Abstract
We aimed to identify caregivers' opinions on the outcome measures that matter in clinical trials in individuals with Dravet syndrome (DS). We conducted a prospective European multicenter study based on an 11 closed questions survey developed by the French reference center for rare epilepsies and DS patients’ advocacy groups. Items included questions on seizures and daily life outcomes that a clinical trial on a therapy for individuals with DS should target. Statistical analyses were performed to evaluate the impact of the country of residence and of the patients’ age. The survey was answered by 153 caregivers (68%: France, 28%: Germany, and 24%: Italy) off individuals with DS. Individuals with DS included 86 males (mean age of 11.4 [interquartile: 7‐20.4] years). Families ranked as important almost all the items proposed. However, items related to daily life had the highest rank in all three countries compared to items about seizures (P = 0.02). Increase in individuals’ age was associated with a higher age at diagnosis (ρ = 0.26, P = 0.02), and a lower impact of seizure duration (ρ = −0.25, P = 0.005) and on the need of hospital referral (ρ = −0.26, P = 0.005). These data can help tailor patient‐centered outcome measures in future clinical and real‐life trials for DS.
Keywords: burden of the disease, families’ expectations, meaningful change, meaningful outcomes, PCOMs
1. INTRODUCTION
Determining what really matters, ie, the meaningful outcomes for the health of individuals, is a key point in clinical trials, especially for rare diseases. Indeed, rare diseases generally involve several organs with high interindividual variability leading to great disparity in terms of clinical presentations and consequences in the daily life. The use of primary outcome measures (POMs) elaborated by practitioners might be simplistic and misrepresents the multiple facets of the impact of a disease. 1 In epilepsy, evaluation of clinical trials is mainly based on POMs targeting efficacy, like responder rate (defined as the number of affected individuals with at least 50% reduction in total seizure frequency) or the proportion of subjects who achieved seizure‐free status, 2 , 3 , 4 , 5 and safety, using incidence of adverse events (AEs) and withdrawals rate due to AEs. 6 , 7 However, it is important to question the meaning of these endpoints, particularly in the context of developmental and epileptic encephalopathy (DEE) characterized by major drug resistance and high incidence of comorbidities beyond seizures. Dravet syndrome (DS), one of the archetype of DEEs, is commonly related to pathogenic variant of SCN1A leading to a loss of function of voltage‐dependent sodium channel. 8 , 9 , 10 , 11 , 12 This DEE is associated with various degrees of intellectual disabilities, autism spectrum disorder in almost one third, and behavioral disorders which incidence increases with age. 8 , 9 , 13 Several additional features are frequently reported: eating and sleep disorders, gait deterioration, dysautonomia, and a higher predisposition to infections. 14 , 15 , 16 Research into the impact of DS beyond seizures increased in the last years and allowed to describe DS whole phenotype based on families and practitioners reports. 14 , 15 , 17 , 18 , 19 , 20 In addition, the burden of illness of individuals with Dravet syndrome also have a high impact on the caregivers. Compared to caregivers of individuals with difficult‐to‐treat epilepsy, caregivers of DS had higher depression scores and were more likely to change their employment status, including leaving their job. 21
The aim of this study is to explore the domains that really matters for individuals with DS and their families emphasizing that a therapy targeting these domains would have a positive meaningful impact on their outcome.
2. METHODS
2.1. Survey development
We developed, with three national patients’ advocacy groups of DS in Europe (“Alliance Syndrome Dravet” in France, “Dravet‐Syndrom e.V.” in Germany, and “Dravet Italia Onlus”), an 11 closed questions survey based on our preliminary surveys 17 , 22 and other literature reports. 14 , 15 , 18 This survey explored different items related to current seizures (frequency, duration, seizures requiring rescue therapy, seizures requiring a referral to emergency room [ER], or intensive care unit [ICU]) and to daily life aspects (sleep, eating disorders, language, motor skills, daily activity, behavior, communication, and interaction) using a Likert's scale from 1 to 5 (not important at all = 1, not important = 2, neutral = 3, important = 4, and highly important = 5).
2.2. Participants
This study was a prospective cohort study with convenience sampling. The survey was filled during annual associations meeting (France and Germany) or shared online with families for a period of 6 weeks (May to June 2019, Italy). For every individual with DS, a unique caregiver completed the survey. Written informed consent to participate in this study was provided by the participants. This study was approved by the ethics committee of our institution (Necker Hospital, APHP).
2.3. Statistics
Results are expressed as the average ± standard deviation in case of Likert's scale data and as median [25th‐75th percentile] otherwise. To study the impact of countries on the responses, we used one‐way ANOVA in case of homogeneity of variance (Levene test). Otherwise, we used a more robust test called Brown Forsythe test with the same factors. 23 Bonferroni post hoc tests after ANOVAs or Tamhane post hoc tests after Brown Forsythe tests were then applied in case of significance. For qualitative data, X 2 tests were used to study the presence or the absence of significant difference between the different countries. We correlated different quantitative answers to affected individuals’ age using Spearman's rank correlation (Rho) coefficients. To illustrate the possible correlation with age, we presented the data in relation to three age groups: <6 years, 6‐12 years, and >12 years.
3. RESULTS
A total of 153 surveys were filled by parents with 96.5% of response rate (missing data: 81/2295). Table 1 summarized the characteristic of the population. There was no significant difference among the three countries regarding demographic characteristics. Concerning the age at diagnosis, a significant correlation with patients’ age was identified (Table 2, ρ = 0.26, P = 0.002). A lower age of diagnosis was associated with the younger age group.
TABLE 1.
Demographic characteristics
| Total | France | Italy | Germany | P | |
|---|---|---|---|---|---|
| N (%) | 153 | 73 (47.7%) | 37 (24.2%) | 43 (28.1%) | |
| Sex (m/f) | 86/67 | 41/32 | 19/18 | 26/17 | ns |
| Current age (y) | 11.4 [7‐20.4] | 11 [5‐20.5] | 10.8 [6.6‐17.7] | 12.8 [9‐23.8] | ns |
| Age at seizure onset (m) | 5 [3.5‐6.5] | 5 [3.5‐7] | 5 [3.5‐6] | 5 [3‐6] | ns |
| Age at diagnosis (y) | 18 [12‐33.6] | 13.2 [9.6‐27.6] | 18 [12‐72] | 21.6 [13.2‐33.6] | ns |
TABLE 2.
Impact of age on the different domains
| Tot | <6 y | From 6 to 12 y | >12 y | Spearman's ρ | P | |
|---|---|---|---|---|---|---|
| Countries (France/Italy/Germany) | 73/37/43 | 22/3/9 | 19/14/14 | 32/20/20 | ‐ | ‐ |
| Current age (year) | 11.4 [7‐20.4] | 4 [3.3‐4.5] | 8.8 [8‐10.7] | 21 [16.3‐26.5] | ‐ | ‐ |
| Age at seizure onset (month) | 5 [3.5‐6] | 5.5 [4‐6.8] | 5 [3‐6.7] | 4 [3.5‐6] | ns | ns |
| Age at diagnosis (month) | 18 [12‐33.6] | 13.2 [9.6‐21.6] | 18 [12‐27.6] | 20.4 [12‐60.5] | 0.26 | .002 |
| Behavior | 4.52 ± 0.7 | 4.55 ± 0.64 | 4.43 ± 0.85 | 4.58 ± 0.56 | ns | ns |
| Communication and interaction | 4.54 ± 0.67 | 4.55 ± 0.71 | 4.47 ± 0.72 | 4.59 ± 0.51 | ns | ns |
| Daily activity | 4.31 ± 0.7 | 4.24 ± 0.75 | 4.39 ± 0.61 | 4.28 ± 0.71 | ns | ns |
| Motor skills | 4.29 ± 0.65 | 4.24 ± 0.7 | 4.36 ± 0.64 | 4.26 ± 0.57 | ns | ns |
| Language | 4.29 ± 0.75 | 4.39 ± 0.81 | 4.32 ± 0.75 | 4.21 ± 0.61 | ns | ns |
| Sleep | 4.27 ± 0.83 | 4.52 ± 0.85 | 4.2 ± 0.96 | 4.21 ± 0.51 | ns | ns |
| Sz frequency | 4.25 ± 0.78 | 4.12 ± 0.87 | 4.36 ± 0.74 | 4.24 ± 0.6 | ns | ns |
| Sz duration | 4.15 ± 1.11 | 4.38 ± 1.16 | 4.22 ± 1.09 | 4 ± 0.99 | −0.25 | .005 |
| Sz (Rescue therapy) | 3.88 ± 1.21 | 4.25 ± 1.31 | 3.96 ± 1.23 | 3.67 ± 0.79 | ns | ns |
| Eating disorders | 3.7 ± 1 | 3.65 ± 1.17 | 3.68 ± 0.88 | 3.72 ± 0.73 | ns | ns |
| Sz (referral to ER or ICU) | 3.55 ± 1.55 | 4.09 ± 1.65 | 3.68 ± 1.49 | 3.22 ± 1.26 | −0.26 | .005 |
The statistical impact of age was identified using Spearman's rank test and illustrated using three groups of affected individuals, namely <6 years, between 6 and 12 years, and >12 years.
Families ranked as important almost all the items proposed (Table 2). They rated similarly communication, sleep, behavior, daily activity, and motor skills (4.05 ± 1.34) compared to lower scores for items related to seizures (3.96 ± 1.22, P = 0.02) (Figure 1A). For items regarding seizures, the highest score was achieved for seizure frequency (4.25 ± 0.78) followed by seizure duration (4.15 ± 1.11).
FIGURE 1.
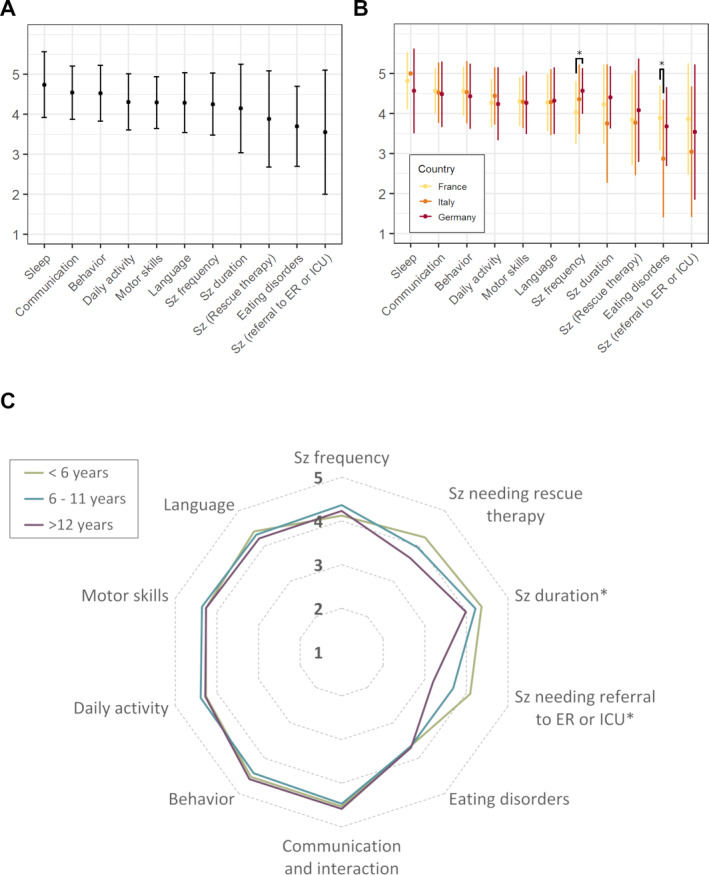
Caregivers’ opinions (Likert's scales from 1: not at all to 5: very important) about the domains that a therapy should improve for their children with Dravet syndrome (A), same results according to the three countries (B), and to the different age groups (C). Sz, seizure
Caregivers in the three countries agreed on the importance with a descending order for sleep, communication, behavior, daily activities, motor skills, language, seizure duration, seizures requiring rescue therapy, and seizures necessitating referral to ER or ICU. In relation to age, only seizure duration and the need of referral to ER or ICU were negatively correlated with individuals’ age, ie, the highest scores were reported in the youngest individuals (ρ = −0.25, P = 0.005 for seizure duration and ρ = −0.26, P = 0.005 for seizure with referral to ER or ICU) (Figure 1C). There were few significant differences in the evaluation of the different items according to countries (Figure 1B). These differences were mainly about seizure frequency, which had higher scores in Italy compared to France (4.57 ± 0.55 in Italy and 4.03 ± 0.77 in France, P = 0.0005, and Germany: 4.36 ± 0.72, P = ns) and those on eating disorders, which was higher in France compared to Germany (3.89 ± 0.8 in France and 2.88 ± 1.45 in Germany, P = 0.004, and Italy: 3.68 ± 0.95, P = ns).
4. DISCUSSION
Caregivers of individuals with DS across three EU countries expressed their needs for therapies that improve behavior, communication, sleep, daily activities, motor skills, and language beyond their efficacy on seizures. These are the first direct results on patients' needs from families across three countries in Europe supporting smaller studies hypothesis. 17 , 22
In order to improve the evidence of efficacy in clinical trials, Food and Drug Agency in 2009 and the European Medicines Agency in 2010 6 , 24 have encouraged the patient‐reported outcomes (PROs) as self‐assessment of affected individuals’ health status, and validated it as a possible secondary endpoint to complement the evaluation of clinical trials. The use of PROs in clinical trials, defined as “any report coming directly from patients, without interpretation by physicians or others, about how they function or feel in relation to a health condition and its therapy,” 25 has increased significantly since 2005. 26 , 27 , 28 The development of specific PROs for DS may seem anecdotic because there are generic PROs, such as the health‐related quality of life. However, generic PROs are not accurate enough to assess the quality of life of individuals with rare diseases and intellectual disability. 29 This is why, given the lack of standardized PROs dedicated to individuals with rare diseases, the International Rare Diseases Consortium has decided to set up the patient‐centered outcome measures (PCOMs) initiatives. 30 Determining the domains that are important to individuals with DS and their families is the first step of PRO development. 31 , 32
Our study showed that different needs can emerge in individuals with DS. In addition, the major needs can vary with age. 12 These results are correlated with the three phases of natural history of individuals with DS. 9 In the first two phases, seizures are at the forefront. During the first 15‐18 months, affected individuals present seizures triggered by fever often prolonged evolving to status epilepticus. Till around 5‐6 years, individuals show different types of seizures as atypical absences, focal and tonic seizures with frequency drug‐resistant epilepsy in addition to the emergence of developmental slowing and behavioral disorders. Finally, in the third phase, seizures often decrease in term of frequency 9 , 18 and might become nocturnal and brief. 33 Intellectual disability and behavior problems move to the front scene with the families struggling for the education and rehabilitation special needs. 19 In this survey, families rated a decreasing need with age for a therapy‐targeting seizures’ reduction and referral to ER or ICU. These data can be interesting in designing age‐related outcomes as ER and ICU needs are significantly more frequent in infants and preschool children with DS compared to adolescents and adults. 18 However, the need of treatment to reduce seizure frequency and of the need to rescue treatment remains stable with age highlighting the persistence of high drug resistance throughout life. 34 Refining the age‐related outcomes in DEEs might be the first step toward a precision design of CTs in such rare diseases.
Another key finding of this study is the age at diagnosis of affected individuals (18 [12‐33.6] months), showing a significant decrease in the age of diagnosis in the youngest individuals. These data confirm the improvement in the early diagnosis of DS over the last years. 18 , 35 , 36 Importantly, this earlier diagnosis age might question a younger age of inclusion in RCTs where the median age of inclusion in recent trials was between 7.6 and 9.3 years. 2 , 4 , 5 , 37 , 38 An earlier therapy can be a clue for a better neurodevelopmental outcome. 39
Some limitations must be highlighted. This is a cross‐sectional study to assess the impact of age on caregivers' expectations regarding what should treatment target. A longitudinal study will be probably more efficient to identify the evolution of caregivers’ perspectives. However, to date, there is no study with this design probably due to its complexity and the rarity of this pathology. The convenient sample of this study might have led to a selection bias. Indeed, the identification of affected individuals through national families’ associations might encourage the recruitment of families with specific profile and individuals with possibly more severe phenotypes. This survey is not accompanied by a qualitative study of the patients' opinions using, eg, Delphi methodology, 40 as we previously reported in DS. 17 , 22 However, the design of this study is complex and time consuming, requires the definition of experts, and does not allow us to have as large a population as in this study. 41
In conclusion, this study highlights the domains that a therapy in development for DS should target in addition to seizures. The next step would be to develop measurable and reproducible scales adding these items to seizures frequency as outcome measures for coming trials. For more accuracy and precision, an age‐related approach might refine these measures. This shift in our thinking in developing outcomes measures with more participatory approaches is urgent to establish in the era of gene therapy. Indeed, these therapies based on correction of the underlying genetic defect aim to rescue the genetic defect and to change the present path of affected individuals achieving disease‐modifying therapies beyond seizures decrease. 39
CONFLICT OF INTERESTS
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
ETHICAL APPROVAL
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
ACKNOWLEDGMENTS
The authors thank the three national associations of Dravet affected individuals and families, namely “Alliance Syndrome Dravet” in France, “Dravet‐Syndrom e.V.” in Germany, and “Dravet Italia Onlus” in Italy, as well as all affected individuals and their families from France, Italy, and Germany for their participation in this study. RN was supported by State funding from the Agence Nationale de la Recherche under “Investissements d'avenir” program (ANR‐10‐IAHU‐01), the “Fondation Bettencourt Schueller”. RN and MK and the European Joint program on rare diseases. This study is a peer‐reviewed modified version of the preprint server publication researchsquare. 42
1. SURVEY PROs DS families
Madam, Sir,
The development of therapies in Dravet syndrome (DS) is currently based on a primary outcome aiming to decrease the frequency of seizures. These therapies are mainly validated as anti‐seizures medications. However, patients with DS have other symptoms that go beyond seizures as speech delay, behavior disorders, gait disorders…. The development of more comprehensive therapies targeting the whole manifestations of the disease are expected and should aim a more holistic efficacy.
This survey aims to understand the domains that a therapy for DS should target identifying what really matters for your child at the time you are filling this survey.
The goal is to give you a possibility to express the needs of your child and to see how we can implement this in future trials of therapies for DS beyond its anti‐seizure efficacy.
2. Thank you to answer this information:
-
‐
Age: …… year(s) and …… months
-
‐
Gender: □ Female/□ Male
-
‐
Age of the first seizure: ………… months
-
‐
Age at which the diagnosis of Dravet syndrome was established: …… year(s) and …… months
What would be the domains where your child needs most an efficient therapy?
For the coming items, please select the degree of importance you give for each point:
About seizures:
-
‐
Reducing seizures frequency
| □ Highly important | □ Important | □ Neutral | □ Not important | □ Not important at all |
-
‐
Decreasing seizures duration
| □ Highly important | □ Important | □ Neutral | □ Not important | □ Not important at all |
-
‐
Decreasing seizures requiring rescue therapy (Any rescue therapy in your emergency protocol)
| □ Highly important | □ Important | □ Neutral | □ Not important | □ Not important at all |
-
‐
Decreasing referral to emergency room or intensive care unit
| □ Highly important | □ Important | □ Neutral | □ Not important | □ Not important at all |
-
‐
Improving communication and interaction (such as peer relationships, sociability, emotional reciprocity)
| □ Highly important | □ Important | □ Neutral | □ Not important | □ Not important at all |
-
‐
Improving language (such as conversational speech intelligibility, articulation, or voice)
| □ Highly important | □ Important | □ Neutral | □ Not important | □ Not important at all |
-
‐
Improving behavior disorders (such aggressivity, attention disorders)
| □ Highly important | □ Important | □ Neutral | □ Not important | □ Not important at all |
-
‐
Improving daily activity (such as having less fatigue, being able to participate in his planned daily activities)
| □ Highly important | □ Important | □ Neutral | □ Not important | □ Not important at all |
-
‐
Improving motor skills (such as fine motor skills, walking)
| □ Highly important | □ Important | □ Neutral | □ Not important | □ Not important at all |
-
‐
Improving eating disorders (such as binge eating or food restriction)
| □ Highly important | □ Important | □ Neutral | □ Not important | □ Not important at all |
-
‐
Improving sleep disorders (such as Sleeping difficulties, insomnia, waking up at night)
| □ Highly important | □ Important | □ Neutral | □ Not important | □ Not important at all |
Chemaly N, Kuchenbuch M, Teng T, et al. A European pilot study in Dravet Syndrome to delineate what really matters for the patients and families. Epilepsia Open. 2024;9:388–396. 10.1002/epi4.12557
Funding information
This research was supported by the Agence Nationale de la Recherche under “Investissements d'avenir” program (ANR‐10‐IAHU‐01) and the “Fondation Bettencourt Schueller”, Paris, France.
REFERENCES
- 1. Morel T, Cano SJ. Measuring what matters to rare disease patients ‐ Reflections on the work by the IRDiRC taskforce on patient‐centered outcome measures. Orphanet J Rare Dis. 2017;12:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Devinsky O, Cross JH, Laux L, Marsh E, Miller I, Nabbout R, et al Trial of cannabidiol for drug‐resistant seizures in the dravet syndrome. N Engl J Med. 2017;376:2011–20. [DOI] [PubMed] [Google Scholar]
- 3. Devinsky O, Patel AD, Cross JH, Villanueva V, Wirrell EC, Privitera M, et al Effect of cannabidiol on drop seizures in the lennox–gastaut syndrome. N Engl J Med. 2018;378:1888–97. [DOI] [PubMed] [Google Scholar]
- 4. Miller I, Scheffer IE, Gunning B, Sanchez‐Carpintero R, Gil‐Nagel A, Perry MS, et al Dose‐ranging effect of adjunctive oral cannabidiol vs placebo on convulsive seizure frequency in dravet syndrome: a randomized clinical trial. JAMA Neurol. 2020;77:613–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nabbout R, Mistry A, Zuberi S, Villeneuve N, Gil‐Nagel A, Sanchez‐Carpintero R, et al Fenfluramine for treatment‐resistant seizures in patients with dravet syndrome receiving stiripentol‐inclusive regimens: a randomized clinical trial. JAMA Neurol. 2020;77:300–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. European Medicine Agency‐Committee for medicinal products for human use . Guideline on clinical investigation of medicinal products in the treatment of epileptic disorders. [Internet]. 2010. [cited 2020]. Available from: https://www.ema.europa.eu/en/documents/scientific‐guideline/guideline‐clinical‐investigation‐medicinal‐products‐treatment‐epileptic‐disorders‐revision‐2_en.pdf
- 7. Ben‐Menachem E, Sander JW, Privitera M, Gilliam F. Measuring outcomes of treatment with antiepileptic drugs in clinical trials. Epilepsy Behav. 2010;18:24–30. [DOI] [PubMed] [Google Scholar]
- 8. Wolff M, Cassé‐Perrot C, Dravet C. Severe myoclonic epilepsy of infants (Dravet syndrome): Natural history and neuropsychological findings. Epilepsia. 2006;47:45–8. [DOI] [PubMed] [Google Scholar]
- 9. Dravet C. The core Dravet syndrome phenotype. Epilepsia. 2011;52:3–9. [DOI] [PubMed] [Google Scholar]
- 10. Claes L, Del‐Favero J, Ceulemans B, Lagae L, Van Broeckhoven C, De Jonghe P. De novo mutations in the sodium‐channel gene SCN1A cause severe myoclonic epilepsy of infancy. Am J Hum Genet. 2001;68:1327–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wu YW, Sullivan J, McDaniel SS, Meisler MH, Walsh EM, Li SX, et al Incidence of dravet syndrome in a US population. Pediatrics. 2015;136:e1310–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Scheffer IE, Nabbout R. SCN1A‐related phenotypes: Epilepsy and beyond. Epilepsia. 2019;60:S17–24. [DOI] [PubMed] [Google Scholar]
- 13. Ragona F, Granata T, Bernardina BD, Offredi F, Darra F, Battaglia D, et al Cognitive development in Dravet syndrome: A retrospective, multicenter study of 26 patients. Epilepsia. 2011;52:386–92. [DOI] [PubMed] [Google Scholar]
- 14. Knupp KG, Scarbro S, Wilkening G, Juarez‐Colunga E, Kempe A, Dempsey A. Parental perception of comorbidities in children with dravet syndrome. Pediatr Neurol. 2017;76:60–5. [DOI] [PubMed] [Google Scholar]
- 15. Villas N, Meskis MA, Goodliffe S. Dravet syndrome: Characteristics, comorbidities, and caregiver concerns. Epilepsy Behav. 2017;74:81–6. [DOI] [PubMed] [Google Scholar]
- 16. Rodda JM, Scheffer IE, McMahon JM, Berkovic SF, Graham HK. Progressive gait deterioration in adolescents with Dravet syndrome. Arch Neurol. 2012;69:873–8. [DOI] [PubMed] [Google Scholar]
- 17. Nabbout R, Auvin S, Chiron C, Irwin J, Mistry A, Bonner N, et al Development and content validation of a preliminary core set of patient‐ and caregiver‐relevant outcomes for inclusion in a potential composite endpoint for Dravet Syndrome. Epilepsy Behav. 2018;78:232–42. [DOI] [PubMed] [Google Scholar]
- 18. Lagae L, Brambilla I, Mingorance A, Gibson E, Battersby A. Quality of life and comorbidities associated with Dravet syndrome severity: a multinational cohort survey. Dev Med Child Neurol. 2018;60:63–72. [DOI] [PubMed] [Google Scholar]
- 19. Sinoo C, de Lange IML, Westers P, Gunning WB, Jongmans MJ, Brilstra EH. Behavior problems and health‐related quality of life in Dravet syndrome. Epilepsy Behav. 2019;90:217–27. [DOI] [PubMed] [Google Scholar]
- 20. Skluzacek JV, Watts KP, Parsy O, Wical B, Camfield P. Dravet syndrome and parent associations: The IDEA League experience with comorbid conditions, mortality, management, adaptation, and grief. Epilepsia. 2011;52:95–101. [DOI] [PubMed] [Google Scholar]
- 21. Strzelczyk A, Schubert‐Bast S, Bast T, Bettendorf U, Fiedler B, Hamer HM, et al A multicenter, matched case‐control analysis comparing burden‐of‐illness in Dravet syndrome to refractory epilepsy and seizure remission in patients and caregivers in Germany. Epilepsia. 2019;60:1697–710. [DOI] [PubMed] [Google Scholar]
- 22. Nabbout R, Auvin S, Chiron C, Thiele E, Cross H, Scheffer IE, et al Perception of impact of Dravet syndrome on children and caregivers in multiple countries: looking beyond seizures. Dev Med Child Neurol. 2019;61:1229–36. [DOI] [PubMed] [Google Scholar]
- 23. Brown MB, Forsythe AB. The anova and multiple comparisons for data with heterogeneous variances. Biometrics. 1974;30:719. [Google Scholar]
- 24. FDA, HHS . Guidance for industry use in medical product development to support labeling claims guidance for industry. Clin Fed Regist. 2009;FDA‐2006‐D‐0362:1–39. [Google Scholar]
- 25. Patrick DL, Burke LB, Powers JH, Scott JA, Rock EP, Dawisha S, et al Patient‐reported outcomes to support medical product labeling claims: FDA perspective. Value Health. 2007;10:S125–37. [DOI] [PubMed] [Google Scholar]
- 26. Vodicka E, Kim K, Devine EB, Gnanasakthy A, Scoggins JF, Patrick DL. Inclusion of patient‐reported outcome measures in registered clinical trials: Evidence from ClinicalTrials.gov (2007‐2013). Contemp Clin Trials. 2015;43:1–9. [DOI] [PubMed] [Google Scholar]
- 27. Mercieca‐Bebber R, Williams D, Tait MA, Roydhouse J, Busija L, Sundaram CS, et al Trials with patient‐reported outcomes registered on the Australian New Zealand Clinical Trials Registry (ANZCTR). Qual Life Res. 2018;27:2581–91. [DOI] [PubMed] [Google Scholar]
- 28. Mercieca‐Bebber R, King MT, Calvert MJ, Stockler MR, Friedlander M. The importance of patient‐reported outcomes in clinical trials and strategies for future optimization. Patient Relat Outcome Meas. 2018;9:353–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Arends M, Hollak CEM, Biegstraaten M. Quality of life in patients with Fabry disease: A systematic review of the literature. Orphanet J Rare Dis. 2015;10:77. 10.1186/s13023-015-0296-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. International rare diseases research Consortium Patient‐Centered Outcome Measures in the Field of Rare Diseases [Internet]. 2016. p. 1‐30. Available from: https://irdirc.org/wp‐content/uploads/2017/12/PCOM_Post‐Workshop_Report_Final.pdf
- 31. Frost MH, Reeve BB, Liepa AM, Stauffer JW, Hays RD, Sloan JA. What is sufficient evidence for the reliability and validity of patient‐reported outcome measures? Value Health. 2007;10:S94–105. [DOI] [PubMed] [Google Scholar]
- 32. Snyder CF, Watson ME, Jackson JD, Cella D, Halyard MY, Sloan JA. Patient‐reported outcome instrument selection: Designing a measurement strategy. Value Health. 2007;10:S76–85. [DOI] [PubMed] [Google Scholar]
- 33. Losito E, Kuchenbuch M, Chemaly N, Laschet J, Chiron C, Kaminska A, et al Age‐related “Sleep/nocturnal” tonic and tonic clonic seizure clusters are underdiagnosed in patients with Dravet Syndrome. Epilepsy Behav. 2017;74:33–40. [DOI] [PubMed] [Google Scholar]
- 34. De Liso P, Chemaly N, Laschet J, Barnerias C, Hully M, Leunen D, et al Patients with dravet syndrome in the era of stiripentol: A French cohort cross‐sectional study. Epilepsy Res. 2016;125:42–6. [DOI] [PubMed] [Google Scholar]
- 35. Strzelczyk A, Kalski M, Bast T, Wiemer‐Kruel A, Bettendorf U, Kay L, et al Burden‐of‐illness and cost‐driving factors in Dravet syndrome patients and carers: A prospective, multicenter study from Germany. Eur J Paediatr Neurol. 2019;23:392–403. [DOI] [PubMed] [Google Scholar]
- 36. de Lange IM, Gunning B, Sonsma ACM, van Gemert L, van Kempen M, Verbeek NE, et al Influence of contraindicated medication use on cognitive outcome in Dravet syndrome and age at first afebrile seizure as a clinical predictor in SCN1A‐related seizure phenotypes. Epilepsia. 2018;59:1154–65. [DOI] [PubMed] [Google Scholar]
- 37. Devinsky O, Patel AD, Thiele EA, Wong MH, Appleton R, Harden CL, et al Randomized, dose‐ranging safety trial of cannabidiol in Dravet syndrome. Neurology. 2018;90:e1204–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Devinsky O, Nabbout R, Miller I, Laux L, Zolnowska M, Wright S, et al Long‐term cannabidiol treatment in patients with Dravet syndrome: An open‐label extension trial. Epilepsia. 2019;60:294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nabbout R, Kuchenbuch M. Impact of predictive, preventive and precision medicine strategies in epilepsy. Nat Rev Neurol. 2020;16:674–88. [DOI] [PubMed] [Google Scholar]
- 40. Jones J, Hunter D. Qualitative Research: Consensus methods for medical and health services research. BMJ. 1995;311:376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Goodman CM. The Delphi technique: a critique. J Adv Nurs. 1987;12:729–34. [DOI] [PubMed] [Google Scholar]
- 42. Teng T, Lo Barco T, Marie E, Hallet A‐S, Brambilla I, Flege S, et al Families driven drug development and clinical trials: a pilot study in Dravet Syndrome to delineate what really matters. 2021. [cited 2021]. Available from: https://www.researchsquare.com/article/rs‐293272/v1 [DOI] [PMC free article] [PubMed]


