Abstract
Cholesterol-sphingolipid rich plasma membrane domains, known as rafts, have emerged as important regulators of signal transduction. The adipocyte insulin receptor (IR) is localized to and signals via caveolae that are formed by polymerization of caveolins. Caveolin binds to IR and stimulates signalling. We report that, in liver-derived cells lacking caveolae, autophosphorylation of the endogenous IR is dependent on raft lipids, being compromised by acute cyclodextrin-mediated cholesterol depletion or by antibody clustering of glycosphingolipids. Moreover, we provide evidence that IR becomes recruited to detergent-resistant domains upon ligand binding and that clustering of GM2 ganglioside inhibits IR signalling apparently by excluding the ligand-bound IR from these domains. Our results indicate that, in cells derived from liver, an important insulin target tissue, caveolae are not required for insulin signalling. Rather, the dynamic recruitment of the ligand-bound IR into rafts may serve to regulate interactions in the initiation of the IR signalling cascade.
INTRODUCTION
Insulin is an anabolic hormone with a central role in carbohydrate and lipid metabolism and in cell growth. Insulin exerts its effects via insulin receptor (IR), a transmembrane receptor tyrosine kinase forming a stable heterotetramer of two α-subunits and two β-subunits (White and Kahn, 1994). The extracellular α-subunits contain the insulin-binding sites, whereas the β-subunits form the transmembrane and intracellular parts of the receptor, including the kinase domain. Upon insulin binding, IR undergoes a conformational change that allows trans-phosphorylation of the catalytic sites on multiple tyrosine residues (Hubbard et al., 1994). IR autophosphorylation is needed for receptor activation. A number of docking proteins, including the insulin receptor substrates (IRSs), are then tyrosine-phosphorylated by the active IR, and this creates recognition sites for binding to the SH2 domains in various signalling proteins (Saltiel, 2001).
The most important tissues for insulin action are liver, adipose tissue and striated muscle. The concentration of IR is high in these tissues, with more than 200 000 receptors on adipocytes and hepatocytes (White and Kahn, 1994). In adipocytes, IRs are sequestered in caveolar invaginations of the plasma membrane and are dependent on caveolae for signalling (Gustavsson et al., 1999). IR has a consensus binding site for interaction with caveolin (Couet et al., 1997), the cholesterol-binding structural protein of caveolae, and interaction of IR with caveolin leads to an increase in IR kinase activity (Yamamoto et al., 1998; Nystrom et al., 1999). Whereas caveolae are present at high concentrations on adipocytes and muscle cells, there are relatively few caveolae in hepatocytes (Fielding and Fielding, 2000; Calvo et al., 2001). Moreover, in hepatocytes, the binding of insulin to its receptor occurs preferentially on microvilli, followed by redistribution of the receptor–ligand complex to non-villous segments of the membrane from where it is internalized via coated pits (Carpentier et al., 1985, 1992).
Increasing evidence suggests that cholesterol-sphingolipid rich membrane microdomains, known as lipid rafts, play an important role in signal transduction by functioning as platforms for signalling receptors (Simons and Toomre, 2000). Because of their presumably small size (beyond the resolution of light microscopy), individual rafts are likely to host only a subset of raft proteins. However, rafts may become clustered and this further enhances protein interactions, thus triggering or amplifying signalling (Janes et al., 1999).
Caveolae represent a subset of rafts (Simons and Toomre, 2000). However, the regulation of specific signalling events by caveolar lipids is difficult to dissect from the dependence of caveolar integrity on caveolin–cholesterol oligomers. In this work, we have analysed the role of the lipid microenvironment in the initiation of the insulin signalling cascade in a hepatic cell line that expresses IRs but lacks caveolae. We demonstrate that acute changes in cholesterol concentration or local sphingolipid distribution affect insulin signalling and provide evidence for the dynamic recruitment of IR into rafts upon activation.
RESULTS
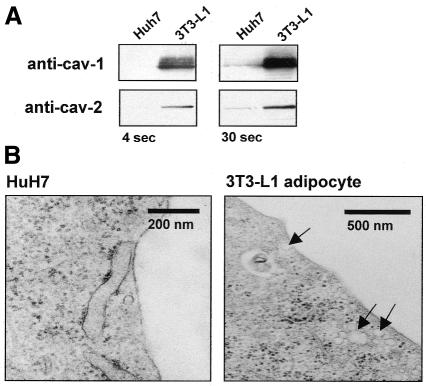
The human hepatoma HuH7 cells express very low levels of caveolin-1 and caveolin-2 in comparison with differentiated 3T3-L1 adipocytes as judged by western blotting (Figure 1A). Moreover, no morphologically identifiable caveolae were found in the HuH7 cell plasma membrane by electron microscopy (Figure 1B). HuH7 cells express biochemically detectable levels of endogenous IR and respond metabolically to insulin, e.g. by increasing lipogenesis (data not shown). Thus, these cells offer a model system for dissecting the role of non-caveolar raft domains in insulin signalling.
Fig. 1. Caveolin expression in HuH7 cells and 3T3-L1 adipocytes. (A) Cell lysate (20 µg) was immunoblotted with anti-caveolin-1 and anti-caveolin-2 antibodies. Two exposure times are shown. (B) Electron microscopy of HuH7 cell and 3T3-L1 adipocyte plasma membrane. Caveolae are abundant in 3T3-L1 adipocytes (arrows) but absent in HuH7 cells.
Cholesterol depletion inhibits IR and IRS-1 phosphorylation
Cholesterol depletion has been shown to inhibit raft-dependent signalling (Simons and Toomre, 2000). Therefore, we extracted cholesterol from HuH7 cells by acute treatment with cyclodextrin and analysed its effect on IR signalling. HuH7 cells were incubated in the presence of 10 mM methyl-β-cyclodextrin for 15 min at 37°C. This resulted in ∼50% depletion of cellular cholesterol as judged by the efflux of [14C]cholesterol from labelled cells (data not shown). During the last 10 min of incubation, the cells were stimulated with insulin. Immunoblotting of the cell lysates with anti-phosphotyrosine (anti-PY) and anti-IRβ antibodies revealed that the cholesterol extraction resulted in an ∼60% decrease in IR phosphorylation in insulin-stimulated cells (Figure 2). The insulin-dependent tyrosine phosphorylation of IRS-1 was also impaired by ∼50%, whereas IRS-2 phosphorylation was not detectable (Figure 2).
Fig. 2. Effect of cholesterol depletion on IR and IRS-1 phosphorylation. (A) HuH7 cells were incubated in the presence (+) or absence (-) of methyl-β-cyclodextrin (CD) for 5 min. Insulin (ins) was then added to the indicated samples and incubation continued for 10 min. Protein (20 µg) from cell lysates was analysed by western blotting using anti-PY antibody (in duplicate). The locations of IRS-1, IRS-2 and IR are indicated and their levels in the same samples shown by western blotting. Incubation with CD alone also affected protein phosphorylation, as indicated by the appearance of an unknown ∼160 kDa band in the +CD samples. (B) Quantitation of anti-PY blotting data from three independent experiments. Error bars = SEM (n = 4–6); *P < 0.05.
Glycosphingolipid clustering inhibits IR phosphorylation
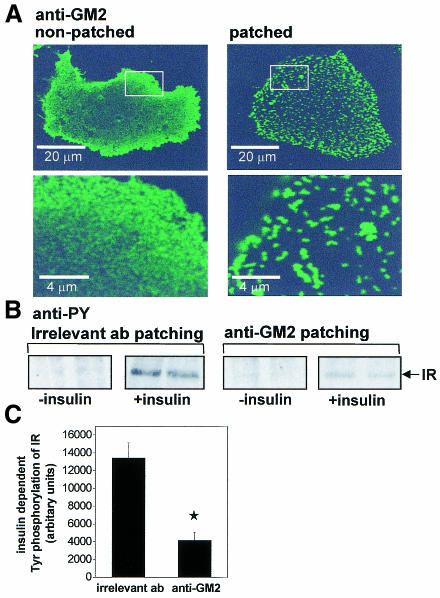
Clustering of raft components has been shown to modulate raft-dependent signalling events. For example, Src-kinase phosphorylation on the cytosolic side of the membrane is stimulated by patching of glycosphingolipids or glycosyl phosphatidylinositol (GPI) anchored proteins on the other side (Horejsi et al., 1998; Kasahara and Sanai, 1999). We next investigated the effect of anti-glycosphingolipid antibody cross-linking on insulin signalling. An anti-ganglioside GM2 IgM antibody was chosen because HuH7 cells were found to contain high levels of GM2 (0.8 nmol/mg protein). The clustering was performed at 12°C, according to Harder et al. (1998), by incubating living cells for 1 h in the presence of the primary anti-glycosphingolipid antibodies followed by 1 h in the presence of FITC-conjugated anti-IgM secondary antibodies. Confocal microscopy revealed that, after GM2 antibody patching, the staining was distributed in micron-size clusters (Figure 3A). No clustering of GM2 was observed when the secondary antibody was incubated after fixation (Figure 3A). When insulin-dependent tyrosine phosphorylation of IR was analysed upon anti-GM2 clustering, we found it to be inhibited by ∼70% compared to incubation with irrelevant antibodies (Figure 3B and C). To test whether this effect of glycolipid clustering is restricted to the hepatoma cells, we carried out the same experiment using primary mouse hepatocytes. Both the patching of GM2 and the parallel reduction in IR phosphorylation were reproduced in these cells (see Supplementary figure 1 available at EMBO reports Online).
Fig. 3. Effect of GM2 clustering on IR phosphorylation. (A) Confocal images of HuH7 cells stained with anti-GM2 antibodies. Cells were incubated in the presence of anti-GM2 for 1 h at 12°C, followed by secondary antibody (patched) or BSA (non-patched) for 1 h at 12°C. After fixing with PFA, non-patched cells were stained with the secondary antibody. Lower panels show the areas marked in the upper panels. (B) Cells were patched with anti-GM2 or irrelevant antibodies (anti LDL-receptor antibody or a mixture of mouse IgM antibodies) for 1 h at 12°C, followed by respective secondary antibodies (1 h at 12°C), and incubated in the presence or absence of insulin for 3 min at 37°C. Cell lysates (20 µg of protein) were analysed by western blotting using anti-PY antibody. (C) Quantitation of the western blotting data. Error bars = SEM (n = 4); *P < 0.05.
Association of IR with detergent-resistant membranes
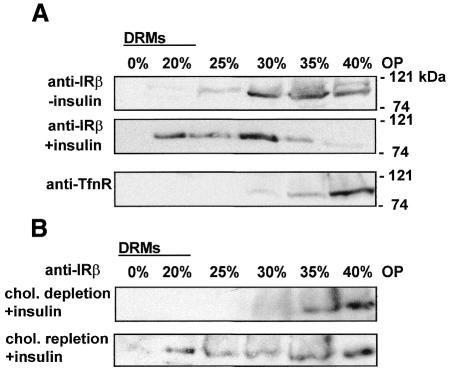
Our observations suggest that an intact raft lipid environment is important for insulin signalling via IR. To further investigate the underlying mechanism, we studied the association of IR with detergent-resistant membranes (DRMs). Triton X-100 insolubility at 4°C is a commonly used criterion for detecting raft association of proteins (London and Brown, 2000). To analyse whether IR associates with DRMs in HuH7 cells, insulin-stimulated and basal cells were lysed in 0.1% Triton X-100 on ice, and the lysates were subjected to Optiprep gradient fractionation in the presence of the detergent. Western blot analysis of the proteins precipitated from the fractions indicated that the distribution of IR in the gradient changed in response to insulin (Figure 4A). In the absence of ligand, the vast majority of the receptors were found at the bottom of the gradient (i.e. solubilized by the detergent), whereas, in the presence of insulin, a significant fraction of IR floated to the upper fractions representing DRMs. The non-raft protein transferrin receptor was completely solubilized by 0.1% Triton X-100 (Figure 4A). Similar results were obtained when using 1% Triton X-100, although the proportion of IR associating with DRMs was slightly smaller (Supplementary figure 2, and data not shown). These results indicate that, in the hepatoma cells, IR can partition into detergent-soluble or detergent-resistant domains, and its association with DRMs depends on ligand binding. Moreover, we found IR association with DRMs to be cholesterol dependent, as it was abolished by the cyclodextrin treatment and recovered after subsequent cholesterol repletion (Figure 4B).
Fig. 4. Association of IR with DRMs. (A) Basal and insulin-stimulated (1.5 min) cells were solubilized in 0.1% Triton X-100 on ice and subjected to Triton–Optiprep (OP) gradient fractionation. Equal volume from each fraction was TCA precipitated and the precipitated proteins analysed by western blotting using anti-IRβ and anti-transferrin receptor (Tfn-R) antibodies. (B) Cells were cholesterol depleted and insulin stimulated as in Figure 2. For cholesterol repletion, depleted cells were incubated for 2 h with CD/cholesterol complex prior to insulin stimulation (10 min). Samples solubilized in 0.1% Triton X-100 were analysed as in (A), using anti-IRβ antibodies.
Effect of glycosphingolipid clustering on the association of IR with rafts
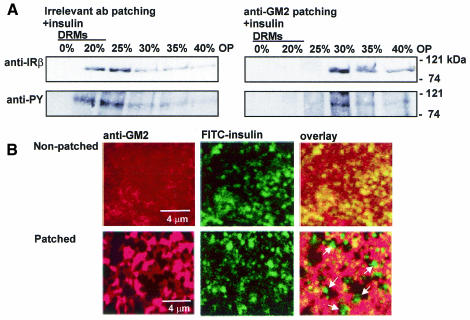
To test whether glycosphingolipid clustering affects the raft association of IR, we studied the distribution of the receptor in the Triton–Optiprep gradient after antibody patching and insulin treatment. Immunoblotting with anti-IRβ antibody revealed that, in cells patched with irrelevant antibodies, the receptor was efficiently recovered from DRMs, whereas, in anti-GM2-patched cells, the receptor was excluded from them (Figure 5A). When analysing the tyrosine phosphorylation of IR, the phosphorylation level in anti-GM2-patched cells was found to be markedly lower than in control cells (Figure 5A), in accordance with the previous experiment (Figure 3).
Fig. 5. The effect of antibody patching on IR raft association. (A) Following antibody patching as in Figure 3B, cells were treated with insulin for 1.5 min, solubilized and fractionated as in Figure 4. The distribution of IR and tyrosine-phosphorylated IR was analysed by western blotting. (B) Distribution of GM2 and FITC-insulin in non-patched and anti-GM2-patched cells by confocal microscopy. GM2 staining was performed as in Figure 3, and insulin was added for 5 min before fixation. Ligand-induced receptor clustering (Jeffrey, 1982) may contribute to the punctate FITC-insulin staining pattern. Insulin preferentially segregates from clustered GM2 (arrows).
Our data suggest that IR becomes excluded from rafts once GM2 is clustered and presumably stabilized in them. To test whether the segregation of IR and GM2 could be observed morphologically in intact cells, we added FITC-coupled insulin onto cells in which GM2 had been clustered with antibodies and visualized the staining by confocal microscopy. We found that insulin labelling localized preferentially to clusters in the intervening areas of GM2 patches (Figure 5B). In non-patched cells, insulin gave a similar punctate staining, whereas the GM2 immunoreactivity seemed essentially uniformly distributed, partially overlapping with that of insulin (Figure 5B). The results suggest that GM2 clustering is accompanied by segregation of IR and the glycolipid and that clustering does not inhibit insulin binding to its receptor.
DISCUSSION
Our results indicate that manipulation of raft lipids (i.e. cholesterol and sphingolipids) modulate IR signalling in the absence of caveolae. Furthermore, distinct differences are found between the caveolae-dependent IR signalling described in adipocytes and the caveolae-independent signalling, analysed here in hepatoma cells. First, IR appears to be restricted to caveolae in adipocytes (Gustavsson et al., 1999), whereas its raft affinity seems to increase upon ligand binding in hepatoma cells. This may be related to the finding that IRs were found preferentially as groups in adipocytes and as single receptors in hepatocytes (Jarett et al., 1980). Secondly, the removal of cholesterol by cyclodextrin treatment inhibits IRS-1 phosphorylation without affecting IR autophosphorylation in adipocytes (Parpal et al., 2001), whereas IR phosphorylation is also inhibited in hepatoma cells. It is possible that the lack of caveolae makes IR more vulnerable to the effects of cholesterol extraction. Interestingly, we have observed that overexpression of caveolin-1 using recombinant Semliki Forest virus augments IR phosphorylation in HuH7 cells (unpublished data), in line with the stimulatory role of caveolin in insulin signalling observed in other cells (Yamamoto et al., 1998; Nystrom et al., 1999).
Based on the available IR crystal structure, the constitutively oligomeric receptor occupies a relatively large area in the plane of the membrane (~80Å in diameter) (Ottensmeyer et al., 2000). Insulin binding changes receptor conformation to one that is permissive for phosphorylation. Although dimerization is not required for activation, unlike in several other transmembrane receptor tyrosine kinases, there is considerable evidence for IR self-aggregation. This has been experimentally observed, e.g. by IR antibodies (Heffetz and Zick, 1986), and also suggested to be mediated by the ligand itself (Jeffrey, 1982). According to our data, the dynamic association of IR with rafts may provide an additional mode of regulation into this framework. The unoccupied receptor seems to have low affinity for rafts, as assessed by detergent solubilization, and, upon insulin binding, its raft affinity increases. It is possible that the nature of the ligand-bound IR may favour its partitioning into DRMs (independent of raft association in vivo). However, ligand binding alone is not sufficient for attaining detergent insolubility, as the ligand-bound IR can be excluded from DRMs by cholesterol depletion or glycolipid patching.
Apart from caveolin interaction, the cue conferring IR raft association could be, for example, fatty acylation as the membrane spanning β-subunit is palmitoylated (Hedo et al., 1987; Magee and Siddle, 1988). Raft association may favour the preservation of the active receptor conformation, and clustering of individual rafts could account for the observed receptor aggregation, helping to potentiate signalling by concentrating downstream components, as has been shown for other signalling cascades. It is also conceivable that IR is constitutively associated with rafts but has only moderate affinity for rafts in vivo that can be disrupted by non-ionic detergents, as suggested for the T-cell receptor (Janes et al., 1999). In this scenario, IR activation could involve aggregation and stabilization of rafts already harbouring the receptor.
METHODS
Cyclodextrin treatments. To deplete cholesterol, HuH7 cells (Nakabayashi et al., 1982) were incubated with 10 mM methyl-β-cyclodextrin for 5 min at 37°C. Insulin (1.0 IU/ml) was added to the indicated samples, and the incubation continued for 10 min. Cells were then lysed for western blotting or gradient fractionation. To measure cholesterol efflux, cells were labelled overnight with 0.05 µCi/ml [14C]cholesterol in growth medium and incubated in the presence of cyclodextrin as above. Radioactivity from cells and media was measured by liquid-scintillation counting. To replete cholesterol, depleted cells were incubated for 2 h with methyl-β-cyclodextrin/cholesterol complex (molar ratio 8:1).
Glycosphingolipid clustering. Cells were incubated at 12°C for 1 h in the presence of 20 µg/ml anti-GM2 [mouse IgM against human GM2 (Karlsson et al., 1990)] or irrelevant antibody (anti-LDL receptor antibody C7 or mouse IgM MOPC 104C), washed and incubated for 1 h at 12°C in the presence of secondary antibodies. After insulin stimulation at 37°C, cells were lysed for western blotting or gradient fractionation. Cells on cover slips were fixed for 2 h in 4% PFA at room temperature after incubation with the secondary antibodies. To visualize GM2 staining without patching, staining with the secondary antibody was done after fixation. To examine the distribution of occupied IR in patched and non-patched cells, FITC-labelled insulin (30 ng/µl) was added for 5 min at 12°C before fixation.
Western blotting, flotation gradients. For western blotting, cells were lysed in buffer containing 1% SDS, 10 mM Tris–HCl, pH 7.5, phosphatase and protease inhibitors and 20 µg of protein resolved by SDS–PAGE. For western blotting with anti-PY antibody, filters were blocked with 3% BSA; otherwise 5% milk was used. The signal visualized by enhanced chemiluminiscence detection was quantified by normalizing the intensity of the anti-PY band with the intensity of the IR band in the same blot. The association of IR with DRMs was studied by lysing the cells in 0.1% Triton X-100 on ice and fractionating in Triton–Optiprep gradients according to Heino et al. (2000). Proteins TCA precipitated from the fractions were analysed by western blotting.
Supplementary data. Supplementary data are available at EMBO reports Online.
Supplementary Material
Acknowledgments
ACKNOWLEDGEMENTS
We thank Antti Virkamäki for valuable comments. This work was financially supported by the Sigrid Juselius Foundation, Jenny and Antti Wihuri Foundation, Swedish Medical Research council and the Academy of Finland.
REFERENCES
- Calvo M., Tebar, F., Lopez-Iglesias, C. and Enrich, C. (2001) Morphologic and functional characterization of caveolae in rat liver hepatocytes. Hepatology, 33, 1259–1269. [DOI] [PubMed] [Google Scholar]
- Carpentier J.L., Fehlmann, M., Van Obberghen, E., Gorden, P. and Orci, L. (1985) Redistribution of 125I-insulin on the surface of rat hepatocytes as a function of dissociation time. Diabetes, 34, 1002–1007. [DOI] [PubMed] [Google Scholar]
- Carpentier J.L., Paccaud, J.P., Gorden, P., Rutter, W.J. and Orci, L. (1992) Insulin-induced surface redistribution regulates internalization of the insulin receptor and requires its autophosphorylation. Proc. Natl Acad. Sci. USA, 89, 162–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couet J., Sargiacomo, M. and Lisanti, M.P. (1997) Interaction of a receptor tyrosine kinase, EGF-R, with caveolins. Caveolin binding negatively regulates tyrosine and serine/threonine kinase activities. J. Biol. Chem., 272, 30429–30438. [DOI] [PubMed] [Google Scholar]
- Fielding C.J. and Fielding, P.E. (2000) Cholesterol and caveolae: structural and functional relationships. Biochim. Biophys. Acta, 1529, 210–222. [DOI] [PubMed] [Google Scholar]
- Gustavsson J. et al. (1999) Localization of the insulin receptor in caveolae of adipocyte plasma membrane. FASEB J., 13, 1961–1971. [PubMed] [Google Scholar]
- Harder T., Scheiffele, P., Verkade, P. and Simons, K. (1998) Lipid domain structure of the plasma membrane revealed by patching of membrane components. J. Cell Biol., 141, 929–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedo J.A., Collier, E. and Watkinson, A. (1987) Myristyl and palmityl acylation of the insulin receptor. J. Biol. Chem., 262, 954–957. [PubMed] [Google Scholar]
- Heffetz D. and Zick, Y. (1986) Receptor aggregation is necessary for activation of the soluble insulin receptor kinase. J. Biol. Chem., 261, 889–894. [PubMed] [Google Scholar]
- Heino S., Lusa, S., Somerharju, P., Ehnholm, C., Olkkonen, V.M. and Ikonen, E. (2000) Dissecting the role of the Golgi complex and lipid rafts in biosynthetic transport of cholesterol to the cell surface. Proc. Natl Acad. Sci. USA, 97, 8375–8380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horejsi V., Cebecauer, M., Cerny, J., Brdicka, T., Angelisova, P. and Drbal, K. (1998) Signal transduction in leucocytes via GPI-anchored proteins: an experimental artefact or an aspect of immunoreceptor function? Immunol. Lett., 63, 63–73. [DOI] [PubMed] [Google Scholar]
- Hubbard S.R., Wei, L., Ellis, L. and Hendrickson, W.A. (1994) Crystal structure of the tyrosine kinase domain of the human insulin receptor. Nature, 372, 746–754. [DOI] [PubMed] [Google Scholar]
- Janes P.W., Ley, S.C. and Magee, A.I. (1999) Aggregation of lipid rafts accompanies signalling via the T cell antigen receptor. J. Cell Biol., 147, 447–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarett L., Schweitzer, J.B. and Smith, R.M. (1980) Insulin receptors: differences in structural organization on adipocyte and liver plasma membranes. Science, 210, 1127–1128. [DOI] [PubMed] [Google Scholar]
- Jeffrey P.D. (1982) The interaction of insulin with its receptor: cross-linking via insulin association as the source of receptor clustering. Diabetologia, 23, 381–385, 391–392. [DOI] [PubMed] [Google Scholar]
- Karlsson G., Mansson, J.E., Wikstrand, C., Bigner, D. and Svennerholm, L. (1990) Characterization of the binding epitope of the monoclonal antibody DMAb-1 to ganglioside GM2. Biochim. Biophys. Acta, 1043, 267–272. [DOI] [PubMed] [Google Scholar]
- Kasahara K. and Sanai, Y. (1999) Possible roles of glycosphingolipids in lipid rafts. Biophys. Chem., 82, 121–127. [DOI] [PubMed] [Google Scholar]
- London E. and Brown, D.A. (2000) Insolubility of lipids in Triton X-100: physical origin and relationship to sphingolipid/cholesterol membrane domains (rafts). Biochim. Biophys. Acta, 1508, 182–195. [DOI] [PubMed] [Google Scholar]
- Magee A.I. and Siddle, K. (1988) Insulin and IGF-1 receptors contain covalently bound palmitic acid. J. Cell. Biochem., 37, 347–357. [DOI] [PubMed] [Google Scholar]
- Nakabayashi H., Taketa, K., Miyano, K., Yamane, T. and Sato, J. (1982) Growth of human hepatoma cells lines with differentiated functions in chemically defined medium. Cancer Res., 42, 3858–3863. [PubMed] [Google Scholar]
- Nystrom F.H., Chen, H., Cong, L.N., Li, Y. and Quon, M.J. (1999) Caveolin-1 interacts with the insulin receptor and can differentially modulate insulin signalling in transfected Cos-7 cells and rat adipose cells. Mol. Endocrinol., 13, 2013–2024. [DOI] [PubMed] [Google Scholar]
- Ottensmeyer F.P., Beniac, D.R., Luo, R.Z. and Yip, C.C. (2000) Mechanism of transmembrane signalling: insulin binding and the insulin receptor. Biochemistry, 39, 12103–12112. [DOI] [PubMed] [Google Scholar]
- Parpal S., Karlsson, M., Thorn, H. and Stralfors, P. (2001) Cholesterol depletion disrupts caveolae and insulin receptor signalling for metabolic control via insulin receptor substrate-1, but not for mitogen-activated protein kinase control. J. Biol. Chem., 276, 9670–9678. [DOI] [PubMed] [Google Scholar]
- Saltiel A.R. (2001) New perspectives into the molecular pathogenesis and treatment of type 2 diabetes. Cell, 104, 517–529. [DOI] [PubMed] [Google Scholar]
- Simons K. and Toomre, D. (2000) Lipid rafts and signal transduction. Nature Rev. Mol. Cell Biol., 1, 31–39. [DOI] [PubMed] [Google Scholar]
- White M.F. and Kahn, C.R. (1994) The insulin signalling system. J. Biol. Chem., 269, 1–4. [PubMed] [Google Scholar]
- Yamamoto M., Toya, Y., Schwencke, C., Lisanti, M.P., Myers, M.G. Jr and Ishikawa, Y. (1998) Caveolin is an activator of insulin receptor signalling. J. Biol. Chem., 273, 26962–26968. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.