Summary
MXenes are promising materials for electrocatalysis due to their excellent metallic conductivity, hydrophilicity, high specific surface area, and excellent electrochemical properties. Herein, we summarize the recent advancement of MXene-based materials for electrocatalysis and highlight their key challenges and opportunities. In particular, this review emphasizes on the major design principles of MXene-based electrocatalysts, including (1) coupling MXene with active materials or heteroatomic doping to create highly active synergistic catalyst sites; (2) construction of 3D MXene structure or introducing interlayer spacers to increase active areas and form fast mass-charge transfer channel; and (3) protecting edge of MXene or in situ transforming the surface of MXene to stable active substance that inhibits the oxidation of MXene and then enhances the stability. Consequently, MXene-based materials exhibit outstanding performance for a variety of electrocatalytic reactions. Finally, the key challenges and promising prospects of the practical applications of MXene-based electrocatalysts are briefly proposed.
Subject areas: Materials chemistry, Energy materials
Graphical abstract
Materials chemistry; Energy materials
Introduction
With ever-increasing concerns of environmental pollution and global energy crisis,1 developing sustainable clean energy is becoming more and more important in our world; in particular, electricity can be coupled with clean energy sources such as wind, tidal, and solar power. Electrocatalytic conversion can effectively use renewable resources to produce clean energy. For example, water splitting can produce hydrogen.2 Carbon dioxide reduction reaction (CO2RR) can reduce the emission of greenhouse gas and produce CO, CH4, formate, etc.3 Electrocatalytic conversion reactions are also the key reaction in the production of fuel cells.4 Therefore, developing electrocatalysts with high activity and long life is crucial to unleash the potential of electrocatalytic conversion reaction and realize industrial production.5
MXenes, a new key family of two-dimensional (2D) transition metal carbides, nitrides, boride, and carbonitrides, were discovered by Naguib et al. in 2011.6,7 MXenes are usually synthesized by selectively etching out the A layers from the MAX phase (Mn+1AXn), where M represents transition metal (M = Sc, Ti, V, Cr, Zr, Nb, Mo, Hf, Ta), A represents group IIIA or IVA element (such as Al, Ga, Si, or Ge), and X represents C, N, and/or B element.8 During the usually etching process in HF or HCl/LiF solution,9 there are abundant surface functional groups on the surface of MXenes, including -O, -OH, and -F. As a result, MXene is usually written as Mn+1XnTx, where T is the surface functional group. Up to date, there are a variety of MXenes, including Ti3C2Tx,7 Ti2CTx,10 Ti4N3Tx,11 Mo2CTx,12 Nb2CTx,13 (Nb0.8Ti0.2)4C3Tx,14 (Nb0.8Zr0.2)4C3Tx,14 V2CTx,15 and Mo4/3B2-xTz,16 that have been synthesized, and more types of MXenes have been predicted according to theoretical calculations.17
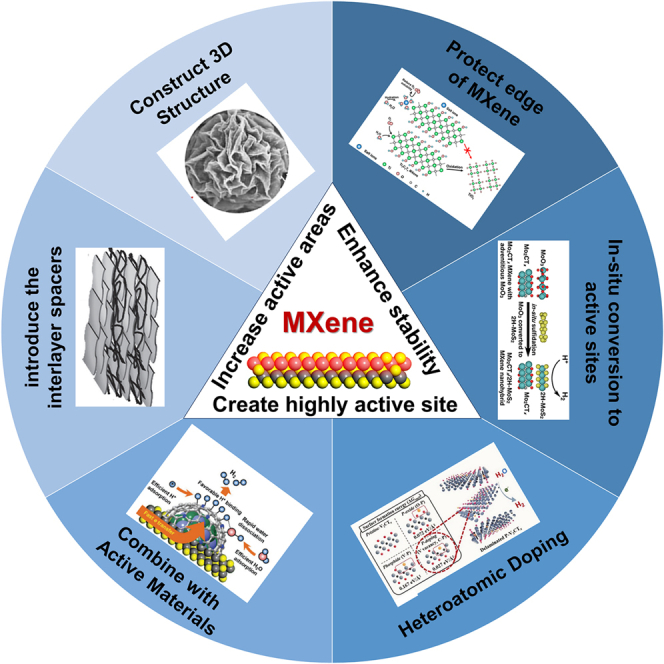
Due to the fascinating properties of MXene, research on MXenes for electrocatalysis has caused a veritable of interest.18,19,20 (1) The high conductivity (6,000–8,000 S cm−1) of MXene can facilitate the fast transport of electrons.21,22 (2) Abundant adjustable surface functional groups give it excellent hydrophilicity, which promotes the infiltration of the electrolyte and contributes to the adsorption of reactive species in water electrolysis reaction.23,24 (3) The abundant surface functional groups make MXene easily coupled with other compounds to form new synergistic catalytic sites.25 (4) The rich composition makes the catalytic properties of MXene adjustable.26 (5) 2D structure enables it abundant active sites and short mass-charge transport paths.27,28 And (6) the low work function and electronegative surface of MXene endow it excellent carrier material, which can regulate the electronic structure of active center. However, there are some key challenges for MXenes in electrocatalysis, such as easy restacking, limited intrinsic catalytic activity, and poor stability at oxygen atmosphere. Recently, many key strategies have been reported to improve their catalytic activity (Scheme 1). The typical strategies to enhance the catalytic activity of MXenes include the following: (1) The introduction of interlayer spacers to prevent MXenes stacking and construction of three-dimensional (3D) MXenes structure is conductive to the exposure of catalytic surface, increasing the amount of catalyst active sites. (2) The MXene-based catalyst with high intrinsic catalytic activity can be designed by doping heteroatom and coupling with other active materials. And (3) oxidation of MXenes can be refrained by avoiding exposure of the edge sites of MXenes to oxygen or in situ conversion of the surface of MXenes to stable substances. As a result, MXene-based catalysts possess large specific surface area, excellent conductivity, hydrophilicity, stability, and intrinsic catalytic activity, which is highly beneficial to develop robust electrocatalysts with high catalytic activity and long-term durability.
Scheme 1.
The key strategies of designing MXene-based electrocatalysts
Structural characteristics and properties of MXenes
Similar to the MAX phase precursors, the crystalline phase of MXenes possesses a hexagonal tightly packed structure with X atoms arranged in the body center of MXenes octahedrons. The transition metal atoms are located in tightly packed structure in MXenes, whereas the nonmetal atoms are packed in the octahedral interstitial positions.29 Consequently, most MXenes are highly conducting. For example, pure Ti3C2Tx shows high electrical conductivities of 2.4 × 105 S m−1,21 which is an important performance parameter in energy storage and conversion. The density of free carries of MXenes is also relatively high, e.g., 8 ± 3×1021 cm−3 of Ti3C2Tx,30 and 3×1020 cm−3 of Mo2CTx,31 and the work function of MXenes is relatively low, e.g., 3.9–4.8 eV of Ti3C2Tx,32 which can enhance charge transfer kinetics.
Usually, HF or LiF/HCl mixed solution is used to etch A component of MAX, thus the surface of MXenes has rich -OH, -F, and -O surface functional groups, which can allow strong interfacial coupling of the ions, thereby facilitating the intercalation of cations and combination with compounds. Moreover, the surface functional group can also render the MXenes’ high hydrophilicity (water contact angle of 21.5–35°), which is conducive to be used in advanced electrocatalyst.10 What is more, the large specific surface area and highly open structure inherited from their 2D structure also make MXenes promising for electrocatalysis.5,30,33 MXenes also shows excellent mechanical properties, e.g., the tensile strengths of a ∼3.3-μm-thick Ti3C2Tx film was 22 ± 2 MPa, with Young’s modules of 3.5 ± 0.01 GPa.21 And the strength of Ti3C2Tx/PVA film was further improved to 91 ± 10 MPa. By mixing Ti3C2Tx film with thermoplastic polyurethane, the tensile strength and elongation at break could rise by 41.2% and 15.4%, respectively.34 The outstanding mechanical properties promote the structural diversity and stability of MXene-based electrocatalyst.
Design strategy of Mxene-based catalyst
Increase catalytic active sites
For electrocatalysis, the number of active sites is an important factor in determining electrocatalytic activity. However, MXene layers tend to the restacking, due to the electrostatic force between the adjacent layers, which hinders the transport of electrolyte ions and limits the full utilization of their surfaces. One effective strategy to inhibit the stacking of 2D materials and improve their electrocatalytic performance is to introduce the interlayer spacers between MXene layers, including the nanoparticles (NPs),35 nanotubes/nanowires,36 and 2D nanosheets.37 In another way, the interlayer spacing of MXenes could increase after intercalating cetyltrimethylammonium bromide and stearyltrimethylammonium bromide pillars.35
Processing MXenes to hierarchical 3D architecture, such as porous films, scaffolds, and networks, is another key strategy to resist the aggregation, which can increase the specific surface area, preserve the intrinsic properties of MXenes, and render the additional characteristics such as fast mass-charge transfer channels, high robustness, and processability. The 3D MXene architecture can be prepared by ultrasonic-assisted aerosol spray drying38; sacrificial poly(methyl methacrylate) spherical templates39; assembly of MXene/rGO aerogel,40,41,42,43 MXene/polyimide aerogel,44 and MXene aerogel45,46,47; and 3D printing.48 For instance, Zhao et al. reported an effective method of electrostatic self-assembly that can assemble 2D MXene nanosheets with negatively charged and 2D layered double hydroxides (LDH) with positively charged into 3D hollow structure.6 Similarly, Xiao et al. reported the flower-like porous Ti3C2Tx by the presence of ethanediamine.49 These results indicated that assembling MXenes into 3D porous and hollow structure was an effective means to enhance the electrochemical performance, which could improve the diffusion and permeation of electrolytes. Further, chemical etching such as acid/alkali treatment50 and catalytic oxidation of MXenes flakes using transition metal salt51 were also the effective ways to prepare porous MXenes. Besides, the deposition of MXenes on 3D integral, e.g., nickel foam (NF), was also a feasible means to maintain its high active area and construct 3D porous skeleton structure for electrolysis directly.52,53
Heteroatomic doping of MXenes
Heteroatomic doping of MXenes can improve the electrochemical performance by providing new active site, changing the electronic structure of MXenes, and affecting the adsorption capacity.54,55,56 Typically, Yoon et al. reported an effective strategy to improve catalytic activity of MXene for hydrogen evolution reaction (HER) by interfacial chemical doping with a nonmetallic P, which was an electron donor. Thus, the P-C bonds in P-V2CTx could work as active sites, which balanced the energy barriers of H+ reduction and Hads desorption well, enhancing the kinetics of HER.54 Further, Kuznetsov et al. reported a Mo2CTx:Co phase that was obtained from Mo2Ga2C:Co, which greatly improved HER activity. It is calculated that the hydrogen adsorption energy on the MXene surface was significantly improved after Co doping.57
Surface modification of MXenes for electrocatalyst
MXenes are an excellent electrocatalytic platform material to design and synthesize high-activity electrocatalyst by in situ growth of catalytic active materials on their surface, thanks to the abundant surface functional groups, excellent hydrophilicity, high surface activity, and conductivity.33,58,59 First, MXene is beneficial to act as the active substance to expose more active sites. Second, it can increase the hydrophilicity of the material and promote the initial adsorption of the active species for water splitting. Third, MXene-based catalyst material can be endowed with high conductivity, accelerating the transport of electrons. Further, the strong interaction between MXene and the active substance can intrinsically optimize the electronic structure of active center, boosting the catalytic activity, such as C3N4/Ti2C3,60 Ti3C2Tx-Co 1,4-benzenedicarboxylate,61 and Co(OH)2@MXene.62 Last but not the least, new high-activity site can be formed by bonding MXenes with active substance. For example, the catalyst containing FeN3O-O-Ti could be synthesized by coupling Fe-chelated polymer-like quantum dots with ultrathin O-terminated MXenes (Ti3C2Ox), enhancing oxygen reduction reaction performance.4 Besides, Pt3Ti alloy was formed on the surface of Ti3C2Tx MXene, showing high activity for HER.63
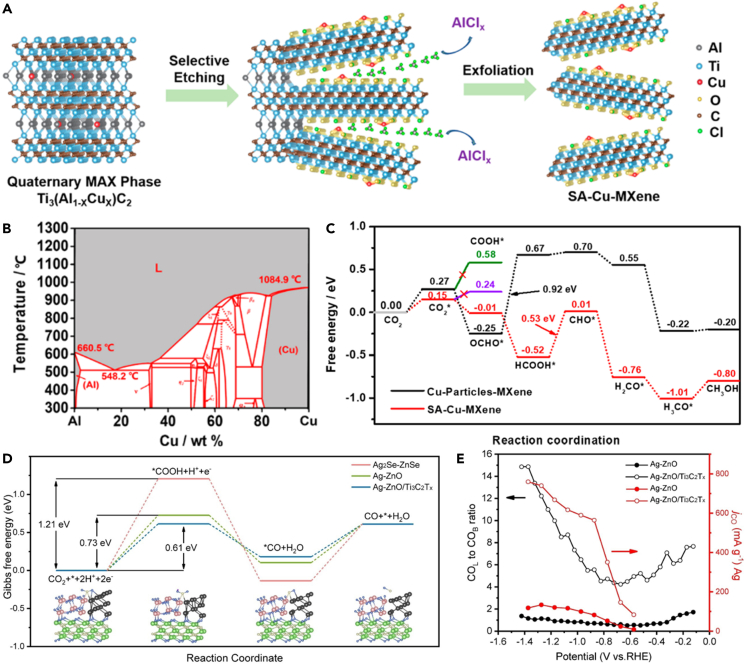
What is more, MXenes are ideal platform materials for preparing single-atom catalysts. (1) Single atom can be uniformly anchored to the defect sites on the surface of MXenes. For instance, the Mo vacancies on the surface of Mo2TiC2Tx MXene can act as the anchoring sites for single Pt atom for HER.64 (2) Via introducing non-metallic elements (e.g., N, S, P) on the surface of MXenes, single atom can be anchored on the surface of MXenes by bonding between non-metallic elements and metallic single atom. In this regard, non-metallic elements can also be used to efficiently regulate the electronic structure of single atom to improve catalytic activity. Representatively, RuSA-N-S-Ti3C2Tx catalyst showed excellent HER performance.65 (3) The composition of MAX phase is rich and adjustable so that single-atom catalysts on MXenes can be prepared by changing the composition of A and choosing a suitable selective etching method. For example, a single-atom Cu-MXene catalyst can be obtained by selective etching of Al from Ti3(Al1-xCux)C2.3
Improves durability of MXenes
With a high-proportion exposure of metal atoms on the surface of MXenes, the MXenes are usually thermodynamically metastable with high surface energy, suffering from poor oxygen tolerance.22,66,67,68,69 Therefore, it is of great importance to prevent the oxidation of MXene so that its structure and properties can be preserved, its preservation time can be extended, and it can undergo high temperature reaction. For instance, a simple carbon coating strategy has been developed to stabilize MXenes against structural collapse caused by spontaneous oxidation.22 Further, it has been reported that adding high-concentration nontoxic inorganic salts, such as NaCl, LiCl, and CaCl2, in MXenes dispersion can inhibit the oxidation of MXenes. The hydration effect of inorganic salts could reduce the ratio of free water molecules in the MXenes dispersion, restricting the oxygen solubility simultaneously, which suppresses MXenes oxidation.70 Similarly, the V2CTx flakes were assembled by Li+ cations (Li-V2CTx), appear to be much stability,71 and capping the edge of MXenes sheets with polyanionic salt could slow down the oxidation process even in aerated water.72 Further, Zhao et al. reported that sodium L-ascorbate could protect the edges of the MXenes, restricting water molecules from otherwise reactive sites.73 It is suggested that covering the edge of MXenes with other molecules can prevent its oxidation effectively. Other methods have been reported, such as hydrogen annealing74 and freezing aqueous MXenes dispersions at a low temperature.75
In another way, the surface oxidation products of MXene can be used as active materials.76,77 As exemplified, Tang et al. developed a controlled anodic oxidation method to improve the rare performance of Ti3C2Tx MXene in acidic electrolyte for pseudocapacitive energy storage.78 Our group reported the designed fabrication of nanoribbons of sodium-titanate (NaTi1.5O8.3) and potassium titanate (K2Ti4O9) for sodium/potassium ion batteries by oxidation and alkalization process of Ti3C2 MXene.79 Later, Fang et al. reported a one-step ethanol thermal oxidation method to in situ convert surface of Ti3C2Tx MXene to oxygen-vacancy-rich TiO2, where the oxygen vacancies worked as the active sites for nitrogen reduction reaction (NRR).76 Meanwhile, the TiO2 of TiO2-MXene (Ti3C2Tx) heterostructures could promote the adsorption and conversion activity of polysulfides in lithium-sulfur batteries.80 Significantly, Yury et al. developed a strategy to avoid the oxidation of Mo2CTx/MXenes by in situ vulcanizing surface of Mo2CTx MXene to form Mo2CTx/2H-MoS2 hybrid structure. The tight coupling between the Mo2CTx and 2H-MoS2 interface guaranteed fast mass-charge transfer, then exhibiting superior HER activities.77
Applications in electrocatalysis
MXenes are considered as potential electrocatalytic material, thanks to their excellent electrical conductivity, hydrophilicity, high surface activity, low work function, and electronegative, which accelerates the transport of electrons, promotes the infiltration of electrolyte, and regulates the electronic structure of active center. As a consequence, MXene-based materials have been developed for various electrocatalysis.
MXene-based materials for oxygen reduction reaction
The oxygen reduction reaction (ORR) is one of the crucial processes in many renewable energy application, including metal-air batteries and fuel cells.81 To meet the needs of practical applications, the development of electrocatalysts with high catalyst activity and long durability has been urgently needed. To this purpose, by combining catalytic active materials and MXenes, the electron density distribution of catalyst active centers can be remarkably enhanced, and new active sites would appear.
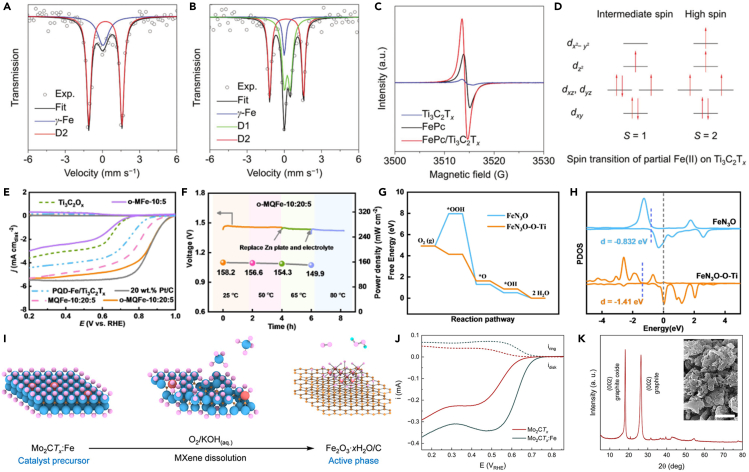
MXenes can be used to readily regulate the electronic structure or change the bonding state of the catalytic activity center to promote catalytic activity in ORR. For instance, the ORR activity of catalyst could be enhanced by coupling FeN4 and Ti3C2Tx MXene, due to the formation of Fe-N-C active sites.82 The binding of FeN4 and MXene could lead to significant Fe 3d electron delocalization and spin-state transition of Fe(II) ions. Then the lower local electron density and higher spin state of the Fe(II) centers significantly favored electron transfer of Fe dz2, making it easier for oxygen adsorption and reduction at FeN4 active center, thereby improving electrocatalytic activity of ORR (Schemes 2A–2D). In addition, Wang et al. coupled Fe-chelated polymer-like quantum dots with Ti3C2Ox MXene, called o-MQFe, to form Fe-O-Ti ligand, which adjust the spin state transition of Fe, thus enhancing the electrocatalytic activity of ORR.4 Compared with the contrast catalysts without Fe-O-Ti ligand, the intrinsic electrocatalytic activity for ORR of o-MQFe was significantly improved, exceeding the commercial Pt/C catalyst (Scheme 2E). Furthermore, Zin-air batteries and H2/O2 fuel cell systems based on o-MQFe catalyst achieved outstanding performance in a wide temperature (Scheme 2F). Further, it is theoretically calculated that Fe-O-Ti ligands in FeN3O-O-Ti could lead to a low-to-medium spin-state transition and optimal oxygen adsorption energy (Schemes 2G and 2H). What is more, Wang et al. reported that the Ti3C2 MXene could be used to stabilize molecularly thin nitride sheets of NiFeMn trimetallic nitride.83 The strong interaction between metallic Ti3C2 MXene sheets and nitrides could promote charge transport redistribution. In addition, the M−N−C or M−N−Ti ligands could be obtained at the interface, which brought about an outstanding electrocatalytic activity of ORR. It is indicated that MXene coupled with electrochemically active materials can produce new active sites with high catalytic activity for ORR. The abovementioned results showed that the electronic structure and electronic spin mode of Fe could be changed when coupled with MXene, due to the strong interaction between MXene and Fe, thereby increasing the ORR activity.
Scheme 2.
MXene-based materials for ORR
(A and B) Fe Mössbauer transmission spectra and their deconvolution of pristine FePc and FePc/Ti3C2Tx.
(C) X-band ESR spectra of pristine Ti3C2Tx, FePc, and FePc/Ti3C2Tx.
(D) Schematic representation of the spin transition of partial Fe(II) on Ti3C2Tx.82 Copyright 2018 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
(E) LSV curves of different catalysts.
(F) The open-circuit voltage and peak power density at different temperatures.
(G) The free energy changes for ORR and (H) the PDOS of Fe 3d orbital for FeN3O and FeN3O-O-Ti. The blue dotted lines denote the d-band centers, while the gray dotted line denotes the Fermi level.4 Copyright 2022 Wiley-VCH GmbH.
(I) In situ reconstruction of Mo2CTx:Fe catalyst during reaction.
(J) Anodic-going polarization curves for the electrodes with deposited Mo2CTx:Fe or Mo2CTx.
(K) XRD patterns of Fh/C that indicate the presence of graphite and graphite oxide phases. The inset shows a SEM image of the Fh/C material (the scale bar is 1 μm).84 Copyright 2021 American Chemical Society
Interestingly, Muller et al. reported Mo2CTx MXene nanosheet with single-atomic iron sites (Mo2CTx:Fe), which showed high catalytic activity for ORR and selective oxygen reduction to hydrogen peroxide.84 However, the Mo2CTx:Fe transformed in situ into a graphitic carbon framework with dispersed iron oxyhydroxide (ferrihydrite, Fh) species (Fh/C) after the catalytic tests, which were the actual active species. Because the metal atoms of MXene are easily decomposed in alkaline environment containing oxygen, it is highly necessary to characterize the in situ reconstruction process of MXene during electrocatalysis to determine the actual catalytic active site.
MXene-based materials for oxygen evolution reaction
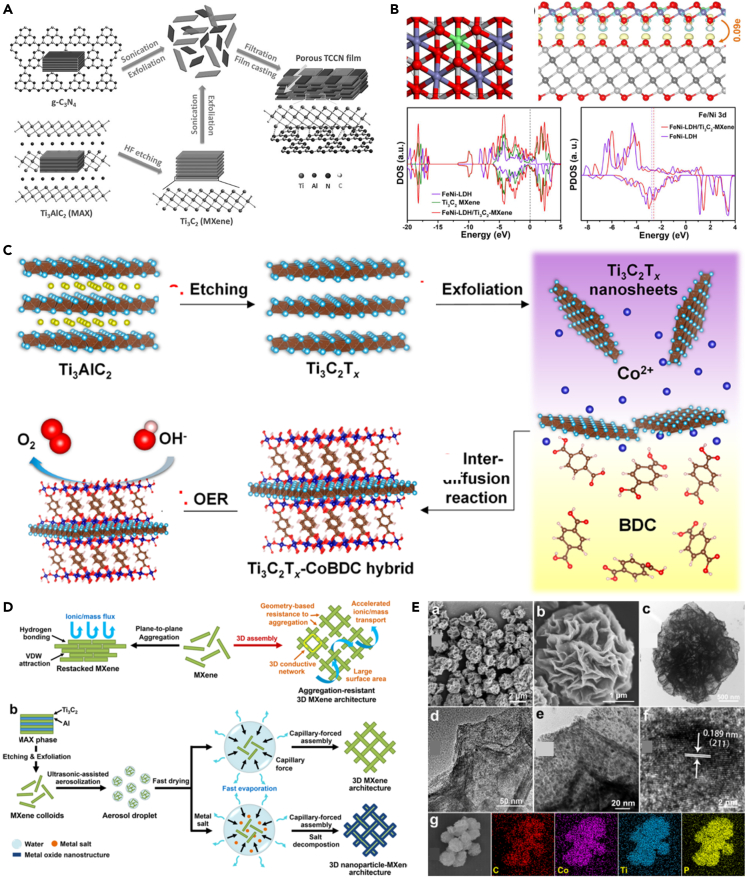
The oxygen evolution reaction (OER) is the cornerstone for many electrocatalytic application, including electrochemical water splitting, fuel cells, and metal-air batteries.61 MXene-based materials also have many applications for OER. For example, 2D Ti3C2 MXene nanosheets were coupled with 2D g-C3N4 via Ti-Nx interaction, which formed a porous free-standing film with high hydrophilic surface and conductive frame (Scheme 3A), thus showing outstanding catalytic activity of OER.60 Except mechanically mixing, 2D materials could be grown on the surface of MXene. As exemplified, Wang et al. validated the fabrication of NiFe-LDH on the surface of MXene for OER.85 The charge analysis showed that there was a 0.09e per unit cell from FeNi-LDH to MXene, indicating the close relationship between FeNi-LDH and MXene (Scheme 3B). The strong interfacial electron interaction between MXene and LDH not only guaranteed fast conductivity and robust structure of the nanohybrid but also accelerated the electrocatalytic process of FeNi-LDH in the OER. It is demonstrated that different 2D materials could be coupled through the interactions to improve their catalytic activity due to the strong interaction.
Scheme 3.
MXene-based materials for OER
(A) Fabrication of the porous Ti3C2-C3N4 film.60 Copyright 2016 Wiley-VCH Verlag GmbH &Co. KGaA, Weinheim.
(B) Model structure, density of states (DOS), and projected DOS of FeNi-LDH/Ti3C2-MXene.85 Copyright 2017 Elsevier Ltd.
(C) Schematic illustration of the preparation process of Ti3C2Tx-CoBDC hybrid for OER.61 Copyright 2017 American Chemical Society.
(D) Capillary-forced assembly of MXene into 3D architecture by spray drying the aerosol droplet of MXene-containing colloids and its application for OER.
(E) SEM, TEM, HRTEM and elemental mapping of CoP@3D Ti3C2-MXene architecture.38 Copyright 2018 American Chemical Society.
In addition to coupling with 2D materials, MXene can also be married with MOF to construct high active catalytic sites. Recently, Huang et al. reported the synthesis of a MXene-MOF composite, Ti3C2Tx-cobalt 1,4-benzenedicarboxylate (Ti3C2Tx-CoBDC), showing excellent electrocatalytic OER activity (Scheme 3C).61 In the electrocatalyst process, the well-defined interface between Ti3C2Tx-CoBDC promotes fast charge and ion transfer. Additionally, the Ti3C2Tx nanosheets with high hydrophilicity could prevent the aggregation of porous CoBDC MOF layers and make the aqueous electrolyte easy to approach the electrocatalyst surface, thus resulting in high electrocatalytic activity for OER. Further, MXene can also be coupled with the NPs to construct highly active composite materials. For instance, it is reported that Co NPs coated with nitrogen-doped multiwalled CNTs were grown on the surface of Ti3C2Tx MXene (Co/N-CNTs@Ti3C2Tx) as bifunctional electrocatalyst for ORR and OER.86 The abovementioned results show that MXene can interact with active substances to modulate the electronic structure of active center, create new active sites, then enhance the catalytic activity of the resulting catalysts. Meanwhile, MXenes can be used as excellent platform materials for electrocatalyst due to its high conductivity and hydrophilicity.
Significantly, 2D MXene can form 3D structure to avoid the restacking between the adjacent layers and then improve the electrocatalytic activity by increasing catalytic active sites and fast charge-mass transport channel. For instance, Wang et al. assembled 2D Ti3C2 MXene into hierarchical 3D architecture using ultrasonic-assisted aerosol spray drying of MXene colloids (Schemes 3D and 3E).38 As a consequence, the 3D MXene structure possessed larger specific surface area, 3D conductive frame, and excellent processability. Importantly, synergistically coupling 3D MXenes with electrocatalytic active materials could yield new composite materials with abundant active sites, fast mass-charge transport channels, and excellent intrinsic catalytic activity. Specifically, CoP@3D Ti3C2-MXene composite material showed higher electrocatalytic activity than CoP@2D Ti3C2-MXene and MXene-free CoP for OER and HER. It is noted that MXene could not only be self-assembled into 3D structures but also be constructed into 3D structures with the help of other 3D skeletons. As demonstrated, this group also reported a strategy for utilizing 3D MXene framework as structural scaffold for water-splitting by coupling NiFe-LDH nanosheets with 3D MXene framework of a microporous NF scaffold.24 It is suggested that the 3D MXene framework with large surface area, high conductivity, reactivity, and hydrophilicity could enhance the charge transfer kinetics and adsorption/activation of the water molecules, thus promoting the OER.
MXene-based electrocatalysts showed excellent catalytic activity for OER. However, the instability of MXene in OER is still difficult to be overcome. MXene, including V2CTx, Ti3C2Tx, and Mo2CTx, will be oxidized in KOH at high oxidation voltage.5,84,87 Thus, the dynamic changes of catalyst during OER should be characterized to determine the true activity site and guide the design of the MXene-based materials, further improving the activity and stability.
MXene-based materials for hydrogen evolution reaction
Water electrolysis is considered to be one of the most economical and cleanest methods for hydrogen production.88 However, lack of cheap, high-activity, and long-life electrocatalysts has limited the rapid development of this technology.2 To this end, MXenes can be used as promising electrocatalyst for HER due to there outstanding physical and chemical properties, including high conductivity, hydrophilicity, reactivity, rich composition, and adjustable surface functional groups.
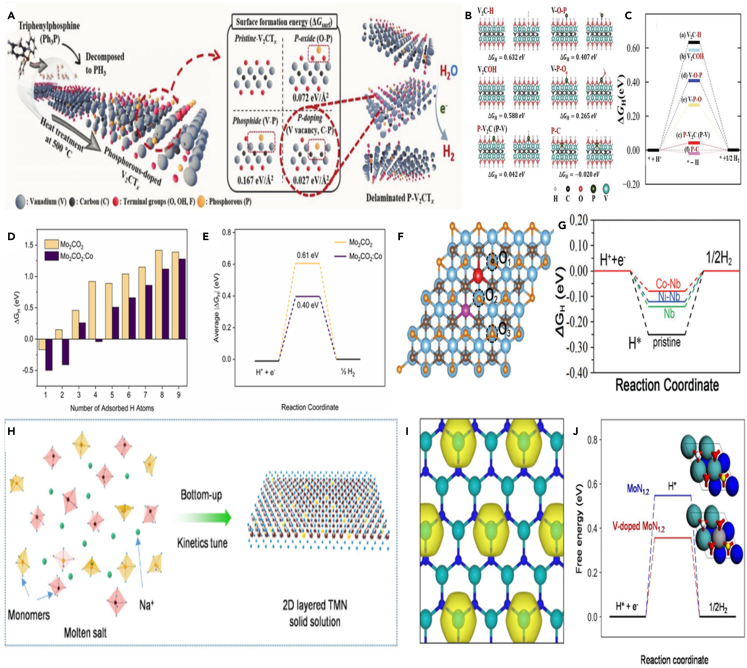
Heteroatomic doping of MXenes is also an effective method to enhance the catalytic activity by optimizing electronic structure of the active center or providing new high active sites.54,89 For instance, the activity of 2D V2CTx MXene has improved after P-element doping (Scheme 4A).54 It is calculated that the P-C bonding showed the lower surface formation energy (ΔGSurf) of 0.027 eV Å−2 and Gibbs free energy (ΔGH) of −0.02 eV (Scheme 4B). The P-V2CTx treated at 500°C showed a higher P-C bond concentration with a smaller overpotential of −163 mV to achieve a current density of 10 mA cm−2 (ηj =10). It is evidenced that the P-C bonds in P-V2CTx could act as the active center, which results in hydrogen binding energy close to zero, balancing the energy barriers of H+ reduction and Hads desorption, then improving HER activity (Scheme 4C). In addition to the doping of nonmetallic element, the doping of metallic elements can also improve the HER activity of MXene. For example, Kuznetsov et al. fabricated Co-doped molybdenum carbide (β-Mo2C:Co) from Mo2CTx:Co phase.57 It is calculated that the thermodynamics of hydrogen biding on the MXene surface has improved after Co coped, which improves the HER activity (Schemes 4D and 4E). Further, Du et al. synthesized NiCo alloy anchored on Nb-doped Ti3C2Tx MXene (named as NiCo@NTM) as HER electrocatalyst.55 It is calculated that the Nb doping could move the Fermi energy level up to the conduction band, thereby improving the electronic conductivity. In addition, the Co-Nb site displayed the more suitable hydrogen binding energy, close to zero, which further enhanced the HER activity (Schemes 4F and 4G). Recently, Qiao et al. prepared a V-Mo bimetallic nitridene solid solution V0.2Mo0.8N1.2 by catalytic molten-salt method (Scheme 4H).89 The synthesis of molten-salt mean could reduce the growth energy barrier of V0.2Mo0.8N1.2, which facilitated the V dissolution. It is theoretically showed that V doping results in the optimal electronic structure for rapid protons coupling to generate hydrogen (Schemes 4I and 4J). It is revealed that heteroatom doping in MXene could optimize the hydrogen adsorption energy, then increasing electrocatalyst activity of HER.
Scheme 4.
Heteroatom doping in MXenes for HER
(A) Schematic illustration of the phosphorization for synthesizing the P-doped V2CTx (P-V2CTx) nanosheets by heat treatment with TPP and their possible chemical compositions which can be determined by calculated surface formation energy.
(B) The hydrogen adsorption free energy when the H-V bond, O-V bond, P-V bond, P-O bond, P-V bond, and P-C bond are formed.
(C) The free energy level diagram for these configurations.54 Copyright 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
(D) Computed values of the free energies of hydrogen adsorption (ΔGH) on Mo2CO2 and Mo2CO2:Co surfaces.
(E) Reaction coordinate for the hydrogen evolution on Mo2CO2 and Mo2CO2:Co with average |ΔGH| values computed for the hydrogen adsorption on six oxygen.57 Copyright 2019 American Chemical Society.
(F) Atomistic configuration of Co/Ni replaced Ti atom on Nb-doped pristine monolayer Ti3C2O2 and the three different H∗ adsorption O sites.
(G) Gibbs free energies for H∗ adsorbed at active site on M-doped Ti3C2O2 monolayer.55 Copyright 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
(H) Schematic for the synthesis of 2D V0.2Mo0.8N1.2 solid solution through nitridation under NH3.
(I) Differential charge density of V-doped MoN1.2.
(J) Free energy diagram for HER.89 Copyright 2022 Wiley-VCH Verlag GmbH &Co. KGaA, Weinheim.
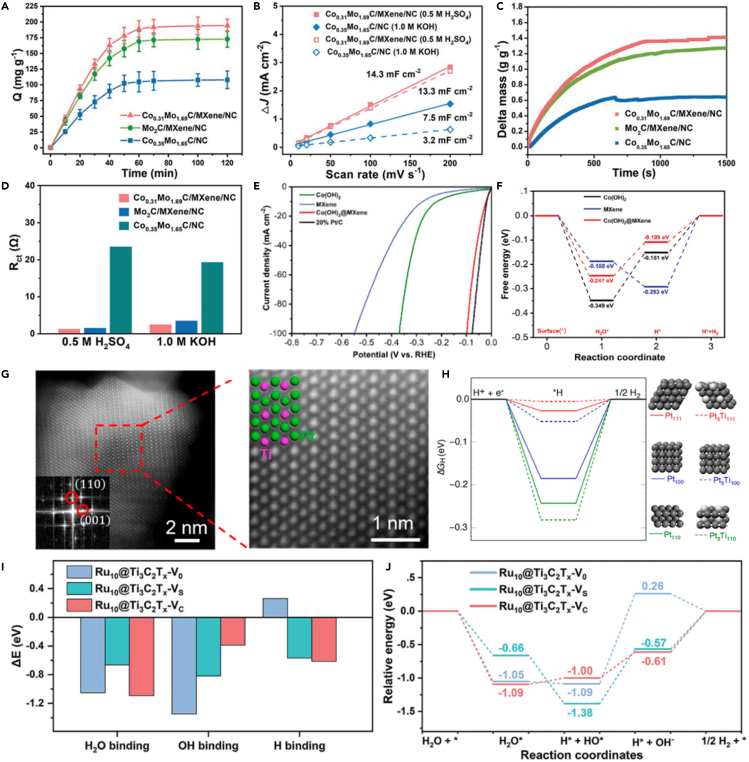
Thanks to the excellent hydrophilicity and electrical conductivity of MXenes, combining the MXene nanosheets with electrocatalytic active materials, such as transition metal compounds and noble metal species, can enhance the catalytic performance. Representatively, Wang et al. developed a key strategy by engineering a multifunctional collaborative electrocatalytic interface among cobalt-molybdenum carbide, N-doped carbon, and Ti3C2Tx MXene (denoted as CoxMo2-XC/MXene/NC) to propel the HER in a wide pH and natural seawater.23 In this case, the MXene could bring advantages of efficient H+/H2O adsorption, large specific surface area, and low charge-transfer impedance, which accelerated the charge transfer kinetics of HER (Schemes 5A–5D). As another example, Peng et al. designed a new strategy of interfacial electron coupling by assembling transition-metal hydroxide with MXenes, including FeOOH@MXene, Co(OH)2@MXene, and Ni(OH)2@MXene, for alkaline HER.62 Consequently, Co(OH)2@MXene showed outstanding catalytic activity for HER with a low overpotential of ηj =10 = 21.0 mV, thanks to the favorable adsorption kinetics of water and hydrogen caused by the synergetic interfacial electron coupling effect between Co(OH)2 and MXene (Schemes 5E and 5F). Also, this group reported the hybrid structure of CoC2O4 coated on MXene nanosheets for alkaline HER with a low overpotential of 28 and 216 mV at 10 and 1000 mA cm−2.90 The loading of active substances on the surface of MXene may also form a new highly active phase. In addition, in situ formation of Pt3Ti NPs on Ti3C2Tx MXenes as catalysts for HER has been reported, in which the Pt underwent a temperature-dependent transition from single atoms into intermetallic compounds (Scheme 5G).63 When the catalyst reduced at 550°C, the Pt/Ti3C2Tx-550 electrocatalyst outperformed commercial Pt/Vulcan and offered a low overpotential of ηj =10 = 32.7 mV. It was suggested that the (111) and (100) surfaces of Pt3Ti NPs exhibited more suitable hydrogen adsorption energy comparable to Pt (111) (Scheme 5H). In addition, Han et al. produced Ti vacancy cluster (Ti3C2Tx-VC)-engineered MXenes through a facile HF etching method. The Ti vacancy clusters in Ti3C2Tx-VC create unique lattice carbon ligand environment toward Ru species, which induces metal-support interaction.91 As a result, compared with nearly vacancy-free (Ti3C2Tx-V0) and single Ti atom vacancy (Ti3C2Tx-VS)-engineered MXenes, the Ti3C2Tx-VC-modulated Ru clusters (Ru10@Ti3C2Tx-VC) exhibit superior electrocatalytic performance in the alkaline HER, due to the optimized balance of H2O adsorption/dissociation and OH/H desorption (Schemes 5I and 5J).
Scheme 5.
MXene-based materials for HER
(A) Dependence of the amount of H+ adsorbed on Co0.31Mo1.69C/MXene/NC, Mo2C/MXene/NC and Co0.35Mo1.65C/NC catalysts on the adsorption time.
(B) The plots of current density differences (Δj) against scan rates at 0.25 V vs. RHE for Co0.31Mo1.69C/MXene/NC and Co0.35Mo1.65C/NC catalysts.
(C) Mass change as a function of time for water adsorption on Co0.31Mo1.69C/MXene/NC, Mo2C/MXene/NC and Co0.35Mo1.65C/NC catalysts.
(D) A comparison in charge-transfer impedance for HER. Copyright 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
(E) HER polarization curves of 20% Pt/C, Co(OH)2, MXene, and Co(OH)2@MXene catalysts.
(F) Gibbs-free energy profiles of the alkaline HER at the equilibrium potential for Co(OH)2, MXene, and Co(OH)2@MXene, respectively.62 Copyright the Royal Society of Chemistry 2021.
(G) Atomic-resolution HAADF-STEM image of Pt/Ti3C2Tx-550.
(H) Simulated STEM image of Pt3Ti along the [110] direction.63 Copyright 2019 American Chemical Society.
(I) The binding energy of H2O, OH, and H on Ru10@Ti3C2Tx models.
(J) Relative energy diagram during HER for Ru10@Ti3C2Tx models.91 Copyright 2023 Wiley-VCH GmbH.
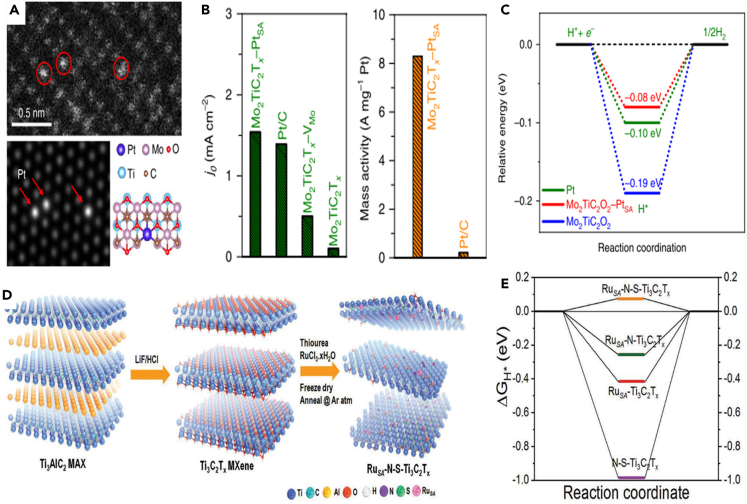
What is more, MXenes can also serve as efficient carrier materials to host single-atom catalysts for HER, thanks to their abundant defect sites and adjustable structure. Typically, Zhang et al. reported double transition metal carbide Mo2TiC2Tx-MXene, with rich exposed base and Mo vacancy in the outer layer by electrochemical stripping.64 The formed Mo vacancy was employed to fix single Pt atom to attain the catalyst of Mo2TiC2Tx-PtSA, thus greatly enhancing the catalytic activity for HER (Scheme 6A). The as-developed catalyst exhibited small overpotential of 30 and 77 mV at 10 and 100 mA cm−2, respectively, and showed high mass activity about 40 times bigger than that of commercial 40% Pt/C (Scheme 6B). Further, it is calculated that the positively charged single Pt atoms loaded on MXene constitute the optimal adsorption position for H+, facilitating the HER (Scheme 6C). Also, Cui et al. developed the heterostructures of Pt-MXene-CNTs as HER catalysts.92 Significantly, nitrogen (N) and sulfur (S) co-doped Ti3C2Tx MXene have been reported to anchor ruthenium single atoms (RuSA) via N/S bonding (Scheme 6D). It is suggested that the RuSA-N-S-Ti3C2Tx electrocatalyst achieved an optimal △GH∗ of close to zero, enhancing the HER activity (Scheme 6E).65 From the abovementioned, it is indicated that MXene is an effective single-atom carrier owing to its abundant defect sites and high surface activity. What is more, the exfoliated Ti3C2Tx-anchored Ru single atom was reported through a wet-chemistry impregnation.93 The as-obtained RuSA@Ti3C2Tx showed excellent catalytic activity in alkaline HER, with a high current density of 1.5 A cm−2 at 464.6 mV.
Scheme 6.
MXene anchoring single-atom catalyst for HER
(A) Magnified HAADF-STEM image of Mo2TiC2Tx-PtSA and its corresponding simulated image and illustration of the structure of Mo2TiC2Tx-PtSA.
(B) Exchange current densities of the catalysts and the mass activity of state-of-the-art Pt/C and Mo2TiC2Tx-PtSA.
(C) Calculated free energy profiles of HER at the equilibrium potential for Mo2TiC2O2, Mo2TiC2Tx-PtSA, and Pt/C.64 Copyright 2018 Springer Nature.
(D) Synthesis and morphological characterizations of RuSA-N-S-Ti3C2Tx catalyst.
(E) The calculated Gibbs hydrogen adsorption free energy (ΔGH∗) diagram.65 Copyright 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
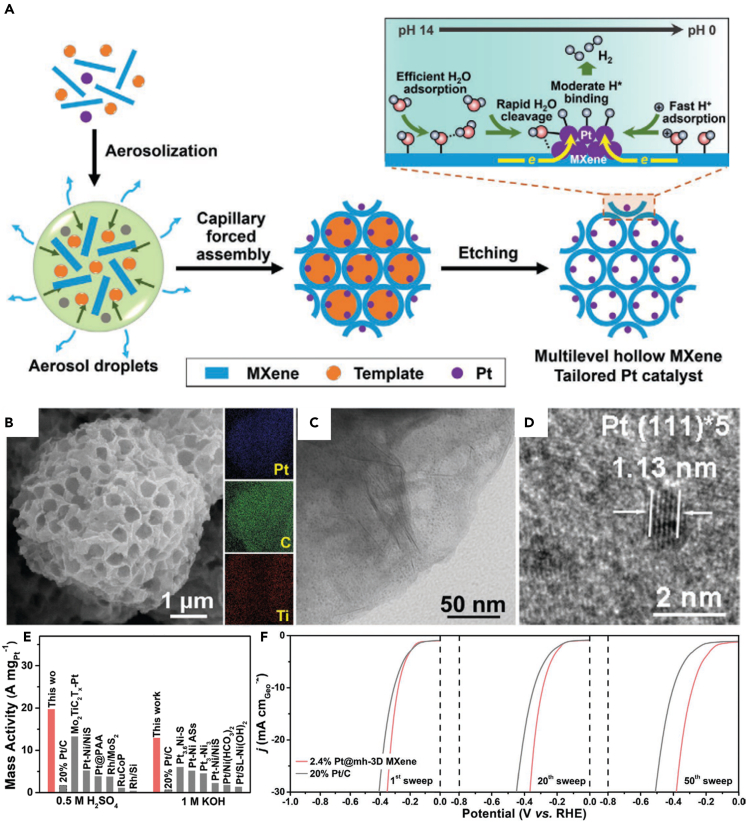
In another way, constructing 3D MXene structure can also promote the activity of HER electrocatalyst due to rich active sites and fast mass-charge transport channel.94 As a typical example, 3D MXene structure with high conductivity, hydrophilicity, and chemical functionalization multilevel hollow structure, defined as mh-3D MXene, has been assembled by an effective template-engaged aerosol drying strategy (Scheme 7A). Coupling mh-3D MXene with Pt could create a high active catalytic interface, showing high stability, excellent H+/water adsorption capacity, and fast charge-mass transport channel (Schemes 7B–7D).95 Therefore, the mass activity of 2.4% Pt@mh-3D MXene was 10–20 times bigger than that of 20% Pt/C in acidic/alkaline solution, and the activity and durability were superior than 20% Pt/C in natural seawater (Schemes 7E and 7F). Similarly, Dang et al. reported a 3D self-supporting electrode by coconstructing the interfaces of Co2P on MXene. As a result, the Co2P grown on the surface of MXene (Ti3C2Tx)-modified Ni foam required a small overpotential of ηj =10 = 29 mV for HER in 1.0 M KOH.52 Moreover, Yu et al. coupled La-doped NiFe-LDH on 3D vertically aligned Ti3C2Tx MXene onto NF skeleton (named as NiFeLa-LDH/v-MXene/NF) for water splitting, which showed higher activity than NiFeLa-LDH/NF without MXene and commercial Pt/C/NF catalyst.53 It is shown that 3D MXene structure could promote HER activity, due to the larger reactive surface area, sufficient meso-/macro-porous channels for mass-charge transport, and 3D continuous fast conductive channel.
Scheme 7.
3D MXene-based catalyst for HER
(A) Schematic illustration of template-engaged ultrafast aerosol drying strategy for yielding multilevel hollow MXene tailored low-Pt catalyst with multifunctional catalytic interface and its benefit in promoting the HER under multi-pH conditions.
(B) SEM image and elemental mapping analysis of Pt@mh-3D MXene.
(C) TEM image of Pt@mh-3D MXene.
(D) HRTEM image of Pt nanocrystallites on mh-3D MXene.
(E) A comparison between 2.4% Pt@mh-3D MXene and reported noble metal catalysts in mass activity at η = 100 mV.
(F) Polarization curves of 2.4% Pt@mh-3D MXene and 20% Pt/C for 1st, 20th, and 50th sweeps in natural seawater.95 Copyright 2020 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
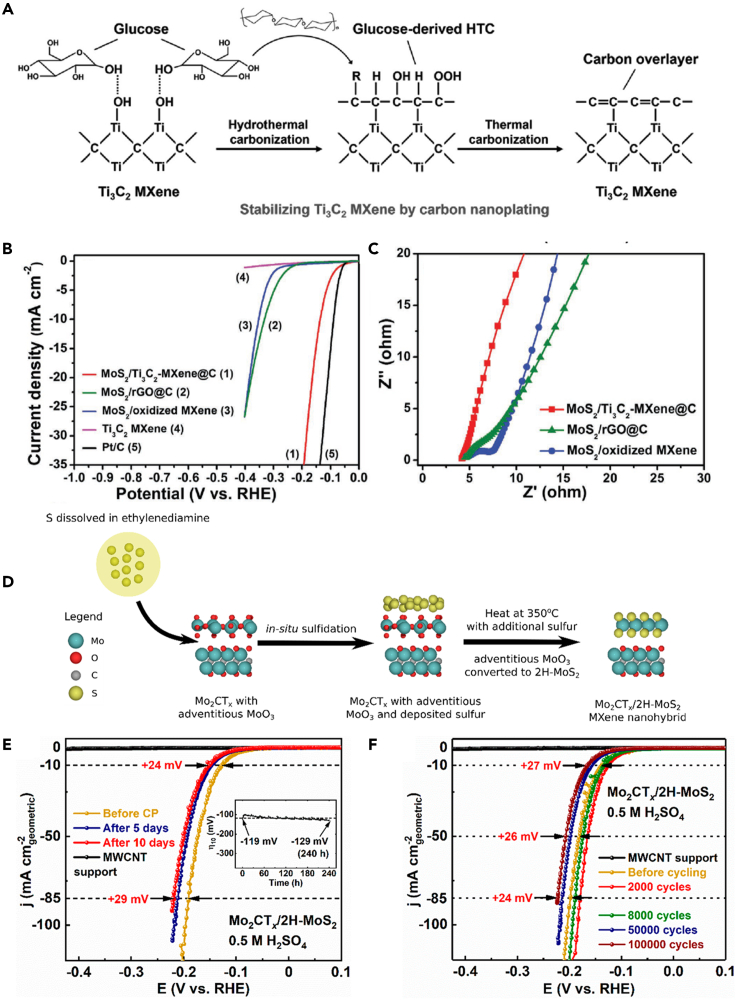
The stability of the catalyst is also an important factor that should be considered in the application of electrocatalysis. Therefore, some key strategies were proposed to inhibit the oxidation of MXenes, maintain excellent physical and chemical properties, and thus improve the durability of the catalyst.77 In order to restrain the problem that MXenes can be oxidized in the hydrothermal process at high temperature, carbon coating on the surface of MXene has been proved to be an effective strategy to inhibit the oxidation of MXene, even in high-temperature hydrothermal environment (Scheme 8A).22 Consequently, the MoS2/Ti3C2@C electrocatalyst exhibited an overpotential of ηj =10 = 135 mV, which was better than that of MoS2/oxidized MXene catalysts due to the well-maintained structure and properties of MXene, such as 2D structural feature, excellent conductivity, and high surface activity (Schemes 8B and 8C).22 In situ conversion of MXene surface to stable active phase could also inhibit the oxidation of MXene effectively. The oxidation of Mo2CTx were avoided by in situ sulfidation to form a Mo2CTx/2H-MoS2 hybrid structure (Scheme 8D). This method not only inhibited the oxidation of MXene but also formed the strong interaction between Mo2CTx and 2H-MoS2 within the nanohybrid structure, accelerated electron transport, in comparison with the physiosorbed nanohybrid. As a result, the Mo2CTx/2H-MoS2 nanohybrid could achieve industrially current density of over −450 mA cm−2geom with outstanding durability. Notably, the Mo2CTx/2H-MoS2 nanohybrid could work stably for 10 days with a less than 30 mV overpotential increase (Schemes 8E and 8F).77 It is validated that reducing the surface-oxygen-containing functional groups and in situ transformation of MXene into high catalytic activity and stability substances can inhibit the oxidation of MXene, thus increasing the stability and expanding the applications of catalysts.
Scheme 8.
Stabilizing MXene-based catalyst for HER
(A) Schematic illustration of the strategy for stabilizing Ti3C2 MXene by carbon nanoplating.
(B) Polarization curves and (C) electrochemical impedance spectroscopy of MoS2/Ti3C2@C and MoS2/oxidized MXene.22 Copyright 2017 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
(D) In situ sulfidation of d-Mo2CTx to produce Mo2CTx/2H-MoS2 nanohybrid. IR-corrected CV polarization curves for Mo2CTx/2H-MoS2 nanohybrid in 0.5 M H2SO4 after (E) 10 days constant current test and (F) 100,000 accelerated CV cycling at 100 mV s−1.77 Copyright 2020 American Chemical Society.
As a result, MXenes present many promising applications in HER, thanks to the excellent physical and chemical properties of MXene that meet the needs of HER catalysts. For example, the excellent electrical conductivity ensures the rapid transport of electrons. And the high hydrophilicity promotes the adsorption of water, which is the initial reactant of HER in neutral/alkaline environment. The following strategies have been considered to improve the activity and stability of MXene-based electrocatalysts for HER. (1) Heteroatomic doping of MXene can tune the electronic structure of active center to boost its catalytic performance. (2) The high surface activity and abundant defect sites of MXenes allow them to be coupled with active materials, including single-atom catalysts, nanoparticles, and 2D nanosheet, which can construct high-activity synergistic catalytic interface. (3) Constructing 3D MXene framework can offer more active sites, as well as charge/mass transport channels, avoiding the stacking of 2D nanosheets. And (4) covering the surface of MXene or in situ converting the surface of MXene to active material can avoid the oxidation of MXene and thus enhance the durability of electrocatalyst. Although many strategies have been developed to greatly boost the HER performance of MXene-based electrocatalysts, the activity and stability of these current catalysts reported are still difficult to meet the needs of industrial production. The electrocatalysts with high current density (>1000 mA cm−2) and long life (>1000 h) can be obtained by developing new MXene with high intrinsic catalytic activity and stability and accelerating the mass transfer process of the catalyst.
MXene-based materials for carbon dioxide reduction reaction
The electrochemical CO2RR is a promising technology to reduce CO2 emissions and obtain high-valued chemicals with a sustainable and economical strategy.3 In this regard, MXene-based materials also show outstanding performance on CO2RR.96 For instance, Yang et al. developed a single-atom copper loaded on MXene layers by selective etching of Al layers from quaternary MAX phases [Ti3(Al1-xCux)C2], due to the easy sublimation properties of AlCl3, remaining the unreacted Cu element on the MXene (Schemes 9A and 9B).3 As a result, the single-atom-Cu-MXene electrocatalyst exhibited a high Faradic efficiency (FE) of 59.1% to produce CH3OH with high stability, delivering a low energy barrier of rate-determining step (HCOOH∗ converted to CHO∗) (Scheme 9C). It was shown that the multi-elements with the alloying feature could randomly took up the single-atom thick A layers in MAX phase, making MAX an ideal platform to produce single-atom catalyst. Meanwhile, Pan et al. predicted the high catalytic activities of single-atom Sc, Ti, and V supported Ti2CN2 to produce CO. Particularly, single-atom Mn and Fe on Ti2CN2 were predicted to be active center for the production of HCOOH.97 In addition, MXene can also regulate the electronic structure of active center to promote the electrochemical CO2 reduction performance of electrocatalyst. For instance, the Ag-ZnO/Ti3C2Tx electrocatalyst achieves an outstanding CO2 conversion performance of a nearly 100% CO Faraday efficiency with a high current density of 22.59 mA cm−2 at −0.87 V vs. RHE, which is attributed to the optimized electronic structure of MXene-regulated Ag-ZnO interfaces having a low intermediate formation energy barrier (Scheme 9D).98 In situ ATR-IR spectra reveal that the dominated linear-bonded CO (COL) intermediate had an accelerated CO desorption rate on Ag-ZnO/Ti3C2Tx (Scheme 9E), which was highly correlated with the partial current of CO formation. What is more, Maryam et al. reported 3D Cu-Pd/MXene aerogels for CO2RR, with formate selectivity >90%,99 whereas Cu-Pd aerogel achieved near-unity CO production without the MXene templating. These results show that MXene can not only promote reaction rate but also change the reaction pathway.
Scheme 9.
MXene-based catalyst for CO2RR
(A) Schematic illustration of the fabrication of SA-Cu-MXene via selective etching quaternary MAX-Ti3(Al1−xCux)C2.
(B) Al−Cu binary phase diagram.
(C) Free energy diagram of CO2 to CH3OH on Cu-O3 structure.3 Copyright 2021 American Chemical Society.
(D) The free energy diagrams of electrochemical CO2 reduction.
(E) COL to bridge-bonded CO (COB) band intensity ratio and mass-specific activity of Ag-ZnO and Ag-ZnO/Ti3C2Tx derived from the ATR-IR spectra.98 Copyright 2023 Wiley-VCH GmbH.
The surface functional groups of MXene also affect the catalytic performance and selectivity of CO2RR. Replacing the O-terminations with F-terminations on Ti2CTx altered the ∗COOH adsorption energy, thus a smaller overpotential could be found at lower amounts of -F termination.100 And Handoko et al. reported that on most O-terminated MXenes, the CO2 reduction pathway to CH4 prefers the formation of the ∗HCOOH intermediate as opposed to the ∗CO intermediate.101
MXene-based materials for nitrogen reduction reaction
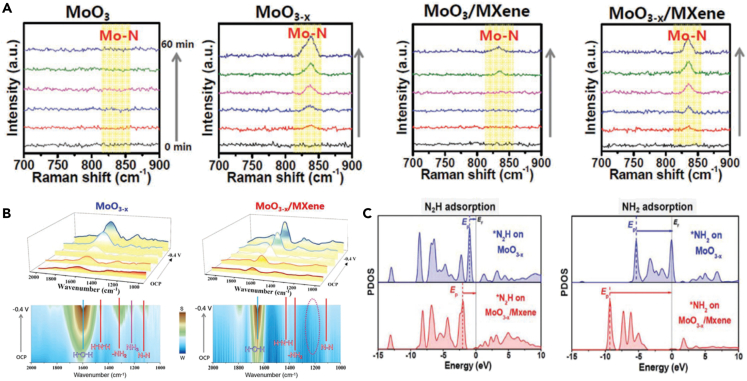
The reduction of nitrogen can produce platform chemical ammonia NH3, which is widely applied in the industry and agriculture, in a sustainable and environmental manner, and help the global nitrogen cycle.102 As reported previously, MXenes can not only be used as platform material for NRR but also can accelerate NRR process. For example, Chu et al. demonstrated the O-vacancy-rich MoO3-x attached on Ti3C2Tx-MXene (MoO3-x/MXene) for NRR, exhibiting outstanding activity for NRR with NH3 yield of 95.8 μg h−1 mg−1 and an FE of 22.3%. In situ Raman spectra showed that the MoO3-x served as the catalytic center for N2 adsorption and activation (Scheme 10A).103 In situ Fourier transform infrared spectroscopy (FTIR) suggested that the produced NH3 was easy desorption from the MoO3-x/MXene surface, facilitating re-exposure of the active sites (Scheme 10B). It was revealed from density functional theory calculation that MXene could further modulate the O-vacancy sites and effectively stabilize ∗N2/∗N2H, while destroying the stability of ∗NH2/∗NH3, thus optimizing the binding energy of NRR intermediates, reducing energy barriers, and enhancing the catalytic activity of MoO3-x/MXene for NRR (Scheme 10C). This work reflected the promoting effect of MXenes for NRR. Further, Hui et al. computational screened single-atom catalysts (i.e., Y, Zr, Nb, Hf, Ta, W, Re, and Os) on the Mo vacancies of MoCO2 MXene (Mo2CO2-MSA) for NRR.102,104 Among them, Mo2CO2-YSA showed the lowest reaction energy barrier of 0.08 eV with high selectivity for NRR. In addition, the formation energy of Mo2CO2-YSA was lower, exhibiting higher feasibility for experimental synthesis of the Mo2CO2-YSA catalyst.
Scheme 10.
MXene-based catalyst for NRR
(A) In situ Raman spectroscopy investigations of various catalysts.
(B) In situ FTIR spectra (top) and corresponding contour maps (bottom) of MoO3-x and MoO3-x/MXene at various potentials.
(C) PDOS of ∗N2H and ∗NH2 intermediates on MoO3/MXene and MoO3-x/MXene.103 Copyright 2021 Wiley-VCH GmbH.
MXene-based materials for other catalytic reactions
Recently, the application of MXene in electrocatalysis has been further extended. For example, MXene-based electrocatalysts can be developed for hydrazine oxidation reaction (HzOR). For example, Ti3C2Tx-MXene has been reported to support Ni single-atom catalysts (Ni SACs/Ti3C2Tx) by the assistance of rich Ti vacancies.105 As a result, the Ni SACs/Ti3C2Tx showed an ultralow onset potential (−0.03 V vs. RHE) for HzOR. It is calculated that surrounding C atoms can optimize the electronic density of states of Ni single atoms, improving the electrocatalytic activity of Ni SACs/Ti3C2Tx. What is more, NiCo@C/MXene/Cu foam required ultralow potentials of −25 and 43 mV to achieve high current densities of 100 and 500 mA cm−2, respectively, for HzOR.106
In addition, MXene-based materials can be applied for nitrate reduction reaction (NO3RR) to produce NH3 and achieve the global nitrogen cycle. The Fe-substituted Mo-based MXene (Mo2CTx:Fe) outperformed excellent activity for NO3RR with FE and NH3 yield rate of 41% and 3.2 μmol h−1 mg−1 in acidic media and 70% and 12.9 μmol h−1 mg−1 in neutral media, respectively.107 The Fe promotes the formation of O vacancy, resulting in high activity of NO3RR. Besides, Fe single atom has anchored on Ti3C2Tx MXene (FeSA/Mxene) for NO3RR with high NH3 FE and selectivity of 82.9% and 99.2%, respectively.108 Moreover, MXene-based materials were also used for other electrocatalytic reactions. For instance, CoS2 nanoparticles with similar size in carbon matrix on MXene-wrapped NF showed excellent electrocatalytic activity for sulfion oxidation reaction (S2−−2e− = S).109 The O-terminated MXene (Ti3C2O2) has been reported for perfluorooctanoic acid oxidation with an oxidation rate constant of 3.9 × 10−2 min−1.110
Conclusions and perspective
The attractive properties of MXenes such as metallic conductivity, hydrophilic nature, and large specific surface area enable it to be a promising class of 2D materials for constructing multifunctional high-performance electrocatalysts with high activity and stability. However, bare MXenes are difficult to meet the needs of practical applications for electrocatalyst, due to easy restacking, poor antioxidant capacity, and limited intrinsic catalytic activity. In this review, many key strategies have been developed to solve these issues, such as (1) introducing the interlayer spacers including CNT, including 0D NPs, 1D nanotubes/nanowires, and 2D nanosheets, (2) processing MXenes into hierarchical 3D architecture by ultrasonic-assisted aerosol spray drying, template method, and preparation of aerogel, (3) heteroatomic doping with metal or nonmetal, (4) surface-modifying MXenes with high active materials including metal ion, transition metal oxides, transition metal dichalcogenides, or transition metal carbide, (5) in situ conversion of MXene surfaces to oxide or sulfide, and (6) covering the edge of MXenes with other molecules, including carbon nanolayer, ion, or organic polymer to prevent oxidation.
As a result, thanks to these promising strategies, MXene-based materials have shown excellent catalytic activity and long durability for ORR, OER, HER, CO2RR, and NRR. And the MXene-based materials for HER, OER, and CO2RR have been summarized in Table 1. The possible key reasons are summarized in the following: (1) reducing the restacking of MXenes can provide larger specific surface area, high porosity, and short ion and mass transport distance, so that electrochemically active sites are easily accessed to facilitate the sufficient electrocatalytic reactions. (2) Surface-modifying or in situ conversion MXenes with high active materials can maintain the advantages of MXenes, such as high electrical conductivity and hydrophily, short ion transport distance, and enhance the activity of the electrocatalysts by providing more active sites, further optimizing the electronic structure of the active centers, and preventing the aggregation of active materials. (3) Heteroatomic doping can optimize the electronic structure of MXenes that provide new active sites for electrocatalytic reaction. And (4) inhibiting the oxidation of MXenes can maintain the excellent electrical conductivity, improve the stability, and broaden the application range of MXenes to high-temperature reactions.
Table 1.
Summary of the reported MXene-based materials for electrocatalysis
| Catalyst | Electrolyte | Reaction | Potential (V vs. RHE) | Reference |
|---|---|---|---|---|
| Mo2CTx/2H-MoS2 | 0.5 M H2SO4 | HER | −0.119 (j = 10) −0.182 (j = 100) |
Lim et al.77 |
| Pt-MXene-single-walled carbon nanotubes | 0.5 M H2SO4 | HER | −0.062 (j = 10) | Cui et al.92 |
| P-doped V2CTx | 0.5 M H2SO4 | HER | −0.163 (j = 10) | Yoon et al.54 |
| RuSA-N-S-Ti3C2Tx | 0.5 M H2SO4 | HER | −0.076 (j = 10) | Ramalingam et al.65 |
| MoS2/Ti3C2-MXene@C | 0.5 M H2SO4 | HER | −0.135 (j = 10) | Wu et al.22 |
| V0.2M0.8N1.2 | 0.5 M H2SO4 | HER | −0.158 (j = 10) | Jin et al.89 |
| Mo2TiC2Tx-PtSA | 0.5 M H2SO4 | HER | −0.030 (j = 10) −0.077 (j = 100) |
Zhang et al.64 |
| Mo2CTx:Co | 1.0 M H2SO4 | HER | −0.18 (j = 10) | Kuznetsov et al.57 |
| Pt/Ti3C2Tx | 0.1 M HClO4 | HER | −0.032 (j = 10) | Li et al.63 |
| Ru@Ti3C2Tx-Vc | 1.0 M KOH | HER | −0.035 (j = 10) −0.463 (j = 1000) −0.488 (j = 1500) |
Wang et al.91 |
| Co0.31Mo1.69C/MXene/NC | 1.0 M KOH 0.5 M H2SO4 |
HER | −0.075 (j = 10) −0.081 (j = 10) |
Wu et al.23 |
| NiCo@Nb-doped Ti3C2Tx MXene | 1.0 M KOH | HER | −0.043 (j = 10) | Du et al.55 |
| Co2P/N@Ti3C2Tx@NF | 1.0 M KOH | HER | −0.015 (j = 10) | Lv et al.52 |
| 2.4% Pt@multilevel hollow-3D MXene | 1.0 M KOH 0.5 M H2SO4 |
HER | −0.027 (j = 10) −0.013 (j = 10) |
Xiu et al.95 |
| RuSA@Ti3C2Tx | 1.0 M KOH | HER | −0.425 (j = 1000) −0.464 (j = 1500) |
Zou et al.93 |
| Co(OH)2@MXene | 1.0 M KOH | HER | −0.021 (j = 10) | Li et al.62 |
| CoC2O4@MXene | 1.0 M KOH | HER | −0.028 (j = 10) −0.216 (j = 1000) |
Wang et al.90 |
| NiFeLa-LDH/vertically-MXene/NF | 1.0 M KOH | HER OER |
−0.233 (j = 500) 1.485 (j = 500) |
Yu et al.53 |
| CoP@3D Ti3C2-MXene | 1.0 M KOH | OER | 1.618 (j = 10) | Xiu et al.38 |
| Ti3C2Tx-CoBDC | 1.0 M KOH | OER | 1.64 (j = 10) | Zhao et al.61 |
| Co/N-CNTs@ Ti3C2Tx | 0.1 M KOH | OER | 1.641 (j = 10) | Zhang et al.86 |
| Overlapped g-C3N4 and Ti3C2 nanosheets | 0.1 M KOH | OER | 1.65 (j = 10) | Ma et al.60 |
| SA-Cu-MXene | 0.1 M KHCO3 | CO2RR to methanol | −1.4 (j = 21.3) | Zhao et al.3 |
| Cu-Pd-MXene | 0.1 M KHCO3 | CO2RR to formate | −0.9 (j = 16.2) | Abdinejad et al.99 |
| Ag-ZnO/Ti3C2Tx | 0.5 M KHCO3 | CO2RR to CO | −0.87 (j = 22.59) | Hao et al.98 |
Despite the significant progress made, we are still facing several key challenges of MXene-based electrocatalysts used in actual industrial production. The following high-priority research directions should be considered fully.
Low-cost mass production of MXene
To expand the practical application of MXenes, it is urgently necessary to develop new MXene production methods with large scale and low cost at mild conditions. The commonly used HF and fluoride salt etching methods are highly toxic and low yield, which limits the large-scale preparation of MXenes. Notably, Hao et al. developed a very promising strategy based on thermal-assisted electrochemical etching to synthesize MXenes (e.g., Ti2CTx, Cr2CTx, and V2CTx).111 Further, more types of MXenes should be investigated using this method. The molten salt etching method is also efficient for producing high-quality 2D MXenes without using toxic reagents.112,113 However, the stripping, surface modification, and defect site construction of MXenes etched by molten salt method still need to be studied. In addition, Yang et al. proposed a supercritical etching method for large-scale and rapid production of MXenes assisted by supercritical carbon dioxide.114 As a result, five typical MXenes (Ti3C2Tx, Nb2CTx, Ti2CTx, Mo2CTx, and Ti3CNTx) can be prepared with the yield of ∼1 kg. Researchers are constantly exploring new low-cost, large-scale preparation methods for MXenes, but there is still a long way to go.
Synthesis of MXene with high catalytic activity
Recently, high-entropy materials exhibit excellent electrocatalytic activity due to their abundant composition and multiple and tunable active sites.115,116 Some high-entropy transition-metal carbide (HE-MXene) have been synthesized, such as (Ti1/5V1/5Zr1/5Nb1/5Ta1/5)2AlC,117 TiVNbMoC3Tx, and TiVCrMoC3Tx.118 HE-MXene performs well in energy storage; however, HE-MXene is rarely used in electrocatalysis so far. DFT calculation was used to compare the reaction energy barrier of HER between HE-MXene TiVNbMoC3 and V4C3, both with the O terminations. It is indicated that the HE-MXene displayed optimal H adsorption, which was much closer to zero (−0.41 eV) compared with V4C3O2 (−0.97 eV), showing more favorable reaction energy barrier when using the HE-MXene. Therefore, it is promising to develop many kinds of HE-MXene for different electrocatalytic reactions. In another way, a new MXene material, boridene, had been reported recently.16 It is predicted that 2D metal borides (MBenes), Mo2B2, Ti2B2, and Cr2B2, could be used for electrocatalytic coupling of N2 and CO2 to produce urea by DFT computations.119 Meanwhile, Sun et al. predicted that Mo2B2-MBene-supported single-atom catalysts (Ti, V, Cr, Mn, Fe, Co, Ni, and Cu) could be used for HER, OER, and ORR.120 However, the stable, large-scale production of MBene still needs to be explored to expand its application in electrocatalysis. In addition, fluorine-free synthesized Ta2C MXene displayed excellent catalytic activity for HER and nitrophenol reduction.121
High-current and long-life MXene-based electrocatalyst
To satisfy the need of industrial production, catalysts are highly needed to offer a high current density of >500 mA cm−2 and long-life performance of >1000 h. For water splitting, the catalysts with high current density and long life need a fast charge and mass transport channels, excellent hydrophilicity and gas-phobicity, high intrinsic catalytic activity, and structural stability.122 MXene-based materials are of great application potential that the current density of reconfiguration-CoC2O4@MXene and RuSA@Ti3C2Tx catalyst could reach up to 1,300 and 1,600 mA cm−2 for HER, respectively.90 In addition, the current density is affected by the electron transport process, especially the electron transport at the interface between the catalyst and the support.100 Thus, the surface of highly conductive MXene can be in situ converted to produce highly active substances. The close link between catalyst and support can effectively reduce the interface resistance. On the other hand, for catalytic reaction with large density current, the mass transfer between gas-liquid-solid is also a decisive factor. The NiCo-MOF on MXene-wrapped Cu foam (NiCo@C/MXene/CF) electrocatalyst has been reported to have larger bubble contact angle of 153o than the NiCo@C/CF electrocatalyst without MXene in seawater for HER, resulting in faster release of smaller gas bubbles, which promoted interfacial stability and facilitates continued exposure of catalytic sites at large current density.106 These results show the potential of MXene for electrocatalysis in large current density. However, the life of MXene in electrocatalysis still needs to be improved. DFT calculation results show that MXene will be poisoned at specific pH and voltage.87 Therefore, characterizing the reconstruction of MXene during electrocatalysis and determining the real active site are helpful to enhance the stability of MXene-based materials. Nevertheless, MXene-based electrocatalysts with high current density and long life should be continually developed. To achieve this goal, the electrocatalysts need to have enough active sites and are conducive to the adsorption of reactants and desorption of products.
In short, we believe that this review will offer close insight to develop highly active and durable MXene-based electrocatalysts and open many promising opportunities for practical application of MXene-based electrocatalysts in the near future.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Grants 22125903, 22209174), Dalian Innovation Support Plan for High Level Talents (2019RT09), Dalian National Laboratory for Clean Energy For Clean Energy (DNL), CAS, DNL Cooperation Fund, CAS (DNL202016, DNL202019), and China Postdoctoral Science Foundation (2021M703143).
Author contributions
Z.-S.W. proposed and supervised the overall project. X.-H.W., Y.W., and Z.-S.W. wrote the manuscript.
Declaration of interests
The authors declare no competing interests.
References
- 1.Seh Z.W., Kibsgaard J., Dickens C.F., Chorkendorff I., Nørskov J.K., Jaramillo T.F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science. 2017;355:eaad4998. doi: 10.1126/science.aad4998. [DOI] [PubMed] [Google Scholar]
- 2.Wang J., Xu F., Jin H., Chen Y., Wang Y. Non-noble metal-based carbon composites in hydrogen evolution reaction: fundamentals to applications. Adv. Mater. 2017;29 doi: 10.1002/adma.201605838. [DOI] [PubMed] [Google Scholar]
- 3.Zhao Q., Zhang C., Hu R., Du Z., Gu J., Cui Y., Chen X., Xu W., Cheng Z., Li S., et al. Selective etching quaternary MAX phase toward single atom copper immobilized MXene (Ti3C2Clx) for efficient CO2 electroreduction to methanol. ACS Nano. 2021;15:4927–4936. doi: 10.1021/acsnano.0c09755. [DOI] [PubMed] [Google Scholar]
- 4.Liu Y., Liu X., Lv Z., Liu R., Li L., Wang J., Yang W., Jiang X., Feng X., Wang B. Tuning the spin state of the iron center by bridge-bonded Fe-O-Ti ligands for enhanced oxygen reduction. Angew. Chem. Int. Ed. 2022;61 doi: 10.1002/anie.202117617. [DOI] [PubMed] [Google Scholar]
- 5.Tsounis C., Kumar P.V., Masood H., Kulkarni R.P., Gautam G.S., Müller C.R., Amal R., Kuznetsov D.A. Advancing MXene electrocatalysts for energy conversion reactions: surface, stoichiometry, and stability. Angew. Chem. Int. Ed. 2023;62 doi: 10.1002/anie.202210828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao X., Xu H., Hui Z., Sun Y., Yu C., Xue J., Zhou R., Wang L., Dai H., Zhao Y., et al. Electrostatically assembling 2D nanosheets of MXene and MOF-derivatives into 3D hollow frameworks for enhanced lithium storage. Small. 2019;15 doi: 10.1002/smll.201904255. [DOI] [PubMed] [Google Scholar]
- 7.Naguib M., Kurtoglu M., Presser V., Lu J., Niu J., Heon M., Hultman L., Gogotsi Y., Barsoum M.W. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 2011;23:4248–4253. doi: 10.1002/adma.201102306. [DOI] [PubMed] [Google Scholar]
- 8.Bai X., Guan J. MXenes for electrocatalysis applications: Modification and hybridization. Chin. J. Catal. 2022;43:2057–2090. [Google Scholar]
- 9.Ghidiu M., Lukatskaya M.R., Zhao M.-Q., Gogotsi Y., Barsoum M.W. Conductive two-dimensional titanium carbide 'clay' with high volumetric capacitance. Nature. 2014;516:78–81. doi: 10.1038/nature13970. [DOI] [PubMed] [Google Scholar]
- 10.Naguib M., Mashtalir O., Carle J., Presser V., Lu J., Hultman L., Gogotsi Y., Barsoum M.W. Two-dimensional transition metal carbides. ACS Nano. 2012;6:1322–1331. doi: 10.1021/nn204153h. [DOI] [PubMed] [Google Scholar]
- 11.Urbankowski P., Anasori B., Makaryan T., Er D., Kota S., Walsh P.L., Zhao M., Shenoy V.B., Barsoum M.W., Gogotsi Y. Synthesis of two-dimensional titanium nitride Ti4N3 (MXene) Nanoscale. 2016;8:11385–11391. doi: 10.1039/c6nr02253g. [DOI] [PubMed] [Google Scholar]
- 12.Halim J., Kota S., Lukatskaya M.R., Naguib M., Zhao M.-Q., Moon E.J., Pitock J., Nanda J., May S.J., Gogotsi Y., Barsoum M.W. Synthesis and characterization of 2D molybdenum carbide (MXene) Adv. Funct. Mater. 2016;26:3118–3127. [Google Scholar]
- 13.Mashtalir O., Lukatskaya M.R., Zhao M.-Q., Barsoum M.W., Gogotsi Y. Amine-assisted delamination of Nb2C MXene for Li-Ion energy storage devices. Adv. Mater. 2015;27:3501–3506. doi: 10.1002/adma.201500604. [DOI] [PubMed] [Google Scholar]
- 14.Yang J., Naguib M., Ghidiu M., Pan L.-M., Gu J., Nanda J., Halim J., Gogotsi Y., Barsoum M.W., Zhou Y. Two-dimensional Nb-based M4C3 solid solutions (MXenes) J. Am. Ceram. Soc. 2016;99:660–666. [Google Scholar]
- 15.Dall'Agnese Y., Taberna P.-L., Gogotsi Y., Simon P. Two-dimensional vanadium carbide (MXene) as positive electrode for sodium-ion capacitors. J. Phys. Chem. Lett. 2015;6:2305–2309. doi: 10.1021/acs.jpclett.5b00868. [DOI] [PubMed] [Google Scholar]
- 16.Zhou J., Palisaitis J., Halim J., Dahlqvist M., Tao Q., Persson I., Hultman L., Persson P.O.Å., Rosen J. Boridene: Two-dimensional Mo4/3B2-x with ordered metal vacancies obtained by chemical exfoliation. Science. 2021;373:801–805. doi: 10.1126/science.abf6239. [DOI] [PubMed] [Google Scholar]
- 17.Perera A.A.P.R., Madhushani K.A.U., Punchihewa B.T., Kumar A., Gupta R.K. MXene-based nanomaterials for multifunctional applications. Materials. 2023;16:1138. doi: 10.3390/ma16031138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ponnada S., Kiai M.S., Gorle D.B., Bose R.S.C., Rajagopal V., Saini B., Kathiresan M., Nowduri A., Singhal R., Marken F., et al. Recent status and challenges in multifunctional electrocatalysis based on 2D MXenes. Catal. Sci. Technol. 2022;12:4413–4441. [Google Scholar]
- 19.Ampong D.N., Agyekum E., Agyemang F.O., Mensah-Darkwa K., Andrews A., Kumar A., Gupta R.K. MXene: fundamentals to applications in electrochemical energy storage. Discov. Nano. 2023;18:3. doi: 10.1186/s11671-023-03786-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ajmal Z., Qadeer A., Khan U., Bilal Hussain M., Irfan M., Mehmood R., Abid M., Djellabi R., Kumar A., Ali H., et al. Current progresses in two-dimensional MXene-based framework: prospects from superficial synthesis to energy conversion and storage applications. Mater. Today Chem. 2023;27 [Google Scholar]
- 21.Ling Z., Ren C.E., Zhao M.Q., Yang J., Giammarco J.M., Qiu J., Barsoum M.W., Gogotsi Y. Flexible and conductive MXene films and nanocomposites with high capacitance. Proc. Natl. Acad. Sci. USA. 2014;111:16676–16681. doi: 10.1073/pnas.1414215111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu X., Wang Z., Yu M., Xiu L., Qiu J. Stabilizing the MXenes by carbon nanoplating for developing hierarchical nanohybrids with efficient lithium storage and hydrogen evolution capability. Adv. Mater. 2017;29 doi: 10.1002/adma.201607017. [DOI] [PubMed] [Google Scholar]
- 23.Wu X., Zhou S., Wang Z., Liu J., Pei W., Yang P., Zhao J., Qiu J. Engineering multifunctional collaborative catalytic interface enabling efficient hydrogen evolution in all pH range and seawater. Adv. Energy Mater. 2019;9 [Google Scholar]
- 24.Yu M., Wang Z., Liu J., Sun F., Yang P., Qiu J. A hierarchically porous and hydrophilic 3D nickel-iron/MXene electrode for accelerating oxygen and hydrogen evolution at high current densities. Nano Energy. 2019;63 [Google Scholar]
- 25.Wang C., Chen S., Song L. Tuning 2D MXenes by surface controlling and interlayer engineering: methods, properties, and synchrotron radiation characterizations. Adv. Funct. Mater. 2020;30 [Google Scholar]
- 26.Wei Y., Zhang P., Soomro R.A., Zhu Q., Xu B. Advances in the synthesis of 2D MXenes. Adv. Mater. 2021;33 doi: 10.1002/adma.202103148. [DOI] [PubMed] [Google Scholar]
- 27.Peng J., Chen X., Ong W.-J., Zhao X., Li N. Surface and heterointerface engineering of 2D MXenes and their nanocomposites: insights into electro- and photocatalysis. Chem. 2019;5:18–50. [Google Scholar]
- 28.Lukatskaya M.R., Mashtalir O., Ren C.E., Dall'Agnese Y., Rozier P., Taberna P.L., Naguib M., Simon P., Barsoum M.W., Gogotsi Y. Cation intercalation and high volumetric capacitance of two-dimensional titanium carbide. Science. 2013;341:1502–1505. doi: 10.1126/science.1241488. [DOI] [PubMed] [Google Scholar]
- 29.Pang J., Mendes R.G., Bachmatiuk A., Zhao L., Ta H.Q., Gemming T., Liu H., Liu Z., Rummeli M.H. Applications of 2D MXenes in energy conversion and storage systems. Chem. Soc. Rev. 2019;48:72–133. doi: 10.1039/c8cs00324f. [DOI] [PubMed] [Google Scholar]
- 30.Miranda A., Halim J., Barsoum M.W., Lorke A. Electronic properties of freestanding Ti3C2Tx MXene monolayers. Appl. Phys. Lett. 2016;108 [Google Scholar]
- 31.Velusamy D.B., El-Demellawi J.K., El-Zohry A.M., Giugni A., Lopatin S., Hedhili M.N., Mansour A.E., Fabrizio E.D., Mohammed O.F., Alshareef H.N. MXenes for plasmonic photodetection. Adv. Mater. 2019;31 doi: 10.1002/adma.201807658. [DOI] [PubMed] [Google Scholar]
- 32.Schultz T., Frey N.C., Hantanasirisakul K., Park S., May S.J., Shenoy V.B., Gogotsi Y., Koch N. Surface termination dependent work function and electronic properties of Ti3C2Tx MXene. Chem. Mater. 2019;31:6590–6597. [Google Scholar]
- 33.Wang Y., Nian Y., Biswas A.N., Li W., Han Y., Chen J.G. Challenges and opportunities in utilizing MXenes of carbides and nitrides as electrocatalysts. Adv. Energy Mater. 2021;11 [Google Scholar]
- 34.Gao Q., Feng M., Li E., Liu C., Shen C., Liu X. Mechanical, thermal, and rheological properties of Ti3C2Tx MXene/thermoplastic polyurethane nanocomposites. Macromol. Mater. Eng. 2020;305 [Google Scholar]
- 35.Luo J., Wang C., Wang H., Hu X., Matios E., Lu X., Zhang W., Tao X., Li W. Pillared MXene with ultralarge interlayer spacing as a stable matrix for high performance sodium metal anodes. Adv. Funct. Mater. 2019;29 [Google Scholar]
- 36.Zhao M.-Q., Ren C.E., Ling Z., Lukatskaya M.R., Zhang C., Van Aken K.L., Barsoum M.W., Gogotsi Y. Flexible MXene/carbon nanotube composite paper with high volumetric capacitance. Adv. Mater. 2015;27:339–345. doi: 10.1002/adma.201404140. [DOI] [PubMed] [Google Scholar]
- 37.Yan J., Ren C.E., Maleski K., Hatter C.B., Anasori B., Urbankowski P., Sarycheva A., Gogotsi Y. Flexible MXene/graphene films for ultrafast supercapacitors with outstanding volumetric capacitance. Adv. Funct. Mater. 2017;27 [Google Scholar]
- 38.Xiu L., Wang Z., Yu M., Wu X., Qiu J. Aggregation-resistant 3D MXene-based architecture as efficient bifunctional electrocatalyst for overall water splitting. ACS Nano. 2018;12:8017–8028. doi: 10.1021/acsnano.8b02849. [DOI] [PubMed] [Google Scholar]
- 39.Zhao M.Q., Xie X., Ren C.E., Makaryan T., Anasori B., Wang G., Gogotsi Y. Hollow MXene spheres and 3D macroporous MXene frameworks for Na-Ion storage. Adv. Mater. 2017;29 doi: 10.1002/adma.201702410. [DOI] [PubMed] [Google Scholar]
- 40.Song J., Guo X., Zhang J., Chen Y., Zhang C., Luo L., Wang F., Wang G. Rational design of free-standing 3D porous MXene/rGO hybrid aerogels as polysulfide reservoirs for high-energy lithium-sulfur batteries. J. Mater. Chem. A. 2019;7:6507–6513. [Google Scholar]
- 41.Ma Y., Yue Y., Zhang H., Cheng F., Zhao W., Rao J., Luo S., Wang J., Jiang X., Liu Z., et al. 3D synergistical MXene/reduced graphene oxide aerogel for a piezoresistive sensor. ACS Nano. 2018;12:3209–3216. doi: 10.1021/acsnano.7b06909. [DOI] [PubMed] [Google Scholar]
- 42.Yue Y., Liu N., Ma Y., Wang S., Liu W., Luo C., Zhang H., Cheng F., Rao J., Hu X., et al. Highly self-healable 3D microsupercapacitor with MXene-graphene composite aerogel. ACS Nano. 2018;12:4224–4232. doi: 10.1021/acsnano.7b07528. [DOI] [PubMed] [Google Scholar]
- 43.Wang Z., Zhang N., Yu M., Liu J., Wang S., Qiu J. Boosting redox activity on MXene-induced multifunctional collaborative interface in high Li2S loading cathode for high-energy Li-S and metallic Li-free rechargeable batteries. J. Energy Chem. 2019;37:183–191. [Google Scholar]
- 44.Liu J., Zhang H.-B., Xie X., Yang R., Liu Z., Liu Y., Yu Z.-Z. Multifunctional, superelastic, and lightweight MXene/polyimide aerogels. Small. 2018;14 doi: 10.1002/smll.201802479. [DOI] [PubMed] [Google Scholar]
- 45.Deng Y., Shang T., Wu Z., Tao Y., Luo C., Liang J., Han D., Lyu R., Qi C., Lv W., et al. Fast gelation of Ti3C2Tx MXene initiated by metal ions. Adv. Mater. 2019;31 doi: 10.1002/adma.201902432. [DOI] [PubMed] [Google Scholar]
- 46.Shi S., Qian B., Wu X., Sun H., Wang H., Zhang H.-B., Yu Z.-Z., Russell T.P. Self-assembly of MXene-surfactants at liquid-liquid interfaces: from structured liquids to 3D aerogels. Angew. Chem. Int. Ed. 2019;58:18171–18176. doi: 10.1002/anie.201908402. [DOI] [PubMed] [Google Scholar]
- 47.Wang X., Fu Q., Wen J., Ma X., Zhu C., Zhang X., Qi D. 3D Ti3C2Tx aerogels with enhanced surface area for high performance supercapacitors. Nanoscale. 2018;10:20828–20835. doi: 10.1039/c8nr06014b. [DOI] [PubMed] [Google Scholar]
- 48.Yang W., Yang J., Byun J.J., Moissinac F.P., Xu J., Haigh S.J., Domingos M., Bissett M.A., Dryfe R.A.W., Barg S. 3D printing of freestanding MXene architectures for current-collector-free supercapacitors. Adv. Mater. 2019;31 doi: 10.1002/adma.201902725. [DOI] [PubMed] [Google Scholar]
- 49.Xiao Z., Yang Z., Li Z., Li P., Wang R. Synchronous gains of areal and volumetric capacities in lithium-sulfur batteries promised by flower-like porous Ti3C2Tx matrix. ACS Nano. 2019;13:3404–3412. doi: 10.1021/acsnano.8b09296. [DOI] [PubMed] [Google Scholar]
- 50.Ming F., Liang H., Zhang W., Ming J., Lei Y., Emwas A.-H., Alshareef H.N. Porous MXenes enable high performance potassium ion capacitors. Nano Energy. 2019;62:853–860. [Google Scholar]
- 51.Ren C.E., Zhao M.-Q., Makaryan T., Halim J., Boota M., Kota S., Anasori B., Barsoum M.W., Gogotsi Y. Porous two-dimensional transition metal carbide (MXene) flakes for high-performance Li-Ion storage. Chemelectrochem. 2016;3:689–693. [Google Scholar]
- 52.Lv Z., Ma W., Wang M., Dang J., Jian K., Liu D., Huang D. Co-constructing interfaces of multiheterostructure on MXene (Ti3C2Tx)-modified 3D self-supporting electrode for ultraefficient electrocatalytic HER in alkaline media. Adv. Funct. Mater. 2021;31 [Google Scholar]
- 53.Yu M., Zheng J., Guo M. La-doped NiFe-LDH coupled with hierarchical vertically aligned MXene frameworks for efficient overall water splitting. J. Energy Chem. 2022;70:472–479. [Google Scholar]
- 54.Yoon Y., Tiwari A.P., Choi M., Novak T.G., Song W., Chang H., Zyung T., Lee S.S., Jeon S., An K.-S. Precious-metal-free electrocatalysts for activation of hydrogen evolution with nonmetallic electron donor: chemical composition controllable phosphorous doped vanadium carbide MXene. Adv. Funct. Mater. 2019;29 [Google Scholar]
- 55.Du C.F., Sun X., Yu H., Liang Q., Dinh K.N., Zheng Y., Luo Y., Wang Z., Yan Q. Synergy of Nb doping and surface alloy enhanced on water-alkali electrocatalytic hydrogen generation performance in Ti-based MXene. Adv. Sci. 2019;6 doi: 10.1002/advs.201900116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Du X., Huang J., Zhang J., Yan Y., Wu C., Hu Y., Yan C., Lei T., Chen W., Fan C., Xiong J. Modulating electronic structures of inorganic nanomaterials for efficient electrocatalytic water splitting. Angew. Chem. Int. Ed. 2019;58:4484–4502. doi: 10.1002/anie.201810104. [DOI] [PubMed] [Google Scholar]
- 57.Kuznetsov D.A., Chen Z., Kumar P.V., Tsoukalou A., Kierzkowska A., Abdala P.M., Safonova O.V., Fedorov A., Müller C.R. Single site cobalt substitution in 2D molybdenum carbide (MXene) enhances catalytic activity in the hydrogen evolution reaction. J. Am. Chem. Soc. 2019;141:17809–17816. doi: 10.1021/jacs.9b08897. [DOI] [PubMed] [Google Scholar]
- 58.VahidMohammadi A., Rosen J., Gogotsi Y. The world of two-dimensional carbides and nitrides (MXenes) Science. 2021;372:1165. doi: 10.1126/science.abf1581. [DOI] [PubMed] [Google Scholar]
- 59.Zhan X., Si C., Zhou J., Sun Z. MXene and MXene-based composites: synthesis, properties and environment-related applications. Nanoscale Horiz. 2020;5:235–258. [Google Scholar]
- 60.Ma T.Y., Cao J.L., Jaroniec M., Qiao S.Z., Qiao S.Z. Interacting carbon nitride and titanium carbide nanosheets for high performance oxygen evolution. Angew. Chem. Int. Ed. 2016;55:1138–1142. doi: 10.1002/anie.201509758. [DOI] [PubMed] [Google Scholar]
- 61.Zhao L., Dong B., Li S., Zhou L., Lai L., Wang Z., Zhao S., Han M., Gao K., Lu M., et al. Interdiffusion reaction-assisted hybridization of two-dimensional metal-organic frameworks and Ti3C2Tx nanosheets for electrocatalytic oxygen evolution. ACS Nano. 2017;11:5800–5807. doi: 10.1021/acsnano.7b01409. [DOI] [PubMed] [Google Scholar]
- 62.Li L., Yu D., Li P., Huang H., Xie D., Lin C.-C., Hu F., Chen H.-Y., Peng S. Interfacial electronic coupling of ultrathin transition-metal hydroxide nanosheets with layered MXenes as a new prototype for platinum-like hydrogen evolution. Energy Environ. Sci. 2021;14:6419–6427. [Google Scholar]
- 63.Li Z., Qi Z., Wang S., Ma T., Zhou L., Wu Z., Luan X., Lin F.-Y., Chen M., Miller J.T., et al. In situ formed Pt3Ti nanoparticles on a two-dimensional transition metal carbide (MXene) used as efficient catalysts for hydrogen evolution reactions. Nano Lett. 2019;19:5102–5108. doi: 10.1021/acs.nanolett.9b01381. [DOI] [PubMed] [Google Scholar]
- 64.Zhang J., Zhao Y., Guo X., Chen C., Dong C.-L., Liu R.-S., Han C.-P., Li Y., Gogotsi Y., Wang G. Single platinum atoms immobilized on an MXene as an efficient catalyst for the hydrogen evolution reaction. Nat. Catal. 2018;1:985–992. [Google Scholar]
- 65.Ramalingam V., Varadhan P., Fu H.-C., Kim H., Zhang D., Chen S., Song L., Ma D., Wang Y., Alshareef H.N., He J.-H. Heteroatom-mediated interactions between ruthenium single atoms and an MXene support for efficient hydrogen evolution. Adv. Mater. 2019;31 doi: 10.1002/adma.201903841. [DOI] [PubMed] [Google Scholar]
- 66.Thakur R., VahidMohammadi A., Moncada J., Adams W.R., Chi M., Tatarchuk B., Beidaghi M., Carrero C.A. Insights into the thermal and chemical stability of multilayered V2CTx MXene. Nanoscale. 2019;11:10716–10726. doi: 10.1039/c9nr03020d. [DOI] [PubMed] [Google Scholar]
- 67.Zhang C.J., Pinilla S., McEvoy N., Cullen C.P., Anasori B., Long E., Park S.-H., Seral-Ascaso A., Shmeliov A., Krishnan D., et al. Oxidation stability of colloidal two-dimensional titanium carbides (MXenes) Chem. Mater. 2017;29:4848–4856. [Google Scholar]
- 68.Rakhi R.B., Ahmed B., Hedhili M.N., Anjum D.H., Alshareef H.N. Effect of postetch annealing gas composition on the structural and electrochemical properties of Ti2CTx MXene electrodes for supercapacitor applications. Chem. Mater. 2015;27:5314–5323. [Google Scholar]
- 69.Chae Y., Kim S.J., Cho S.-Y., Choi J., Maleski K., Lee B.-J., Jung H.-T., Gogotsi Y., Lee Y., Ahn C.W. An investigation into the factors governing the oxidation of two-dimensional Ti3C2 MXene. Nanoscale. 2019;11:8387–8393. doi: 10.1039/c9nr00084d. [DOI] [PubMed] [Google Scholar]
- 70.Wang X., Wang Z., Qiu J. Stabilizing MXene by hydration chemistry in aqueous solution. Angew. Chem. Int. Ed. 2021;60:26587–26591. doi: 10.1002/anie.202113981. [DOI] [PubMed] [Google Scholar]
- 71.VahidMohammadi A., Mojtabavi M., Caffrey N.M., Wanunu M., Beidaghi M. Assembling 2D MXenes into highly stable pseudocapacitive electrodes with high power and energy densities. Adv. Mater. 2019;31 doi: 10.1002/adma.201806931. [DOI] [PubMed] [Google Scholar]
- 72.Natu V., Hart J.L., Sokol M., Chiang H., Taheri M.L., Barsoum M.W. Edge capping of 2D-MXene sheets with polyanionic salts to mitigate oxidation in aqueous colloidal suspensions. Angew. Chem. Int. Ed. 2019;58:12655–12660. doi: 10.1002/anie.201906138. [DOI] [PubMed] [Google Scholar]
- 73.Zhao X., Vashisth A., Prehn E., Sun W., Shah S.A., Habib T., Chen Y., Tan Z., Lutkenhaus J.L., Radovic M., Green M.J. Antioxidants unlock shelf-stable Ti3C2T (MXene) nanosheet dispersions. Matter. 2019;1:513–526. [Google Scholar]
- 74.Lee Y., Kim S.J., Kim Y.-J., Lim Y., Chae Y., Lee B.-J., Kim Y.-T., Han H., Gogotsi Y., Ahn C.W. Oxidation-resistant titanium carbide MXene films. J. Mater. Chem. A. 2020;8:573–581. [Google Scholar]
- 75.Zhang J., Kong N., Hegh D., Usman K.A.S., Guan G., Qin S., Jurewicz I., Yang W., Razal J.M. Freezing titanium carbide aqueous dispersions for ultra-long-term storage. ACS Appl. Mater. Interfaces. 2020;12:34032–34040. doi: 10.1021/acsami.0c06728. [DOI] [PubMed] [Google Scholar]
- 76.Fang Y., Liu Z., Han J., Jin Z., Han Y., Wang F., Niu Y., Wu Y., Xu Y. High-performance electrocatalytic conversion of N2 to NH3 using oxygen-vacancy-rich TiO2 in situ grown on Ti3C2Tx MXene. Adv. Energy Mater. 2019;9 [Google Scholar]
- 77.Lim K.R.G., Handoko A.D., Johnson L.R., Meng X., Lin M., Subramanian G.S., Anasori B., Gogotsi Y., Vojvodic A., Seh Z.W. 2H-MoS2 on Mo2CTx MXene nanohybrid for efficient and durable electrocatalytic hydrogen evolution. ACS Nano. 2020;14:16140–16155. doi: 10.1021/acsnano.0c08671. [DOI] [PubMed] [Google Scholar]
- 78.Tang J., Mathis T.S., Kurra N., Sarycheva A., Xiao X., Hedhili M.N., Jiang Q., Alshareef H.N., Xu B., Pan F., et al. Tuning the electrochemical performance of titanium carbide MXene by controllable in situ anodic oxidation. Angew. Chem. Int. Ed. 2019;58:17849–17855. doi: 10.1002/anie.201911604. [DOI] [PubMed] [Google Scholar]
- 79.Dong Y., Wu Z.-S., Zheng S., Wang X., Qin J., Wang S., Shi X., Bao X. Ti3C2 MXene-derived sodium/potassium titanate nanoribbons for high-performance sodium/potassium ion batteries with enhanced capacities. ACS Nano. 2017;11:4792–4800. doi: 10.1021/acsnano.7b01165. [DOI] [PubMed] [Google Scholar]
- 80.Jiao L., Zhang C., Geng C., Wu S., Li H., Lv W., Tao Y., Chen Z., Zhou G., Li J., et al. Capture and catalytic conversion of polysulfides by in situ built TiO2-MXene heterostructures for lithium-sulfur batteries. Adv. Energy Mater. 2019;9 [Google Scholar]
- 81.Liu S., Wang Z., Zhou S., Yu F., Yu M., Chiang C.Y., Zhou W., Zhao J., Qiu J. Metal-organic-framework-derived hybrid carbon nanocages as a bifunctional electrocatalyst for oxygen reduction and evolution. Adv. Mater. 2017;29 doi: 10.1002/adma.201700874. [DOI] [PubMed] [Google Scholar]
- 82.Li Z., Zhuang Z., Lv F., Zhu H., Zhou L., Luo M., Zhu J., Lang Z., Feng S., Chen W., et al. The marriage of the FeN4 moiety and MXene boosts oxygen reduction catalysis: Fe 3d electron delocalization matters. Adv. Mater. 2018;30 doi: 10.1002/adma.201803220. [DOI] [PubMed] [Google Scholar]
- 83.Wu Z., Wang H., Xiong P., Li G., Qiu T., Gong W.-B., Zhao F., Li C., Li Q., Wang G., Geng F. Molecularly thin nitride sheets stabilized by titanium carbide as efficient bifunctional electrocatalysts for fiber-shaped rechargeable zinc-air batteries. Nano Lett. 2020;20:2892–2898. doi: 10.1021/acs.nanolett.0c00717. [DOI] [PubMed] [Google Scholar]
- 84.Kuznetsov D.A., Chen Z., Abdala P.M., Safonova O.V., Fedorov A., Müller C.R. Single-atom-substituted Mo2CTx:Fe-layered carbide for selective oxygen reduction to hydrogen peroxide: tracking the evolution of the MXene phase. J. Am. Chem. Soc. 2021;143:5771–5778. doi: 10.1021/jacs.1c00504. [DOI] [PubMed] [Google Scholar]
- 85.Yu M., Zhou S., Wang Z., Zhao J., Qiu J. Boosting electrocatalytic oxygen evolution by synergistically coupling layered double hydroxide with MXene. Nano Energy. 2018;44:181–190. [Google Scholar]
- 86.Zhang Y., Jiang H., Lin Y., Liu H., He Q., Wu C., Duan T., Song L. In situ growth of cobalt nanoparticles encapsulated nitrogen-doped carbon nanotubes among Ti3C2Tx (MXene) matrix for oxygen reduction and evolution. Adv. Mater. Interfaces. 2018;5 [Google Scholar]
- 87.Ashton M., Trometer N., Mathew K., Suntivich J., Freysoldt C., Sinnott S.B., Hennig R.G. Predicting the electrochemical synthesis of 2D materials from first principles. J. Phys. Chem. C. 2019;123:3180–3187. [Google Scholar]
- 88.Lagadec M.F., Grimaud A. Water electrolysers with closed and open electrochemical systems. Nat. Mater. 2020;19:1140–1150. doi: 10.1038/s41563-020-0788-3. [DOI] [PubMed] [Google Scholar]
- 89.Jin H., Yu H., Li H., Davey K., Song T., Paik U., Qiao S.Z. MXene analogue: a 2D nitridene solid solution for high-rate hydrogen production. Angew. Chem. Int. Ed. 2022;61 doi: 10.1002/anie.202203850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang L., Hao Y., Deng L., Hu F., Zhao S., Li L., Peng S. Rapid complete reconfiguration induced actual active species for industrial hydrogen evolution reaction. Nat. Commun. 2022;13:5785. doi: 10.1038/s41467-022-33590-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang X., Ding J., Song W., Yang X., Zhang T., Huang Z., Wang H., Han X., Hu W. Cation vacancy clusters in Ti3C2Tx MXene induce ultra-strong interaction with noble metal clusters for efficient electrocatalytic hydrogen evolution. Adv. Energy Mater. 2023;13 [Google Scholar]
- 92.Cui C., Cheng R., Zhang H., Zhang C., Ma Y., Shi C., Fan B., Wang H., Wang X. Ultrastable MXene@Pt/SWCNTs' nanocatalysts for hydrogen evolution reaction. Adv. Funct. Mater. 2020;30 [Google Scholar]
- 93.Zou Y., Kazemi S.A., Shi G., Liu J., Yang Y., Bedford N.M., Fan K., Xu Y., Fu H., Dong M., et al. Ruthenium single-atom modulated Ti3C2Tx MXene for efficient alkaline electrocatalytic hydrogen production. Ecomat. 2023;5 [Google Scholar]
- 94.Li K., Liang M., Wang H., Wang X., Huang Y., Coelho J., Pinilla S., Zhang Y., Qi F., Nicolosi V., Xu Y. 3D MXene architectures for efficient energy storage and conversion. Adv. Funct. Mater. 2020;30 [Google Scholar]
- 95.Xiu L., Pei W., Zhou S., Wang Z., Yang P., Zhao J., Qiu J. Multilevel hollow MXene tailored low-Pt catalyst for efficient hydrogen evolution in full-pH range and seawater. Adv. Funct. Mater. 2020;30 [Google Scholar]
- 96.Ajmal S., Kumar A., Selvaraj M., Alam M.M., Yang Y., Das D.K., Gupta R.K., Yasin G. MXenes and their interfaces for the taming of carbon dioxide & nitrate: A critical review. Coord. Chem. Rev. 2023;483 [Google Scholar]
- 97.Li F., Ai H., Shi C., Lo K.H., Pan H. Single transition metal atom catalysts on Ti2CN2 for efficient CO2 reduction reaction. Int. J. Hydrogen Energy. 2021;46:12886–12896. [Google Scholar]
- 98.Hao Y., Hu F., Zhu S., Sun Y., Wang H., Wang L., Wang Y., Xue J., Liao Y.F., Shao M., Peng S. MXene-regulated metal-oxide interfaces with modified intermediate configurations realizing nearly 100% CO2 electrocatalytic conversion. Angew. Chem. Int. Ed. 2023;62 doi: 10.1002/anie.202304179. [DOI] [PubMed] [Google Scholar]
- 99.Abdinejad M., Subramanian S., Motlagh M.K., Noroozifar M., Duangdangchote S., Neporozhnii I., Ripepi D., Pinto D., Li M., Tang K., et al. Insertion of MXene-based materials into Cu–Pd 3D aerogels for electroreduction of CO2 to formate. Adv. Energy Mater. 2023;13 [Google Scholar]
- 100.Handoko A.D., Chen H., Lum Y., Zhang Q., Anasori B., Seh Z.W. Two-dimensional titanium and molybdenum carbide MXenes as electrocatalysts for CO2 reduction. iScience. 2020;23 doi: 10.1016/j.isci.2020.101181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Handoko A.D., Khoo K.H., Tan T.L., Jin H., Seh Z.W. Establishing new scaling relations on two-dimensional MXenes for CO2 electroreduction. J. Mater. Chem. A. 2018;6:21885–21890. [Google Scholar]
- 102.Wang S., Li L., San Hui K., Bin F., Zhou W., Fan X., Zalnezhad E., Li J., Hui K.N. Computational screening of single atoms anchored on defective Mo2CO2 MXene nanosheet as efficient electrocatalysts for the synthesis of ammonia. Adv. Eng. Mater. 2021;23 [Google Scholar]
- 103.Chu K., Luo Y., Shen P., Li X., Li Q., Guo Y. Unveiling the synergy of O-vacancy and heterostructure over MoO3-x/MXene for N2 electroreduction to NH3. Adv. Energy Mater. 2022;12 [Google Scholar]
- 104.Johnson L.R., Sridhar S., Zhang L., Fredrickson K.D., Raman A.S., Jang J., Leach C., Padmanabhan A., Price C.C., Frey N.C., et al. MXene materials for the electrochemical nitrogen reduction-functionalized or not? ACS Catal. 2020;10:253–264. [Google Scholar]
- 105.Zhou S., Zhao Y., Shi R., Wang Y., Ashok A., Héraly F., Zhang T., Yuan J. Vacancy-rich MXene-immobilized Ni single atoms as a high-performance electrocatalyst for the hydrazine oxidation reaction. Adv. Mater. 2022;34 doi: 10.1002/adma.202204388. [DOI] [PubMed] [Google Scholar]
- 106.Sun F., Qin J., Wang Z., Yu M., Wu X., Sun X., Qiu J. Energy-saving hydrogen production by chlorine-free hybrid seawater splitting coupling hydrazine degradation. Nat. Commun. 2021;12:4182. doi: 10.1038/s41467-021-24529-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Abbott D.F., Xu Y.Z., Kuznetsov D.A., Kumar P., Müller C.R., Fedorov A., Mougel V. Understanding the synergy between Fe and Mo sites in the nitrate reduction reaction on a bio-inspired bimetallic MXene electrocatalyst. Angew. Chem. Int. Ed. 2023;62:e202313746. doi: 10.1002/anie.202313746. [DOI] [PubMed] [Google Scholar]
- 108.Ren Y., Tian F., Jin L., Wang Y., Yang J., You S., Liu Y. Fluidic MXene Electrode Functionalized with Iron Single Atoms for Selective Electrocatalytic Nitrate Transformation to Ammonia. Environ. Sci. Technol. 2023;57:10458–10466. doi: 10.1021/acs.est.3c02520. [DOI] [PubMed] [Google Scholar]
- 109.Zhang L., Wang Z., Qiu J. Energy-saving hydrogen production by seawater electrolysis coupling sulfion degradation. Adv. Mater. 2022;34 doi: 10.1002/adma.202109321. [DOI] [PubMed] [Google Scholar]
- 110.Ma Q., Gao J., Moussa B., Young J., Zhao M., Zhang W. Electrosorption, desorption, and oxidation of perfluoroalkyl carboxylic acids (PFCAs) via MXene-based electrocatalytic membranes. ACS Appl. Mater. Interfaces. 2023;15:29149–29159. doi: 10.1021/acsami.3c03991. [DOI] [PubMed] [Google Scholar]
- 111.Pang S.Y., Io W.F., Wong L.W., Zhao J., Hao J. Efficient energy conversion and storage based on robust fluoride-free self-assembled 1D niobium carbide in 3D nanowire network. Adv. Sci. 2020;7 doi: 10.1002/advs.201903680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shen M., Jiang W., Liang K., Zhao S., Tang R., Zhang L., Wang J.Q. One-pot green process to synthesize MXene with controllable surface terminations using molten salts. Angew. Chem. Int. Ed. 2021;60:27013–27018. doi: 10.1002/anie.202110640. [DOI] [PubMed] [Google Scholar]
- 113.Li Y., Shao H., Lin Z., Lu J., Liu L., Duployer B., Persson P.O.Å., Eklund P., Hultman L., Li M., et al. A general Lewis acidic etching route for preparing MXenes with enhanced electrochemical performance in non-aqueous electrolyte. Nat. Mater. 2020;19:894–899. doi: 10.1038/s41563-020-0657-0. [DOI] [PubMed] [Google Scholar]
- 114.Chen N., Duan Z., Cai W., Wang Y., Pu B., Huang H., Xie Y., Tang Q., Zhang H., Yang W. Supercritical etching method for the large-scale manufacturing of MXenes. Nano Energy. 2023;107 [Google Scholar]
- 115.Sun Y., Dai S. High-entropy materials for catalysis: a new frontier. Sci. Adv. 2021;7 doi: 10.1126/sciadv.abg1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang Y., Mi J., Wu Z.-S. Recent status and challenging perspective of high entropy oxides for chemical catalysis. Chem Catal. 2022;2:1624–1656. [Google Scholar]
- 117.Du Z., Wu C., Chen Y., Cao Z., Hu R., Zhang Y., Gu J., Cui Y., Chen H., Shi Y., et al. High-entropy atomic layers of transition-metal carbides (MXenes) Adv. Mater. 2021;33 doi: 10.1002/adma.202101473. [DOI] [PubMed] [Google Scholar]
- 118.Nemani S.K., Zhang B., Wyatt B.C., Hood Z.D., Manna S., Khaledialidusti R., Hong W., Sternberg M.G., Sankaranarayanan S.K.R.S., Anasori B. High-entropy 2D carbide MXenes: TiVNbMoC3 and TiVCrMoC3. ACS Nano. 2021;15:12815–12825. doi: 10.1021/acsnano.1c02775. [DOI] [PubMed] [Google Scholar]
- 119.Zhu X., Zhou X., Jing Y., Li Y. Electrochemical synthesis of urea on MBenes. Nat. Commun. 2021;12:4080. doi: 10.1038/s41467-021-24400-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang T., Zhang B., Peng Q., Zhou J., Sun Z. Mo2B2 MBene-supported single-atom catalysts as bifunctional HER/OER and OER/ORR electrocatalysts. J. Mater. Chem. A. 2021;9:433–441. [Google Scholar]
- 121.Vijayaprabhakaran A., Kathiresan M. Fluorine-free synthesized tantalum carbide (Ta2C Mxene) as an efficient electrocatalyst for water reduction and nitro compound reduction. Mater. Adv. 2023;4:3593–3602. [Google Scholar]
- 122.Luo Y., Zhang Z., Chhowalla M., Liu B. Recent advances in design of electrocatalysts for high-current-density water splitting. Adv. Mater. 2022;34 doi: 10.1002/adma.202108133. [DOI] [PubMed] [Google Scholar]