Abstract
The Toll family of transmembrane proteins participates in signaling infection during the innate immune response. We analyzed the nine Drosophila Toll proteins and found that wild-type Toll-9 behaves similar to gain-of-function Toll-1. Toll-9 activates strongly the expression of drosomycin, and utilizes similar signaling components to Toll-1 in activating the antifungal gene. The predicted protein sequence of Toll-9 contains a tyrosine residue in place of a conserved cysteine, and this residue switch is critical for the high activity of Toll-9. The Toll-9 gene is expressed in adult and larval stages prior to microbial challenge, and the expression correlates with the high constitutive level of drosomycin mRNA in the animals. The results suggest that Toll-9 is a constitutively active protein, and implies its novel function in protecting the host by maintaining a substantial level of antimicrobial gene products to ward off the continuous challenge of microorganisms.
INTRODUCTION
Insects possess a self-defense system equivalent to the innate immune system of humans (Imler and Hoffmann, 2001; Kimbrell and Beutler, 2001). Similar to the acquired immune response, an important aspect of the innate immune system is the recognition of pathogens and the stimulation of downstream events. A central component for recognition is the so-called pattern recognition receptors (Medzhitov and Janeway, 2000; Takeuchi and Akira, 2001). These receptors function to distinguish molecules that are common within a group of pathogens, rather than to recognize specific antigens present in individual microorganisms.
Several lines of evidence suggest that the Toll family of transmembrane proteins may serve as parts of pattern recognition receptors (Medzhitov and Janeway, 2000; Imler and Hoffmann, 2001; Kimbrell and Beutler, 2001; Takeuchi and Akira, 2001). The first Toll protein was identified in Drosophila, where Toll functions to transmit extracellular signals into the cytoplasm to control dorsal–ventral patterning in the early embryo. Toll and many components in this signaling pathway also mediate antifungal response at post-embryonic stages. The identification of Toll-like receptors (TLRs) in mammals further demonstrates that the Toll family play important roles in innate immunity (Medzhitov and Janeway, 2000; Takeuchi and Akira, 2001). TLR proteins including TLR2, TLR4, TLR5, TLR6 and TLR9 are essential in mediating the effects of Gram-positive and -negative bacterial, mycobacterial and fungal products, as well as bacterial DNA (Medzhitov and Janeway, 2000; Takeuchi and Akira, 2001).
The Drosophila genome has nine genes that encode Toll-related proteins (Tauszig et al., 2000), while 10 TLRs have been identified in humans (Medzhitov and Janeway, 2000; Takeuchi and Akira, 2001). However, it is not clear whether the detailed pathogen recognition mechanism is conserved in Drosophila and humans (Imler and Hoffmann, 2001). Antimicrobial response in Drosophila utilizes two largely separable pathways. The antifungal response depends on the Toll/Cactus signaling pathway (Imler and Hoffmann, 2001; Kimbrell and Beutler, 2001), and the antibacterial response pathway is composed of Imd, TAK1, Dredd, IKK and Relish, with the receptor yet to be identified (Hedengren et al., 1999; Elrod-Erickson et al., 2000; Leulier et al., 2000; Rutschmann et al., 2000b; Silverman et al., 2000; Vidal et al., 2001). To better understand the functions of Drosophila Toll receptors, we tested whether full-length Toll proteins could regulate the expression of immunity genes. We found that Toll-9 can activate constitutively the expression of the antifungal gene drosomycin, similar to those of gain-of-function mutants of Toll-1.
RESULTS
Toll proteins in the activation of antimicrobial peptide genes
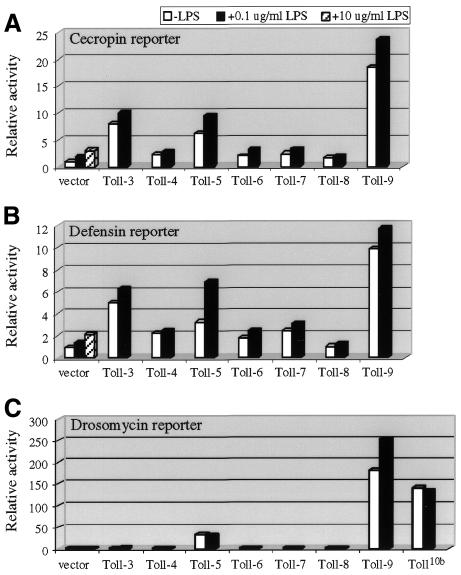
The prototypic Toll and 18Wheeler, called here Toll-1 and Toll-2, respectively, have been well characterized (Imler and Hoffmann, 2001; Kimbrell and Beutler, 2001). Toll-3 to Toll-9 are predicted genes based on the Drosophila genome sequence (Tauszig et al., 2000; www.fruitfly.org). In the experiments presented in this report, we used Toll constructs that contain both extracellular and cytoplasmic domains to ask whether any of them could augment the lipopolysaccharide (LPS) response or activate antimicrobial peptide gene expression in S2 culture cells. In transient transfection assays, we found that Toll-3 and Toll-5 caused a 3- to 7-fold increase in cecropin and defensin promoter-reporter gene activities (Figure 1A and B). Toll-9 activated both cecropin and defensin reporters 10- to 20-fold higher than the vector. However, none of these proteins could enhance the LPS response significantly above that of the vector alone. We also tested whether transfection of combinations of Toll proteins, particularly Toll-3, -5 and -9, would have a synergistic effect. We observed no augmentation of the LPS response (data not shown).
Fig. 1. Activation of antimicrobial peptide reporter genes by Toll proteins. Transient transfections of the Actin5C promoter-driven Toll and the luciferase reporters under the control of cecropin (A), defensin (B) or drosomycin (C) promoter were performed. The copia–lacZ plasmid was included as a transfection control. Three hours prior to harvest, LPS (L2637; Sigma) was added to the samples as indicated. The luciferase activity was normalized with the β-galactosidase activity. Each normalized luciferase activity was divided by that of the vector alone without LPS treatment. The results obtained were plotted as relative activity. The results are the average of three independent transfection experiments. Expression of Toll-3 and Toll-5 have modest effects on cecropin and defensin-luciferase expression, whereas Toll-9 shows higher activity. LPS treatment does not have a synergistic effect on the reporter activity when compared to the vectors alone. Toll-5 and Toll-9 strongly activate drosomycin reporter expression.
When assayed for the drosomycin reporter gene, we found that Toll-5 and Toll-9 could highly activate the expression (Figure 1C). Toll-5 activated the reporter by ∼30-fold, which is similar to that reported using a chimeric Toll-5 construct (Tauszig et al., 2000). Toll-9 showed even better stimulation, and increased the luciferase activity by >150-fold. The activating capability of Toll-9 is similar to that of a gain-of-function allele of Toll-1, Toll10b (Figure 1C). It has been clearly demonstrated in whole animals that Toll10b activates constitutively the antifungal response, mostly through the dorsal–ventral signaling components and the NF-κB factor Dif (Lemaitre et al., 1996; Meng et al., 1999; Rutschmann et al., 2000a).
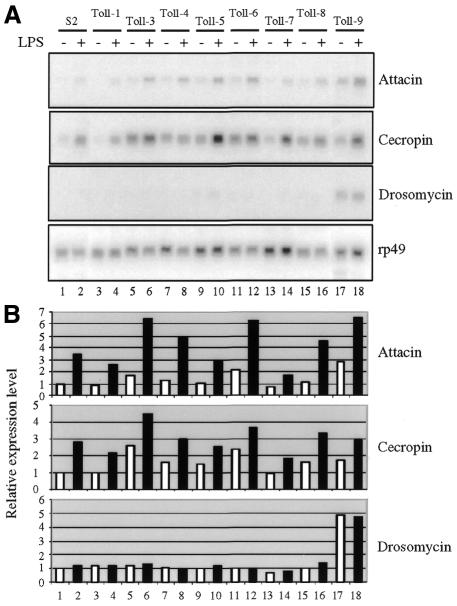
Stably transfected cell lines harboring individual Toll genes were also established to examine their ability to activate endogenous antimicrobial peptide genes. While some Toll proteins (e.g. Toll-3) caused slightly increased expression of the antibacterial peptide genes, none of the stably transfected Toll cell lines had enhanced LPS response when compared to that of parental cells (Figure 2). On the other hand, we observed significant drosomycin gene activation from the expression of Toll-9. Quantification of the signals showed a 5-fold increase in the expression of the antifungal gene in the cells. Therefore, both transient and stable transfection demonstrated that Toll-9 is a modest activator of antibacterial peptide genes and a strong activator of an antifungal peptide gene.
Fig. 2. Stable transfection of Toll-9 activates endogenous drosomycin expression. The Actin5C–Toll constructs were transfected to obtain stable cell lines. RNA expression of endogenous immunity genes was analyzed and the results are shown in (A). They are representative blots of two independent northern experiments. The hybridized blots were quantified using a phosphoimager. The expression of the immunity genes was normalized with that of the ribosomal protein 49 gene. The average result of two independent experiments was plotted and is shown in (B). Expression of some Toll proteins, such as Toll-3, can lead to a slight increase in attacin and cecropin gene expression. The addition of LPS to the stably transfected cell lines has little effect when compared with the parental S2 cells. The stable expression of Toll-9, in contrast, clearly increases the endogenous drosomycin gene expression.
Toll-9 is a constitutively active allele
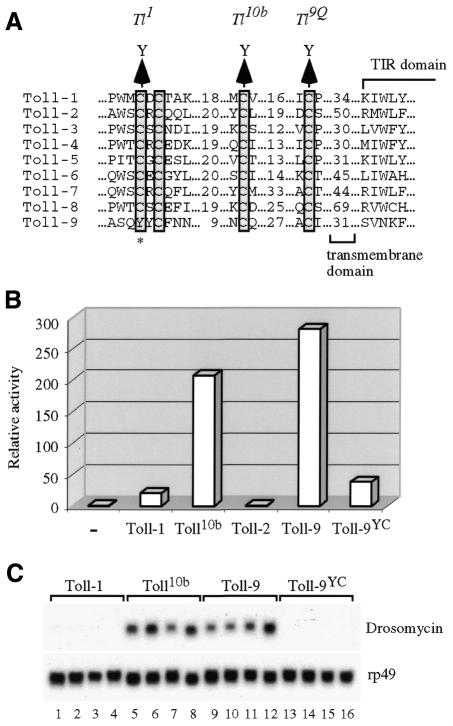
Based on the observation that Toll-9 has an activity similar to Toll10b, we surmized that the naturally existing Toll-9 could be a constitutively active protein. Previous genetic screens have isolated a class of three strong gain-of-function mutants of Toll-1. Each of these three mutants has one of the cysteine residues, located just outside the transmembrane domain, changed to tyrosine (Schneider et al., 1991). We aligned the Drosophila Toll protein sequences in this region and found that all except Toll-9 have strict conservation of these cysteine residues (Figure 3A). In particular, the N-terminal CXC motif is clearly absent from the predicted Toll-9 protein. Due to the sequence divergence of Toll-9, there are different possible alignments in this cysteine-rich region. One of the possible alignments, as shown in Figure 3A, illustrates a tyrosine residue (656) in place of a conserved cysteine. The Toll1 gain-of-function mutant allele of Toll-1 has an equivalent change of cysteine to tyrosine (Schneider et al., 1991). Therefore, it is possible that the high activity of Toll-9 is due to the loss of the cysteine or the presence of the tyrosine residue.
Fig. 3. Sequence alignment and mutagenesis reveal a constitutive activating potential of Toll-9. (A) Sequence alignment of Toll-1 to -9 in the cysteine-rich region. The four conserved cysteine residues proximal to the transmembrane domain are boxed. Toll-9 has four cysteine residues in this region but they are scattered and clearly do not form a CXC motif. One of the possible alignments as shown in (A) indicates a tyrosine residue in place of the first conserved cysteine residue. This change is similar to an induced gain-of-function mutation in Toll-1, named Toll1. (B) Changing the tyrosine to cysteine causes loss of activity of Toll-9. The Toll-9YC mutant activity was examined in a transient transfection assay using the drosomycin reporter. The Y to C mutation allows Toll-9 to behave similar to Toll-1 and has much lower activity than that of Toll10b and wild-type Toll-9. (C) Stably transfected cells containing Toll-1, Toll10b, Toll-9 or Toll-9YC were established in parallel and the endogenous drosomycin expression examined. Four individual lines of each construct exhibit the same results, such that Toll10b and Toll-9 activates the endogenous target gene efficiently but Toll-1 and Toll-9YC do not have such ability. The lower panel is the rp49 loading control.
We first confirmed Toll-9 clones from different strains by sequencing, and the tyrosine codon was identical to that of the Berkeley Drosophila Genome Project published sequence. Therefore, it is unlikely to be a sequencing error or a polymorphism only present in the strain used for genome sequencing. We then introduced a nucleotide substitution to change the amino acid to a cysteine (the construct is named Toll-9YC) and examined the activity in a transfection assay. The result showed that this mutation made Toll-9 inactive in regulating all three luciferase reporters (Figure 3B for drosomycin; data not shown for defensin and cecropin). The tyrosine to cysteine substitution lowered the activity of Toll-9 to resemble that of Toll-1, rather than that of Toll10b.
To further demonstrate the importance of the tyrosine/cysteine change in Toll-9, we established cell lines stably transfected with various Toll constructs including Toll-9YC. Four independent transfection of each construct were analyzed for the expression of the endogenous drosomycin gene (Figure 3C). While the Toll-1 containing cells exhibited no activation of drosomycin, all four lines of Toll10b showed obvious activation. The Toll-9 containing cells established in parallel had expression levels of drosomycin similar to those of the Toll10b lines. All four Toll-9YC lines had no elevated expression of the target gene, similar to that of Toll-1. These results strongly support the idea that Toll-9 is a constitutively active receptor protein.
Signaling components in the Toll-9 pathway
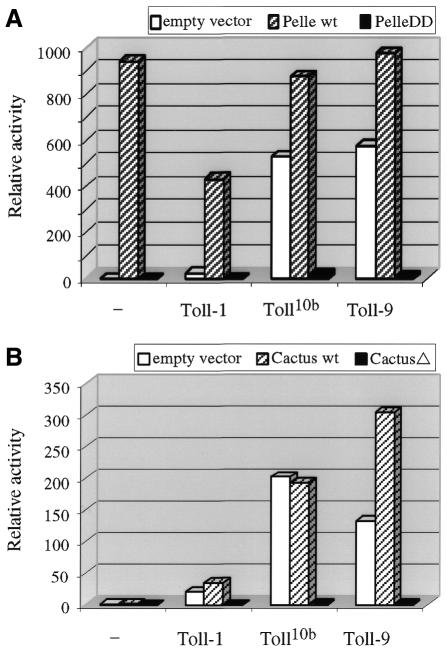
In both dorsal–ventral development and antifungal response, activated Toll-1 recruits Tube and Pelle to initiate signaling (Imler and Hoffmann, 2001; Kimbrell and Beutler, 2001). Both Tube and Pelle contain death domains, and Pelle is a kinase. Recruitment of Pelle somehow leads to degradation of the inhibitor Cactus and release of the transcription factors, Dorsal and Dif. We examined whether Toll-9 employs the same signaling components to activate drosomycin expression. A construct for Pelle containing only the death domain (PelleDD), but lacking the kinase domain, was generated. This mutated Pelle protein should function as dominant negative by binding to the death domain of Tube but cannot phosphorylate downstream substrates (Xiao et al., 1999). Transfection of wild-type Pelle activated the reporter gene efficiently, consistent with an important role of the protein in antifungal response (Figure 4). As expected, PelleDD did not activate the reporter. On the other hand, the PelleDD construct inhibited all the Toll-1-, Toll10b- and Toll-9-mediated drosomycin reporter activities.
Fig. 4. Toll-9 activation is mediated by dorsal–ventral signaling components. (A) The Actin5C vector alone, Actin5C–Pelle or Actin5C–PelleDD were co-transfected with different Toll expression plasmids. Wild-type (wt) Pelle activates strongly the drosomycin-luciferase reporter. PelleDD cannot activate the reporter. In the presence of wt Pelle, the activation by the Toll proteins remain high. However, in the presence of PelleDD, activation by the Toll constructs is abolished. (B) Similar experiments were performed using the Actin5C–Cactus and Actin5C–CactusΔ125ΔPEST mutants. The results reveal that while wt Cactus has little effect on the activation of drosomycin, the CactusΔ125ΔPEST (CactusΔ) mutant blocks the signaling.
Cactus uses its ankyrin repeats to bind to the Rel homology domains of Dif and Dorsal (Tatei and Levine, 1995). The Cactus protein degradation is regulated both by signal dependent and signal independent mechanisms, through the N-terminal serine residues and C-terminal PEST sequence, respectively (Belvin and Anderson, 1996). Therefore, we utilized a construct CactusΔ125ΔPEST that contained only the ankyrin repeats. This mutant Cactus should stably bind to and inhibit Dif and Dorsal, even when the signaling pathway is stimulated. Co-transfection of wild-type Cactus did not lead to significant changes in the activation of drosomycin by Toll-1, Toll10b and Toll-9. In contrast, the CactusΔ125ΔPEST construct abolished all these Toll signaling activities (Figure 4B). Therefore, Cactus and Pelle, and probably the binding partners Dif and Tube, are likely signaling components that mediate the activation of drosomycin by Toll-9.
Expression of Toll-9 in whole animals and during infection
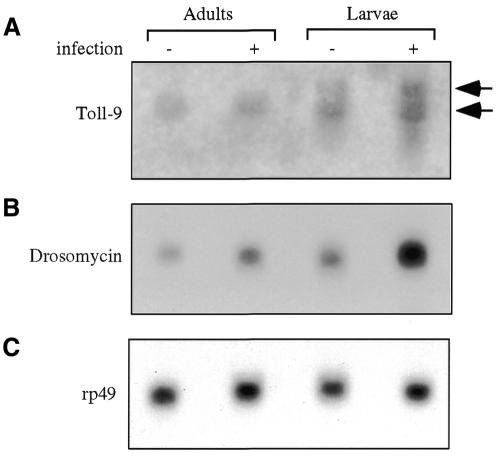
If Toll-9 has a constitutive function in activating antimicrobial defense, there should be detectable expression at the larval and adult stages, when the animals are free moving in the environment and need continuous protection. Thus, we isolated RNA from wild-type flies and wandering third instar larvae to analyze the Toll-9 gene expression. There are two detectable Toll-9 transcripts on the northern blot, with approximate sizes 3 and 5 kb (Figure 5A). The 3 kb transcript is present in both larval and adult stages, and did not seem to change upon bacterial challenge. The 5 kb transcript was detected only in larvae, and also had no significant change on infection. The two bands on the northern blot may represent the mature and the non-spliced transcripts, based on the annotated sequence prediction (CG5528, Gadfly; Berkeley Drosophila Genome Project). Other possible explanations include alternative promoter usage and alternative splicing. Regardless of these, there is constitutive expression of Toll-9 at relevant stages. This constitutive expression of Toll-9 correlates with an easily detectable level of drosomycin mRNA at the same stages even in the absence of microbial challenge (Figure 5B), as observed previously (Lemaitre et al., 1996; Meng et al., 1999). The exposure time for the drosomycin blot shown in Figure 5B was 1.5 h, compared with 3 days for that of Toll-9, highlighting the relatively high level of constitutive drosomycin expression. These results support the idea that Toll-9 may function to maintain a constant production of antimicrobial substances in whole animals.
Fig. 5. Expression of Toll-9 correlates with constitutive drosomycin in larvae and adults. Poly(A) mRNA was isolated from uninjected and injected (left to recover for 3 h) wild-type animals at larval and adult stages. The samples were analyzed by northern blotting and hybridization. (A) The autoradiograph is shown. The upper band resulting from Toll-9 hybridization is ∼5 kb and the lower band is ∼3 kb. The larval and adult mRNA both contain the 3 kb band and the signal does not change significantly after Escherichia coli injection. The upper band is only detected in larvae, and also has no significant change upon injection. (B) The same blot was hybridized with a drosomycin probe, revealing an ∼0.5 kb band. The drosomycin mRNA can be detected easily in uninduced animals and have higher levels in induced animals (C). The blot was re-hybridized with rp49 as the loading control.
DISCUSSION
We have demonstrated that the Drosophila Toll-9 protein is a potent regulator of the antifungal gene drosomycin. The results corroborate with a biological function of continuous stimulation of antimicrobial peptide production by Toll-9. As illustrated in Figure 5, drosomycin has both constitutive and inducible expression in whole animals (Lemaitre et al., 1996; Ferrandon et al., 1998; Meng et al., 1999). Toll-1 is required for the inducible expression of drosomycin, but in Toll-1 loss-of-function mutants the constitutive expression persisted (Lemaitre et al., 1996; Ferrandon et al., 1998). Therefore, Toll-9 may be a key factor in maintaining the constitutive expression in whole animals.
Toll proteins have been demonstrated to function as parts of pathogen recognition receptors to signal infection (Imler and Hoffmann, 2001; Takeuchi and Akira, 2001). Our results suggest a novel function for Toll proteins: some of them may provide constant protection prior to systemic infection. The digestive tracts and epidermis of complex organisms are in constant contact with microbes. The epithelial tissues besides serving as a passive barrier, also provide active protection against infection by secreting antimicrobial molecules (Ferrandon et al., 1998; Tzou et al., 2000). Therefore, proteins such as Toll-9 would function as a constitutive stimulator of the regulatory pathways to achieve this. The in vivo requirement of Toll-9 in the self defense process can be examined once mutant alleles of Toll-9 are available. In addition, we have tested pelle385 loss-of-function mutant flies but found no change in the basal expression level of drosomycin (data not shown). Further genetic experiments are required to examine whether null alleles of pelle may have a defect or whether other signaling components, such as the newly identified DMyD88 (Horng and Medzhitov, 2001), may provide redundant functions.
We have not been able to unveil a functional Toll receptor that could mediate the LPS response in Drosophila. A possible explanation is that hetero-multimerization of different Toll proteins is required to form a functional receptor. Alternatively, it is possible that the Toll protein that functions in LPS recognition is not among the nine different genes we tested. It is also noteworthy that the mammalian LPS receptor complex includes at least three proteins: TLR4, CD14 and MD2 (da Silva Correia et al., 2001). The Drosophila genome sequence has not revealed obvious CD14 and MD2 homologs so far. LPS recognition in insects may utilize different mechanism because of an independent evolutionary course.
While our experiments reveal a constitutive function of Toll-9, whether it can be stimulated by molecules derived from pathogens remains unknown. The Toll-9 protein contains the characteristic leucine-rich repeats in the extracellular domain and therefore may function similarly to other Toll proteins in pathogen recognition. Furthermore, since Toll-1 and Toll-9 both activate the antifungal defense and may share signaling intermediates, whether Toll-9 dimerizes with Toll-1 or with other Toll proteins to signal infection, is another interesting question that remains to be answered.
METHODS
Molecular cloning. The Toll-1 and Toll10b cDNA clones in the Actin5C vector have been described previously (Norris and Manley, 1996). The Toll-2 to Toll-9 clones were PCR amplified using wild-type genomic DNA as template, thus some clones contain intron sequences. The accession numbers of the Toll genes have been published previously (Tauszig et al., 2000). The PCR products were first cloned into the Bluescript KS vector and sequenced to confirm the identity. The fragments were then subcloned into the pGem3–Actin5C vector for subsequent transfection assays.
To generate the Actin5C–PelleDD construct, the Pelle cDNA was digested with BspEI enzyme and the 700 bp fragment (from position –58 to +642, where +1 is the translation start site) was purified. The fragment was blunt-end cloned into the EcoRV site of the pGem3–Actin5C vector. The Actin5C–Pelle wild-type clone was as described previously (Norris and Manley, 1996). To generate Actin5C–cactus, a HindIII–NotI fragment from the pNB40–cactus cDNA was subcloned into the same sites of the Bluescript KS vector. A KpnI–SacI fragment was then excised and cloned into the same sites of the Actin5C vector. The cactusΔ125ΔPEST cloning started with a cactus cDNA that has the first 125 amino acids deleted and has a stop codon in front of the PEST sequence. A HindIII–NotI fragment was excised and cloned into the Bluescript KS vector. Next, a KpnI–SacI fragment was similarly subcloned into the Actin5C vector.
Site-directed mutagenesis was used to generate the Toll-9YC construct. A NotI–SalI fragment of the Bluescript Toll-9 clone was excised and subcloned into the same sites of the Bluescript KS vector. Mutagenesis was conducted using double-stranded plasmid and QuikChange mutagenesis kit from Stratagene. Two oligonucleotides were utilized to change codon 656 from TAT to TGT. The region was fully sequenced to confirm the targeted mutation and the lack of the other undesired mutation. Actin5C–Toll-9YC was generated by swapping the NotI–SalI fragment with that of the wild-type clone.
Cell culture and transfection assay. The Drosophila Schneider-2 and the various Toll-expressing cell lines were maintained at 25°C in Schneider’s medium (Life Technologies) supplemented with 10% heat-treated fetal bovine serum (Hyclone), 100 U/ml of penicillin and 100 mg/ml of streptomycin sulfate. Transient transfections were performed as described in the protocol for the Lipofectin transient transfection (Life Technologies). The cells were transfected with 1 µg of the Actin5C constructs, along with 0.1 µg of luciferase reporter plasmids and 1 µg of copia–lacZ plasmids. The cecropin-, defensin- and drosomycin-luciferase reporters contained 0.8, 0.9 and 2.4 kb of upstream sequences, respectively. The transfected cells were grown for 48 h and harvested. The cells were lysed in 100 µl lysis buffer containing 1% Triton, 25 mM glycylglycine pH 7.8, 15 mM MgSO4, 4 mM EGTA and 1 mM dithiothreitol. Ten microliters of the lysate were used for luciferase assays while 50 µl were used for β-galactosidase assay. Stable transfectants were generated as described previously (Han and Ip, 1999). The cells were assayed for RNA expression of the respective Toll genes to confirm the integration and expression (data not shown). The RNA preparation and northern blot analysis were performed as described previously (Han and Ip, 1999).
Acknowledgments
ACKNOWLEDGEMENTS
We thank Dominique Ferrandon and Ruslan Medzhitov for the drosomycin reporter plasmid, David Stein and Carl Thummel for the cactus plasmid, Steve Wasserman for the pelle plasmid and Jim Manley for the Toll-1 and Toll10b plasmids. This publication was made possible by grant GM53269 from National Institute of General Medical Sciences (NIGMS); its contents are the sole responsibility of the authors and do not necessarily represent the official views of the NIGMS.
REFERENCES
- Belvin M.P. and Anderson, K.V. (1996) A conserved signaling pathway: the Drosophila Toll-dorsal pathway. Annu. Rev. Cell. Dev. Biol., 12, 393–416. [DOI] [PubMed] [Google Scholar]
- da Silva Correia J., Soldau, K., Christen, U., Tobias, P.S. and Ulevitch, R.J. (2001) Lipopolysaccharide is in close proximity to each of the proteins in its membrane receptor complex. Transfer from cd14 to tlr4 and md-2. J. Biol. Chem., 276, 21129–21135. [DOI] [PubMed] [Google Scholar]
- Elrod-Erickson M., Mishra, S. and Schneider, D. (2000) Interactions between the cellular and humoral immune responses in Drosophila. Curr. Biol., 10, 781–784. [DOI] [PubMed] [Google Scholar]
- Ferrandon D., Jung, A.C., Criqui, M., Lemaitre, B., Uttenweiler-Joseph, S., Michaut, L., Reichhart, J. and Hoffmann, J.A. (1998) A drosomycin–GFP reporter transgene reveals a local immune response in Drosophila that is not dependent on the Toll pathway. EMBO J., 17, 1217–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Z.S. and Ip, Y.T. (1999) Interaction and specificity of Rel-related proteins in regulating Drosophila immunity gene expression. J. Biol. Chem., 274, 21355–21361. [DOI] [PubMed] [Google Scholar]
- Hedengren M., Asling, B., Dushay, M.S., Ando, I., Ekengren, S., Wihlborg, M. and Hultmark, D. (1999) Relish, a central factor in the control of humoral but not cellular immunity in Drosophila. Mol. Cell, 4, 827–837. [DOI] [PubMed] [Google Scholar]
- Horng T. and Medzhitov, R. (2001) Drosophila MyD88 is an adapter in the Toll signaling pathway. Proc. Natl Acad. Sci. USA, 98, 12654–12658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imler J. and Hoffmann, J.A. (2001) Toll receptors in innate immunity. Trends Cell Biol., 11, 304–311. [DOI] [PubMed] [Google Scholar]
- Kimbrell D.A. and Beutler, B. (2001) The evolution and genetics of innate immunity. Nature Rev. Genet., 2, 256–267. [DOI] [PubMed] [Google Scholar]
- Lemaitre B., Nicolas, E., Michaut, L., Reichhart, J.M. and Hoffmann, J.A. (1996) The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell, 86, 973–983. [DOI] [PubMed] [Google Scholar]
- Leulier F., Rodriguez, A., Khush, R.S., Abrams, J.M. and Lemaitre, B. (2000) The Drosophila caspase Dredd is required to resist Gram-negative bacterial infection. EMBO rep., 1, 353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R. and Janeway, C.J. (2000) Innate immunity. N. Engl. J. Med., 343, 338–344. [DOI] [PubMed] [Google Scholar]
- Meng X., Khanuja, B.S. and Ip, Y.T. (1999) Toll receptor-mediated Drosophila immune response requires Dif, an NF-κB factor. Genes Dev., 13, 792–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris J.L. and Manley, J.L. (1996) Functional interactions between the pelle kinase, Toll receptor, and tube suggest a mechanism for activation of dorsal. Genes Dev., 10, 862–872. [DOI] [PubMed] [Google Scholar]
- Rutschmann S., Jung, A.C., Hetru, C., Reichhart, J.M., Hoffmann, J.A. and Ferrandon, D. (2000a) The Rel protein DIF mediates the antifungal but not the antibacterial host defense in Drosophila. Immunity, 12, 569–580. [DOI] [PubMed] [Google Scholar]
- Rutschmann S., Jung, A.C., Zhou, R., Silverman, N., Hoffmann, J.A. and Ferrandon, D. (2000b) Role of Drosophila IKK γ in a toll-independent antibacterial immune response. Nature Immunol., 1, 342–347. [DOI] [PubMed] [Google Scholar]
- Schneider D.S., Hudson, K.L., Lin, T.-Y. and Anderson, K.V. (1991) Dominant and recessive mutations define functional domains of Toll, a transmembrane protein required for dorsal-ventral polarity in the Drosophila embryo. Genes Dev., 5, 797–807. [DOI] [PubMed] [Google Scholar]
- Silverman N., Zhou, R., Stoven, S., Pandey, N., Hultmark, D. and Maniatis, T. (2000) A Drosophila IκB kinase complex required for Relish cleavage and antibacterial immunity. Genes Dev., 14, 2461–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi O. and Akira, S. (2001) Toll-like receptors: their physiological role and signal transduction system. Int. Immunopharmacol., 1, 625–635. [DOI] [PubMed] [Google Scholar]
- Tatei K. and Levine, M. (1995) Specificity of Rel-inhibitor interactions in Drosophila embryos. Mol. Cell. Biol., 15, 3627–3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauszig S., Jouanguy, E., Hoffmann, J.A. and Imler, J.L. (2000) Toll-related receptors and the control of antimicrobial peptide expression in Drosophila. Proc. Natl Acad. Sci. USA, 97, 10520–10525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzou P., Ohresser, S., Ferrandon, D., Capovilla, M., Reichhart, J.M., Lemaitre, B., Hoffmann, J.A. and Imler, J.L. (2000) Tissue-specific inducible expression of antimicrobial peptide genes in Drosophila surface epithelia. Immunity, 13, 737–748. [DOI] [PubMed] [Google Scholar]
- Vidal S., Khush, R.S., Leulier, F., Tzou, P., Nakamura, M. and Lemaitre, B. (2001) Mutations in the Drosophila dTAK1 gene reveal a conserved function for MAPKKKs in the control of rel/NF-κB-dependent innate immune responses. Genes Dev., 15, 1900–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao T., Towb, P., Wasserman, S.A. and Sprang, S.R. (1999) Three-dimensional structure of a complex between the death domains of Pelle and Tube. Cell, 99, 545–555. [DOI] [PMC free article] [PubMed] [Google Scholar]