Abstract
rpc160-112, a mutant of the RNA polymerase III active site, is corrected in vivo by six second-site mutants obtained by random mutagenesis. These mutants introduce single-site amino acid replacements at the two large subunits of the enzyme. The mutated motifs are conserved in RNA polymerases I and II and, for some of them, in the bacterial enzyme, thus delineating key elements of the active site in eukaryotic RNA polymerases.
INTRODUCTION
In bacterial and yeast RNA polymerases (Pols) (Zhang et al., 1999; Cramer et al., 2000, 2001), the two large subunits form a broad DNA channel leading to an internal catalytic pocket. This pocket harbours the nucleoside triphosphate (NTP) binding site (Mustaev et al., 1991; Treich et al., 1992) and a Mg2+-chelating NADFDGD motif forming the catalytic site of polymerization (Dieci et al., 1995; Zaychikov et al., 1996). The similarity of these spatial structures leaves no doubt that the basic mechanism of transcription is universally conserved. Nevertheless, eukaryotic Pols differ from the bacterial ones by their more complex subunit structure, with a core enzyme of ten subunits (Cramer et al., 2000) also conserved in the archaeal subunit (Langer et al., 1995). Their catalytic function can be conveniently studied in yeast Pol III, where faithful transcription operates in purified preparations containing only Pol III itself and its two initiation factors TFIIIB and TFIIIC (Kumar et al., 1998). Previous work had shown that a temperature-sensitive (ts) Pol III mutant at the NADFGDG motif (rpc160-112) specifically impairs elongation (Dieci et al., 1995). We describe here second-site suppressors of rpc160-112, obtained by random mutagenesis and all located at conserved domains shared by the Pol II and Pol III enzymes.
RESULTS AND DISCUSSION
rpc160-112 is suppressed by mutations at conserved motifs on the two large subunits
rpc160-112 is a T506I,N509Y double mutant where the lethal mutation N509Y at the first position of the NADFGD motif is suppressed by the silent T506I replacement. On Pol II, the corresponding positions (S476 and N479) are only separated by 4.5 Å, indicating that suppression involves a local change in the NADFDGD motif. Dosage-dependent suppressors (FHL1 and RPS20) of rpc160-112 affect the synthesis of ribosomes and may overcome a general defect of Pol III mutants with regard to the accumulation of rRNA (Hermann-Le Denmat et al., 1994a,b). The suppressors analysed here were obtained by random UV mutagenesis of the rpc160-112 strain MW670 (Figure 1). Although the entire yeast genome was mutagenized, suppressors were only found at five positions (L750S, L792S, L889I, P923S and E1292K) of the largest subunit. The yield in mutants was very low (nine out of 5×107 cells), about one hundred times less than canavanine resistant mutants obtained in the same experiment and resulting from the inactivation of the CAN1 gene (Grenson et al., 1966). Yet, their absence in untreated controls implies that they result from different mutational events. Moreover, rpc160-P923S and rpc160-L750S were isolated two and four times, indicating a fairly saturating mutagenesis. UV mutagenesis is sequence-dependent and additional suppressors might thus be obtained by other treatments. Indeed, ret1-V955M was obtained by random mutagenesis of the second largest subunit in a mutator Escherichia coli.
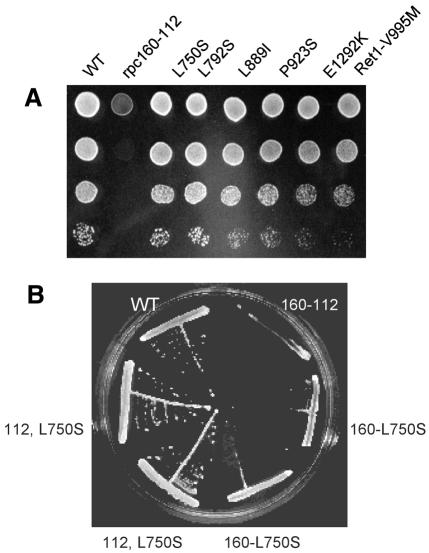
Fig. 1. Suppression of rpc160-112. (A) MW409 (wild type), MW670 (rpc160-112), SRY212 (rpc160-112,L750S) and other suppressors of rpc160-112 (as indicated) were serially diluted on YPD and grown for 4 days at 34°C. All strains are isogenic. (B) MW409, MW670, SRY12 and SRY213 (rpc160-L750S) were streaked on YPD and grown for 4 days at 34°C, demonstrating the mutual suppression effect of rpc160-112 and rpc160-L750S. The other suppressors have no growth defect in their own right.
Suppression restores a quasi wild-type growth pattern at 34°C (Figure 1). It also corrects a moderate 6-azauracil sensitivity of rpc160-112 at 25°C, consistent with the elongation defect of rpc160-112 as 6-azauracil reduces the cellular pool in NTPs (Exinger and Lacroute, 1992). rpc160-L750S is ts when separated from rpc160-112, but the other suppressors are indistinguishable from wild type. ret1-V955M also suppresses rpc160-N518Q, a ts mutant located three amino acids downstream of NADFDGD and probably very similar to rpc160-112 in its effect on elongation (Dieci et al., 1995). Except in this special case, rpc160-112 suppressors are allele-specific, with no effect on rpc31-236 (affecting initiation; Thuillier et al., 1995) or the rpc160-270 elongation mutant (Thuillier et al., 1996). Unlike rpc160-112, rpc160-270 enhances the RNase activity of Pol III with no effect on transcriptional slippage. Moreover, its synthetic lethality with rpc160-112 suggests cumulative effects on elongation (not shown).
rpc160-L750S, a termination mutant antagonizing the elongation defect of rpc160-112
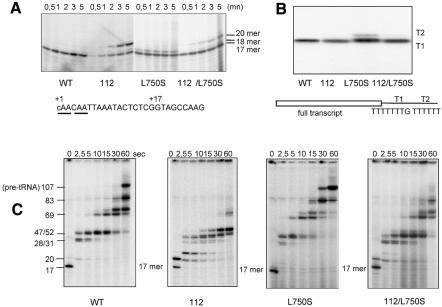
Since rpc160-L750S is the only suppressor to have a growth defect, we compared the enzymatic properties of Pol III in isogenic wild-type, rpc160-112, rpc160-L750S and rpc160-112,L750S strains. They have similar chromatographic behaviour, enzyme yield, subunit composition and activity on poly-d(A-T) templates. However, they differ in their faithful transcription properties, when tested for the synthesis of a nascent 17-mer transcript in the absence of GTP (the first G on the SUP4 template is at position 18), for the elongation of this nascent transcript following addition of GTP, and for complete transcription and termination in a multiple round assay (Figure 3). rpc160-112 initiates properly but is prone to transcriptional slippage, generating a 20-mer transcript by 5′ extension of the 17-mer, and has a severe elongation defect (Dieci et al., 1995). rpc160-L750S elongates somewhat faster than the wild-type control, with a partial readthrough of the termination signal (T1) at the 3′ end of SUP4. In the nascent transcription assay, this mutant also produces a small amount of an 18-mer product, which may correspond to an aberrant termination by-product (see Chédin et al., 1998) The rpc160-112,L750S double mutant transcribes essentially like wild type, indicating that the single mutants compensate each other in vitro, in their mutual suppression in vivo.
Fig. 3. Faithful RNA synthesis in vitro. (A) Synthesis of the nascent SUP4 17-mer. (B) Synthesis of the full transcript and termination in multiple round transcription. (C) Elongation from the nascent 17-mer complexes. (See Methods for the transcription assays; Dieci et al. 1995; Brun et al. 1997; Chédin et al., 1998).
Like all heteromultimeric Pols, Pol III has an RNA cleaving activity, critical for the processivity of elongation and depending on the Rpc11p subunit (Whitehall et al., 1994; Thuillier et al., 1996; Chédin et al., 1998). This activity was assayed in halted elongation complex (Figure 4). rpc160-112 forms the 15-mer and 18-mer expected from the cleavage of its 17-mer and 20-mer nascent transcripts. Thus, a mutation in the NADFDGD motif discriminates between polymerization and cleavage, although both are Mg2+ dependent. Likewise, rpc160-L750S is competent for cleavage, as shown by the formation of the 15-mer product. However, a faint 16-mer product not found in wild type may reflect a minor effect on the cleavage kinetics.
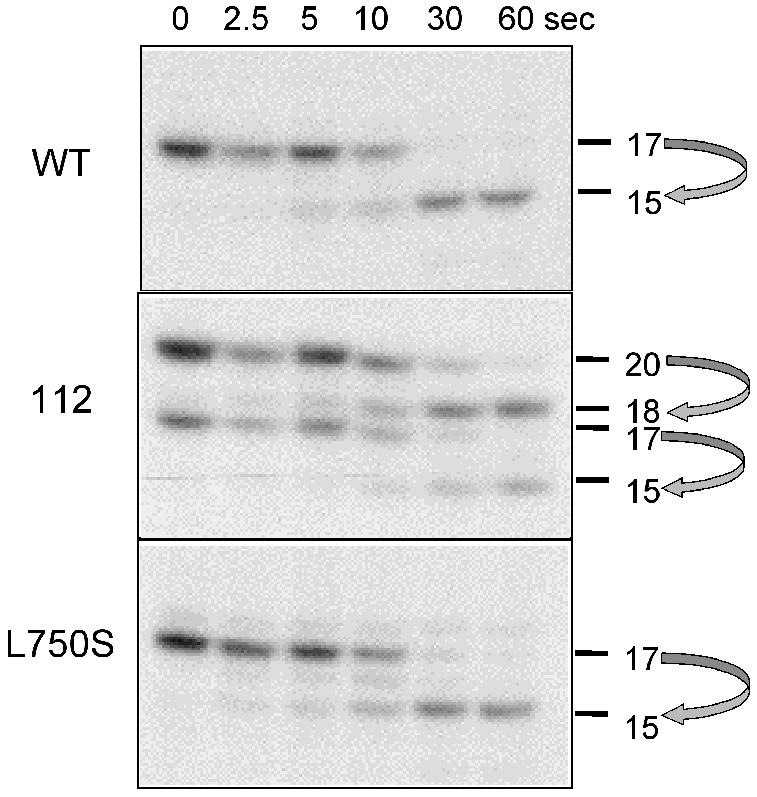
Fig. 4. RNA cleavage in vitro. Ternary elongation complexes were purified on Sepharose CL-2B and incubated in 8 mM MgCl2 transcription buffer without nucleotide (Thuillier et al., 1996). Arrows relate the primary transcripts (20- and 17-mer) to their cleavage products.
Sequence conservation and spatial distribution of rpc160-112 suppressors
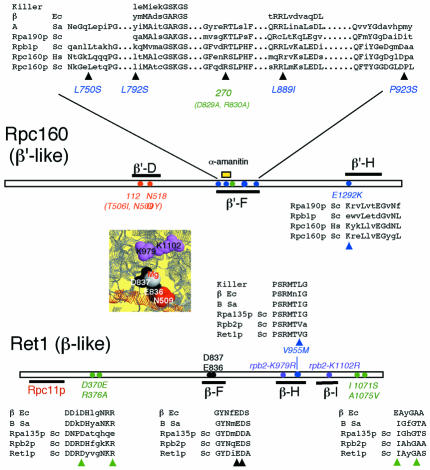
rpc160-112 suppressors correspond to positions invariant between Pol II and III (rpc160-L750S, rpc160-L889I, ret1-V955M) or belong to conserved motifs (rpc160-L792S, rpc160-P923S, rpc160-E1292K) shared by these enzymes (Figure 2). Moreover, rpc160-L889I and rpc160-P923S are in a 65 amino acids segment (863/927 in Rpc160p) interchangeable in vivo between Pol II and III (Thuillier et al., 1996). rpc160-L889I, rpc160-L792S, and ret1-V995M are in motifs present from bacteria to man.
Fig. 2. Sequence conservation of Pol III mutations. Bars represent highly conserved domains on Rpc160p and Ret1p, and the target domain of subunit Rpc11p on Ret1p, based on two-hybrid interactions (Flores et al.,1999). Red: elongation mutants rpc160-112(T506I, N509Y) and rpc160-N518Q. N509 and N518 correspond to N479 and N488 on Rpb1p. Blue: rpc160-112 suppressors. Positions L750, L792, L889, P923 and E1292 (Rpc160p) correspond to L701, K744, L841, A875 and V1305 (Rpb1p). V955 (Ret1p) is V1023 on Rpb2p. Green: RNase up mutants rpc160-270(D829A,R830A), ret1-D370E, ret1-R376A, ret1-I1071S and ret1-A1075V corresponding to D781-R782 on Rpb1p and D399, R405, I1139, A1144 on Rpb2p. Violet: lethal (rpb2-K979R) and viable (rpb2-K1102R) Pol II mutants at the NTP priming site (K911 and K1034 on Ret1p). Black: invariant carboxylic acids forming an hypothetical Mg2+ binding site on domain βF (E836, D837 in Rpb2p, E768, D769 in Ret1p). The yellow box denotes a domain corresponding to mouse α-amanitin resistant mutants (positions 726, 756, 764 and 772 in yeast Rpb1p; Bartolomei and Corden, 1995). Alignments are based on the Blosum 62 matrix and correspond to the E. coli (Ec), Sulfolobus acidocaldarius (Sa), Homo sapiens (Hs), Saccharomyces cerevisiae (Sc) Pols and to the putative monomeric enzyme encoded by the Kluyveromyces lactis killer factor. A spatial view of the catalytic Mg2+ and its immediate surrounding is taken from the bacterial data (Zhang et al., 1999).
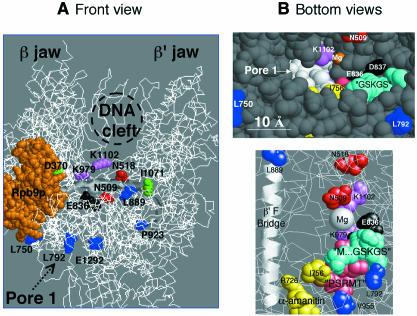
The spatial distribution of these mutants can be extrapolated from the Pol II structure, based on this sequence conservation. This is illustrated in Figure 5, which also shows yeast Pol II mutants at the NTP priming site (Treich et al., 1992) and mouse mutants resistant to α-amanitin (Bartolomei and Corden, 1995). Pol III initiation mutants have been described at subunits Rpc31p and Rpc34p (Thuillier et al., 1995; Brun et al., 1997). Their spatial position is unknown, but DNA cross-linking (Bartholomew et al., 1992) and two-hybrid (Flores et al., 1999) data suggest that they are close to the DNA channel. Mutants at the common subunits Rpc40p, Rpc19p, Rpb10p, Rpc10p, located at the enzyme rear and forming an ‘α-like’ domain equivalent to the bacterial α2 dimer (Lalo et al., 1993; Cramer et al., 2000) interfere with enzyme assembly or stability, but have no direct effect on its activity (Mann et al., 1987; Gadal et al., 1999; Rubbi et al., 1999).
Fig. 5. Spatial distribution of Pol III mutations. (A) Front view (Cramer et al., 2000) of the spatial distribution of Pol III mutants, with the approximate position of the DNA cleft and Pore 1. The rpb2-K979R and rpb2-K1102R Pol II mutants and two invariant carboxylic amino acids (E836 and D837 in Rpb2p) are also shown. Figure prepared with the RASMOL software (ftp://ftp.dcs.ed.ac.uk/pub/rasmol), using yeast Pol II co-ordinates (http://kornberg.stanford.edu and P. Cramer, D.A. Bushnell and R. Kornberg, personal communication). Rpb9p and individual amino acids are spacefilled. The β′F domain of Rpb1p (815/879) and Rpc160p (863/927) is shown as a helical ribbon. Colour key as in Figure 2. The correspondance of individual amino acids in Pol III and Pol II is the following: N509, N518, L750, L792, L889, P923 and E1292 (Rpc160p) = N479, N488, L701, K744, L841, A875 and V1305 (Rpb1p). D370, R376, E768, D769, V955, I1071 and A1075 (Ret1p) = D399, R405, E836, D837, I1139, A1144 and V1023 (Rpb2p). rpc160-112(T506I,N509Y) is only denoted by its critical position N509. rpc160-270(D829A,R830A) corresponds to positions D781-R782 on Rpb2p. (B) Detail of the catalytic pocket (bottom view, Cramer et al., 2000). Colour key and nomenclature as above. The PSRMT (1018/1022 in Rpb2p) and M…GSKGS (748 and 750/755 in Rpb1p) motifs are in pink and cyan, respectively. The spacefilled view above shows the outlet of Pore 1, with the outer enzyme surface (part of Rpb1p) in grey.
None of the rpc160-112 suppressors is immediately close to the catalytic Mg2+ (Figure 5A). Hence, they do not result from a short distance structural effect on rpc160-112(T5O6I,N509Y), but probably compensate the kinetic defect of that mutation by stimulating elongation, as shown above for rpc160-L750S. Only one of them (rpc160-E1292K) belongs to a eukaryote-specific motif. Its location in the DNA cleft suggests an interaction with the duplex DNA, downstream of the transcription bubble. rpc160-L750S corresponds to a motif conserved between archaeal and eukaryotic Pols, but not in the bacterial enzyme. The corresponding amino acid is located near Rpb9p (homologous to the Rpc11p Pol III subunit).
rpc160-L889I, ret1-V955M and rpc160-L792S are part of highly conserved motifs shared by all Pols from bacteria to man. On the spatial structure, they correspond to a sector comprising the ‘bridge’ and ‘wall’ elements of the catalytic pocket and a secondary channel referred to as Pore 1, connecting the catalytic side to the enzyme ‘floor’ (Cramer et al., 2000). rpc160-L889I maps to the β′F helix, bridging the DNA cleft ahead of Mg2+ and functionally interchangeable between yeast Pol II and III (Thuillier et al., 1996). Half of its amino acids are invariant from bacteria to man. The fact that a mere Leu–Ile replacement fully overcomes the rpc160-112(T5O6I,N509Y) defect underscores its importance in transcriptional elongation. rpc160-P923S also belongs to this interchangeable region, albeit at a less conserved position, and could thus be functionally related to the β′F helix.
ret1-V955M affects a PSRMT motif present in the second largest subunit of all Pols, including the momomeric enzyme encoded by yeast killer plasmid (Wilson and Meacock, 1988). Through its Arg, this motif is adjacent to D837, one of two invariant carboxylic acids of the second largest subunit that may form a second Mg2+ chelating site (see below). Its Met is close (between 5 and 11 Å) to R726 and I756, which can be mutated to α-amanitin resistance in mouse Pol II (Bartolomei and Corden, 1995). PSRMT is also spatially very close to the M…GSKGS motif also conserved in all Pols, including the monomeric ‘killer’ enzyme (Figure 2). The latter motif is very near rpc160-L792S in the sequence (Figure 2) and spatial structure of Pol II (Figure 5B). Thus, ret1-V955M and rpc160-L792S affect a highly conserved stucture connecting two invariant carboxylic amino acids to the amanatin pocket of the largest subunit (Bartolomei and Corden, 1995) and to rpc160-L792S. This structure belongs to a secondary channel (referred to as ‘Pore 1’, Cramer et al., 2000) linking the catalytic pocket to the outer surface of the enzyme. This channel could allow the entry of NTPs and/or lodge the bactracking RNA prior to its cleavage, thus playing a critical role in elongation (Cramer et al., 2000). rpc160-L750S corresponds to the same sector of the enzyme outer surface, but is located away from the secondary channel itself.
In summary, rpc160-L889I, ret1-V955M, rpc160-L792S (and, to some extent, rpc160-P923S) define key amino acid positions of the active site ‘pocket’, its β′F bridge and its secondary channel, as initially defined by bacterial studies (Zhang et al., 1999; Korzheva et al., 2000), which also contain the catalytic Mg2+ ion(s) (Dieci et al., 1995; Zaychikov et al., 1996), an NTP priming site (Mustaev et al., 1991; Treich et al., 1992) and the α-amanitin site as defined by resistant mutants (Bartolomei and Corden, 1995). No equivalent mutants exist in Pol I, Pol II or in the E. coli Pol, but their strong suppressor effect in vivo and the high conservation of the corresponding amino acid motifs leaves little doubt as to their general relevance to the catalytic process of transcription. rpc160-L750S and rpc160-E1292K, on the other hand, belong to less strongly conserved domains with no counterpart in the bacterial enzyme.
Spatial distribution of Pol III mutants interfering with the cleaving RNase activity
The Mg2+ dependent cleaving RNase of Pol III is not altered in rpc160-112 itself, although the latter alters the Mg2+ chelating motif shared by all Pols (Figure 4). However, this activity is fully lacking in a Pol III devoid of Rpc11p, homologous to the Pol II subunit Rpb9p (Chédin et al., 1998), and is strongly enhanced in rpc160-270 (Thuillier et al., 1996) and in mutants of the second largest subunit (ret1-D370E, ret1-R376A, ret1-I1071S and ret1-A1075V; Bobkova et al., 1999). rpc160-270, ret1-D370E and ret1-R376A belong to the β jaw, close to the C-end domain of Rpc11p (the position corresponding to rpc160-270 is hidden behind Rpb9p in Figure 5A) and to the rpc160-L750S suppressor, whilst ret1-I071S and ret1-A1075V are near the basis of the β′ jaw. The two jaws are probably mobile in Pol II (Cramer et al., 2000) and could therefore undergo concerted structural changes, tightening and relaxing their grip on DNA as they shift between a polymerizing and a cleaving conformation. Both activities modify the RNA 3′ end in an Mg-dependent way. They can be discriminated by mutations, since Rpc11p-less Pol III is competent for polymerization (Chédin et al., 1998), whilst rpc160-112(T506I,N509Y) impairs elongation with no effect on cleavage. Mg2+ may thus be chelated by the NADFGDFD motif to promote polymerization, and by another site nearby to determine cleavage. We propose that this second chelating site corresponds to the two invariant carboxylic amino acids (E768-D769 on Ret1p, D836-E837 on Rpb2p) discussed above.
METHODS
Yeast strains, growth conditions and genetic techniques were previously described (Dieci et al., 1995). Suppressors mutants were isolated on YPD after five days at 34°C, after UV mutagenesis of MW670 (bearing RPC160 on the pC160 plasmid) at 30 and 60% of survival (Rozenfeld and Thuriaux, 2001). Plasmids were extracted from the mutant clones, transferred back to yeast to reconstitute MW670. Growth was restored at 34°C in all cases, indicating that the UV-induced mutations had occurred on the plasmid itself. The corresponding mutations were determined by sequencing the RPC160 coding sequence of the mutant plasmids. ret1-V955M was generated by random mutagenesis of pYEP24-RET1 (Bobkova et al., 1999) in an E. coli mutator strain XL1-Red (Stratagene). The rpc160-L750S single-mutant derivative of pC160 (strain SRY13) was constructed by substituting a 2619 nt Spe I C-terminal fragment of RPC160 (containing the L750S mutation) to the corresponding wild type fragment of pC160.
Transcription was assayed on SUP4 templates, as previously described (Dieci et al., 1995; Brun et al., 1997; Chédin et al., 1998). Briefly, nascent transcripts were synthesized in the absence of GTP, using 40 µCi of [α-32P]UTP (3 µM). In addition to the main 17-mer signal, an 18-mer RNA was formed by misincorporation at position G18 and a 20-mer signal resulted from initiation slippage, due to the tandemly repeated CAA located between –1 and +5. The elongation of the nascent transcription complexes was followed by adding 600 µM GTP and 3 µM non-radioactive UTP, in the presence of heparin (0.3 mg/ml). Multiple round transcription (Dieci et al., 1995) was assayed with 10 µCi of UTP (30 µM).
Acknowledgments
ACKNOWLEDGEMENTS
We thank Isabelle Brun, Christophe Carles, Stéphane Chédin, Michel Riva, André Sentenac and Michel Werner for good advice; Roger Sayle for the Rasmol software; Emmanuel Favry for TFIIIB and TFIIIC fractions and Fabien Wattiez for help in isolating suppressors. P. Cramer, D.A. Bushnell and R. Kornberg generously sent their Pol II co-ordinates before publication. S.R. was supported by the Fondation pour la Recherche Médicale.
REFERENCES
- Bartholomew B., Durkovitch, D., Kassavetis, G.A. and Geiduschek, E.P. (1992) Orientation and topography of RNA polymerase III in transcription complexes. Mol. Cell. Biol., 13, 942–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolomei M.S. and Corden, J.L. (1995) Clustered α-amanitin resitance mutations in mouse. Mol. Gen. Genet., 246, 778–782. [DOI] [PubMed] [Google Scholar]
- Bobkova E.V., Habib, N., Alexander, G. and Hall, B.D. (1999) Mutational analysis of the hydrolytic activity of yeast RNA polymerase III. J. Biol. Chem., 274, 21342–21348. [DOI] [PubMed] [Google Scholar]
- Brun I., Sentenac, A. and Werner, M. (1997) Dual role of the C34 subunit of RNA polymerase III in transcription initiation. EMBO J., 16, 5730–5741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chédin S., Riva, M., Schultz, P., Sentenac, A. and Carles, C. (1998) The RNA cleavage activity of RNA polymerase III is mediated by an essential TFIIS-like subunit and is important for transcription termination. Genes Dev., 12, 3857–3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer P. et al. (2000) Architecture of RNA polymerase II and implications for the transcription mechanism. Science, 288, 640–649. [DOI] [PubMed] [Google Scholar]
- Cramer P., Bushnell, D.A. and Kornberg, R.D. (2001) Science, in press. [Google Scholar]
- Dieci G., Hermann-Le Denmat, S., Lukhtanov, E., Thuriaux, P., Werner, M. and Sentenac, A. (1995) A universally conserved region of the largest subunit participates in the active site of RNA polymerase III. EMBO J., 14, 3766–3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exinger F. and Lacroute, F. (1992) 6-azauracil inhibition of GTP biosynthesis in Saccharomyces cerevisiae. Curr. Genet., 22, 9–11. [DOI] [PubMed] [Google Scholar]
- Flores A. et al. (1999) A protein–protein interaction map of yeast RNA polymerase III. Proc. Natl Acad. Sci. USA, 96, 7815–7820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadal O., Shapkovski, G.V. and Thuriaux, P. (1999) Mutants in ABC10β, a conserved subunit shared by all three yeast RNA polymerases, specifically affect RNA polymerase I assembly. J. Biol. Chem., 274, 8421–8427. [DOI] [PubMed] [Google Scholar]
- Grenson M., Mousset, M., Wiame, J.M. and Béchet, J. (1966) Multiplicity of the amino acid permeases in Saccharomyces cerevisiae. Biochim. Biophys. Acta, 127, 325–338. [DOI] [PubMed] [Google Scholar]
- Hermann-Le Denmat S., Sipiczki, M. and Thuriaux, P. (1994a) Suppression of yeast RNA polymerase III mutations by the URP2 gene encoding a protein homologous to the mammalian ribosomal protein S20. J. Mol. Biol., 240, 1–7. [DOI] [PubMed] [Google Scholar]
- Hermann-Le Denmat S., Werner, M., Sentenac, A. and Thuriaux, P. (1994b) Suppression of yeast RNA polymerase III mutations by FHL1, a gene coding for a forkhead protein involved in rRNA processing. Mol. Cell. Biol., 14, 2905–2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korzheva N., Mustaev, A., Kozlov, M., Malhotra, A., Nikiforov, V., Goldfarb, A. and Darst, S.A. (2000) A structural model of transcription elongation. Science, 289, 619–625. [DOI] [PubMed] [Google Scholar]
- Kumar A., Grove, A., Kassavetis, G.A. and Geiduschek, E.P. (1998) Transcription factor IIIB: the architecture of its DNA complex, and its roles in initiation of transcription by RNA polymerase III. Cold Spring Harb. Symp. Quant. Biol., 63, 121–129. [DOI] [PubMed] [Google Scholar]
- Lalo D., Carles, C., Sentenac, A. and Thuriaux, P. (1993) Interactions between three common subunits of yeast RNA polymerases I and III. Proc. Natl Acad. Sci. USA, 90, 5524–5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer D., Hain, J., Thuriaux, P. and Zillig, W. (1995) Transcription in Archaea: similarity to that in Eucarya. Proc. Natl Acad. Sci. USA, 92, 5768–5772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann C., Buhler, J.M., Treich, I. and Sentenac, A. (1987) RPC40, a unique gene for a subunit shared between yeast RNA polymerase A and C. Cell, 48, 627–637. [DOI] [PubMed] [Google Scholar]
- Mustaev A.A., Kashlev, M., Lee, J.Y., Polyakov, A., Lebedev, A., Zalenskaya, K., Grachev, M., Goldfarb, A. and Nikiforov, V. (1991) Mapping of the priming substrate contacts in the active center of Escherichia coli RNA polymerase. J. Biol. Chem., 266, 23927–23931. [PubMed] [Google Scholar]
- Rozenfeld S. and Thuriaux, P. (2001) Genetic interactions within TFIIIC, the promoter-binding factor of yeast RNA polymerase III. Mol. Gen. Genet., in press. [DOI] [PubMed] [Google Scholar]
- Rubbi L., Labarre, S., Chédin, S. and Thuriaux, P. (1999) Functional characterization of ABC10α, an essential polypeptide shared by all three forms of eukaryotic DNA-dependent RNA polymerases. J. Biol. Chem., 274, 31485–31492. [DOI] [PubMed] [Google Scholar]
- Thuillier V., Stettler, S., Sentenac, A., Thuriaux, P. and Werner, M. (1995) A mutation in the C31 subunit of Saccharomyces cerevisiae RNA polymerase III affects transcription initiation. EMBO J., 14, 351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuillier V., Brun, I., Sentenac, A. and Werner, M. (1996) Mutations in the α-amanitin conserved domain of the largest subunit of yeast RNA polymerase III affect pausing, RNA cleavage and transcriptional transitions. EMBO J., 15, 618–629. [PMC free article] [PubMed] [Google Scholar]
- Treich I., Carles, C., Sentenac, A. and Riva, M. (1992) Determination of lysine residues affinity-labeled in the active site of yeast RNA polymerase II(B) by mutagenesis. Nucleic Acids Res., 20, 4721–4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehall S.K., Bardeleben, C. and Kassavetis, G.A. (1994) Hydrolytic cleavage of nascent RNA in RNA polymerase III ternary complex. J. Biol. Chem., 269, 2299–2306. [PubMed] [Google Scholar]
- Wilson D.W. and Meacock, P.A. (1988) Extranuclear gene expression in yeast: evidence for a plasmid-encoded RNA polymerase of unique structure. Nucleic Acids Res., 16, 9097–9112. [PMC free article] [PubMed] [Google Scholar]
- Zaychikov E., Martin, E., Denissova, L., Kozlov, M., Heuman, H., Nikiforov, V., Goldfarb, A. and Mustaev, A. (1996) Mapping of catalytic residues in the RNA polymerase active center. Science, 273, 107–109. [DOI] [PubMed] [Google Scholar]
- Zhang G., Campbell, E.A., Minakhin, L., Richter, C., Severinov, K. and Darst, S.A. (1999) Crystal structure of Thermus aquaticus core RNA polymerase at 3.3 Å resolution. Cell, 98, 811–824. [DOI] [PubMed] [Google Scholar]