol. ts20 cells were shifted to the restrictive temperature of 40°C for 6 h, or the indicated amount of time, using MEMα medium containing a HEPES buffer. Cells were split into three fractions, one for luciferase determination, one for CAT assay and one for western blotting.
Western blots. 106 cells were directly lysed in 200 µl of boiling 2¥ SDS–PAGE sample buffer. Equal amounts of protein were separated on a 10% polyacrylamide gel. β-catenin protein was detected by probing the blot first with α-ABC or a commercially available α-β-catenin antibody (Transduction Laboratories, clone 14), followed by an HRP-conjugated rabbit anti-mouse IgG antibody (Pierce), and visualized by enhanced chemiluminescence (Amersham).
Acknowledgments
ACKNOWLEDGEMENTS
We thank members of the Clevers laboratory and Dr B. Burgering and Dr M. Peifer for stimulating discussions. F.J.T.S. is a fellow of the Dutch Royal Academy of Sciences (KNAW); H.C.C. is supported by Pionier and Program grants from the Dutch Organisation for Scientific Research (NWO).
REFERENCES
- Aberle H., Bauer, A., Stappert, J., Kispert, A. and Kemler, R. (1997) β-catenin is a target for the ubiquitin-proteasome pathway. EMBO J., 16, 3797–3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens J., von Kries, J.P., Kuhl, M., Bruhn, L., Wedlich, D., Grosschedl, R. and Birchmeier, W. (1996) Functional interaction of b-catenin with the transcription factor LEF-1. Nature, 382, 638–642. [DOI] [PubMed] [Google Scholar]
- Behrens J., Jerchow, B.A., Wurtele, M., Grimm, J., Asbrand, C., Wirtz, R., Kuhl, M., Wedlich, D. and Birchmeier, W. (1998) Functional interaction of an axin homolog, conductin, with β-catenin, APC, and GSK3β. Science, 280, 596–599. [DOI] [PubMed] [Google Scholar]
- Brunner E., Peter, O., Schweizer, L. and Basler, K. (1997) Pangolin encodes a Lef-1 homologue that acts downstream of Armadillo to transduce the Wingless signal in Drosophila. Nature, 385, 829–833. [DOI] [PubMed] [Google Scholar]
- Devereux T.R., Anna, C.H., Foley, J.F., White, C.M., Sills, R.C. and Barrett, J.C. (1999) Mutation of β-catenin is an early event in chemically induced mouse hepatocellular carcinogenesis. Oncogene, 18, 4726–4733. [DOI] [PubMed] [Google Scholar]
- Easwaran V., Song V., Polakis P. and Byers S. (1999) The ubiquitin-proteasome pathway and serine kinase activity modulate adenomatous polyposis coli protein-mediated regulation of β-catenin-lymphocyte enhancer-binding factor signaling. J. Biol. Chem., 274, 16641–16645. [DOI] [PubMed] [Google Scholar]
- Fagotto F., Gluck, U. and Gumbiner, B.M. (1998) Nuclear localization signal-independent and importin/karyopherin-independent nuclear import of β-catenin. Curr. Biol., 8, 181–190. [DOI] [PubMed] [Google Scholar]
- Guger K.A. and Gumbiner, B.M. (2000) A mode of regulation of β-catenin signaling activity in Xenopus embryos independent of its levels. Dev. Biol., 223, 441–448. [DOI] [PubMed] [Google Scholar]
- Hart M.J., de los Santos, R., Albert, I.N., Rubinfeld, B. and Polakis, P. (1998) Downregulation of β-catenin by human Axin and its association with the APC tumor suppressor, β-catenin and GSK3 β. Curr. Biol., 8, 573–581. [DOI] [PubMed] [Google Scholar]
- Hart M. et al. (1999) The F-box protein beta-TrCP associates with phosphorylated β-catenin and regulates its activity in the cell. Curr. Biol., 9, 207–210. [DOI] [PubMed] [Google Scholar]
- Jiang J. and Struhl, G. (1998) Regulation of the Hedgehog and Wingless signalling pathways by the F-box/WD40-repeat protein Slimb. Nature, 391, 493–496. [DOI] [PubMed] [Google Scholar]
- Kishida S., Yamamoto, H., Ikeda, S., Kishida, M., Sakamoto, I., Koyama, S. and Kikuchi, A. (1998) Axin, a negative regulator of the wnt signaling pathway, directly interacts with adenomatous polyposis coli and regulates the stabilization of β-catenin. J. Biol. Chem., 273, 10823–10826. [DOI] [PubMed] [Google Scholar]
- Korinek V., Barker, N., Morin, P.J., van Wichen, D., de Weger, R., Kinzler, K.W., Vogelstein, B. and Clevers, H. (1997) Constitutive transcriptional activation by a β-catenin–Tcf complex in APC-/- colon carcinoma. Science, 275, 1784–1787. [DOI] [PubMed] [Google Scholar]
- Kulka R.G., Raboy, B., Schuster, R., Parag, H.A., Diamond, G., Ciechanover, A. and Marcus, M. (1988) A Chinese hamster cell cycle mutant arrested at G2 phase has a temperature-sensitive ubiquitin-activating enzyme, E1. J. Biol. Chem., 263, 15726–15731. [PubMed] [Google Scholar]
- Liu C., Kato, Y., Zhang, Z., Do, V.M., Yankner, B.A. and He, X. (1999) β-Trcp couples β-catenin phosphorylation–degradation and regulates Xenopus axis formation. Proc. Natl Acad. Sci. USA, 96, 6273–6278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J.R. and Moon, R.T. (1996) Signal transduction through β-catenin and specification of cell fate during embryogenesis. Genes Dev., 10, 2527–2539. [DOI] [PubMed] [Google Scholar]
- Molenaar M., van de Wetering, M., Oosterwegel, M., Peterson-Maduro, J., Godsave, S., Korinek, V., Roose, J., Destree, O. and Clevers, H. (1996) XTcf-3 transcription factor mediates β-catenin-induced axis formation in Xenopus embryos. Cell, 86, 391–399. [DOI] [PubMed] [Google Scholar]
- Morin P.J., Sparks, A.B., Korinek, V., Barker, N., Clevers, H., Vogelstein, B. and Kinzler, K.W. (1997) Activation of β-catenin-Tcf signaling in colon cancer by mutations in β-catenin or APC. Science, 275, 1787–1790. [DOI] [PubMed] [Google Scholar]
- Nelson R.W. and Gumbiner, B.M. (1999) A cell-free assay system for β-catenin signaling that recapitulates direct inductive events in the early Xenopus laevis embryo. J. Cell Biol., 147, 367–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R. and Varmus, H.E. (1992) Wnt genes. Cell, 69, 1073–1087. [DOI] [PubMed] [Google Scholar]
- Nusse R., Brown, A., Papkoff, J., Scambler, P., Shackleford, G., McMahon, A., Moon, R. and Varmus, H. (1991) A new nomenclature for int-1 and related genes: the Wnt gene family. Cell, 64, 231. [DOI] [PubMed] [Google Scholar]
- Orford K., Crockett, C., Jensen, J.P., Weissman, A.M. and Byers, S.W. (1997) Serine phosphorylation-regulated ubiquitination and degradation of β-catenin. J. Biol. Chem., 272, 24735–24738. [DOI] [PubMed] [Google Scholar]
- Papkoff J., Rubinfeld, B., Schryver, B. and Polakis, P. (1996) wnt1 regulates free pools of catenins and stabilizes APC–catenin complexes. Mol. Cell. Biol., 16, 2128–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peifer M., Pai, L.M. and Casey, M. (1994a) Phosphorylation of the Drosophila adherens junction protein Armadillo: roles for wingless signal and zeste-white 3 kinase. Dev. Biol., 166, 543–556. [DOI] [PubMed] [Google Scholar]
- Peifer M., Sweeton, D., Casey, M. and Wieschaus, E. (1994b) wingless signal and Zeste-white 3 kinase trigger opposing changes in the intracellular distribution of Armadillo. Development, 120, 369–380 [DOI] [PubMed] [Google Scholar]
- Riese J., Yu, X., Munnerlyn, A, Eresh, S., Hsu, S.C., Grosschedl, R. and Bienz, M. (1997) LEF-1, a nuclear factor coordinating signaling inputs from wingless and decapentaplegic. Cell, 88, 777–787. [DOI] [PubMed] [Google Scholar]
- Sakanaka C., Weiss, J.B. and Williams, L.T. (1998) Bridging of β-catenin and glycogen synthase kinase-3β by axin and inhibition of β-catenin-mediated transcription. Proc. Natl Acad. Sci. USA, 95, 3020–3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staal F.J.T., Burgering, B.T., van de Wetering, M. and Clevers, H. (1999) Tcf-1 mediated transcription in T lymphocytes: diffrential role for glycogen synthase kinase-3 in firoblasts and T cells. Int. Immunol., 11, 312–317. [DOI] [PubMed] [Google Scholar]
- Strous G.J., van Kerkhof, P., Govers, R., Ciechanover, A. and Schwartz, A.L. (1996) The ubiquitin conjugation system is required for ligand-induced endocytosis and degradation of the growth hormone receptor. EMBO J., 15, 3806–3812. [PMC free article] [PubMed] [Google Scholar]
- Strous G.J., van Kerkhof, P., Govers, R., Rotwein, P. and Schwartz, A.L. (1997) Growth hormone-induced signal tranduction depends on an intact ubiquitin system. J. Biol. Chem., 272, 40–43. [DOI] [PubMed] [Google Scholar]
- van de Wetering M. et al. (1997) Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell, 88, 789–799. [DOI] [PubMed] [Google Scholar]
- Yost C., Torres, M., Miller, J.R., Huang, E., Kimelman, D. and Moon, R.T. (1996) The axis-inducing activity, stability, and subcellular distribution of β-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev., 10, 1443–1454. [DOI] [PubMed] [Google Scholar]
- Young C.S., Kitamura, M., Hardy, S. and Kitajewski, J. (1998) wnt1 induces growth, cytosolic β-catenin, and Tcf/Lef transcriptional activation in Rat-1 fibroblasts. Mol. Cell. Biol., 18, 2474–2485. [DOI] [PMC free article] [PubMed] [Google Scholar]