Summary
Background
RYR1-related myopathies (RYR1-RM) are caused by pathogenic variants in the RYR1 gene which encodes the type 1 ryanodine receptor (RyR1). RyR1 is the sarcoplasmic reticulum (SR) calcium release channel that mediates excitation-contraction coupling in skeletal muscle. RyR1 sub-conductance, SR calcium leak, reduced RyR1 expression, and oxidative stress often contribute to RYR1-RM pathogenesis. Loss of RyR1-calstabin1 association, SR calcium leak, and increased RyR1 open probability were observed in 17 RYR1-RM patient skeletal muscle biopsies and improved following ex vivo treatment with Rycal compounds. Thus, we initiated a first-in-patient trial of Rycal S48168 (ARM210) in ambulatory adults with genetically confirmed RYR1-RM.
Methods
Participants received 120 mg (n = 3) or 200 mg (n = 4) S48168 (ARM210) daily for 29 days. The primary endpoint was safety and tolerability. Exploratory endpoints included S48168 (ARM210) pharmacokinetics (PK), target engagement, motor function measure (MFM)-32, hand grip and pinch strength, timed functional tests, PROMIS fatigue scale, semi-quantitative physical exam strength measurements, and oxidative stress biomarkers. The trial was registered with clinicaltrials.gov (NCT04141670) and was conducted at the National Institutes of Health Clinical Center between October 28, 2019 and December 12, 2021.
Findings
S48168 (ARM210) was well-tolerated, did not cause any serious adverse events, and exhibited a dose-dependent PK profile. Three of four participants who received the 200 mg/day dose reported improvements in PROMIS-fatigue at 28 days post-dosing, and also demonstrated improved proximal muscle strength on physical examination.
Interpretation
S48168 (ARM210) demonstrated favorable safety, tolerability, and PK, in RYR1-RM affected individuals. Most participants who received 200 mg/day S48168 (ARM210) reported decreased fatigue, a key symptom of RYR1-RM. These results set the foundation for a randomized, double-blind, placebo-controlled proof of concept trial to determine efficacy of S48168 (ARM210) in RYR1-RM.
Funding
NINDS and NINR Intramural Research Programs, NIH Clinical Center Bench to Bedside Award (2017-551673), ARMGO Pharma Inc., and its development partner Les Laboratoires Servier.
Keywords: Central core disease, Congenital myopathies
Research in context.
Evidence before this study
PubMed was searched from database inception through February 21, 2023, for articles that included the keywords “RYR1” and “Rycal” or “ARM210”. The search identified several nonclinical proof-of-concept articles; however, no clinical trials were found on use of Rycal ARM210 in patients with RYR1-related myopathy.
Added value of this study
This is the first clinical trial to test a Rycal molecule in patients, including RYR1-related myopathy. The results indicate that Rycal S48168 (ARM210) has a favorable safety and tolerability profile at the doses tested over 29 days. There is evidence that S48168 ARM210 reaches the target tissue in RYR1-RM patients and may decrease fatigue at the highest dose level tested.
Implications of all the available evidence
Studies incorporating randomization and masking of treatment allocation are needed to further assess the long-term safety, tolerability, and potential efficacy of Rycal S48168 (ARM210) in patients with RYR1-RM.
Introduction
RYR1-related myopathies (RYR1-RM) are a heterogenous group of monogenic neuromuscular disorders caused by pathogenic variants in the RYR1 gene (OMIM#180901, 19q13.2). RYR1-RM have historically been categorized based on clinical and histological features and can be separated into two main phenotypic categories: 1) Phenotypes with dynamic and episodic manifestations without interceding myopathy between exacerbations, including susceptibility to malignant hyperthermia (MH), a potentially fatal hypermetabolic crisis in response to certain environmental and pharmacologic triggers, exertional rhabdomyolysis, and atypical periodic paralysis. 2) Phenotypes with frank myopathy of variable severity including severe fetal akinesia, late-onset axial myopathy, and congenital myopathies.1 The latter can be further subclassified into central core disease, multi-minicore disease, congenital fiber type disproportion, and centronuclear myopathy based on histological features. RYR1-RM are the most common congenital myopathies, affecting at least 1:90,000 children in the United States, though this is likely an underestimate of true prevalence.2 MH incidence is estimated at between 1:10,000 and 1:250,000 though Bayesian population modelling assessing the posterior probabilities of pathogenicity and variant frequencies in the Genome Aggregation Database (gnomAD) suggests that the RYR1-related malignant hyperthermia susceptibility (MHS) trait may be carried in as high as 1:300 to 1:1075 individuals.3
There is notable clinical overlap between the two main categories of RYR1-RM. Some affected individuals with RYR1-RM are at risk of developing MH.4 An overlapping syndrome with myopathy, MHS, and dysmorphic features (King-Denborough syndrome) has also been described.5 It is increasingly recognized that individuals with MHS or exertional rhabdomyolysis may develop a progressive myopathy in older age, further blurring this dichotomy.6 Thus, a molecular nosological system that relies on presence of pathogenic variant(s) in RYR1 with compatible clinical and histological features provides a more consistent categorization system for RYR1-RM.
Both autosomal dominant (monoallelic, including de novo pathogenic variants) and recessive (biallelic) patterns of inheritance are reported to cause RYR1-RM. Classic central core disease is dominantly inherited and typically reflects the relatively milder end of the clinical spectrum, albeit with a wide range of clinical severity and penetrance.7,8 On the other hand, recessive RYR1-RM is clinically more severe in most patients, particularly in those carrying a hypomorphic allele in compound heterozygosity with one or more missense variants, and can present with profound muscle weakness, respiratory insufficiency, necessitating ventilatory support, ophthalmoplegia, and at times, feeding difficulties, requiring the use of a feeding tube.9 Although RYR1-RM disease course was originally described as static or slowly progressive by comparison to muscular dystrophies, they can be quite disabling and further worsen with aging and result in accrual of notable disability over time. Clinical management of RYR1-RM is supportive and there are currently no approved treatments.
RYR1 encodes the type 1 ryanodine receptor (RyR1) that forms a 2.2 MDa homotetrameric ion channel localized to the skeletal muscle sarcoplasmic reticulum (SR) membrane and functions as a gatekeeper of intracellular calcium stores.10 Upon depolarization of the transverse tubule membrane, the RyR1 channel, in complex with other proteins, orchestrates a coordinated release of SR calcium to the myofiber cytosol, enabling excitation-contraction coupling and force generation. Pathogenic RYR1 variants can have distinct consequences on RyR1 channel function. These include channel hypersensitivity in response to subthreshold stimuli (for example, in MH), blocking excitation-contraction coupling and calcium release, subconductance SR calcium leak (leaky state irrespective of a triggering action potential), and decreased RyR1 protein stability or expression.7 Only a minority of pathogenic RYR1 variants are functionally characterized. In individuals with biallelic variants, more than one mechanism may contribute to disease pathogenesis, which further complicates correlating disease phenotypes with underlying molecular pathomechanisms. Intracellular calcium dysregulation has been associated with mitochondrial oxidative/nitrosative stress in RYR1-RM.11, 12, 13 In addition to pathogenic variants, post-translational modifications of RyR1, can result in its dissociation from one of its known channel stabilizers, FKBP12 (calstabin1), which is thought to further exacerbate myofiber dysfunction.11,14,15
RYR1 variants shown to result in subconductance SR calcium leak are the principal target of Rycal compounds. We previously reported that skeletal muscle samples from 17 RYR1-RM patients show increased SR calcium leak, have diminished RyR1-calstabin1 association, and increased RyR1 channel open probability. In these specific patient samples, the above parameters were improved by ex vivo treatment with the Rycal molecule S107.16 Rycal molecule S48168 (ARM210) was thus designed, manufactured, and developed for clinical trials. Recently, cryo-EM studies have shown that S48168 (ARM210) preferentially binds the RyR1 channel in the leaky state, changes its conformation, and therefore directly stabilizes the RyR1 closed state.17 S48168 (ARM210) underwent a series of investigational new drug (IND)-enabling toxicology studies. In the absence of appropriate animal models of RYR1-RM, pharmacodynamic studies were performed in the mdx murine model of Duchenne muscular dystrophy (these mice exhibit RyR1 and calstabin1 dissociation).14 Subsequently, S48168 (ARM210) was administered to otherwise healthy male volunteers in four phase one clinical trials. In 2018, ARMGO Pharma Inc. received FDA Orphan Drug Designation of S48168 (ARM210) for treatment of RYR1-RM. Here, we report on a phase one, first-in-patient, open-label, dose-escalation trial testing the safety, pharmacokinetics, pharmacodynamics, and preliminary efficacy of S48168 (ARM210) in adult men and women affected with RYR1-RM.
Methods
Study design and participants
The single-site, open-label, dose-escalation trial of S48168 (ARM210) was conducted at the National Institutes of Health Clinical Center between October 28, 2019, to December 12, 2021. The trial was registered with clinicaltrials.gov (NCT04141670) and sponsored by ARMGO Pharma Inc. All procedures were approved by the National Institutes of Health Institutional Review Board and participants provided written informed consent prior to enrollment. The trial was conducted in accordance with the Declaration of Helsinki and ICH-GCP guidelines. An independent data and safety monitoring board was appointed by the study team to oversee trial safety and adjudicate adaptive dose escalation. The trial comprised three visits to the National Institutes of Health Clinical Center over a 29-day S48168 (ARM210) dosing period [visit one (baseline; Day 0), visit two (mid-point; Day 14), and visit three (post-intervention; Days 28–29)]. This was followed by a two-week follow-up period for drug withdrawal safety monitoring. Following completion of prespecified interim analyses, all participants who completed treatment with the low dose were eligible to be re-enrolled in the high dose cohort, to maximize the prospect of potential benefit and provide an opportunity for paired analyses. In the single participant who opted for re-enrollment, duration of efficacy following drug withdrawal was explored by replacing the mid-point (Day 14) study visit with a study visit two weeks post-intervention (Day 42), which included the full battery of exploratory efficacy assessments.
Participants
Eligible participants had genetically confirmed RYR1-RM (documented pathogenic or likely pathogenic RYR1 variant(s)), were between 18 and 65 years of age, and able to walk at least ten meters with or without assistance. All enrolled individuals with likely pathogenic RYR1 variant(s) had a clinical phenotype characteristic of RYR1-RM (per medical record review and baseline physical and neurological examinations). For five of the six enrolled participants, including the two participants with biallelic RYR1 variants, prior muscle biopsies had been previously characterized in vitro/ex vivo to result in SR calcium leak.16 One monoallelic participant had a RYR1 variant (p.Arg975Trp) previously reported in association with malignant hyperthermia susceptibility and central core disease that had not been assessed for its impact on intracellular calcium flux at the time of enrollment.18,19 Exclusion criteria included a history of seizures and any of the following at screening: HbA1c > 7%, forced vital capacity <50% predicted, estimated creatinine clearance <30 mL/min, and significant elevations in baseline transaminases. An initial requirement for participants to have a prior muscle biopsy with demonstrated loss of RyR1-Calstabin1 association proved to be overly restrictive on recruitment and was subsequently removed following DSMB review of interim target engagement assay data, and IRB approval. Full eligibility criteria are available at clinicaltrials.gov (NCT04141670). As an open-label trial, no masking of treatment allocation was required.
Procedures
S48168 (ARM210) was self-administered orally over 29 days. Participants were provided with instructions on drug administration and a diary to aid compliance and symptom reporting. Adherence to intervention was verified by pill counts conducted at trial mid-point and at the end of the dosing period. The 20 mg enterically coated S48168 (ARM210) tablet formulation was produced according to good manufacturing practice by Les Laboratoires Servier Industrie, France. Cohorts one (n = 3) and two (n = 4) received 120 and 200 mg/day S48168 (ARM210), respectively. As informed by nonclinical studies and interim analyses of the 120 mg dose cohort, the highest dose level was selected based on ensuring that S48168 (ARM210) plasma Cmax would not exceed a FDA-mandated dose cap.
Safety assessments included physical and neurological examinations (conducted by the same neuromuscular neurologist), vital signs, electrocardiograms and echocardiograms, clinical laboratory tests, Columbia Suicide Severity Rating Scale (C-SSRS), and treatment-emergent adverse event (TEAE) monitoring. Prespecified adverse events of special interest (AESI) were transaminases at least three times the upper limit of normal, tremors or seizures, severe gastric/epigastric pain or severe vomiting, and plasma S48168 (ARM210) Cmax >35 μg/mL. Severity and seriousness of adverse events were defined per the National Cancer Institute Common Terminology Criteria for Adverse Events (version 5.0) and FDA 21CFR 312.32, respectively. Stopping criteria were as follows: SAE possibly or definitely related to S48168 (ARM210), non-serious adverse but severe event (grade 3 on CTCAE version 5.0) possibly or definitely related to S48168 (ARM210), and serious AESI.
Detailed methodology for exploratory endpoints is provided in the Supplementary Appendix. Blood was drawn for pharmacokinetic (PK) and exploratory biomarker analyses. Plasma concentrations of S48168 (ARM210) were determined by mass spectrometry (Nuvisan Pharma, Germany). All participants (n = 6) underwent ultrasound-guided tibialis anterior needle biopsies for assessment of S48168 (ARM210) target engagement; five participants underwent muscle biopsies at baseline and at the end of dosing period while one participant underwent a single muscle biopsy procedure at the end of the dosing period. The side of the baseline muscle biopsy was determined by block randomization and the opposite side was sampled at the end of the dosing period. The random allocation sequence was generated by a statistician independent to the study team. S48168 (ARM210) concentrations were also determined in post-intervention skeletal muscle tissue samples by mass spectrometry (200 mg/day dose group only, n = 3). Participants also completed physical therapy assessments (Motor Function Measure-32 [MFM-32], timed functional tests, Myotools handgrip and pinch dynamometry) and the PROMIS-fatigue quality of life scale.
Endpoints
The primary endpoint was S48168 (ARM210) safety and tolerability comprising all TEAEs ≥ grade two in severity, serious adverse events (SAE), and AESI. Exploratory S48168 (ARM210) PK endpoints included maximum plasma concentration at steady state (Cmax[ss]), Day 14 trough plasma concentration (Ctrough), terminal elimination half-life (t½), and area under the curve over the 29-day dosing period (AUC[0-tau]). Target engagement was assessed in skeletal muscle by RyR1-calstabin1 co-immunoprecipitation and Western blot. In two patients with remaining muscle samples, a proximity ligation assay was conducted to assess RyR1-calstabin1 association in situ. Additionally, skeletal muscle S48168 (ARM210) concentration was assessed as an exploratory endpoint in the high dose group from available needle biopsy specimens. Exploratory efficacy endpoints included change from baseline to Day 29 in total MFM-32 (% maximum score), dominant hand grip and pinch strength (kg), time to ascend and descend four stairs, supine to stand, and walk ten meters (seconds), muscle strength (MRC grade), and patient-reported PROMIS-fatigue (t-score). Exploratory biomarkers were assessed at each timepoint and included plasma isoprostane, pro- and anti-inflammatory cytokines, and protein carbonyl concentrations.
Statistical analyses
Primary endpoint
As a first-in-patient, open-label safety trial, sample size was not determined by an a priori statistical power calculation. The safety population comprised participants who received at least one dose of S48168 (ARM210). Descriptive safety data included frequencies of TEAE ≥ grade two in severity, all SAEs, and all AESIs tabulated by dose group and MedDRA system organ class and preferred term.
Exploratory endpoints
Summary statistics were generated for all nominal PK and target engagement data. PK data were presented as mean concentration–time profiles by dose group. Exploratory efficacy and biomarker endpoints were analyzed by change from baseline or absolute values without inferential statistical tests. Natural history and placebo arm data on efficacy endpoints were obtained from a prior study.20 Prior natural history data were obtained at a six-month interval; therefore, estimated trajectories were adjusted to reflect expected change over the one-month dosing period in this trial. Using prior natural history data (NCT02362425) on trial endpoints that were assessed in this trial (PROMIS-fatigue, MFM, hand grip and pinch strength and timed functional tests), preliminary distribution-based minimum detectable change (MDC) thresholds were determined using a combination of standard error of measurement (SEM) and 1/3 standard deviation at baseline, as previously described,21 with average MDC of these approaches used to define the threshold value. Reference equations to determine percent predicted values for handgrip and pinch strength were derived from aggregate data on individuals >17 years of age (compiled by Institute of Myology).22 SAS (Version 9.4) and GraphPad Prism (Version 9.0) were used to conduct statistical analyses and generate graphs, respectively.
Role of funding
The sponsor (ARMGO Pharma Inc.) collaborated with authors during study design, drafting of the study protocol and its amendments, data collection, data analysis, data interpretation, and reviewed the manuscript prior to submission. All authors had full access to all the data in the study and the corresponding author had final responsibility for the decision to submit for publication. The clinical trial team was funded through the Intramural Research Programs of the National Institute of Neurological Disorders and Stroke (NIH/NINDS) and the National Institute of Nursing Research (NIH/NINR). A NIH Clinical Center Bench to Bedside Award (2017-551673) also provided part of the funding for the study. The study was also funded in part by the Sponsor through its development partner Les Laboratoires Servier.
Results
Participant characteristics
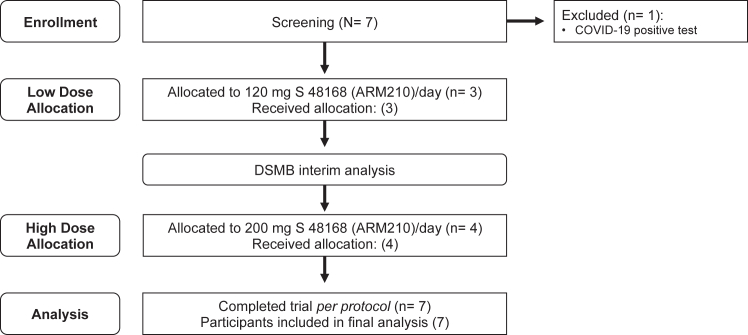
Overall, seven individuals were screened for participation. Six individuals who met the stringent eligibility criteria and could travel for all study visits to the NIH were enrolled and received either a low dose (120 mg, n = 3) or high dose (200 mg, n = 4) of S48168 (ARM210) daily for one month (Fig. 1). Re-enrollment was allowable for all low dose participants; however, only one individual opted to do so by the time of study closure. There was a 14-month washout period between first exposure and re-enrollment and the re-enrolled participant completed baseline assessments before initating treatment at each dose level. For five of the six enrolled participants, RYR1 variants had been previously characterized to result in SR calcium leak.16 The study population comprised men and women with monoallelic and biallelic RYR1 pathogenic or likely pathogenic variants (Table 1). Genotype and clinical characteristics of participants are provided in Table 2. Representative histopathology, T1-weighted MRI, and RYR1 variants mapped to the rabbit RyR1 3D cryogenic electron microscopy (cryo-EM) structure is provided in Supplementary Fig. S1.
Fig. 1.
Study flow diagram. A total of seven individuals were screened for eligibility. Six individuals were enrolled in the clinical trial and initially assigned to either a low dose (120 mg S48168 [ARM210] daily, n = 3) or high dose (200 mg S48168 [ARM210] daily, n = 4) group. One individual who received the low dose was re-enrolled and received the high dose after an appropriate washout period. The total dosing period for both groups was 29 days, and all individuals were included in the final analysis.
Table 1.
Participant demographics and baseline functional characteristics.
| Measure | Natural history cohort |
S48168 (ARM210) dose group |
|
|---|---|---|---|
| (N = 20) | 120 mg/day (N = 3) | 200 mg/day (N = 4) | |
| Age at enrollment, years | 39 ± 12 | 38 ± 7 | 43 ± 5 |
| Mode of inheritance, dominant | 5 (33) | 1 (33) | 3 (75) |
| Sex, male | 13 (65) | 2 (67) | 2 (50) |
| Height, cm | 163 ± 12 | 171 ± 20 | 173 ± 10 |
| Weight, kg | 68 ± 18 | 68 ± 12 | 90 ± 10 |
| Body mass index, kg/m2 | 26 ± 7 | 24 ± 5 | 31 ± 5 |
| Total MFM-32, % maximum score | 75 ± 11 | 75 ± 20 | 85 ± 17 |
| Hand grip strength, % predicted | 58 ± 29 | 64 ± 35 | 87 ± 25 |
| Hand pinch strength, % predicted | 78 ± 31 | 43 ± 21 | 67 ± 16 |
| 10-m run, seconds | 6 ± 2 | 9 ± 5 | 8 ± 5 |
Data are n (%) or mean ± SD.
Table 2.
Genotypes and clinical features of participants.
| Case | Dose level | Nucleotide changea | Amino acid change | Mode of inheritance | Neurological | Respiratory | Ophthalmologic | Malignant hyperthermia | Other findings |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 120 mg | c.6721C>T c.325C>T c.2122G>A c.1453A>G |
p.Arg2241∗ p.Arg109Trp p.Asp708Asn p.Met485Val |
Recessive | Muscle atrophy Proximal weakness (severe) Rigid spine |
↓ Forced vital capacity 60% predicted |
Ophthalmoplegia Ptosis |
No | High arched palate Retrognathia Jaw opening contracture Scoliosis |
| 2 | 120 mgb | c.5140_512del c.14126C>T c.4999C>T |
p.Leu1714del p.Thr4709Met p.Arg1667Cys |
Recessive | Muscle atrophy Proximal weakness (severe) Rigid spine |
↓ Forced vital capacity 63% predicted |
Ophthalmoplegia Ptosis |
No | High arched palate Joint contractures |
| 3 | 120 mg | c.2923C>T | p.Arg975Trp | Dominant | Proximal weakness (minimal) Myalgia |
Forced vital capacity 101% predicted |
No | ||
| 4 | 200 mg | c.7354C>T | p.Arg2452Trp | Dominant | Proximal weakness (mild) Myalgia Muscle cramps Tremor |
↓ Forced vital capacity 64% predicted |
Yes | Migraine headaches | |
| 5 | 200 mg | c.14731G>A | p.Glu4911Lys | Dominant | Proximal weakness (mild) Myalgia |
Forced vital capacity 90% predicted |
Yesc | ||
| 6 | 200 mg | c.14818G>A | p.Ala4940Thr | Dominant | Proximal weakness (mild) Myalgia |
Forced vital capacity 82% predicted |
No |
Pathogenic and likely pathogenic variants are highlighted in bold for participants with biallelic RYR1 variants.
RYR1, NM_00540.3.
Subject was re-enrolled and received high dose of 200 mg/day following washout period.
MH-like episode.
Safety and tolerability
S48168 (ARM210) exhibited a favorable safety profile at both dose levels as suggested by absence of SAEs, AESIs, and discontinuations (Table 3). There were also no changes from baseline in C-SSRS. S48168 (ARM210) was well-tolerated at the administered doses, reflected by a 100% compliance rate in both treatment arms. In total, 27 TEAEs were recorded of which three were moderate in severity and therefore met primary endpoint criteria (Supplementary Table S1). All moderate TEAEs were deemed unrelated to S48168 (ARM210) and comprised of vomiting (200 mg dose group), laceration (120 mg dose group), and elevated creatine phosphokinase (120 mg dose group). Intermittent grade one gastroesophageal reflux occurred in a participant with underlying gastroesophageal reflux disease (120 mg dose group). Due to non-clinical gastric toxicity data, this event was deemed possibly related to S48168 (ARM210); however, the event self-resolved and was not observed in any additional participants. An isolated grade one incidence of hyperglycemia occurred in one participant (120 mg dose group). As fasting glucose was previously within normal limits, this event was considered possibly related to S48168 (ARM210) but was resolved by the next follow-up visit and within the active dosing period. The safety results of this trial are consistent with four prior dose ranging studies conducted in otherwise healthy adult men, in which no dose-limiting toxicity was observed (repeated dosing up to 240 mg daily for 14 days) and do not deviate from the AE profile reported in RYR1-RM natural history (Supplementary Table S2).
Table 3.
Treatment-emergent adverse events.
| Safety event | S48168 (ARM210) dose group |
|||
|---|---|---|---|---|
| 120 mg/day (N = 3) |
200 mg/day (N = 4) |
|||
| Events |
Participants |
Events |
Participants |
|
| N | N | N | N | |
| Total treatment-emergent adverse events | 17 | 3 | 9 | 3 |
| Treatment-emergent adverse events ≥ grade two in severitya | 2 | 2 | 1 | 1 |
| Treatment-emergent adverse events at least possibly related to S48168 (ARM210) | 2 | 2 | 0 | 0 |
| Adverse events of special interest | 0 | 0 | 0 | 0 |
| Serious adverse events | 0 | 0 | 0 | 0 |
| Deaths | 0 | 0 | 0 | 0 |
Defined per NCI Common Terminology Criteria for Adverse Events (version 5.0).
Pharmacokinetics and target tissue engagement and distribution
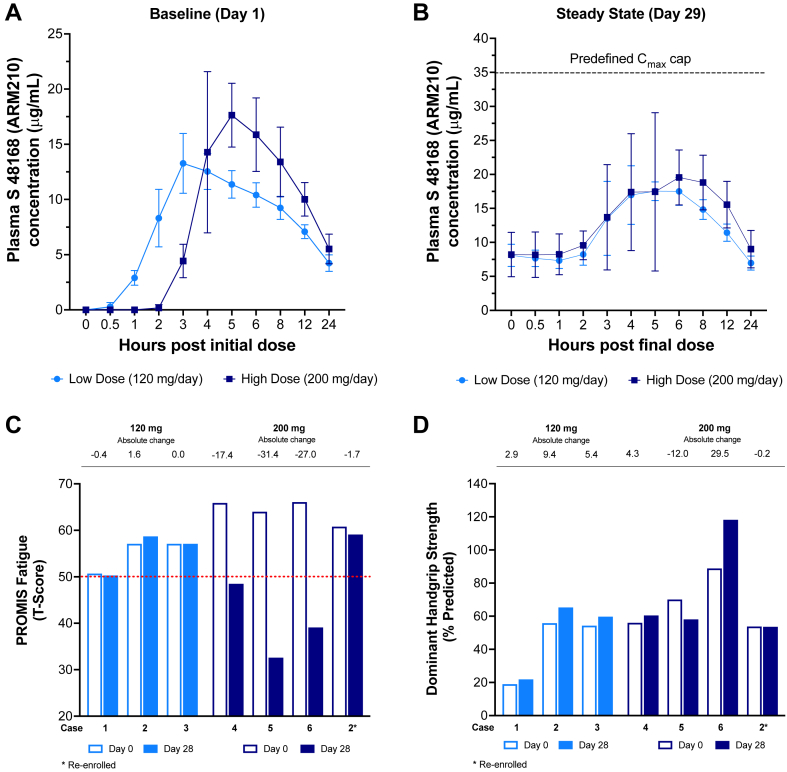
S48168 (ARM210) exhibited a dose-dependent PK profile (Table 4). In RYR1-RM affected individuals, S48168 (ARM210) maximum concentration and exposure at steady state (Day 29), Fig. 2 A-B, were consistent with expected PK based on projections from prior male adult healthy volunteer studies. No participant exceeded the pre-specified Cmax[ss] limit of 35 μg/mL. Assessment of S48168 (ARM210) target engagement by the pre-specified RyR1-calstabin1 co-immunoprecipitation with Western blot was not achievable due to substantial assay test-retest variability leading to inconsistent signal in control tissue and pre-dose muscle homogenates. Exploratory analyses using pre- and post-dose skeletal muscle tissue from two participants who received 200 mg/day S48168 (ARM210) demonstrated that when processed correctly, muscle needle biopsies can be used for assays of target engagement in situ (Supplementary Fig. S2). Nonetheless, additional experimental studies should be conducted with a larger sample size to evaluate the reliability of the proximity ligation assay for the purposes of assessing RyR1-calstabin1 association. S48168 (ARM210) was detected in all available skeletal muscle tissue samples derived from three participants in the high-dose group (909 ± 404 ng/mg tissue, N = 3), suggesting appropriate target tissue penetration.
Table 4.
Pharmacokinetic parameters (Mean).
| Pharmacokinetic measure | Timepoint and S48168 (ARM210) dose group |
|||
|---|---|---|---|---|
| Baseline (Day 0) |
Steady state (Day 29) |
|||
| 120 mg/day (N = 3) | 200 mg/day (N = 3) | 120 mg/day (N = 3) | 200 mg/day (N = 4) | |
| Cmax, μg/mL | 13.1 | 17.6 | 19.2 | 21.6 |
| Tmax, hours | 3.0 | 5.0 | 3.95 | 5.08 |
| t½, hours | 14.1 | 12.8 | ND | ND |
| AUC0-∞, hours × μg/mL | 256.6 | 309.0 | – | – |
| AUC0-tau, hours × μg/mL | – | – | 271.0 | 329.0 |
Data are geometric mean.
Cmax, maximum plasma concentration; Tmax, time to maximum plasma concentration; t ½, apparent plasma terminal half-life; AUC0-∞, area under the curve (total exposure) from time 0 extrapolated to infinite time; AUC0-tau, area under the curve (total exposure) from time 0 to the last measurable concentration.
Fig. 2.
(A) S48168 (ARM210) PK time-concentration curves at Day 1 and (B) Day 29. (C) Change over time in PROMIS fatigue and (D) percent predicted dominant handgrip strength. PK data are presented as Mean ± SD. For panels C and D, each bar represents absolute change from baseline in an individual participant. S48168 (ARM210) exhibited a dose-dependent PK profile at Day 1 and Day 29. Neither dose group exceeded the pre-defined Cmax limit of 35 μg/mL following treatment for 29 days. We found no notable difference in the S48168 (ARM210) PK profile between RYR1-RM affected individuals and otherwise healthy men in prior studies (data not shown). RYR1-RM natural history data were obtained from six-month lead-in phase of NCT02362425 and adjusted to reflect predicted change over a one-month period.20 Participants in the 200 mg dose group exhibited a decrease in PROMIS fatigue (t score) over the one-month dosing period. Mean percent predicted dominant handgrip strength was increased from baseline in both dose groups at Day 28. Error bars indicate SD.
Preliminary efficacy
Consistent with prior natural history studies of this disease, at baseline, six participants demonstrated minimal to moderate increased physical fatigue compared to the United States general population (PROMIS-fatigue t-score normal limits range: 20–55; RYR1-RM mean ± SD: 55 ± 4 and 64 ± 3, for low and high dose groups respectively).23 In the high dose group, PROMIS fatigue t-scores declined over the course of the trial (mean ± SD Day 14, −15 ± 8; Day 28 −19 ± 13), Fig. 2C. In three high dose participants the observed decline in fatigue from baseline was greater than what was observed in a prior natural history study (Supplementary Table S3). Moreover, this exceeded the RYR1-RM MDC threshold (t-score change ≥ 2.83) and estimated placebo effect (−2.93, N = 7) based on a previous clinical trial in this patient population,20 and was not observed in the low dose group (Fig. 2C). Average percent predicted dominant handgrip strength was increased from baseline in both dose groups at Day 28, (120 mg/day dose group: +5.9%, range +2.9 to +9.4%; 200 mg/day dose group: +5.4%, range −12.0 to +29.5%), Fig. 2D. On physical examination, it was noted that shoulder abduction Medical Research Council (MRC) grades improved at Day 28 in three of four participants who received the high dose (Supplementary Table S4). These were the same participants who showed improvement in PROMIS fatigue scores. Two participants had shoulder abduction MRC grade assessed at Day 42 (Supplementary Table S4), two weeks after dosing was completed. In one of these two participants who had shown improvements, Day 42 shoulder abduction MRC grade had returned to baseline levels. Results for all other exploratory efficacy endpoints were mixed (Supplementary Figs. S3 and S4 and Table S5).
Exploratory biomarkers
All participants demonstrated elevated plasma lipid peroxidation at baseline compared to established reference norms (15-F2t-isoprostane 266.2 ± 65.9, N = 7 versus 52.5 ± 9.3 pg/mL, N = 110)24 though to a lesser extent than collagen VI-related muscular dystrophy (COL6-RD) disease controls (415.6 ± 79.0 pg/mL, n = 6). S48168 (ARM210) treatment did not modify plasma 15-F2t-isoprostane concentrations at the doses tested (Day 28: 120 mg/day, −0.03 ± 0.11 pg/mL versus 200 mg/day 0.05 ± 0.20 pg/mL), Supplementary Fig. S5. At baseline and following treatment with S48168 (ARM210), plasma protein carbonyl concentrations were comparable between participants and age and sex-matched controls (N = 7, 2.47 ± 0.87 and N = 18, 2.40 ± 0.84 nmol/mg protein, respectively), Supplementary Fig. S5. At baseline and following treatment with S48168 (ARM210), participants exhibited a higher average plasma interleukin (IL)-6 concentration and IL-6/IL-10 ratio compared to age and sex-matched controls, albeit with notable variability (Supplementary Fig. S6).
Discussion
In this open-label dose escalation trial, we demonstrate that S48168 (ARM210) has a favorable safety profile and is well-tolerated in the RYR1-RM population at 120 and 200 mg per day for 29 days. This is the first-in-patient report on Rycals, a new class of small molecules with a novel mechanism of action. This trial is also the first to test S48168 (ARM210) in women. S48168 (ARM210) Cmax[ss] remained within pre-defined, PK safety margins at both dose levels. The adaptive design enabled dose-escalation which was informed by results of the low dose cohort and thus limited potential exposure of participants to a S48168 (ARM210) Cmax over the limit, an AESI based on prior nonclinical studies. Establishing target tissue penetration and target engagement are important considerations in drug development.25 S48168 (ARM210) was measurable in skeletal muscle, the disease-relevant target tissue. Nonetheless, target engagement could not be assessed in this trial due to technical reasons.
This trial further corroborates that fatigue is a prominent symptom that contributes to disease burden and negatively impacts quality of life in RYR1-RM.26 A large decrease (∼20 points in the t-score = two standard deviations from the reference population mean) in self-reported fatigue was observed in three of four participants in the high dose group, an unexpected but encouraging finding considering that RYR1-RM is a slowly progressive disease and despite the short treatment duration in this trial. Self-reported fatigue continued to decrease between Days 14 and Day 28. Notably, the observed decrease in self-reported fatigue was also much greater than the estimated placebo effect from natural history studies and placebo arm of a prior clinical trial in this patient population (adjusted to one month, Supplementary Table S3).20 Nonetheless, self-report bias and an exacerbated placebo effect for PROMIS-fatigue cannot be ruled out due to the open-label design. Overall, these findings highlight the feasibility and importance of incorporating validated patient reported outcome measures of fatigue in future RYR1-RM trials. Given the prominence of self-reported fatigue in RYR1-RM, it would also be of interest to incorporate measures of free-living physical activity into future natural history studies and clinical trials.
Increased handgrip strength was observed in five trial participants (low dose, n = 3; high dose n = 2). The small sample size and intra- and inter-subject variability precludes conclusive interpretation of handgrip strength responses to S48168 (ARM210) treatment. Furthermore, these measurements are focused on distal muscle strength, while RYR1-RM patients more often show proximal muscle weakness.8 Our exploratory efficacy studies did not include quantitative measurements of proximal muscle strength. However, using the shoulder abduction Medical Research Council (MRC) grading on physical examination, proximal muscle weakness trended higher at Day 28 in the high dose group in the three participants with monoallelic variants. Overall, the exploratory efficacy data on self-reported fatigue, handgrip strength and proximal muscle weakness warrant further investigation in an appropriately powered, randomized, masked, placebo-controlled proof of concept trial.
Our observation of elevated plasma lipid peroxidation in all participants at baseline aligns with prior findings in urine samples of RYR1-RM affected individuals.20 S48168 (ARM210) treatment did not decrease 15-F2t isoprostane concentrations over a one-month dosing period. This is not entirely unexpected as the primary mechanism of action of S48168 (ARM210) is stabilization of the RyR1 closed state and any impact on lipid peroxidation would likely be downstream through dampening of intracellular calcium dysregulation. Thus, it is possible that an extended treatment duration may be necessary to detect a treatment effect of S48168 (ARM210) on such biomarkers. Further exploration of 15-F2t isoprostane as a noninvasive biomarker in RYR1-RM is warranted given its consistent elevation above well-established normative values and long-term chemical stability.24 Plasma protein carbonyl content, a general biomarker of protein oxidation, was comparable to control; however, on average, participants did exhibit increased IL-6 concentrations and IL-6/IL-10 ratios. Similar results, namely elevation of IL-6, have been reported previously in RYR1-RM patient-derived primary myotubes and is associated with muscle inflammation and regeneration in the context of other neuromuscular disorders.27,28 Given the limited sample size of this trial and variability of these cytokines, it would be prudent to verify these observations by incorporating pro- and anti-inflammatory cytokine analyses in natural history studies of RYR1-RM.
For phase two design in this population, a multi-site, randomized, masked, placebo-controlled, crossover trial is an attractive approach as within-subjects analyses maximize statistical power. In this phase one trial, several participants with monoallelic RYR1 variants had a baseline ceiling effect on MFM-32. To address this challenge, it will be important to focus on endpoints that are more likely to capture significant improvement in RYR1-RM affected individuals, such as quantitative assessment of muscle strength in proximal muscle groups (e.g., shoulder abductors), and the PROMIS-fatigue scale with appropriate statistical considerations to reduce bias in sample size estimation.29
Diminished RyR1 protein expression has been reported in some RYR1-RM patients with biallelic RYR1 variants, and may occur alongside SR calcium leak depending on the precise genotype.16,30 Indeed, the biallelic RYR1 participants who were included in this trial had also shown SR calcium leak, with favorable effect of Rycals in prior in vitro and ex vivo studies of their archival muscle biopsies.16 However, both participants did not demonstrate decreased PROMIS-fatigue following treatment with 120 mg/day (n = 2) or 200 mg/day (n = 1, re-enrolled) for 29 days. The cause for the discrepancy between ex vivo studies and apparent lack of efficacy in these participants is not clear. First, only one participant with biallelic RYR1 variants was treated at the 200 mg dose level. Additionally, in individuals with biallelic variants resulting in decreased RyR1 expression, it is possible that higher doses, or a longer treatment period may be required to elicit a clinically meaningful treatment effect.
This study has several limitations. The small sample size, partially owing to the rarity of RYR1-RM and our stringent eligibility criteria, precludes definitive conclusions, especially from exploratory efficacy endpoints. Eligibility criteria were carefully tailored to mitigate unwarranted risk to individuals with severe disease burden without compromising the generalizability of findings relating to the primary safety and tolerability endpoint. Inclusion of monoallelic and biallelic individuals resulted in a heterogeneous cohort yet also enabled wider investigation of disease-specific safety considerations not possible from healthy volunteer studies. For example, since S48168 (ARM210) targets RyR function and had not been tested in RYR1-RM affected individuals before, potential to induce malignant hyperthermia was a theoretical safety consideration in susceptible individuals. On the other hand, inclusion of biallelic participants enabled safety and tolerability assessment of S48168 (ARM210) in this subpopulation, who typically have more severe disease. Nonetheless, more homogeneous participant selection based on genotype and/or baseline functional status may be warranted in future studies. The open-label design together with the fluctuant nature of physical fatigue affected the interpretation of improved PROMIS-fatigue and muscle strength (MRC) results. Thus, these favorable but preliminary findings would benefit from further characterization in a prospective longitudinal natural history study and more rigorous testing in a placebo-controlled, masked, and randomized study. Lastly, since S48168 (ARM210) was only tested over a 29 day period, the long-term safety and tolerability of S48168 (ARM210) warrants further investigation.
In conclusion, S48168 (ARM210) demonstrated favorable safety, tolerability, PK, and target tissue penetration in RYR1-RM affected individuals. A majority of participants who received 200 mg/day S48168 (ARM210) reported decreased fatigue and increased proximal muscle strength but, given the methodological limiations of this study, these findings warrant further investigation in a randomized, masked, placebo-controlled trial of S48168 (ARM210) for treatment of RYR1-RM.
Contributors
JJT: Conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, writing–original draft. TAL: investigation, writing–review and editing. ICC, AK,CG, MSJ, MRW, VB, SP, ME-B,: data curation, project administration, investigation, writing–review and editing, KB: project administration, writing–review and editing, CJM: project administration. ARF: project administration, investigation, writing–review and editing, PV, WR, GN, KC, CC-P: data curation, methodology, writing–review and editing, KGM: Conceptualization, funding acquisition, project administration, investigation, writing–review and editing, ARM, YW, EEM: Conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, writing–original draft. CGB: Conceptualization, methodology, project administration, writing–original draft, writing–review and editing. PM: Conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, writing–original draft. All authors had full access to all the data in the study and the corresponding author had final responsibility for the decision to submit for publication. PM, JJT, and EEM had directly accessed and verified the underlying data reported in the manuscript.
Data sharing statement
Key elements of the protocol and statistical analysis plan and the main study results will be made available at clinicaltrials.gov (NCT04141670). After regulatory approval in the United States or other regulatory region in the respective indication and publication of the primary article, ARMGO Pharma Inc. will share deidentified data that relate to the results reported in this article with qualified researchers who provide a valid research question. An executed data transfer agreement is a requirement and scope of data use is limited to noncommercial purposes. Proposals should be submitted to (info@armgo.com).
Declaration of interests
E.E.M is an employee of ARMGO Pharma, Inc., during the conduct of the study; an employee of ARMGO Pharma, Inc., outside the submitted work; In addition, E.E.M has a patent US2023/0372358 pending. Y.W is an employee of ARMGO Pharma, Inc. In addition, Y.W has a patent US8853198 issued. A.R.M. is a co-founder of ARMGO Pharma Inc, chairs the Scientific Advisory Board of ARMGO Pharma Inc., and holds stock in the company. All other authors declare no conflict of interest with the research in this manuscript.
Acknowledgements
Foremost, the authors would like to thank the study participants for their commitment to this trial. The authors also thank the RYR-1 Foundation for their continued advocacy on behalf of the RYR1-RM community. The authors acknowledge the following individuals: Karez Hawkins (NINR) for coordinating study visits; Alexander Ross (NINR) for support with PROMIS questionnaires, Drs. Tahseen Mozaffar (University of California, Irvine), Nicol Voermans (Radboud Medical Center), Jomy George, Edwin Lam, (Clinical Pharmacology, NIH Clinical Center) and Paul Wakim (Statistician, NIH Clinical Center), for serving as DSMB members; NINDS Clinical Trials Unit for regulatory and operational support; NIH Clinical Center 7SWN inpatient unit staff for care of study participants during their visits; NIH Clinical Center Department of Transfusion Medicine for obtaining control plasma samples. Eicosanoid (plasma 15-F2t isoprostane) analyses were performed in the Vanderbilt University Eicosanoid Core Laboratory. The clinical trial team was funded through the Intramural Research Programs of the National Institute of Neurological Disorders and Stroke, NIH/NINDS), the National Institute of Nursing Research, NIH/NINR), and a NIH Clinical Center Bench to Bedside Award (2017-551673). The study was also funded in part by the Sponsor through its development partner Les Laboratoires Servier.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2024.102433.
Appendix ASupplementary data
References
- 1.Lawal T.A., Todd J.J., Witherspoon J.W., et al. Ryanodine receptor 1-related disorders: an historical perspective and proposal for a unified nomenclature. Skeletal Muscle. 2020;10(1):32. doi: 10.1186/s13395-020-00243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amburgey K., McNamara N., Bennett L.R., McCormick M.E., Acsadi G., Dowling J.J. Prevalence of congenital myopathies in a representative pediatric United States population. Ann Neurol. 2011;70(4):662–665. doi: 10.1002/ana.22510. [DOI] [PubMed] [Google Scholar]
- 3.Johnston J.J., Dirksen R.T., Girard T., et al. Updated variant curation expert panel criteria and pathogenicity classifications for 251 variants for RYR1-related malignant hyperthermia susceptibility. Hum Mol Genet. 2022;31(23):4087–4093. doi: 10.1093/hmg/ddac145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riazi S., Kraeva N., Hopkins P.M. Malignant hyperthermia in the post-genomics era: new perspectives on an old concept. Anesthesiology. 2018;128(1):168–180. doi: 10.1097/ALN.0000000000001878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dowling J.J., Lillis S., Amburgey K., et al. King-Denborough syndrome with and without mutations in the skeletal muscle ryanodine receptor (RYR1) gene. Neuromuscul Disord. 2011;21(6):420–427. doi: 10.1016/j.nmd.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 6.van den Bersselaar L.R., Jungbluth H., Kruijt N., et al. Neuromuscular symptoms in patients with RYR1-related malignant hyperthermia and rhabdomyolysis. Brain Commun. 2022;4(6) doi: 10.1093/braincomms/fcac292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Treves S., Jungbluth H., Muntoni F., Zorzato F. Congenital muscle disorders with cores: the ryanodine receptor calcium channel paradigm. Curr Opin Pharmacol. 2008;8(3):319–326. doi: 10.1016/j.coph.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Shy G.M., Magee K.R. A new congenital non-progressive myopathy. Brain. 1956;79(4):610–621. doi: 10.1093/brain/79.4.610. [DOI] [PubMed] [Google Scholar]
- 9.Amburgey K., Bailey A., Hwang J.H., et al. Genotype-phenotype correlations in recessive RYR1-related myopathies. Orphanet J Rare Dis. 2013;8:117. doi: 10.1186/1750-1172-8-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lanner J.T., Georgiou D.K., Joshi A.D., Hamilton S.L. Ryanodine receptors: structure, expression, molecular details, and function in calcium release. Cold Spring Harb Perspect Biol. 2010;2(11) doi: 10.1101/cshperspect.a003996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andersson D.C., Betzenhauser M.J., Reiken S., et al. Ryanodine receptor oxidation causes intracellular calcium leak and muscle weakness in aging. Cell Metab. 2011;14(2):196–207. doi: 10.1016/j.cmet.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Durham W.J., Aracena-Parks P., Long C., et al. RyR1 S-nitrosylation underlies environmental heat stroke and sudden death in Y522S RyR1 knockin mice. Cell. 2008;133(1):53–65. doi: 10.1016/j.cell.2008.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Michelucci A., Liang C., Protasi F., Dirksen R.T. Altered Ca(2+) handling and oxidative stress underlie mitochondrial damage and skeletal muscle dysfunction in aging and disease. Metabolites. 2021;11(7):424. doi: 10.3390/metabo11070424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bellinger A.M., Reiken S., Carlson C., et al. Hypernitrosylated ryanodine receptor calcium release channels are leaky in dystrophic muscle. Nat Med. 2009;15(3):325–330. doi: 10.1038/nm.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Capogrosso R.F., Mantuano P., Uaesoontrachoon K., et al. Ryanodine channel complex stabilizer compound S48168/ARM210 as a disease modifier in dystrophin-deficient mdx mice: proof-of-concept study and independent validation of efficacy. FASEB J. 2018;32(2):1025–1043. doi: 10.1096/fj.201700182RRR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kushnir A., Todd J.J., Witherspoon J.W., et al. Intracellular calcium leak as a therapeutic target for RYR1-related myopathies. Acta Neuropathol. 2020;139(6):1089–1104. doi: 10.1007/s00401-020-02150-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Melville Z., Dridi H., Yuan Q., et al. A drug and ATP binding site in type 1 ryanodine receptor. Structure. 2022;30(7):1025–1034.e4. doi: 10.1016/j.str.2022.04.010. [DOI] [PubMed] [Google Scholar]
- 18.Todd J.J., Sagar V., Lawal T.A., et al. Correlation of phenotype with genotype and protein structure in RYR1-related disorders. J Neurol. 2018;265(11):2506–2524. doi: 10.1007/s00415-018-9033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brandom B.W., Bina S., Wong C.A., et al. Ryanodine receptor type 1 gene variants in the malignant hyperthermia-susceptible population of the United States. Anesth Analg. 2013;116(5):1078–1086. doi: 10.1213/ANE.0b013e31828a71ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Todd J.J., Lawal T.A., Witherspoon J.W., et al. Randomized controlled trial of N-acetylcysteine therapy for RYR1-related myopathies. Neurology. 2020;94(13):e1434–e1444. doi: 10.1212/WNL.0000000000008872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McDonald C.M., Henricson E.K., Abresch R.T., et al. The 6-minute walk test and other clinical endpoints in duchenne muscular dystrophy: reliability, concurrent validity, and minimal clinically important differences from a multicenter study. Muscle Nerve. 2013;48(3):357–368. doi: 10.1002/mus.23905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Annoussamy M., Lilien C., Gidaro T., et al. X-linked myotubular myopathy: a prospective international natural history study. Neurology. 2019;92(16):e1852–e1867. doi: 10.1212/WNL.0000000000007319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rothrock N.E., Hays R.D., Spritzer K., Yount S.E., Riley W., Cella D. Relative to the general US population, chronic diseases are associated with poorer health-related quality of life as measured by the Patient-Reported Outcomes Measurement Information System (PROMIS) J Clin Epidemiol. 2010;63(11):1195–1204. doi: 10.1016/j.jclinepi.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van 't Erve T.J., Kadiiska M.B., London S.J., Mason R.P. Classifying oxidative stress by F(2)-isoprostane levels across human diseases: a meta-analysis. Redox Biol. 2017;12:582–599. doi: 10.1016/j.redox.2017.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thornton C.A., Moxley R.T., III, Eichinger K., et al. Antisense oligonucleotide targeting DMPK in patients with myotonic dystrophy type 1: a multicentre, randomised, dose-escalation, placebo-controlled, phase 1/2a trial. Lancet Neurol. 2023;22(3):218–228. doi: 10.1016/S1474-4422(23)00001-7. [DOI] [PubMed] [Google Scholar]
- 26.van Ruitenbeek E., Custers J.A.E., Verhaak C., et al. Functional impairments, fatigue and quality of life in RYR1-related myopathies: a questionnaire study. Neuromuscul Disord. 2019;29(1):30–38. doi: 10.1016/j.nmd.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 27.Ducreux S., Zorzato F., Müller C., et al. Effect of ryanodine receptor mutations on interleukin-6 release and intracellular calcium homeostasis in human myotubes from malignant hyperthermia-susceptible individuals and patients affected by central core disease∗. J Biol Chem. 2004;279(42):43838–43846. doi: 10.1074/jbc.M403612200. [DOI] [PubMed] [Google Scholar]
- 28.Gros M., Nunes A.M., Daoudlarian D., et al. Identification of serum interleukin 6 levels as a disease severity biomarker in facioscapulohumeral muscular dystrophy. J Neuromuscul Dis. 2022;9(1):83–93. doi: 10.3233/JND-210711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rothwell J.C., Julious S.A., Cooper C.L. Adjusting for bias in the mean for primary and secondary outcomes when trials are in sequence. Pharm Stat. 2022;21(2):460–475. doi: 10.1002/pst.2180. [DOI] [PubMed] [Google Scholar]
- 30.Zhou H., Jungbluth H., Sewry C.A., et al. Molecular mechanisms and phenotypic variation in RYR1-related congenital myopathies. Brain. 2007;130(Pt 8):2024–2036. doi: 10.1093/brain/awm096. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.