INTRODUCTION
Osteoporosis is a complex disease characterized by a reduction in bone mass with associated bone microarchitectural deterioration and a high risk of fractures. Although environmental factors like nutrition and mechanical load, as well as lifestyle, may influence the development of the disease, family and twin pair studies have suggested that there is also a strong genetic component in a predisposition to osteoporosis.
Two separate approaches have guided the research of scientists interested in understanding the genetics of osteoporosis. On one side, basic molecular biologists are unravelling the regulatory cascades that control osteoblast and osteoclast differentiation and thereby bone mass. On the other, geneticists, epidemiologists and clinical researchers are looking for genetic mutations and factors that can predispose individuals to the development of the disease. The objective of this EMBO workshop on the genetics of osteoporosis (http://ermes.cba.unige.it/genospora/EMBO.htm) was to bring together clinicians and basic scientists and to stimulate mutual discussion and the transfer of ideas.
The regulation of bone mass depends on the balance between the amount of bone formed by osteoblasts and the amount of bone resorbed by osteoclasts. Insight into the genetic factors predisposing to the disease depends on understanding the mechanisms that control the differentiation and the function of these cells. This knowledge is emerging from three major areas. First, many functional data on the transcriptional regulatory cascades that control differentiation of bone cells are accumulating. Some of the major highlights and new discoveries presented at this meeting were indeed reports of new bone-specific trans and cis regulatory elements. Secondly, the signalling pathways involved in osteoclast and osteoblast differentiation, including those that mediate estrogen receptor (ER) functions are gradually being elucidated, opening new therapeutic perspectives. Finally, the interface between the clinic and basic biology could come in the area of bone growth. It was shown that bone size is a major determinant of bone strength, and therefore genes involved in the control of bone growth and apposition could have a role in the development of osteoporosis and their study could ultimately lead to the development of new therapies.
What role do genes play in osteoporosis?
The relative impacts of environmental and genetic factors on a predisposition to osteoporosis is still a matter of debate and was discussed at length. John Kanis (Sheffield, UK) presented epidemiological data that argue that the environment plays a major role. For instance, feeding behaviour and differences in lifestyle can account for variation in the prevalence of osteoporosis in different European countries. In contrast, Tim Spector (London, UK) presented twin studies in which bone mass was more similar in monozygotic than dizygotic twins, arguing for a role of genetic factors that persists even in aged women. However, in this particular model, shared environment can be responsible for part of the resemblance. Marie-Christine de Vernejoul (Paris, France) showed data indicating that osteoporosis in young men might have a stronger genetic than environmental component, whereas in post-menopausal women environmental factors might be more important. High dominant heriditability could be shown from large pedigrees of young subjects with osteoporosis. Although this has permitted the definition of several genomic regions of interest, the specific genes involved have not yet been identified.
Transcriptional regulation of bone differentiation
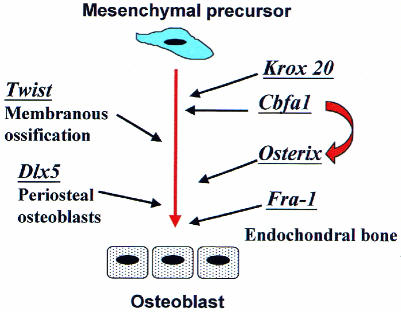
To understand how bone homeostasis is regulated, it is essential to elucidate the transcriptional regulatory cascades that govern the proliferation and differentiation of the cellular components of bone. Figure 1 summarizes the actions of some of the major transcriptional regulators presented in this meeting.
Fig. 1. Schematic diagram of the genetic control of osteoblast differentiation, in which the contributions of the different transcription factors presented at this meeting are summarized. All the transcription factors represented have a stimulatory role on osteoblast differentiation. Those on the right side of the figure have a prominent role in periosteal or membranous bone formation; those on the left are mostly involved in endochondral bone formation.
Cbfa-1 is one of the key transcriptional activators of osteoblast differentiation (Ducy et al., 1997) and it is known to control the expression of major structural proteins of the bone matrix. However, many other transcription factors seem to be involved in the control of bone formation. Benoit de Crombrugghe (Houston, TX) presented a new transcriptional regulator named Osterix. The gene encoding Osterix was identified by performing a subtraction hybridization screen using C2C12 muscle progenitor cells that differentiate into osteoblasts upon treatment with the cytokine bone morphogenetic protein 2 (BMP2). Osterix is expressed in chondrocytes of the endochondral bones and persists later in most osteoblasts in all ossification centres. Targeted inactivation of the Osterix gene led to a complete absence of bone formation in all skeletal elements, and to the loss of most markers of bone differentiation. However, Cbfa-1 is still present in these mutants, suggesting that Osterix acts at a later step than Cbfa-1 in the pathway of osteoblast differentiation.
The zinc-finger transcription factor Krox-20 is expressed in developing bone and its inactivation severely affects endochondral ossification. A detailed analysis of the regulatory sequences controlling tissue-specific expression of Krox-20 in bone has been performed by Monique Frain (Paris, France) and has led to the identification of a bone-specific enhancer characterized by the presence of many Cbfa-1 responsive elements and Krox-20 autoregulatory sites. This bone-specific enhancer also contains specific elements that are neither Cbfa-1 nor Krox-20 responsive.
In contrast to Cbfa-1, Osterix or Krox-20, which mainly affect endochondral ossification, Dlx5 and Twist act on osteoblasts involved in periosteal and membranous ossification. Dlx5 is a homeobox-containing transcription factor expressed in all developing bones. Inactivation of the Dlx5 gene (Giovanni Levi, Genova, Italy) leads to a defect in periosteal bone formation and to defective growth of primary cultures of mutant osteoblasts. Twist is a b-HLH transcription factor specifically expressed in mesoderm, and Jacky Bonaventure (Paris, France) showed that an inactivating mutation of twist affects membranous ossification.
Members of the Fos family of transcription factors (including c-Fos, FosB, Δ-FosB, Fra1 and Fra2) heterodimerize with Jun family proteins to form active AP1 (activator protein 1) transcription factors that play a prominent role in bone cell function and differentiation. Targeted inactivation of c-Fos leads to osteopetrosis with deficiencies in bone remodelling. Two talks indicated that other members of the Fos family might also be involved in bone formation. Fra-1 is a Fos-related protein encoded by Fosl1, a gene induced by c-Fos. Erwin Wagner (Vienna, Austria) reported that mice over-expressing Fra-1 have increased bone formation related to accelerated osteoblast differentiation from osteoprogenitors (Jochum et al., 2000). Roland Baron (New Haven, CT) showed that in transgenic mice conditionally overexpressing Δ-FosB, a naturally occurring truncated form of FosB, bone formation is stimulated and bone mass keeps increasing with time, ultimately leading to a severe form of osteosclerosis. Turning the gene off in vivo leads to a rapid return of bone mass to normal values. In both situations, bone resorption parameters were not modified, despite the major changes in bone mass. The involvement of AP-1 related transcription factors in osteogenesis could therefore be very complex.
Bone defects and osteoporosis can also derive from mutation in the trans regulatory elements of key structural bone genes. The most important example is that of Collagen type I (COL1), a major structural component of bone. Certain mutations in the COL1AI coding sequence induce ostogenesis imperfecta, a phenotype of extremely severe osteoporosis. Furthermore, the group of Stuart Ralston (Aberdeen, UK) reported that a polymorphism in an Sp1-responsive element in the first intron of COL1AI is associated with low bone mass and higher risk of fracture. Val Mann from the Ralston group presented a functional study on cultured osteoblasts from patients carrying different COL1A1 Sp1 alleles (S and s), demonstrating that the COL1A1 ‘s’ allele, which is associated with low bone mass, induces a high level of COL1A1 expression relative to COL1A2. Since the balance of these proteins is critical for the proper formation of bone, the presence of the ‘s’ allele could result in low collagen quality and reduced bone strength (Mann et al., 2001).
Bone growth and macrostructure
Bone mineral density (BMD) is one of the best predictors of fracture risk and is determined by the amount of bone accumulated at the end of skeletal growth, the so called peak bone mass, and by the amount of bone lost subsequently. Factors affecting bone growth have a profound effect on peak bone mass and on BMD, and can ultimately cause osteoporosis. René Rizzoli (Geneva, Switzerland) and Ego Seeman (Melbourne, Australia) discussed the clinical determinants of BMD and peak bone mass (Rizzoli et al., 2001; Tabensky et al., 2001). Large studies on the BMD in families affected by osteoporosis and in growing children indicate that 70% of the peak bone mass reached at 20 years of age is genetically determined. However, environmental factors, mainly calcium and protein nutrition, can modulate this genetic potential through mechanisms that might involve the regulation of IGF-1 (insulin-like growth factor-1) and PTH (parathyroid hormone). Another important consideration in the search for genes responsible for osteoporosis is that bone strength depends on bone size. The bone size increase that occurs during growth is more evident in boys, who generally have larger bones than girls. Changes to bone macrostructure that occur during ageing also depend on gender: in males periosteal apposition continues throughout life, leading to an increase in the diameter of long bones; in ageing females, an increase in endosteal resorption leads to a decrease in cortical thickness. To understand osteoporosis, it is therefore important to consider not only the factors affecting endochondral bone formation and resorption, but also those that can affect periosteal cell proliferation and differentiation.
Stem cells and bone repair
Bone marrow mesenchymal stem cells (BMSCs) are multipotent precursors that can be isolated and cultured from bone marrow. The possibility of inducing their differentiation into osteoblasts provides a potential new strategy for the promotion of bone regeneration and repair. Dafna Benayahu (Tel Aviv, Israel) reported the characterization of a new early marker for the differentiation of stromal cells into osteoblasts and explained its potential use in selecting cells for tissue grafting. An interesting application of these cells to regenerative therapy was shown by Ranieri Cancedda (Genova, Italy) who analysed the osteo-chondro-adipogenic potential of non-immortalized clones from human bone marrow cultures. He also showed very promising results of grafting autologous BMSC/bioceramic composites to regenerate large missing parts of the tibia of adult mice and sheep. These studies showed complete integration of ceramic with bone and good functional recovery; their extension to the treatment of human patients is ongoing.
Estrogen receptors and bone
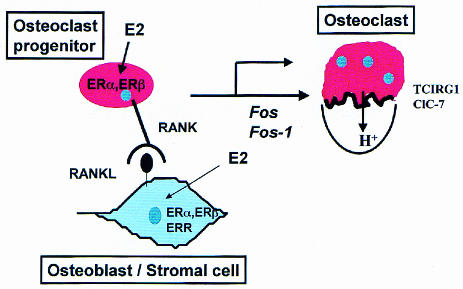
Gonadal hormones are important determinants of bone homeostasis. The roles of these, and other proteins which are thought to modulate osteoclast differentiation and function and that were presented at this meeting, are summarized in Figure 2.
Fig. 2. Factors regulating osteoclast differentiation and function presented at this meeting. Stromal cells express RANKL, which activates RANK expressed in osteoclast progenitors. Activation of RANK induces expression of Fos and Fos-1, both of which are required for osteoclast differentiation. Estradiol-(E2) acts both on osteoblast and osteoclast precursors through ERα and ERβ. Osteoblasts also express the orphan receptor ERR, which could be regulated by E2. Osteoclasts express TCIRG1 and ClC-7, which are required for acidification and therefore resorption by osteoclasts.
In post-menopausal women, the decrease in serum estrogen levels due to cessation of ovarian function is accompanied by a negative bone remodelling balance, with subsequent bone loss and increased fracture risk. In women, polymorphisms in estrogen receptor alpha (ERα) have been associated with the rate of bone loss after menopause. No association between ERα polymorphism and bone mass has been observed in men, although a mutation that completely inactivates ERα dramatically affected bone mass in a male patient. The group of Frank Gannon (Heidelberg, Germany) showed that several ERα isoforms are expressed as a result of alternative splicing of the ERα gene in human osteoblasts. These data suggest that, in osteoblasts, estradiol (E2) can act in part through an ERα isoform that is markedly different from the 66 kDa receptor, and that ERβ plays only a minor role in human osteoblasts.
Goran Andersson from the Karolinska Institute (Stockholm, Sweden) presented a study on male and female mice in which either the ERα or the ERβ gene had been inactivated by homologous recombination. He showed that lack of either affects the adult bone, but in a distinct manner. Loss of ERα was associated with decreased longitudinal and radial limb growth in male mice, whereas the opposite effect, i.e. increased limb length and increased cortical BMD, was observed in ERβ female mice. These data are difficult to interpret, as the genetic lesions involved are known to induce major changes in the circulating levels of the ligand hormones, and because the two receptors might be redundant. Another molecule which might prove to be very important for hormonal bone regulation is the estrogen-related receptor α (ERRα). As was shown by Jean-Marie Vanacker (Lyon, France), this protein is highly expressed in osteoblasts and regulates bone-specific targets. ERRα is activated by ER and could have a role in bone loss following estrogen deprivation. Finally, not only osteoblast, but also osteoclast, precursors are targets of estradiol in bone, as shown by Martine Cohen-Solal (Paris, France), who presented a study in which estradiol decreased osteoclast differentiation and function from human monocytes and induced a 31-fold decrease in c-Jun expression.
Mechanism of bone resorption
To understand osteoporosis, it is critical to clarify the genetic basis of bone resorption. The opposite phenotype, osteopetrosis, is present in rare human disorders that are caused by a number of osteoclast dysfunctions. Erwin Wagner showed that mice lacking c-Fos are osteopetrotic due to a differentiation block in bone-resorbing cells. Furthermore, Fra-1 is also implicated in osteoclast differentiation, as the encoding transgene can rescue c-fos–/– mice, making possible the development of osteoclasts in these animals. Interestingly, fra-1 was induced in hematopoietic precursors by RANKL, the osteoclast-differentiating factor. This induction is c-fos-dependent, and thus establishes a link between RANK signalling and the expression of AP1 proteins during osteoclast differentiation.
The resorptive function of the osteoclast is mostly dependent on its capacity to acidify the bone matrix. As shown by Annalisa Frattini (Milan, Italy), an osteoclast-specific subunit of the vacuolar proton pump, TCIRG1, is mutated in 50% of patients with recessive osteopetrosis. Mice in which the endogenous gene has been deleted also have defective osteoclastic resorption. The search for a polymorphism associated with osteoporosis is underway. Voltage chloride channels (CLC) are also necessary for acidification and ClC-7, a member of this family, is highly expressed in osteoclasts. Dagmar Kasper-Biermann (Hamburg, Germany) showed that mice in which ClC-7 is deleted have osteopetrosis, and that a patient with recessive osteopetrosis was compound heterozygous for both nonsense and missense mutations in this gene (Kornak et al., 2001).
Regulation of bone mass: do osteoblasts and osteoclasts interact?
Both Patricia Ducy (Houston, TX) and Valerie Geoffroy (Paris, France) described the phenotype caused by overexpression of Cbfa-1 in bone. Interestingly, the two groups obtained apparently contradictory results. When Ducy over-expressed Cbfa-1 under the control of the promoter of osteocalcin, a gene expressed in highly differentiated osteoblasts, she observed a high bone mass phenotype. This was due to an increase in the amount of bone matrix produced, and not to an increase in osteoblast number. In contrast, Geoffroy overexpressed Cbfa-1 under the control of the Collagen I promotor and observed bone loss mimicking human osteoporosis. In this case, both bone formation and bone resorption were increased, inducing high bone turnover and remodelling rates. Most likely, the overexpression of Cbfa-1 had increased osteoclast recruitment induced by osteoblast precursors, and resulted in higher bone resorption through increased production of factors inducing osteoclast differentiation. This is a nice example of opposite phenotypes resulting from the forced expression of a transcriptional regulator in cells at different stages of differentiation.
The keynote lecture of Roland Baron was particularly provocative in the same vein. He described several mutant and transgenic animals exhibiting a failure in cross-regulation between bone mass and bone formation/resorption. The examples he discussed included two models of transgenic mice in which gene targeting into mature osteoblasts had been used to attempt to either enhance (DfosB) or ablate (tk gene under the control of osteocalcin promoter) bone formation. He pointed out that in both cases, bone resorption was unchanged. Baron’s conclusions were a matter of debate, as clinicians are aware of several therapeutic situations (treatments with inhibitors of bone resorbtion) where there is cross-regulation of bone mass. In fact, they are expecting new agents to be capable of continually increasing bone formation while simultaneously decreasing bone resorption, as has been observed in mice harbouring the src deletion.
Conclusions
The etiology of osteoporosis is clearly due to a multiplicity of factors in nature. While many of the non-genetic factors contributing to the risk for the disorder have been widely investigated in recent decades, the search for genetic determinants is relatively new, albeit very intense. From family histories, twin studies and molecular genetics, it is clear that the predisposition for osteoporosis can be inherited. However, it is also clear that genetic control of osteoporosis is polygenic, and the specific genes involved are just beginning to be identified. Both structural and regulatory genes have been implicated in the propensity toward osteoporosis. Mutations in genes that control bone mass (and its mineral content) and/or bone turnover are obvious candidate genes. Estimation of the genetic contribution to the variance found in BMD, for example, ranges from 60 to 90%.
The challenge of providing new approaches towards the treatment of osteoporosis requires a worldwide effort in which the progress and insights from basic research are joined with the experience from clinical and pharmacological work.

Between the 31st March and 3rd April 2001 the EMBO workshop ‘Genetics of Osteoporosis: from Basic to Clinical Research’ was held in Sestri Levante, Italy. The complete programme of the meeting and all the abstracts can be found online (http://ermes.cba.unige.it/genospora/EMBO.htm).

The organizers of the meeting: Giovanni Levi & Marie-Christine de Vernejoul.
Acknowledgments
ACKNOWLEDGEMENTS
We apologize to the many participants whose work has not been discussed due to space limitations. The authors are grateful to EMBO for having provided the economical means for this workshop and to Servier (France) and Lilly (France) for having sponsored this meeting. The support of the European Union to the GENOSPORA Consortium (Grant: QLK-1999-20108 to G.L. and M.C.D.) and of Telethon, Italy (GP0218/01) to G.L. is gratefully acknowledged.
References
- Ducy P., Zhang, R., Geoffroy, V., Ridall, A.L. and Karsenty, G. (1997) Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell, 89, 747–754. [DOI] [PubMed] [Google Scholar]
- Jochum W., David, J.P., Elliott, C., Wutz, A., Plenk, H., Matsuo, K. and Wagner, E.F. (2000) Increased bone formation and osteosclerosis in mice overexpressing the transcription factor Fra-1. Nature Med., 6, 980–984. [DOI] [PubMed] [Google Scholar]
- Kornak U., Kasper, D., Bosl, M.R., Kaiser, E., Schweizer, M., Schulz, A., Friedrich, W., Delling, G. and Jentsch, T.J. (2001) Loss of the ClC-7 chloride channel leads to osteopetrosis in mice and man. Cell, 104, 205–215. [DOI] [PubMed] [Google Scholar]
- Mann V., Hobson, E.E., Li, B., Stewart, T.L., Grant, S.F., Robins, S.P., Aspden, R.M. and Ralston, S.H. (2001) A COL1A1 Sp1 binding site polymorphism predisposes to osteoporotic fracture by affecting bone density and quality. J. Clin. Invest., 107, 899–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzoli R., Bonjour, J.P. and Ferrari, S.L. (2001) Osteoporosis, genetics and hormones. J. Mol. Endocrinol., 26, 79–94. [DOI] [PubMed] [Google Scholar]
- Tabensky A., Duan, Y., Edmonds, J. and Seeman, E. (2001) The contribution of reduced peak accrual of bone and age-related bone loss to osteoporosis at the spine and hip: insights from the daughters of women with vertebral or hip fractures. J. Bone Miner. Res., 16, 1101–1107. [DOI] [PubMed] [Google Scholar]