Abstract
Colon cancer cells frequently display minisatellite instability (MIN) or chromosome instability (CIN). While MIN is caused by mismatch repair defects, the lesions responsible for CIN are unknown. The observation that CIN cells fail to undergo mitotic arrest following spindle damage suggested that mutations in spindle checkpoint genes may account for CIN. However, here we show that CIN cells do undergo mitotic arrest in response to spindle damage. Although the maximum mitotic index achieved by CIN lines is diminished relative to MIN lines, CIN cells clearly have a robust spindle checkpoint. Consistently, mutations in spindle checkpoint genes are rare in human tumours. In contrast, the adenomatous polyposis coli (APC) gene is frequently mutated in CIN cells. Significantly, we show here that expression of an APC mutant in MIN cells reduces the mitotic index following spindle damage to a level observed in CIN cells, suggesting that APC dysfunction may contribute to CIN.
INTRODUCTION
Prior to cell division, the replicated genome is segregated such that the daughter cells receive all the genetic information required for further growth and development. The fate of the daughters is dependent on the accuracy of this process: missegregation dramatically alters gene dosage and can result in cell death or birth defects such as Down’s syndrome (Nicklas, 1997). Human tumour cells often have abnormal chromosome complements suggesting that aneuploidy may also cause cancer. However, whether aneuploidy is a contributing factor, or merely a consequence of tumorigenesis has remained controversial (Li et al., 2000).
It is now apparent that the majority of aneuploid colon cancer cells missegregate their chromosomes with high frequency, that is they exhibit chromosome instability (CIN) (Lengauer et al., 1997). In contrast, diploid colon cancer lines missegregate their chromosomes with much lower frequency, and thus while not CIN they frequently exhibit minisatellite instability (MIN). Although a minority of colon cancers display both CIN and MIN, or neither, these two phenotypes are generally mutually exclusive (Lengauer et al., 1997; Georgiades et al., 1999; Abdel-Rahman et al., 2001), suggesting that one or the other is required for the evolution of the majority of colon cancers (Lengauer et al., 1998; Cahill et al., 1999b).
While it is now widely accepted that MIN is due to mutations in mismatch repair genes (Kinzler and Vogelstein, 1996), the genetic lesions which give rise to CIN remain undefined. However, the very nature of the CIN phenotype suggests that the cause may be mutations in genes required to maintain the fidelity of chromosome segregation (Lengauer et al., 1997). Support for this notion came from the observation that, in contrast to the MIN lines, CIN lines fail to undergo mitotic arrest in response to spindle damage (Cahill et al., 1998). However, we show here that the CIN lines do undergo mitotic arrest in response to spindle damage. Interestingly, the maximum mitotic index achieved by the CIN lines is diminished relative to MIN lines suggesting that although the CIN cells have a robust checkpoint it may well be compromised. Significantly, we also show that expression of an APC mutant can compromise the spindle checkpoint response in MIN cells to a level observed in CIN cells, thus raising the possibility that APC dysfunction may contribute to CIN.
RESULTS
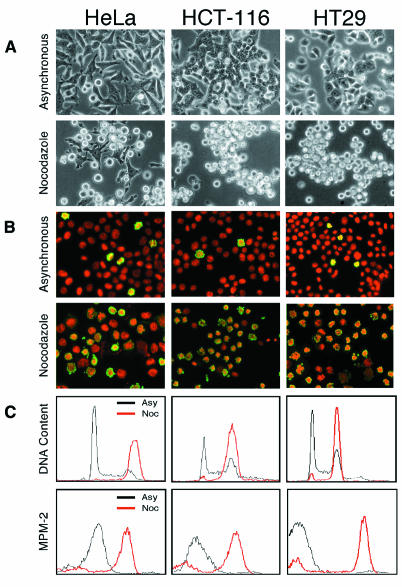
Following observations that aneuploid colon cancer cells exhibit CIN (Lengauer et al., 1997), we analysed the spindle checkpoint status of the MIN lines HCT-116 and DLD-1; the CIN lines HT-29, LoVo, SW480 and SW837; and HeLa cells. When grown in plastic dishes and viewed by phase-contrast microscopy, the vast majority of the cells in each line remained flat and adhered to the dish (Figure 1A). Following treatment with 0.2 µg/ml nocodazole for 18 h, the cells rounded up and detached from the dish (Figure 1A). Immunofluorescence analysis of asynchronous cultures showed that the vast majority of the cells had round nuclei with decondensed chromatin which did not express phospho-histone H3 (Figure 1B). Following nocodazole treatment however, most of the cells in all the lines had condensed chromosomes positive for phospho-histone H3 (Figure 1B). When analysed by flow cytometry, the untreated cells exhibited typical asynchronous DNA content histograms (Figure 1C) and few cells expressed mitotic specific MPM-2 epitopes. DNA content histograms of nocodazole-treated cells displayed large 4N peaks, consistent with a G2/M arrest. The majority of cells also expressed MPM-2 epitopes consistent with these cells being in mitosis. Taken together, these observations suggest that all the lines analysed undergo mitotic arrest following spindle damage.
Fig. 1. CIN cells undergo mitotic arrest following spindle damage. HeLa, HCT-116 (MIN) and HT-29 (CIN) cells were treated without or with 0.2 µg/ml nocodazole for 18 h. (A) Phase-contrast microscopy shows the majority of the cells in all lines round up following nocodazole treatment. (B) Immunofluorescence microscopy shows that in nocodazole, the majority of cells have condensed chromosomes (red) which are positive for phospho-histone H3 (green). (C) Flow cytometry shows that in the absence of nocodazole all three lines exhibit typical DNA content profiles (upper panels) with few MPM-2 positive cells (lower panels). In the presence of nocodazole all the lines exhibit large G2/M peaks and the majority of the cells are MPM-2 positive indicating that they are mitotic.
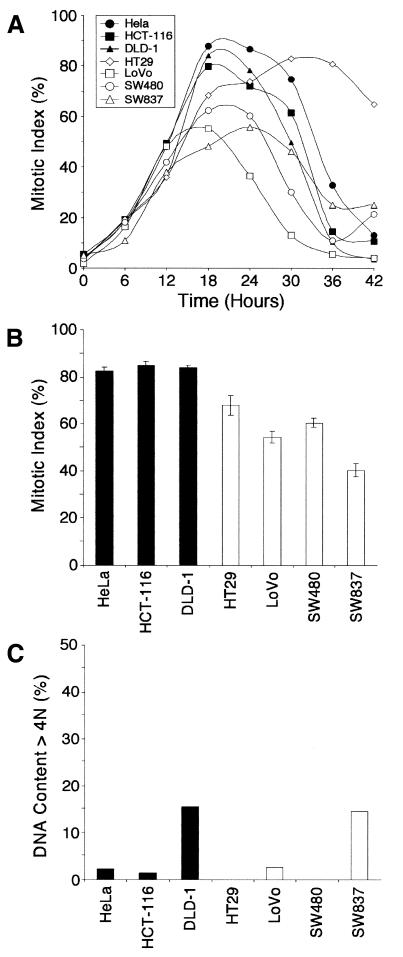
To characterize the spindle checkpoint response in more detail, we treated the lines with nocodazole and determined the mitotic index every 6 h (Figure 2A). The mitotic index of each line increased ∼10-fold during the first 12 h and in each case there is a clear peak between 18–24 h, which falls significantly by 36–42 h. Thus, in contrast to a previous report (Cahill et al., 1998), we conclude that both the MIN and CIN lines do undergo a transient mitotic arrest in response to spindle damage. Interestingly, there do appear to be differences in the maximum mitotic index achieved by the various lines. To characterize this further, the mitotic index of each line was determined in four separate experiments following an 18 h nocodazole block (Figure 2B). While the mitotic indices achieved by the HeLa and MIN cells was typically >80%, the CIN cells achieved mitotic indices of only 40 to 68%.
Fig. 2. CIN lines exhibit a robust spindle checkpoint. The lines indicated were plated out and treated with nocodazole to prevent spindle assembly. (A) Plot of mitotic index every 6 h. (B) Mitotic index at 18 h with each value representing the average and standard error from four independent experiments. (C) Percentage of cells with DNA contents >4N after 48 h.
Previously, we showed that cells with an abrogated spindle checkpoint can bypass apoptosis following prolonged mitotic arrest (Taylor and McKeon, 1997), suggesting that aneuploidy may not be due to elevated chromosome loss in the CIN cells but rather due to the ability of the CIN cells to survive an aberrant mitosis. However, only DLD-1 and SW837 cells showed any significant signs of DNA re-replication in the presence of nocodazole with ∼15% of the cells having DNA contents >4N after 48 h (Figure 2C). In contrast, when the spindle checkpoint is abrogated in either HeLa cells by expressing a dominant-negative Bub1 or in HCT-116 cells by mutating the MAD2 gene, >70% of the cells accumulate DNA contents >4N after 48 h in nocodazole (Taylor and McKeon, 1997; Michel et al., 2001).
Taken together our results show that the CIN cells do indeed have a robust spindle checkpoint. Consistent with this notion, mutations in known spindle checkpoint genes are extremely rare in human tumours (Cahill et al., 1998; Cahill et al., 1999a; Imai et al., 1999; Yamaguchi et al., 1999; Myrie et al., 2000; Sato et al., 2000). However, our data suggest that the spindle checkpoint may be partially compromised in CIN cells, raising the possibility that mutations in genes not directly involved in the spindle checkpoint but rather involved in microtubule dynamics and/or spindle positioning may be mutated in these cells. Interestingly, the APC gene is frequently mutated in CIN cells (Cahill et al., 1999b; Rowan et al., 2000). Furthermore, APC can stabilize microtubule ends (Zumbrunn et al., 2001) and also binds EB1 (Su et al., 1995), a protein involved in spindle positioning (Tirnauer and Bierer, 2000).
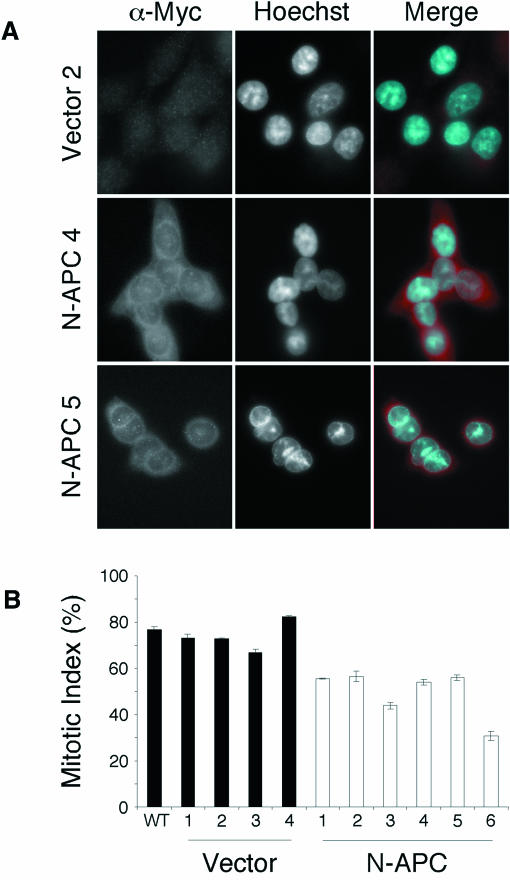
To test whether APC dysfunction may play a causal role in CIN, we stably expressed an N-terminal APC mutant in HCT-116 cells which are MIN and have a wild-type APC gene (Lengauer et al., 1997; Rowan et al., 2000). The mutant expressed encodes the first 750 amino acids of APC and is thus typical of APC mutants expressed in colon cancer cells (Fearnhead et al., 2001). Consistently, expression of N-APC was detectable in lines transfected with the N-APC vector but not a control vector (Figure 3A). When treated with nocodazole for 18 h, the mitotic index of the four control cell lines ranged from 67 to 83% (Figure 3B), similar to that of parental HCT-116 cells. In contrast, the mitotic index of the six cell lines expressing N-APC ranged from 31 to 57% (Figure 3B). Thus, expression of an N-terminal APC mutant in a MIN line can reduce the mitotic index following spindle damage to levels observed in CIN cells.
Fig. 3. Expression of N-APC compromises spindle checkpoint function. (A) HCT-116 cells stably transfected with control or N-APC vectors stained with anti-Myc antibodies and Hoechst dye. Only background staining is observed in the control line whereas extensive cytoplasmic staining is visible in the N-APC lines. (B) Stably transfected HCT-116 lines were treated with nocodazole to prevent spindle assembly. After 18 h the mitotic index was determined. Each value represents the average and standard error from three independent experiments. The mitotic index of lines expressing N-APC is reduced compared with the controls.
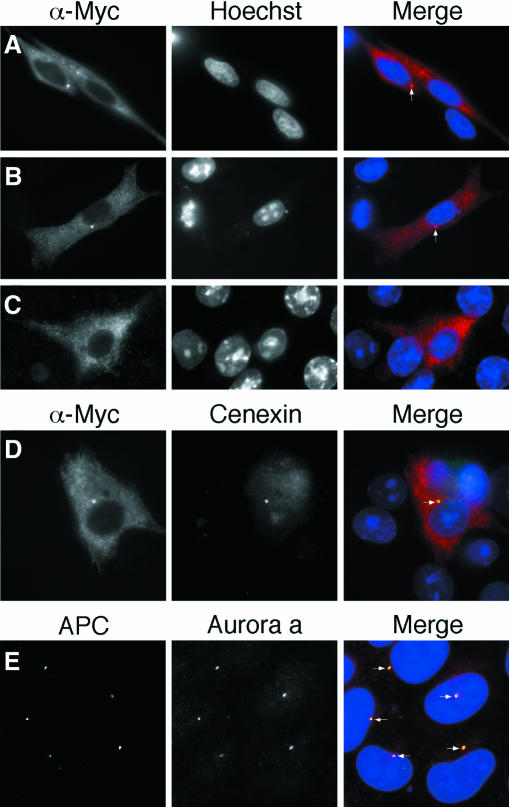
To gain insight into the mechanism by which APC dysfunction might compromise spindle checkpoint function, we overexpressed N-APC mutants in BHK and HeLa cells. Significantly, the N-terminal 750 amino acids of APC localized to the cytoplasm and to a structure which resembled the centrosome (Figure 4A and B). The N-terminal 1314 amino acids exhibited a similar pattern (data not shown). In contrast, full length APC localized only in the cytoplasm (Figure 4C). Co-staining with antibodies against cenexin (Lange and Gull, 1995) confirmed that N-APC does indeed localize to the centrosome (Figure 4D). Furthermore, the endogenous N-APC mutant expressed in DLD-1 cells, corresponding to amino acids 1 to 1416 (Homfray et al., 1998), localizes to the centrosome (Figure 4E).
Fig. 4. N-APC localizes to the centrosome. (A–D) Transfected cells stained with anti-Myc antibodies and Hoechst as indicated. (A) HeLa and (B) BHK cell showing localization of N-APC to the cytoplasm and centrosome. (C) BHK cell showing localization of full length APC to the cytoplasm. (D) BHK cell showing co-localization of N-APC and cenexin. (E) DLD-1 cells stained with antibodies against APC and aurora a (Bischoff et al., 1998) showing co-localization to centrosomes. White arrows identify the centrosomes.
DISCUSSION
We show here that CIN colon cancer cells undergo transient mitotic arrest in response to spindle damage. However, the maximum mitotic indices achieved by the CIN cells is lower than that of the MIN cells. Thus, we argue that the aneuploid colon cancer cells examined here have a robust spindle checkpoint. Consistently, it now appears that mutations in known spindle checkpoint genes are extremely rare in human tumours (Cahill et al., 1998, 1999a; Imai et al., 1999; Yamaguchi et al., 1999; Myrie et al., 2000; Sato et al., 2000). The reason for the apparent lack of such mutations may be that they are lethal. Although spindle checkpoint genes are non-essential in yeast (reviewed in Taylor, 1999) mutations in MAD2 and BUB3 result in embryonic lethality in mice (Dobles et al., 2000; Kalitsis et al., 2000). Furthermore, disrupting Bub1 and Mad2 function accelerates mitotic exit in human cells suggesting that spindle checkpoint function may be required to complete normal somatic cell divisions (Taylor and McKeon, 1997; Gorbsky et al., 1998; Michel et al., 2001).
Thus, it appears unlikely that mutations in spindle checkpoint genes underlie the aneuploidies associated with human tumours. In contrast, the APC gene is frequently mutated in aneuploid colon cancer cells. APC is integral to the Wnt signalling pathway where it downregulates β-catenin dependent transcription of genes which promote cellular proliferation (reviewed in Fearnhead et al., 2001; van Es et al., 2001) and significantly, mutations in either APC or β-catenin are frequently associated with colon cancer. Although not all colon cancer cells have mutations in APC or β-catenin (Salahshor et al., 1999), there is evidence that β-catenin mutations are often an alternative to APC mutations (Lamlum et al., 2000). Furthermore, whereas MIN cells generally have wild-type APC (Salahshor et al., 1999), CIN cells frequently have truncating mutations which result in expression of N-terminal fragments (Cahill et al., 1999b; Rowan et al., 2000). Taken together these observations provide interesting but circumstantial evidence that mutations in APC may play a causal role in CIN.
Significantly, we show here that expression of an N-terminal APC mutant in a MIN line reduces the maximum mitotic index achieved following spindle damage to a level typical of the CIN lines, providing direct evidence that APC dysfunction may interfere with accurate chromosome segregation. Furthermore, we show that N-APC mutants localize to the centrosome, a key component of the chromosome segregation machinery. These observations suggest that, perhaps as a consequence of an inability to interact with microtubules and/or EB1, N-terminal APC mutants accumulate at the centrosome thus compromising but not abrogating the mechanisms involved in ensuring accurate chromosome segregation. While some segregation errors are likely to be lethal, others may provide a growth advantage. In this model, the original truncating mutation in APC acts as a ‘double whammy’, destabilizing the genome and setting the stage for dysregulated cellular proliferation upon loss of the second APC allele. In contrast, mutations in spindle checkpoint genes may have such disastrous effects on mitosis that the daughter cells are not viable, explaining why even highly aneuploid colon cancer cells have a robust spindle checkpoint.
METHODS
Cell Culture. HCT-116, DLD-1, HT-29, SW480, SW837, LoVo and BHK cell lines were obtained from the ATCC. TA-HeLa cells were as described (Taylor and McKeon, 1997). All lines were cultured in DMEM plus 10% fetal calf serum, 2 mM glutamine, penicillin and streptomycin, all from Gibco, at 37°C in a humidified 5% CO2 incubator. Nocodazole (Sigma) was dissolved in DMSO at 5 mg/ml then used at a final concentration of 0.2 µg/ml. To determine checkpoint status, cells were seeded into plastic dishes and 24 h later nocodazole added. At the time points indicated, loosely attached mitotic cells were isolated and adherent interphase cells harvested by trypsinization. The mitotic and interphase cells were pooled, washed with PBS then processed to determine mitotic index (see below).
Immunofluorescence. For immunofluorescence, cells were centrifuged onto microscope slides then fixed in 3% formaldehyde in PBS (Figure 2B) or grown on coverslips then fixed in methanol (Figures 3 and 4B). Cells were washed in PBS plus 0.1% Triton X-100 (PBST), blocked in 5% non-fat dried milk then incubated at room temperature for 30 min with primary antibodies diluted in PBST. Primary antibodies used were: rabbit anti-phospho-histone H3 (1:200; Upstate Biotech); rabbit anti-cenexin (1:1000; a gift from Keith Gull); rabbit anti-aurora a (1:2000; a gift from Nick Keen); rabbit anti-APC (1:1000; a gift from Inke Nathke); 9E10 mouse anti-Myc tag (1:500); mouse anti-N-APC (1:20; Oncogene). Following washes with PBST, cells were then stained with Cy2 or Cy3 conjugated donkey anti-rabbit and/or anti-mouse secondary antibodies (Jackson Immunoresearch) diluted 1:500 in PBST for 30 min at room temperature. Following washes, cells were stained with Hoechst at 1 µg/ml in PBST then mounted in 90% glycerol plus 20 mM Tris–HCl pH 8.0. Fluorescence microscopy was performed using a Leica DMR equipped with epifluorescence and a Photometrics CCD camera driven by IP Lab software. Greyscale images were pseudocoloured using PhotoShop 5.0 (Adobe) before printing. To determine the mitotic index at each time point, at least 1000 cells were counted and the state of chromosome condensation used to score the cell as either mitotic or interphase.
Flow cytometry. For FACS analysis, cells were fixed in ethanol, rehydrated in PBS then incubated on ice for 30 min with an FSE conjugated MPM-2 antibody (Upstate Biotech) diluted 1:65 in PBS. Following washing with PBS, the cells were treated with 40 µg/ml propidium iodide and 10 µg/ml RNase for 30 min at room temperature then analysed on a FACSCAN (Becton Dickinson).
Expression of N-APC. N-terminal fragments of APC encoding amino acids 2–750 and 2–1314 were amplified with Pfu polymerase (Stratagene), cloned into pcDNA-3 Myc-tagged expression vectors (Taylor and McKeon, 1997) and sequenced. Plasmids were purified using ion exchange chromatography (Qiagen) then transfected into BHK and TA-HeLa cells using the ProTransfection calcium phosphate kit (Promega) or into HCT-116 cells by electroporation. To generate stable lines, HCT-116 cells were selected in 800 µg/ml geneticin, single colonies were picked and expanded.
Acknowledgments
ACKNOWLEDGEMENTS
We are grateful to Inke Nathke, Nick Keen and Keith Gull for reagents. This work was supported by the Association for International Cancer Research. S.S.T. is a BBSRC David Phillips Research Fellow.
REFERENCES
- Abdel-Rahman W.M. et al. (2001) Spectral karyotyping suggests additional subsets of colorectal cancers characterized by pattern of chromosome rearrangement. Proc. Natl Acad. Sci. USA, 98, 2538–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff J.R. et al. (1998) A homologue of Drosophila aurora kinase is oncogenic and amplified in human colorectal cancers. EMBO J., 17, 3052–3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill D.P., Lengauer, C., Yu, J., Riggins, G.J., Willson, J.K., Markowitz, S.D., Kinzler, K.W. and Vogelstein, B. (1998) Mutations of mitotic checkpoint genes in human cancers. Nature, 392, 300–303. [DOI] [PubMed] [Google Scholar]
- Cahill D.P., da Costa, L.T., Carson-Walter, E.B., Kinzler, K.W., Vogelstein, B. and Lengauer, C. (1999a) Characterization of MAD2B and other mitotic spindle checkpoint genes. Genomics, 58, 181–187. [DOI] [PubMed] [Google Scholar]
- Cahill D.P., Kinzler, K.W., Vogelstein, B. and Lengauer, C. (1999b) Genetic instability and Darwinian selection in tumours. Trends Cell Biol., 9, M57–M60. [PubMed] [Google Scholar]
- Dobles M., Liberal, V., Scott, M.L., Benezra, R. and Sorger, P.K. (2000) Chromosome missegregation and apoptosis in mice lacking the mitotic checkpoint protein Mad2. Cell, 101, 635–645. [DOI] [PubMed] [Google Scholar]
- Fearnhead N.S., Britton, M.P. and Bodmer, W.F. (2001) The abc of apc. Hum. Mol. Genet., 10, 721–733. [DOI] [PubMed] [Google Scholar]
- Georgiades I.B., Curtis, L.J., Morris, R.M., Bird, C.C. and Wyllie, A.H. (1999) Heterogeneity studies identify a subset of sporadic colorectal cancers without evidence for chromosomal or microsatellite instability. Oncogene, 18, 7933–7940. [DOI] [PubMed] [Google Scholar]
- Gorbsky G.J., Chen, R.H. and Murray, A.W. (1998) Microinjection of antibody to Mad2 protein into mammalian cells in mitosis induces premature anaphase. J. Cell Biol., 141, 1193–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homfray T.F., Cottrell, S.E., Ilyas, M., Rowan, A., Talbot, I.C., Bodmer, W.F. and Tomlinson, I.P. (1998) Defects in mismatch repair occur after APC mutations in the pathogenesis of sporadic colorectal tumours. Hum. Mutat., 11, 114–120. [DOI] [PubMed] [Google Scholar]
- Imai Y., Shiratori, Y., Kato, N., Inoue, T. and Omata, M. (1999) Mutational inactivation of mitotic checkpoint genes, hsMAD2 and hBUB1, is rare in sporadic digestive tract cancers. Jpn J. Cancer Res., 90, 837–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalitsis P., Earle, E., Fowler, K.J. and Choo, K.H. (2000) Bub3 gene disruption in mice reveals essential mitotic spindle checkpoint function during early embryogenesis. Genes Dev., 14, 2277–2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinzler K.W. and Vogelstein, B. (1996) Lessons from hereditary colorectal cancer. Cell, 87, 159–170. [DOI] [PubMed] [Google Scholar]
- Lamlum H., Papadopoulou, A., Ilyas, M., Rowan, A., Gillet, C., Hanby, A., Talbot, I., Bodmer, W. and Tomlinson, I. (2000) APC mutations are sufficient for the growth of early colorectal adenomas. Proc. Natl Acad. Sci. USA, 97, 2225–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange B.M. and Gull, K. (1995) A molecular marker for centriole maturation in the mammalian cell cycle. J. Cell Biol., 130, 919–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengauer C., Kinzler, K.W. and Vogelstein, B. (1997) Genetic instability in colorectal cancers. Nature, 386, 623–627. [DOI] [PubMed] [Google Scholar]
- Lengauer C., Kinzler, K.W. and Vogelstein, B. (1998) Genetic instabilities in human cancers. Nature, 396, 643–649. [DOI] [PubMed] [Google Scholar]
- Li R., Sonik, A., Stindl, R., Rasnick, D. and Duesberg, P. (2000) Aneuploidy vs. gene mutation hypothesis of cancer: recent study claims mutation but is found to support aneuploidy. Proc. Natl Acad. Sci. USA, 97, 3236–3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel L.S. et al. (2001) MAD2 haplo-insufficiency causes premature anaphase and chromosome instability in mammalian cells. Nature, 409, 355–359. [DOI] [PubMed] [Google Scholar]
- Myrie K.A., Percy, M.J., Azim, J.N., Neeley, C.K. and Petty, E.M. (2000) Mutation and expression analysis of human BUB1 and BUB1B in aneuploid breast cancer cell lines. Cancer Lett., 152, 193–199. [DOI] [PubMed] [Google Scholar]
- Nicklas R.B. (1997) How cells get the right chromosomes. Science, 275, 632–637. [DOI] [PubMed] [Google Scholar]
- Rowan A.J., Lamlum, H., Ilyas, M., Wheeler, J., Straub, J., Papadopoulou, A., Bicknell, D., Bodmer, W.F. and Tomlinson, I.P. (2000) APC mutations in sporadic colorectal tumors: A mutational ‘hotspot’ and interdependence of the ‘two hits’. Proc. Natl Acad. Sci. USA, 97, 3352–3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salahshor S., Kressner, U., Pahlman, L., Glimelius, B., Lindmark, G. and Lindblom, A. (1999) Colorectal cancer with and without microsatellite instability involves different genes. Genes Chromosomes Cancer, 26, 247–252. [PubMed] [Google Scholar]
- Sato M., Sekido, Y., Horio, Y., Takahashi, M., Saito, H., Minna, J.D., Shimokata, K. and Hasegawa, Y. (2000) Infrequent mutation of the hBUB1 and hBUBR1 genes in human lung cancer. Jpn J. Cancer Res., 91, 504–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su L.K., Burrell, M., Hill, D.E., Gyuris, J., Brent, R., Wiltshire, R., Trent, J., Vogelstein, B. and Kinzler, K.W. (1995) APC binds to the novel protein EB1. Cancer Res., 55, 2972–2977. [PubMed] [Google Scholar]
- Taylor S.S. (1999) Chromosome segregation: dual control ensures fidelity. Curr. Biol., 9, R562–R564. [DOI] [PubMed] [Google Scholar]
- Taylor S.S. and McKeon, F. (1997) Kinetochore localization of murine Bub1 is required for normal mitotic timing and checkpoint response to spindle damage. Cell, 89, 727–735. [DOI] [PubMed] [Google Scholar]
- Tirnauer J.S. and Bierer, B.E. (2000) EB1 proteins regulate microtubule dynamics, cell polarity and chromosome stability. J. Cell Biol., 149, 761–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Es J.H., Giles, R.H. and Clevers, H.C. (2001) The many faces of the tumor suppressor gene apc. Exp. Cell Res., 264, 126–134. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K., Okami, K., Hibi, K., Wehage, S.L., Jen, J. and Sidransky, D. (1999) Mutation analysis of hBUB1 in aneuploid HNSCC and lung cancer cell lines. Cancer Lett., 139, 183–187. [DOI] [PubMed] [Google Scholar]
- Zumbrunn J., Kinoshita, K., Hyman, A.A. and Nathke, I.S. (2001) Binding of the adenomatous polyposis coli protein to microtubules increases microtubule stability and is regulated by GSK3β phosphorylation. Curr. Biol., 11, 44–49. [DOI] [PubMed] [Google Scholar]