Abstract
The mammalian host response to infection includes the production and secretion of antimicrobial peptides from phagocytes and epithelial cells. Protegrins, a group of broadly microbicidal peptides isolated originally from porcine neutrophil lysates, were found to be stored as inactive proforms in porcine neutrophil granules but could be activated extracellularly by neutrophil elastase. We assessed the biological role of protegrins and other elastase-activated polypeptides in the microbicidal activity of neutrophil secretions and inflammatory fluids. When stimulated with phorbol myristate acetate (PMA), neutrophils generated stable microbicidal activity against both Escherichia coli and Listeria monocytogenes under normal-salt conditions and in the presence of 0 to 10% serum. The generation of these antimicrobial substances was dependent on neutrophil elastase, since it was inhibited by 1 mM N-methoxysuccinyl-Ala-Ala-Pro-Val chloromethyl ketone when it was present during activation, but not when this inhibitor was added afterwards. However, elastase-dependent activation of proprotegrins to protegrins in PMA-stimulated neutrophils was not inhibited by the presence of 1 to 2% serum. Porcine neutrophils also released antibacterial activity during phagocytosis of latex beads, and this too was dependent in large part on elastase-activated polypeptides, including protegrins. Moreover, protegrins were found at bactericidal concentrations in cell-free abscess fluid from naturally infected pigs. Taken together, these studies show that protegrins and other elastase-activated polypeptides are important stable antibacterial factors in porcine neutrophil secretions. The potential host defense role of elastase as an activating enzyme for the precursors of microbicidal peptides must be taken into account when therapeutic inhibitors of neutrophil elastase are evaluated for clinical use as anti-inflammatory agents.
The host defense armamentarium of mammals includes various antimicrobial peptides and proteins released from phagocytes and epithelia (4). In addition to larger antimicrobial proteins that function as enzymes (e.g., lysozyme or phospholipase A2) or contain binding sites for specific microbial macromolecules (e.g., the bactericidal–permeability-inducing protein [BPI]), mammalian phagocytes and epithelia produce two families of small microbicidal peptides, defensins and cathelicidins. Unlike defensins, which share a three-dimensional structure, cathelicidins are defined by a common N-terminal precursor motif, cathelin, joined to a highly variable C-terminal domain that appears to be the active microbicidal moiety. Many, but not all, cathelicidins contain a characteristic elastase cleavage site between the anionic cathelin domain and the cationic C-terminal peptide (28). Proteolytic processing at this site has been observed in activated bovine (29) and porcine (16) neutrophils and was required for microbicidal activity. In contrast, the rabbit cathelicidins p15a and p15b lack this consensus site, their cathelin domains are less anionic, and they are microbicidal without proteolytic processing (26). Cathelicidins are located in the types of neutrophil granules that are readily released into the extracellular fluid, i.e., the specific (secondary) granules in human and murine neutrophils and the large granules in bovine neutrophils (3, 12, 20, 22, 29), suggesting that the peptides may function primarily in extracellular spaces.
Neutrophils engaged in host defense also release a variety of important short-lived microbicidal substances, such as reactive oxygen and nitrogen intermediates, but in this study we focused on secretions that remain stable in inflammatory fluids over a period of days. This stable material will be referred to as neutrophil secretion. We hypothesized that elastase-activated cathelicidins contribute substantially to the extracellular microbicidal activity of neutrophil secretions and inflammatory fluids. We have chosen the pig as an experimental model, since porcine neutrophils lack defensins (9) but are unusually well endowed with cathelicidins, including protegrins (8), prophenins (5), PR-39 (19), and porcine myeloid antimicrobial peptides (PMAPs) (24). The precursors for all these peptides contain a characteristic elastase cleavage site, but, with the exception of protegrins, their processing by elastase has not yet been demonstrated. Of the porcine cathelicidins, the protegrins PG1, PG2, and PG3 are among the most active and abundant (9). Their amino acid sequences have been determined to be RGGRLCYCRRRFCVCVGRam, RGGRLCYCRRRFCICVam, and RGGGLCYCRRRFCVCVGRam, re- spectively (boldfaced residues differ among the three peptides, and “am” stands for C-terminal amidation). We previously showed that porcine neutrophils, when stimulated with phorbol myristate acetate (PMA), generated extracellular microbicidal activity against Listeria monocytogenes by elastase-mediated activation of secreted proprotegrins (pPGs) (16). In the present study, we assess the biological role of protegrins and other elastase-activated polypeptides in the antibacterial activity of neutrophil secretions and inflammatory fluids.
MATERIALS AND METHODS
Neutrophil secretions.
Porcine peripheral-blood neutrophils were isolated from 8- to 10-week-old healthy pigs as described earlier (18). Briefly, 30 ml of EDTA-treated blood (final concentration, 5 mM EDTA) was mixed with 30 ml of 3% dextran in phosphate-buffered saline (PBS) and was incubated for 30 min at room temperature. The supernatant was carefully removed from the dextran-sedimented blood, overlaid on 10 ml of Histopaque (Sigma), and centrifuged at 300 × g for 25 min at room temperature. Neutrophils were collected from the bottom of the tube, and contaminating red blood cells were lysed with 10 ml of 0.2% NaCl for 30 s. Isotonicity was restored with 10 ml of 1.6% NaCl. Neutrophils (>95% of the cells) were washed once in ice-cold PBS solution without divalent cations and kept on ice.
For degranulation experiments, freshly isolated neutrophils were resuspended at 108/ml in PBS containing 1 mM Ca2+ and 1 mM Mg2+, with or without serum. Neutrophils were then incubated with PMA (100 ng/ml) at 37°C for 30 min in the presence or absence of a specific inhibitor for neutrophil elastase, N-methoxysuccinyl-Ala-Ala-Pro-Val chloromethyl ketone (CMK) (1 mM; Sigma). Neutrophil secretions were collected as supernatant after the removal of neutrophils by centrifugation at 13,000 × g for 1 min.
For phagocytosis experiments, zymosan (Sigma) and latex beads (Difco) were opsonized as described earlier (6, 7). Briefly, zymosan (109 particles/ml in PBS) was tumbled with the same volume of freshly prepared porcine serum from a 6- to 8-week-old healthy pig at 37°C for 30 min, then washed twice with PBS, and resuspended in PBS at 109 particles/ml. Latex beads were opsonized by mixing 0.25 ml of Difco 0.81 latex beads, 0.05 ml of porcine serum, and 0.4 ml of PBS and incubating at 37°C for 30 min; then they were washed twice with PBS and resuspended in PBS at 109 particles/ml. Freshly isolated neutrophils were incubated with opsonized zymosan or latex beads (cell/particle ratio, 1:10 for both particles) in the presence or absence of 1 mM CMK or 0.5 mM diisopropylfluorophosphate (DFP; Sigma), a cell-permeant serine protease inhibitor, at 37°C for 30 min. The association of phagocytes with particles was verified by light microscopy. Neutrophil secretions induced by zymosan phagocytosis were collected after 1 min of centrifugation at 13,000 × g to remove both neutrophils and zymosan particles. In the phagocytosis experiments with latex beads, neutrophils were collected by centrifugation at 200 × g for 5 min and latex beads were then collected by centrifugation at 13,000 × g for 1 min, leaving the neutrophil secretions as supernatant.
Collection and analysis of abscess fluids.
Two otherwise healthy pigs with abscesses (one subcutaneous, the other in the abdominal wall) were identified in the slaughterhouse. Both abscesses were collected and centrifuged immediately at 13,000 × g for 10 min to remove cells and cell debris. The supernatants (abscess fluids) were subjected to a radial diffusion microbicidal assay, then analyzed by acid-urea-polyacrylamide gel electrophoresis (AU-PAGE), gel-overlay bactericidal assay, and Western blot analysis with anti-PG3 antibody as described below.
Antibacterial assays. (i) Bacteria.
L. monocytogenes EGD and Escherichia coli ML35p were used in the antibacterial assays. Overnight cultures of bacteria in 3% Trypticase soy broth (TSB) were subcultured for 2.5 h to exponential-growth phase in a shaking water bath at 37°C. Bacterial concentrations were estimated photometrically (an optical density of 1 at 620 nm corresponds to ∼2.5 × 108 bacteria/ml). Bacteria were washed and diluted with PBS to the desired concentrations.
(ii) Radial diffusion assay.
The radial diffusion assay was performed as described previously (21). Briefly, the underlay consisted of 1% agarose and 0.03% TSB in 10 mM sodium phosphate with 100 mM NaCl (normal salt medium), pH 7.4. The overlay consisted of 6% TSB and 1% agarose in PBS for all assays. Bacteria (5 × 106) were mixed with 10 ml of underlay gel solution (43°C) and poured into 10- by 10-cm petri dishes. A series of wells (diameter, 3 mm) were made after the agarose solidified. Peptide solutions or neutrophil secretions (5 μl per well) were added to designated wells. Plates were incubated at 37°C for 3 h. The bacterial agars were then covered with 10 ml of overlay. After 18 h of incubation at 37°C to allow visible bacterial growth, the plates were stained with 0.001% Coomassie blue for 10 h. Antibacterial activity was indicated by the clear zone (no bacterial growth) around the well. The activity was represented in radial diffusion units, defined as (diameter of clear zone in millimeters, − 3.0 mm) × 10. All assays were performed in duplicate and repeated at least once.
(iii) CFU assay.
The CFU assay was performed as described earlier (16). Briefly, 25 μl of neutrophil secretion and 2 μl of bacterial solution (108 CFU/ml) were mixed and incubated at 37°C in a shaking water bath for 30 min and then were diluted 500-fold in PBS, and aliquots were plated in triplicate on Trypticase soy agar plates. After incubation for 16 h at 37°C, the colonies were counted and the numbers of CFU per milliliter were calculated.
(iv) Gel overlay assay.
The gel-overlay assay was performed as described previously (16). Briefly, proteins and peptides were separated by AU-PAGE, and the gel was neutralized by washing for 15 min in 0.01 M PBS (pH 7.4) with 0.01 N NaOH, then in 0.01 M PBS only for 15 min. The gel was then placed on a premade 1% agarose plate containing 10 ml of 10 mM sodium phosphate, 100 mM NaCl, 0.1% TSB, 1% porcine serum, and 106 L. monocytogenes or E. coli organisms and was incubated at 37°C for 3 h to allow the proteins and peptides in the polyacrylamide gel to diffuse into the bacterial layer. The polyacrylamide gel was then removed, and the bacterial layer was overlaid with a nutrient layer that contained 6% TSB in 1% agarose. After 18 h of incubation at 37°C to allow visible bacterial growth, the agarose plate was stained with 0.001% Coomassie blue for 10 h. Antibacterial activity was indicated by the clear zone (no bacterial growth).
ELISA for pPGs and Western blot analysis for protegrins.
For the enzyme-linked immunosorbent assay (ELISA), 96-well plates (Becton Dickinson Labware, Oxnard, Calif.) were coated with 1 μg of monoclonal anti-PG3 antibody/ml (16) at 37°C for 2 h. Plates were then washed and blocked with 0.1% fetal bovine serum at 37°C for 30 min; then they were washed and incubated with 100 μl of pPG3 (0.02, 0.04, 0.08, 0.16, 0.32, 0.64, or 0.128 μg/ml) or neutrophil secretion samples (1:10 or 1:100 dilution) for 1 h at 37°C. At the end of the incubation, plates were washed and incubated with a monoclonal anti-pPG antibody-biotin conjugate (16) (1:1,000 dilution) at 37°C for 30 min, and then they were washed and incubated with avidin-horseradish peroxidase conjugate (1:1,000 dilution) at 37°C for 30 min. After washing, plates were developed with o-phenylenediamine substrate (Sigma) and the optical densities at 492 nM were measured with a microplate reader. Standards and samples were run in triplicate.
For Western blot analysis, after electrophoresis, proteins were electroblotted to an Immobilon-P membrane in 0.7% acetic acid and the blots were probed with a 1:1,000 dilution of the monoclonal anti-PG3 antibody and a 1:1,000 dilution of rabbit anti-mouse immunoglobulin G-alkaline phosphatase conjugate, then developed in a 5-bromo-4-chloro-3-indolylphosphate–nitroblue tetrazolium solution. Recombinant PG3 (16) was used as a positive control.
Elastase activity and elastase inhibitory capacity assays.
The elastase activity assay and elastase inhibitory capacity assay were performed by using Alphasin elastase diffusion plates (Elastin Products Co., Owensville, Mo.) as described by the manufacturer. An Alphasin elastase diffusion plate is a square plastic petri dish containing 10 ml of buffered agar gel that has been uniformly impregnated with elastin-fluorescein with a particle size smaller than 37 μm. The gel contains 25 wells, each 4 mm in diameter. Elastase and purified α1 protease inhibitor (α1-PI) were dissolved in 0.15 M NaCl. An aliquot (10 μl) of elastase (400 ng), alone or mixed with a series of concentrations of either α1-PI or serum, or with neutrophil secretion, was added to each well. Samples were run in duplicate. Plates were incubated at 37°C for 6 h, and the diameter of the clear zone (resulting from the solubilization of elastin particles by elastase) was measured. The elastase inhibitory capacities of neutrophil secretion and serum were determined by using α1-PI as the standard.
RESULTS
Elastase is required for the generation of neutrophil secretions bactericidal to E. coli.
Initially, we determined the contribution of elastase-cleaved polypeptides to bactericidal activity released from freshly isolated porcine neutrophils. The neutrophils were incubated at 108/ml in PBS containing 1 mM Ca2+ and 1 mM Mg2+ and were stimulated for 30 min with PMA (100 ng/ml) in the presence or the absence of the specific inhibitor for neutrophil elastase, CMK. The bactericidal activity of the cell-free secretions was assayed after overnight storage at 4°C.
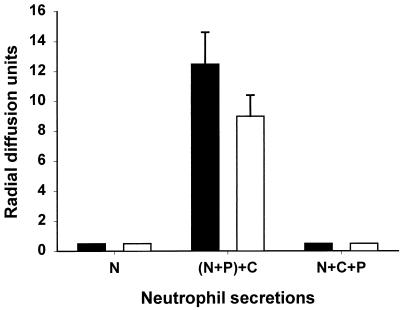
Secretions of unstimulated neutrophils were not bactericidal to either L. monocytogenes or E. coli (Fig. 1), but upon stimulation by PMA, porcine neutrophils secreted antibacterial substances that were bactericidal to both L. monocytogenes and E. coli. The antibacterial activity of neutrophil secretions against both bacteria was completely blocked if CMK, the specific elastase inhibitor, was added before neutrophils were stimulated by PMA, but not if CMK was added after incubation with PMA. We had shown previously (16) that CMK had no detectable effect on bacteria and no effect on antibacterial activity when added to cell-free polymorphonuclear leukocyte (PMN) secretions. The inhibition of bactericidal activity by CMK is consistent with the proposed role of elastase as an activator of secreted cathelicidins for extracellular killing of bacteria.
FIG. 1.
Bactericidal activity of neutrophil secretions in a radial diffusion assay. Five microliters of neutrophil secretion (equivalent to that from 5 × 105 neutrophils) was added into each well. Secretions were obtained from unstimulated neutrophils (N), neutrophils stimulated with PMA (100 ng/ml) for 30 min in the absence of CMK, with CMK added to the secretion after neutrophils were removed [(N+P)+C], or neutrophils stimulated with PMA in the presence of CMK (N+C+P). Zones of clearance were measured with a calibrated microscope. The microbicidal activity was expressed in arbitrary units (0.1 mm = 1 U) by subtracting the diameter of the well (3 mm) from the diameter of the clear zone. Open bars, E. coli; solid bars, L. monocytogenes. Data are means ± standard deviations from assays performed twice in duplicate.
Antibacterial activity of neutrophil secretions persists in the presence of normal salt medium and 1 to 2% serum.
While the effect of porcine serum on the antibacterial activity of neutrophil secretions is potentially very complex (10, 11, 15, 17), the microbicidal activity of PG1 has been reported to be resistant to serum (21, 27). Indeed, we confirmed that the bactericidal activity of PG3 against both L. monocytogenes and E. coli was also unchanged or even increased in the presence of 1 to 2% porcine serum and normal salt medium compared to that under low-salt, serum-free conditions (data not shown).
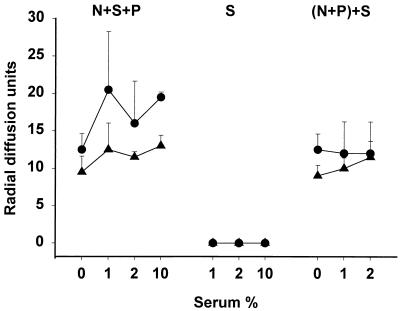
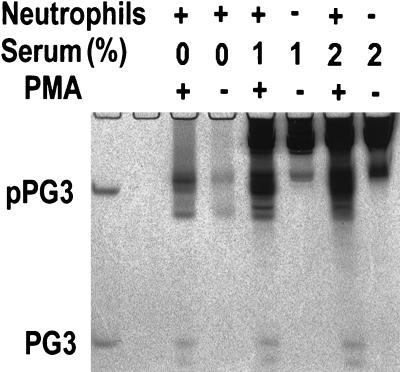
An important effect of serum is mediated by its neutrophil elastase inhibitors, e.g., α1-PI and α2-macroglobulin, which could impair the proteolytic processing of protegrins and other elastase-activated polypeptides and could decrease the antibacterial activity of neutrophil secretions. To assess the effect of serum on the microbicidal activity of neutrophil secretions, serum was added either before the PMA-induced secretion of granule contents or, as a control, after the degranulation was completed and the neutrophils were removed. Regardless of when the serum was added, it did not inhibit, and in some cases it enhanced, the microbicidal activity of neutrophil secretions against both E. coli and L. monocytogenes (Fig. 2). Porcine serum by itself was inactive against both bacteria under these assay conditions. The protein composition of neutrophil secretions was assessed by AU-PAGE (Fig. 3). In the presence of 1 to 2% serum, the proteolytic generation of protegrins from pPGs was not inhibited.
FIG. 2.
Influence of serum on the antibacterial activity of neutrophil secretions in a radial diffusion assay. N+S+P, neutrophils were stimulated by PMA (100 ng/ml) in the presence or absence of serum; S, serum only, diluted in PBS; (N+P)+S, serum was added into neutrophil secretions after neutrophils were removed. Symbols: •, E. coli; ▴, L. monocytogenes. Data are means ± standard deviations from assays performed twice in duplicate.
FIG. 3.
AU-PAGE of neutrophil secretions stimulated by PMA in the presence or absence of serum. Proteins and peptides from neutrophil secretions or serum solutions were subjected to AU-PAGE and stained with Coomassie blue after electrophoresis. Recombinant pPG3 (upper band) and synthetic PG3 (lower band) (0.5 μg of each) were used as standards (leftmost lane). In neutrophil secretions, the bands comigrating with the PG3 standard contain PG2 and PG3, and the slightly faster migrating band corresponds to PG1. Neutrophil secretions were induced by PMA in the presence or absence of serum. For each lane, 100 μl of neutrophil secretions (equivalent to that from 107 cells) or 100 μl of serum solution (1 or 2%) was extracted with 10 μl of StrataClean resin (Stratagene) and the resin beads were loaded into each well for electrophoresis.
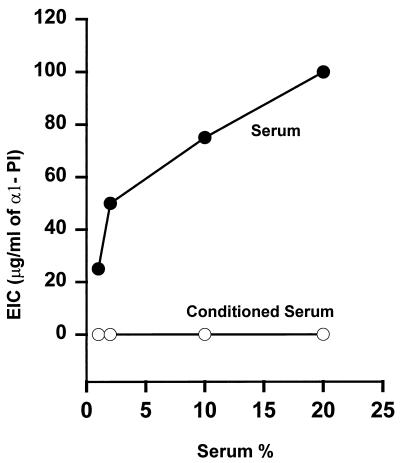
We next asked whether the inability of serum to inhibit the processing of protegrins by neutrophil elastase could be due to the lack of elastase inhibitory activity in porcine serum. The elastase inhibitory capacities of serum and of neutrophil secretions were assayed on elastin plates relative to human α1-PI as a standard inhibitor of elastase. As shown in Fig. 4, the elastase inhibitory capacity in 10% normal fresh porcine serum was equivalent to that of 70 μg of α1-PI/ml, a finding similar to reported data (23). However, exposure of 1 to 20% serum to neutrophils activated by PMA completely ablated the elastase inhibitory capacity. The loss of elastase inhibitory capacity is likely due to the inactivation of α1-PI and other elastase inhibitors by reactive oxygen intermediates generated during the respiratory burst of neutrophils (2).
FIG. 4.
The elastase inhibitory capacity (EIC) of porcine serum. Human neutrophil elastase (400 ng) in 10 μl of saline (0.15 M NaCl) or saline-serum mixture (serum), or a mixture of elastase with serum exposed to activated porcine neutrophils (conditioned serum), was assayed on an elastin-agar plate. Mixtures of human neutrophil elastase (400 ng) with varying amounts of purified α1-PI were used as standards for the inhibition of elastase activity. Data are means from two experiments.
During phagocytosis, protegrins are generated from pPGs by elastase.
To study the role of protegrins and other elastase-activated peptides in secretions generated during phagocytosis, we first incubated porcine neutrophils with zymosan that had been opsonized with porcine serum. Phagocytosis was verified by light microscopy. However, mature protegrins were not detected in the neutrophil secretions by AU-PAGE analysis (data not shown), and to our surprise, no bactericidal activity was found in the zymosan-stimulated neutrophil secretions by the radial diffusion assay and CFU assay (data not shown). In a previous study, we found that the extracellular processing of pPGs by PMA was mediated by neutrophil elastase, since it could be inhibited extracellularly by the specific inhibitor CMK (16). To determine whether elastase cleaved pPGs during neutrophil phagocytosis of zymosan, we used the cell-permeant serine protease inhibitor DFP to inhibit neutrophil elastase (and other serine proteases) during phagocytosis (1), or we used CMK as an extracellular elastase-specific inhibitor. Light microscopy revealed that DFP- or CMK-treated PMNs phagocytized the same number of zymosan particles as untreated PMNs (data not shown), in agreement with observations reported by others (13). To detect the processed and unprocessed protegrin forms, we performed both Western blot analysis and an ELISA using anti-pPG and antiprotegrin antibodies. In Western blot analysis, only pPGs, not protegrins, were detected in neutrophil secretions when DFP or CMK was added with zymosan, but without DFP and CMK, neither pPGs nor protegrins were detected (data not shown). By an ELISA designed to detect pPGs only, the amounts of pPGs released from neutrophils during phagocytosis of zymosan were 22.29 ± 2.28 μg/108 PMNs/ml and 23.90 ± 0.70 μg/108 PMNs/ml in the presence of DFP and CMK respectively, which were similar to those resulting from PMA stimulation in the presence of CMK (21.57 ± 3.10 μg/108 PMNs/ml). In the absence of CMK and DFP, only small amounts of pPGs were detectable in secretions generated by either PMA- or zymosan-treated neutrophils (1.35 ± 0.13 μg/108 PMNs/ml for PMA versus 1.55 ± 0.07 μg/108 PMNs/ml for zymosan). We concluded that elastase did process secreted pPGs during phagocytosis of zymosan.
We hypothesized that our inability to detect processed protegrins was due to their high affinity for zymosan, leading to the adsorption of protegrins from the neutrophil secretions. To test this hypothesis, we added zymosan either to neutrophil secretions that had been generated by PMA-treated neutrophils or to a solution of synthetic PG3. After 30 min of incubation at 37°C, zymosan particles were removed by brief centrifugation, and the supernatants were tested for antibacterial activity against both E. coli and L. monocytogenes. While solutions not exposed to zymosan were microbicidal to both bacteria, no antibacterial activity was found in the zymosan-extracted neutrophil secretions or PG3 solutions (data not shown). The interaction of protegrins with zymosan was so strong that protegrins could not be detected even when the zymosan-protegrin complex was subjected to AU-PAGE and sodium dodecyl sulfate-PAGE under reducing conditions (data not shown).
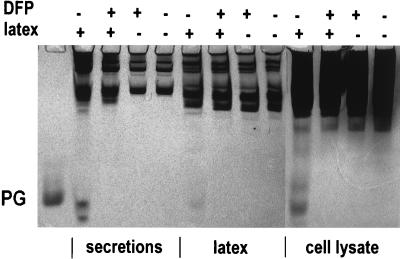
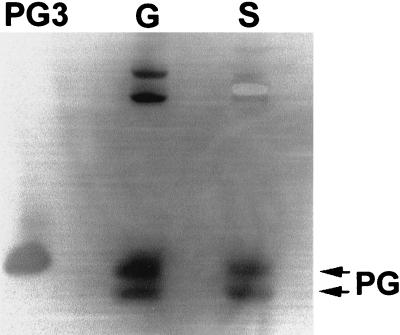
To overcome the difficulty caused by protegrins binding to zymosan particles, we used latex beads as an alternative particulate stimulus. First, we assayed the binding of PG3 to latex beads and found that PG3 bound to the latex less avidly than to zymosan and that bound PG3 was released when the latex-PG3 complex was subjected to AU-PAGE (data not shown). After phagocytosis of latex, neutrophils were sedimented by low-speed centrifugation (at 200 × g for 6 min), leaving neutrophil secretions and the uningested latex beads in the supernatant. Latex beads were then removed from the neutrophil secretions by high-speed centrifugation at 13,000 × g for 1 min, leaving latex-free neutrophil secretions. The fractions were analyzed by AU-PAGE. As shown in Fig. 5, protegrins were detected in all the fractions from neutrophils that phagocytized latex beads but received the cell-permeant serine protease inhibitor DFP only at the completion of phagocytosis, but no protegrins were detected if DFP was added before latex beads were incubated with neutrophils. No protegrins were detected in unstimulated neutrophils where latex beads were added at the end of the incubation. The activation of protegrins during neutrophil phagocytosis of latex beads was further confirmed by Western blot analysis using a monoclonal antiprotegrin antibody (Fig. 6). Porcine neutrophil granules, when isolated without protease inhibitors, contain both mature protegrins and their precursors. However, only mature protegrins were detected in the neutrophil secretions induced by phagocytosis of latex beads. The presence of mature protegrins in the cell lysates of neutrophils that phagocytized latex beads suggested that either protegrins were activated from their precursors inside or on the surfaces of neutrophils or they were adsorbed secondarily from the medium (Fig. 5).
FIG. 5.
AU-PAGE of neutrophil secretions stimulated by latex beads. Gels were stained with Coomassie blue. Each lane contains either 107 cell equivalents of either neutrophil secretions, extracellular latex beads after phagocytosis, or neutrophil lysates. The symbols + and − indicate which materials were added into each tube before the phagocytosis started. At the end of the incubation, DFP and/or latex beads were added into tubes that did not have them during phagocytosis. The PG standard is 0.5 μg of PG3.
FIG. 6.
Western blot analysis of neutrophil secretions stimulated by latex beads. After AU-PAGE, proteins were blotted to an Immobilon-P membrane, and the membrane was probed with a monoclonal anti-PG3 antibody and rabbit anti-mouse immunoglobulin G-alkaline phosphatase conjugate, then developed in 5-bromo-4-chloro-3-indolylphosphate–nitroblue tetrazolium solution. PG3, 0.5 μg of synthetic PG3; G, porcine neutrophil granule lysate (5 × 106 cell equivalents); S, neutrophil secretions generated during phagocytosis of latex beads in the absence of DFP (107 cell equivalents).
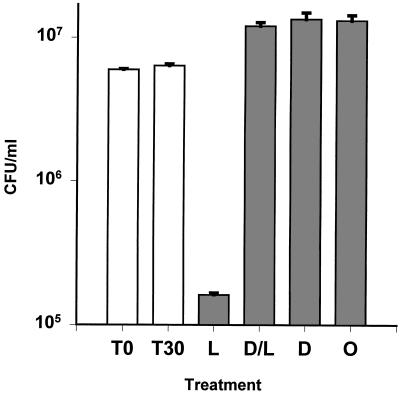
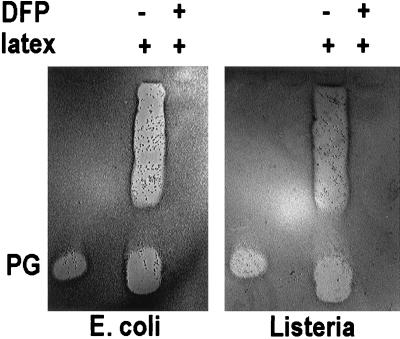
The antibacterial activity of neutrophil secretions stimulated by latex beads was evaluated by a CFU assay and a gel-overlay assay. As shown in Fig. 7, neutrophils secreted antibacterial substances that were active against L. monocytogenes, and this activity was inhibited when DFP was added before the neutrophils phagocytized latex beads. In the gel overlay assay, when either E. coli or L. monocytogenes was used to detect antimicrobial activity, the results were very similar (Fig. 8). Activity corresponding to protegrins was seen only in secretions generated by neutrophils that had not been pretreated with DFP. Additional antibacterial bands were detected in the neutrophil secretions, and these were slower migrating than protegrins. Because the activity of these substances was also blocked by pretreatment with DFP, we suspect that these bands represent other elastase-activated cathelicidins, such as PR-39 (19), prophenins (5), and PMAPs (24).
FIG. 7.
Bactericidal activity of latex-stimulated neutrophil secretions in a CFU assay. Shown are the results of a CFU assay with L. monocytogenes. T0, initial bacterial inoculum; T30, bacteria in PBS after 30 min at 37°C. Bacteria were incubated as follows: with neutrophil secretions stimulated with latex beads for 30 min and then treated with DFP (L); with neutrophil secretions stimulated with latex beads for 30 min in the presence of DFP (D/L); with neutrophil secretions elicited with DFP, incubated for 30 min, and then treated with latex beads at the end of incubation (D); or with neutrophil secretions treated with DFP and latex beads at the end of 30 min of incubation (O).
FIG. 8.
Bactericidal activity of latex-stimulated neutrophil secretions in a gel-overlay bactericidal assay. Shown are results of two gel-overlay assays with E. coli and L. monocytogenes. Neutrophil secretions were prepared as described for Fig. 5. Ten million cell equivalents of neutrophil secretion was used in each lane of the overlay assays. PG, 0.5 μg of synthetic PG3, used as positive control for the activity and identification of protegrins. Antibacterial activity was indicated by a clear zone (no bacterial growth).
Protegrins contribute to the bactericidal activity of abscess fluid.
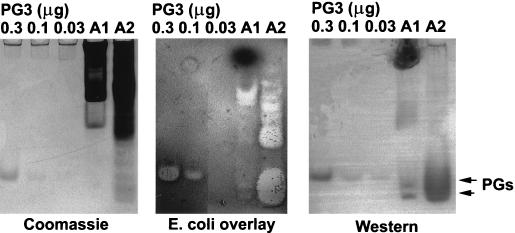
Abscesses contain accumulations of activated neutrophils and serum proteins. To determine whether cell-free porcine abscess fluid is microbicidal, we collected fresh abscess fluid from two pigs, one with an abdominal wall abscess (A1) and one with a dermal abscess (A2), rapidly removed the particulate fraction by centrifugation, and assayed the microbicidal activity of the supernatant by radial diffusion assay. One abscess fluid sample (A2) displayed potent activity (100 to 120 radial diffusion units) against both E. coli and L. monocytogenes; however, the activity of the other sample was very weak (10 to 15 radial diffusion units). To detect microbicidal protegrins, we then analyzed the supernatant using AU-PAGE, a gel-overlay bactericidal assay, and Western blot analysis (Fig. 9). The protein profiles of abscess fluids on AU-PAGE indicated that protegrins were present in both samples. The activity and the immunoreactivity of protegrins in the abscess fluids were confirmed by gel-overlay bactericidal assay and Western blot analysis using a monoclonal antiprotegrin antibody (Fig. 9). Based on the intensity of the Western blot, we estimated that the concentrations of mature protegrins in abscess fluids were 15 to 150 μg/ml, well within the range of concentrations known to be bactericidal to E. coli and L. monocytogenes (9). The Western blot also indicated that most of the protegrins in the less-active abscess fluid were strongly associated with substances that prevented the normal migration of protegrins in AU-PAGE. Additional studies will be necessary to identify these substances and to determine whether they originate in the microbes or the host.
FIG. 9.
Microbicidal activity of porcine abscess fluids. Porcine abdominal-wall abscess fluid (A1) (20 μl), porcine skin abscess fluid (A2) (20 μl), and synthetic PG3 (0.03, 0.1, and 0.3 μg) were subjected to AU-PAGE. (Left) The gel was stained with Coomassie blue. (Center) Gel-overlay assay with E. coli. Antibacterial activity was indicated by a clear zone (no bacterial growth). (Right) Western blot analysis was performed as for Fig. 6.
DISCUSSION
The role of an individual peptide in the neutrophil antimicrobial activity has been difficult to determine because several families of antimicrobial proteins and peptides typically coexist in the neutrophils of each mammalian species. Thus, both defensins and cathelicidins have been identified in the neutrophils of humans, rabbits, and cattle. We selected porcine neutrophils as a simple model for the study of cathelicidins, since the porcine neutrophils lack defensins (9). Evidence supporting the hypothesis that neutrophil cathelicidins are important host defense factors is emerging. We previously showed that protegrins were stored as inactive proforms in porcine neutrophil granules and could be activated extracellularly by elastase. Stimulation of neutrophil secretions with PMA rendered them bactericidal to L. monocytogenes in low-salt medium (16). In other studies, rabbit cathelicidins referred to as p15’s potently synergized with BPI to inhibit the growth of E. coli in rabbit peritoneal exudate (25). These two rabbit cathelicidins, p15a and p15b, have less-anionic cathelin domains than other cathelicidins, lack the characteristic elastase cleavage sites, and do not require elastase-mediated activation. In the present study, we demonstrated that the antibacterial activity of porcine neutrophil secretions generated during neutrophil phagocytosis of latex beads was dependent in large part on elastase-activated polypeptides, including protegrins. The elastase-dependent activity was detected against both E. coli and L. monocytogenes under normal-salt conditions. Protegrins, active and abundant porcine cathelicidins, were found at bactericidal concentrations in neutrophil secretions and in abscess fluid. Further studies will be necessary to identify the other elastase-activated polypeptides, to ascertain whether they are also cathelicidins, and to assess their contribution to the antibacterial activity of porcine inflammatory fluid.
In vivo, neutrophils are commonly exposed to varying concentrations of serum, which contains elastase inhibitors. The extracellular processing of pPGs and other procathelicidins to active forms must depend on the release of elastase in functional excess of its inhibitors. Despite the strong elastase inhibitory activity in fresh porcine serum, the maturation of protegrins was observed even when the serum was added before neutrophil activation. We found that the elastase inhibitory activity of fresh porcine serum was totally abolished during neutrophil activation, probably by respiratory-burst products (2). Thus, in the neighborhood of phagocytizing neutrophils, elastase may exceed its inhibitors and activate cathelicidins. The unimpaired processing of protegrins could also be explained by the adsorption of elastase to the neutrophil surface, which can protect elastase from its inhibitors (14).
Protegrins, and other elastase-activated polypeptides, were the predominant antibacterial factors in porcine neutrophil secretions generated during phagocytosis. Although species differences in the antimicrobial arsenal of neutrophils preclude direct extrapolation to humans, the potential host defense role of elastase as an activating enzyme for the precursors of microbicidal peptides must be taken into account when therapeutic inhibitors of neutrophil elastase are evaluated for clinical use as anti-inflammatory agents (25).
ACKNOWLEDGMENTS
We thank Shawn McGill, Fernando Vinuela, Jr., and John Robert for their excellent technical support and Edith Martin Porter for helpful discussions.
This work was supported by grant HL46809 from the National Institutes of Health and a postdoctoral fellowship from the Cystic Fibrosis Foundation to J. Shi.
REFERENCES
- 1.Amrein P C, Stossel T P. Prevention of degradation of human polymorphonuclear leukocyte proteins by diisopropylfluorophosphate. Blood. 1980;56:442–447. [PubMed] [Google Scholar]
- 2.Carp H, Janoff A. Potential mediator of inflammation. Phagocyte-derived oxidants suppress the elastase-inhibitory capacity of alpha 1-proteinase inhibitor in vitro. J Clin Investig. 1980;66:987–995. doi: 10.1172/JCI109968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ganz T, Lehrer R I. Antimicrobial peptides of leukocytes. Curr Opin Hematol. 1997;4:53–58. doi: 10.1097/00062752-199704010-00009. [DOI] [PubMed] [Google Scholar]
- 4.Ganz T, Weiss J. Antimicrobial peptides of phagocytes and epithelia. Semin Hematol. 1997;34:343–354. [PubMed] [Google Scholar]
- 5.Harwig S S, Kokryakov V N, Swiderek K M, Aleshina G M, Zhao C, Lehrer R I. Prophenin-1, an exceptionally proline-rich antimicrobial peptide from porcine leukocytes. FEBS Lett. 1995;362:65–69. doi: 10.1016/0014-5793(95)00210-z. [DOI] [PubMed] [Google Scholar]
- 6.Henson P M. The immunologic release of constituents from neutrophil leukocytes. II. Mechanisms of release during phagocytosis, and adherence to nonphagocytosable surfaces. J Immunol. 1971;107:1547–1557. [PubMed] [Google Scholar]
- 7.Jandl R C, Andre-Schwartz J, Borges-DuBois L, Kipnes R S, McMurrich B J, Babior B M. Termination of the respiratory burst in human neutrophils. J Clin Investig. 1978;61:1176–1185. doi: 10.1172/JCI109033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kokryakov V N, Harwig S S, Panyutich E A, Shevchenko A A, Aleshina G M, Shamova O V, Korneva H A, Lehrer R I. Protegrins: leukocyte antimicrobial peptides that combine features of corticostatic defensins and tachyplesins. FEBS Lett. 1993;327:231–236. doi: 10.1016/0014-5793(93)80175-t. [DOI] [PubMed] [Google Scholar]
- 9.Lehrer R I, Ganz T. Endogenous vertebrate antibiotics. Defensins, protegrins, and other cysteine-rich antimicrobial peptides. Ann N Y Acad Sci. 1996;797:228–239. doi: 10.1111/j.1749-6632.1996.tb52963.x. [DOI] [PubMed] [Google Scholar]
- 10.Lehrer R I, Szklarek D, Barton A, Ganz T, Hamann K J, Gleich G J. Antibacterial properties of eosinophil major basic protein and eosinophil cationic protein. J Immunol. 1989;142:4428–4434. [PubMed] [Google Scholar]
- 11.Lehrer R I, Szklarek D, Ganz T, Selsted M E. Correlation of binding of rabbit granulocyte peptides to Candida albicans with candidacidal activity. Infect Immun. 1985;49:207–211. doi: 10.1128/iai.49.1.207-211.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moscinski L C, Hill B. Molecular cloning of a novel myeloid granule protein. J Cell Biochem. 1995;59:431–442. doi: 10.1002/jcb.240590404. [DOI] [PubMed] [Google Scholar]
- 13.Musson R A, Becker E L. The role of an activatable esterase in immune-dependent phagocytosis by human neutrophils. J Immunol. 1977;118:1354–1365. [PubMed] [Google Scholar]
- 14.Owen C A, Campbell M A, Sannes P L, Boukedes S S, Campbell E J. Cell surface-bound elastase and cathepsin G on human neutrophils: a novel, non-oxidative mechanism by which neutrophils focus and preserve catalytic activity of serine proteinases. J Cell Biol. 1995;131:775–789. doi: 10.1083/jcb.131.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Panyutich A, Ganz T. Activated alpha 2-macroglobulin is a principal defensin-binding protein. Am J Respir Cell Mol Biol. 1991;5:101–106. doi: 10.1165/ajrcmb/5.2.101. [DOI] [PubMed] [Google Scholar]
- 16.Panyutich A, Shi J, Boutz P L, Zhao C, Ganz T. Porcine polymorphonuclear leukocytes generate extracellular microbicidal activity by elastase-mediated activation of secreted proprotegrins. Infect Immun. 1997;65:978–985. doi: 10.1128/iai.65.3.978-985.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Panyutich A V, Hiemstra P S, Van Wetering S, Ganz T. Human neutrophil defensin and serpins form complexes and inactivate each other. Am J Respir Cell Mol Biol. 1995;12:351–357. doi: 10.1165/ajrcmb.12.3.7873202. [DOI] [PubMed] [Google Scholar]
- 18.Shi J, Goodband R D, Chengappa M M, Nelssen J L, Tokach M D, McVey D S, Blecha F. Influence of interleukin-1 on neutrophil function and resistance to Streptococcus suis in neonatal pigs. J Leukocyte Biol. 1994;56:88–94. doi: 10.1002/jlb.56.1.88. [DOI] [PubMed] [Google Scholar]
- 19.Shi J, Ross C R, Chengappa M M, Blecha F. Identification of a proline-arginine-rich antibacterial peptide from neutrophils that is analogous to PR-39, an antibacterial peptide from the small intestine. J Leukocyte Biol. 1994;56:807–811. doi: 10.1002/jlb.56.6.807. [DOI] [PubMed] [Google Scholar]
- 20.Sorensen O, Arnljots K, Cowland J B, Bainton D F, Borregaard N. The human antibacterial cathelicidin, hCAP-18, is synthesized in myelocytes and metamyelocytes and localized to specific granules in neutrophils. Blood. 1997;90:2796–2803. [PubMed] [Google Scholar]
- 21.Steinberg D A, Lehrer R I. Designer assays for antimicrobial peptides. In: Shafer W M, editor. Antimicrobial peptide protocols. Totowa, N.J: Humana Press, Inc.; 1997. pp. 169–187. [DOI] [PubMed] [Google Scholar]
- 22.Storici P, Tossi A, Lenarcic B, Romeo D. Purification and structural characterization of bovine cathelicidins, precursors of antimicrobial peptides. Eur J Biochem. 1996;238:769–776. doi: 10.1111/j.1432-1033.1996.0769w.x. [DOI] [PubMed] [Google Scholar]
- 23.Stratil A, Cizova-Schroffelova D, Gabrisova E, Pavlik M, Coppieters W, Peelman L, Van de Weghe A, Bouquet Y. Pig plasma alpha-protease inhibitors PI2, PI3 and PI4 are members of the antichymotrypsin family. Comp Biochem Physiol B. 1995;111:53–60. doi: 10.1016/0305-0491(94)00232-j. [DOI] [PubMed] [Google Scholar]
- 24.Tossi A, Scocchi M, Zanetti M, Storici P, Gennaro R. PMAP-37, a novel antibacterial peptide from pig myeloid cells. cDNA cloning, chemical synthesis and activity. Eur J Biochem. 1995;228:941–946. doi: 10.1111/j.1432-1033.1995.tb20344.x. [DOI] [PubMed] [Google Scholar]
- 25.Vender R L. Therapeutic potential of neutrophil-elastase inhibition in pulmonary disease. J Investig Med. 1996;44:531–539. [PubMed] [Google Scholar]
- 26.Weinrauch Y, Foreman A, Shu C, Zarember K, Levy O, Elsbach P, Weiss J. Extracellular accumulation of potently microbicidal bactericidal/permeability-increasing protein and p15s in an evolving sterile rabbit peritoneal inflammatory exudate. J Clin Investig. 1995;95:1916–1924. doi: 10.1172/JCI117873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yasin B, Harwig S S, Lehrer R I, Wagar E A. Susceptibility of Chlamydia trachomatis to protegrins and defensins. Infect Immun. 1996;64:709–713. doi: 10.1128/iai.64.3.709-713.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zanetti M, Gennaro R, Romeo D. Cathelicidins: a novel protein family with a common proregion and a variable C-terminal antimicrobial domain. FEBS Lett. 1995;374:1–5. doi: 10.1016/0014-5793(95)01050-o. [DOI] [PubMed] [Google Scholar]
- 29.Zanetti M, Litteri L, Griffiths G, Gennaro R, Romeo D. Stimulus-induced maturation of probactenecins, precursors of neutrophil antimicrobial polypeptides. J Immunol. 1991;146:4295–4300. [PubMed] [Google Scholar]