Abstract
Fas, a member of the tumor necrosis factor receptor family, can upon ligation by its ligand or agonistic antibodies trigger signaling cascades leading to cell death in lymphocytes and other cell types. Such signaling cascades are initiated through the formation of a membrane death-inducing signaling complex (DISC) that includes Fas, the Fas-associated death domain protein (FADD) and caspase-8. We report here that a considerable fraction of Fas is constitutively partitioned into sphingolipid- and cholesterol-rich membrane rafts in mouse thymocytes as well as the L12.10-Fas T cells, and Fas ligation promotes a rapid and specific recruitment of FADD and caspase-8 to the rafts. Raft disruption by cholesterol depletion abolishes Fas-triggered recruitment of FADD and caspase-8 to the membrane, DISC formation and cell death. Taken together, our results provide the first demonstration for an essential role of membrane rafts in the initiation of Fas-mediated cell death signaling.
INTRODUCTION
Members of the tumor necrosis factor receptor (TNFR) superfamily are involved in a number of physiological and pathological responses. The Fas (CD95/APO-1) receptor belongs to the death receptor subgroup of this superfamily characterized by the presence of a death domain (DD) in its cytoplasmic portion (Ashkenazi and Dixit, 1998; Wallach et al., 1999; Krammer, 2000; Locksley et al., 2001). Upon engagement by FasL, Fas rapidly recruits to the membrane Fas-associated death domain protein (FADD) and caspase-8 proenzyme, which together form the death-inducing signaling complex (DISC) (Kischkel et al., 1995). This will result in activation of caspases leading to cell death. Indeed, following Fas oligomerization, the FADD adapter protein binds to the intracellular DD of Fas through a homologous domain in its COOH terminus (Boldin et al., 1995; Chinnaiyan et al., 1995). In addition to its DD, FADD bears at its NH2 terminus a death-effector domain (DED), another protein–protein interaction module. FADD can therefore recruit caspase-8 to the DISC by homotypic interactions between the DEDs of each protein (Muzio et al., 1996; Medema et al., 1997). The essential role of FADD and caspase-8 in Fas-mediated cell death has been revealed by the complete block of Fas signaling in thymocytes and embryonic fibroblasts of mice deficient in these proteins (Varfolomeev et al., 1998; Yeh et al., 1998; Zhang et al., 1998).
Rafts are tightly packed, ordered membrane microdomains rich in sphingolipids and cholesterol, as well as in different lipid-anchored proteins and transmembrane proteins (Simons and Ikonen, 1997; Brown and London, 1998). Recent studies have shown that rafts play an important role in cell signaling, in particular through the organization of surface receptors, signaling enzymes and adaptor molecules into membrane complexes at specific sites in the membrane (Simons and Toomre, 2000). Indeed, raft association has been shown to be essential for the initiation of signaling from a number of receptors, especially in immune cells (Langlet et al., 2000).
In this study, we aimed to investigate the role of membrane rafts in the initiation of the Fas-triggered cell death signaling.
RESULTS AND DISCUSSION
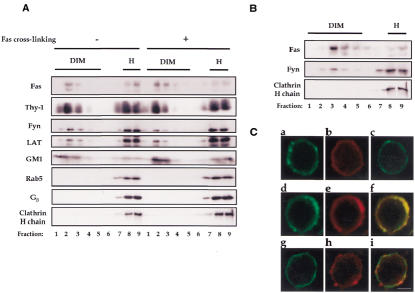
The association between Fas and rafts was investigated in mouse thymocytes, using a newly developed raft isolation procedure (P. Drevot, C. Langlet, X.J. Guo, A.M. Bernard, O. Colard, J.P. Chauvin, R. Lasserre and H.T. He, submitted). In this procedure, cell membranes are first treated with Brij 98 detergent at 37°C. Rafts, ordered membrane domains resistant to this treatment (Simons and Ikonen, 1997; Brown and London, 1998), can then be separated from the disordered membrane environments in a sucrose density gradient. The former structures float to the light fractions while the latter remain in the heavy fractions of the gradient due to solubilization. One of the advantages of this procedure over the previous ones (that most often utilize Triton X-100 detergent at low temperature) lies in the fact that it allows the analysis of membrane rafts at physiological temperature. To analyze the association of Fas with rafts, post-nuclear supernatants (PNS) from the thymocytes that were either non-stimulated or stimulated by Fas cross-linking mAb were solubilized by Brij 98 and lysates were subjected to ultracentrifugation onto a sucrose density gradient. Each fraction from the density gradient was analyzed on immunoblots for the presence of Fas as well as the raft and non-raft markers. The raft markers included (GPI-anchored) Thy-1, (myristoylated and palmitoylated) Fyn, (dual palmitoylated) LAT and GM1 glycosphingolipid. The non-raft markers included Rab5 and Gβ, which are modified by a branched bulky prenyl group precluding their partitioning to ordered domains (Melkonian et al., 1999), as well as clathrin heavy chain. Figure 1A shows a strong partitioning of Fas to rafts (which can exceed that of Fyn and LAT) for both non-stimulated and Fas-stimulated thymocytes (with a slight increase occasionally noted for the latter). In addition, we found a similar partition of Fas into rafts when they were isolated using Triton X-100 at 4°C (Figure 1B). Finally, a raft-partition of Fas was also found in L12.10-Fas murine T cells (see below). Thus, Fas is constitutively and strongly associated with membrane rafts.
Fig. 1. Constitutive partitioning of Fas in membrane rafts. (A) Brij 98-solubilized PNS from unstimulated (protein A) or Fas-stimulated (Jo2 + protein A) thymocytes (2 × 108) were fractionated on a sucrose gradient. Molecules in each fraction were resolved by SDS–PAGE and analyzed on western blot with the specific probes, as indicated. Fas, Rab5, Gβ and clathrin heavy chain were analyzed under reducing conditions while Thy-1, Fyn, LAT and GM1 under non-reducing conditions. (B) Cold Triton X-100-solubilized PNS from unstimulated thymocytes (2 × 108) were fractionated on a sucrose gradient. Molecules in each fraction were resolved by SDS–PAGE and analyzed on western blot with specific antibodies, as indicated. (C) Fas (a, d, f), GM1 (b, e, f, h, i) and CD45 (c, g, i) membrane expression in thymocytes, before (a–c) or after (d–i) raft patching induction. Cell labeling with FITC-conjugated anti-Fas and anti-CD45 mAbs and rhodamin-conjugated CTB as well as the raft patching induction were carried out as described in Methods. Confocal micrographs of a single plane show colocalization of Fas (d and f, green), but not CD45 (g and i, green), with GM1 (e and f; h and i, red) upon raft patching. Bar in i: 3 µm. The results shown are representative of three different experiments.
We also performed fluorescence microscopy analysis on intact mouse thymocytes (Figure 1C). The cell surface expression of Fas was uneven with the presence of small clusters (panel a), in contrast to the more homogenous distribution of GM1 (panel b). In addition, GM1 cross-linking by cholera toxin subunit B (CTB)/anti-CTB induced the formation of GM1-containing patches that are also highly enriched in Fas (panels d–f), but not in CD45 (panels g–i), a non-raft membrane protein (Janes et al., 1999). Indeed, cross-linking of raft components has been shown to promote coalescence of individual rafts (Harder et al., 1998), the sizes of which are below the resolution of optical microscopy (Simons and Toomre, 2000). These observations are therefore consistent with biochemical studies, and further demonstrate the association between Fas and membrane rafts.
Fas ligation leads to the recruitment of FADD and caspase-8 to the membrane, forming the DISC (Chinnaiyan et al., 1995; Kischkel et al., 1995). Therefore, we next investigated whether FADD and caspase-8 are recruited to rafts upon Fas cross-linking. To this end, membrane rafts from non-stimulated and Fas-stimulated thymocytes were concentrated and blotted with anti-FADD and caspase-8 antibodies. We found that FADD and caspase-8 are absent from the rafts in non-stimulated cells, but immediately recruited to these membrane compartments upon Fas cross-linking (Figure 2). As the membrane targeting of both FADD and caspase-8 is, at least in mouse thymocytes and embryonic fibroblasts, both necessary and sufficient to initiate Fas-induced cell death signaling (Varfolomeev et al., 1998; Yeh et al., 1998; Zhang et al., 1998), these results suggest that rafts represent the membrane site from which Fas initiates signaling cascade upon binding to its ligand.
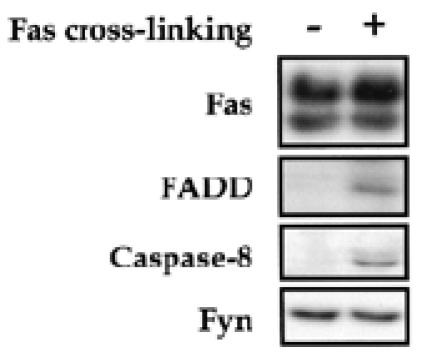
Fig. 2. Recruitment of early death signaling molecules in rafts upon Fas engagement. Thymocytes were incubated for 5 min at 37°C with 5 µg/ml protein A (–) or with 2.5 µg/ml Jo2 mAb plus 5 µg/ml protein A (+), lysed in Brij 98 and subjected to density gradient centrifugation. Fractions (1–5) containing DIMs were pooled and examined for known constituents of DISC (Fas, FADD and caspase-8) and for Fyn by western blot analysis.
We performed further experiments to directly evaluate the contribution of membrane rafts in Fas signal initiation. To this end, thymocytes were pre-incubated with methyl-β-cyclodextrin (MβCD), which is capable of inducing cholesterol efflux from the cell membrane and consequently raft disruption (Simons and Toomre, 2000). Such disruption could be monitored by the shift of raft markers from the buoyant fractions to heavy fractions in the sucrose density gradient. Figure 3A shows that pre-incubation with 10 mM MβCD for 12 min at 37°C drastically reduces the amount of rafts in thymocytes. Fas was also shifted to the heavy fractions following cholesterol depletion, further confirming its association with membrane rafts. Importantly, raft disruption by MβCD severely inhibited the membrane recruitment of FADD and caspase-8 following Fas ligation (Figure 3B), albeit without modifying the surface expression of Fas (data not shown). Furthermore, we found that MβCD blocked the Fas cross-linking-induced formation of DISC, composed by Fas, FADD and caspase-8, which represents one earliest step in the Fas-mediated cell death process (Figure 3C). These results demonstrated that membrane rafts are required for the initiation of Fas-triggered cell death.
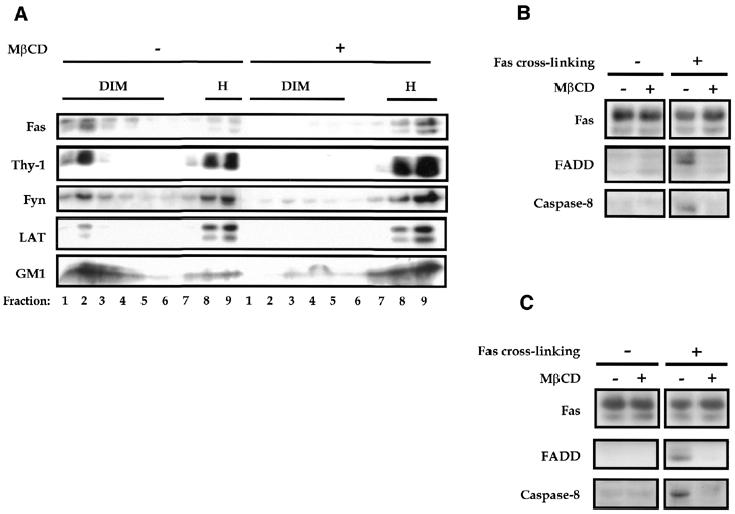
Fig. 3. Raft disruption interferes with the initiation of Fas signaling. (A) Disruption of rafts following cholesterol depletion by MβCD. Brij 98-solubilized PNS of thymocytes (2 × 108) untreated or treated with MβCD (10 mM for 12 min at 37°C) were fractionated on a sucrose gradient. Molecules in each fraction were resolved by SDS–PAGE and blotted with the indicated probes. Fas, Fyn and GM1 were analyzed under reducing conditions while Thy-1 and LAT under non-reducing conditions. (B) Blockade of the membrane recruitment of FADD and caspase-8 by MβCD. Thymocytes (1.6 × 108) pretreated or not with MβCD as in (A) were left unstimulated or stimulated with 2.5 µg/ml Jo2 mAb plus 5 µg/ml protein A for 5 min at 37°C. Membrane fractions were then prepared from PNS, resolved by SDS–PAGE and blotted with Fas-, FADD- and caspase-8-specific antibodies, respectively. (C) Inhibition of the Fas-triggered DISC formation by MβCD. Thymocytes (1.6 × 108) were left untreated or treated with MβCD as in (A), before being stimulated with 2.5 µg/ml biotinylated Jo2 mAb plus 5 µg/ml streptavidin for 5 min at 37°C. Following membrane solubilization by 1% Triton X-100 at 4°C, the DISC was immunoprecipitated with protein A–Sepharose via the Jo2 mAb used for stimulation. As a control for the DISC isolation, lysate from unstimulated cells was immunoprecipitated with 0.25 µg/ml Jo2 mAb plus 0.5 µg/ml streptavidin and protein A–Sepharose as described in Methods. Fas immunoprecipitates were resolved by SDS–PAGE and blotted with Fas-, FADD- and caspase-8-specific antibodies, respectively.
The experiments above predict that the cholesterol depletion under MβCD treatment, by preventing DISC formation, should inhibit Fas-induced cell death. Experiments were thus carried out to address this. Experimental conditions were first calibrated to ensure optimal detection of Fas-triggered cell death. Figure 4A shows that indeed MβCD strongly inhibited Fas-mediated cell death in mouse thymocytes, whereas αCD, which does not promote cholesterol efflux from the membrane, did not exhibit any effects. Moreover, MβCD-mediated cholesterol depletion did not interfere generally with cell death processes, inasmuch as cell death induced by CD45/CD3 co-cross-linking was not inhibited (Figure 4B). We also examined the requirement of rafts in Fas-mediated cell death signaling in the L12.10-Fas T cell line (Rouvier et al., 1993). In addition to MβCD, the cell cholesterol content was also reduced by lovastatin and cholesterol oxidase. Lovastatin is an inhibitor of hydroxymethylglutaryl-CoA reductase that interferes with cholesterol synthesis and cholesterol oxidation has recently been shown to inhibit formation of ordered domains in model membranes (Xu et al., 2001). As shown in Figure 5, cholesterol depletion in L12.10-Fas T cells by each of these compounds was found to markedly disrupt membrane rafts and to inhibit cell death induced by cross-linked, soluble FasL. Taken together, the results above strongly suggest an essential role for cholesterol-rich membrane rafts in the initiation of Fas signaling cascades leading to cell death.
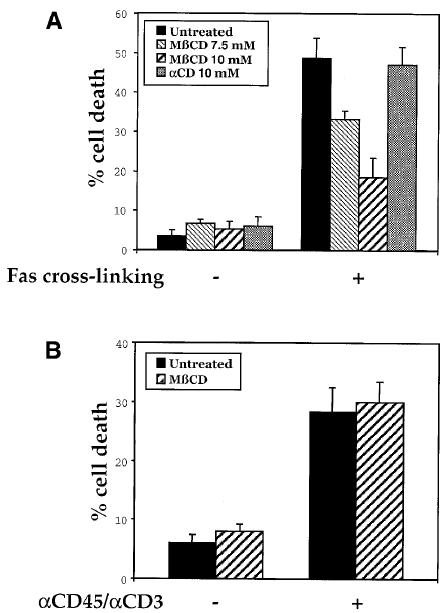
Fig. 4. Inhibition of Fas-mediated cell death upon cholesterol depletion in thymocytes. (A) Thymocytes were mock-treated or treated with MβCD or αCD for 12 min at 37°C, then washed twice and either left unstimulated (–) or stimulated (+) with 100 ng/ml anti-Fas mAb (Jo2) plus 100 ng/ml protein A. Cell death was determined 9 h later by propidium iodide staining and flow cytometry analysis. Percentage means ± SE of the sub-G1 peak, obtained from at least four independent experiments, are provided. (B) Thymocytes were incubated in tubes pre-coated or not with anti-CD45 and anti-CD3 mAbs. Cell death was examined 16 h later as in (A).
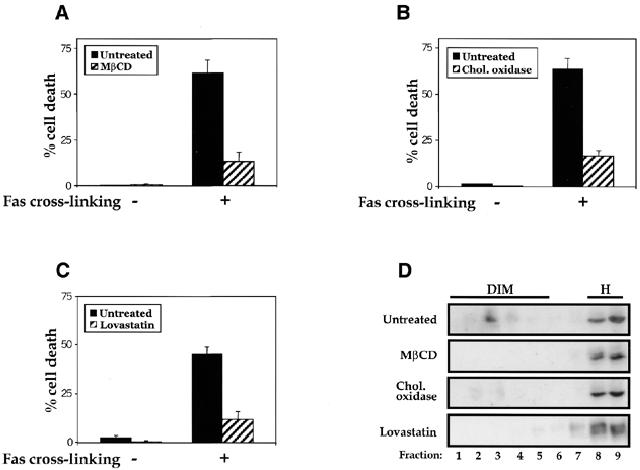
Fig. 5. Inhibition of Fas-mediated cell death upon cholesterol depletion in L12.10-Fas T cells. L12.10-Fas cells were mock-treated or treated at 37°C with 10 mM MβCD for 12 min (A), 5 µM lovastatin for 24 h (B) or 2 U/ml cholesterol oxidase for 1 h (C), then washed twice and either left unstimulated (–) or stimulated (+) with 50 ng/ml soluble Fas ligands plus 1 µg/ml anti-Flag antibodies. Cell death was determined 9 h later by propidium iodide staining and flow cytometry analysis. Percentage means ± SE of the sub-G1 peak, obtained from at least four independent experiments, are shown. (D) Raft disruption in L12.10-Fas T cells following cholesterol depletion. Brij 98-solubilized PNS of L12.10-Fas (0.5 × 108) untreated or treated with MβCD (10 mM, 12 min), cholesterol oxidase (2 U/ml, 1 h) or lovastatin (5 µM, 24 h) were fractionated on a sucrose gradient. Proteins in each fraction were resolved by SDS–PAGE and blotted with anti-Fas antibodies.
Recently, Grassmé et al. (2001) reported that ceramide production following Fas cross-linking could be responsible for the formation of Fas-containing membrane patches that might represent large raft aggregates. Our observation of the constitutive raft partitioning of Fas prior to any ligand binding indicates that de novo production of ceramide following Fas cross-linking is not necessary for Fas association with rafts. In addition, the fact that Z-Vad, a caspase inhibitor known to block Fas-induced ceramide production, did not interfere with the Fas-triggered recruitment of FADD and caspase-8 to the rafts (our unpublished observations) strongly corroborates that ceramide production upon Fas cross-linking is not involved in the earliest steps of raft-dependent Fas signaling, at least in the cells we studied here. Nevertheless, one could envisage that ceramide amplifies the Fas signaling cascade following DISC formation in membrane rafts, for instance via induction of raft aggregation.
The present study has provided evidence for a constitutive association of Fas with rafts and for the requirement of such an association for the DISC-dependent initiation of Fas signaling cascades. Raft association of Fas could be a fundamental and general prerequisite for its signaling capability in physio-pathological conditions. Supporting this are our preliminary results indicating a constitutive association of Fas with rafts taking place also in SKW 6.4 human B lymphomas and Swiss 3T3 murine fibroblasts (our unpublished data). Furthermore, membrane rafts could play an essential role in the signal transduction mediated by other members of the TNFR superfamily. For instance, Vidalain et al. have recently reported that CD40 signaling in human dendritic cells is initiated in membrane rafts (Vidalain et al., 2000). The confined lateral diffusion of membrane proteins in ordered domains is expected to favor the formation and stabilization of supra-molecular signaling complexes to trigger signaling cascades. Interestingly, it has recently been reported that Fas forms trimers via a self-association domain called the pre-ligand assembly domain and that this trimerization is a prerequisite for the signaling initiation (Papoff et al., 1999; Siegel et al., 2000). Therefore, specific molecular pre-organization at both protein–protein and protein–membrane levels could be a basic feature for Fas, and likely for the other members of TNF death receptor family to initiate signaling cascades upon ligation.
METHODS
Cells and antibodies. Single cell suspension of thymus from 4–6-week-old C57BL/6 mice were prepared in serum-free DMEM. L12.10-Fas T cell line was provided by P. Golstein (CIML, Marseille, France) (Rouvier et al., 1993) and cultured in DMEM containing 5% FCS. The antibodies used in this study were as follows: anti-FADD mAb, anti-caspase 8 and anti-CTB pAbs from Calbiochem; anti-Fas (M20), anti-Fyn and anti-Gβ (T20) pAbs from Santa Cruz Biotechnology; unconjugated, FITC-conjugated and biotinylated anti-Fas mAb (Jo2) from Pharmingen; anti-Thy-1 (H194-92), anti-CD3 (145.2C11), unconjugated and FITC-conjugated anti-CD45 (H193-16) mAbs produced in our laboratory; anti-Rab-5 rabbit antiserum provided by P. Chavrier (CNRS UMR144, Paris, France); anti-LAT rabbit antiserum provided by J. Nunez (INSERM U119, Marseille, France); Horseradish peroxidase (HRP)-coupled secondary antibodies from Jackson Immunoresearch Laboratories. CTB-HRP was from Sigma-Aldrich.
PNS and membrane preparation. Fresh thymocytes were gently sonicated (5 s bursts, 5 W; Vibracell, Bioblock Scientific) in 1 ml of ice-cold buffer A (25 mM HEPES, 150 mM NaCl, 1 mM EGTA, 1 µg/ml leupeptin, 1 µg/ml pepstatin, 2 µg/ml chymostatin and 5 µg/ml α2 macroglobulin). The PNS was obtained after centrifugation at 800 g and 4°C for 10 min. The membrane fraction was obtained by pelleting the PNS at 100 000 g for 1 h at 4°C.
Raft isolation. Briefly, PNS from mouse thymocytes (2 × 108) was solubilized in 1 ml buffer A containing 1% Brij 98 for 5 min at 37°C and diluted with 2 ml buffer A containing 2 M sucrose (final sucrose concentration: 1.33 M; final Brij 98 concentration: 0.33%) and chilled on ice before being placed at the bottom of a step sucrose gradient (0.9–0.8–0.75–0.7–0.6–0.5–0.4–0.2 M sucrose, 1 ml each) in buffer A. Samples were centrifuged at 38 000 r.p.m. for 16 h in a SW41 rotor (Beckman Instruments Inc.) at 4°C. One milliliter fractions were harvested from the top, except for the last one (No. 9) that contains 3 ml. When not specified, DIM fraction is pooled fractions 1–5 and H fraction is pooled fractions 8 and 9. For DIM isolation in cold Triton X-100, PNS from mouse thymocytes was solubilized in 1% Triton X-100 at 4°C for 1 h before been subjected to centrifugation onto a sucrose density gradient (Garcia et al., 1993).
Immunofluorescence confocal microscopy and raft patching. Cells were washed and disposed on TESPA (3-aminopropyltriethoxysilane; Sigma-Aldrich)-coated slides. Cell staining was performed at 4°C for 45 min with FITC-conjugated anti-Fas mAb (Jo2), FITC-conjugated anti-CD45 antibody (H193-16) and rhodamine-conjugated CTB (List Biological Laboratories), respectively. To analyze raft patching, cells were first incubated with rhodamine-conjugated CTB for 45 min at 4°C, followed by anti-CTB antibody for 20 min at 37°C (Janes et al., 1999). After three washes with PBS, cells were pre-saturated in PBS, 2% BSA for 10 min and incubated with FITC-conjugated anti-Fas and anti-CD45 mAbs, respectively. Cells were then fixed with 3% paraformaldehyde in PBS for 30 min and mounted in moviol (Calbiochem). Confocal microscopy was performed with a Leitz DMBRE and 100× objective lens.
Fas stimulation in mouse thymocytes. Thymocytes (2 × 108) were incubated with 2.5 µg/ml of anti-Fas mAb Jo2 plus 5 µg/ml of protein A (Amersham Pharmacia) in complete medium for 1 min at 37°C. Cells were then centrifuged at 10 000 g for 8 s. One milliliter of ice-cold buffer A was immediately added to cell pellets and gently sonicated for PNS preparation.
DISC isolation. Thymocytes (1.6 × 108) were stimulated with 2.5 µg/ml biotinylated Jo2 mAb plus 5 µg/ml strepavidin (Pierce) for 5 min at 37°C. The PNS was prepared and solubilized in buffer A containing 1% Triton X-100 and 10% glycerol (buffer B) at 4°C. The solubilizate was then subjected to immunoprecipitation via the Jo2 anti-Fas antibodies used for stimulation with protein A–Sepharose beads (Amersham Pharmacia) at 4°C for 2 h to isolate DISC (Kischkel et al., 1995). The immunoprecipitates were washed four times before eluted from the beads by heating in SDS–PAGE sample buffer at 95°C for 5 min. Control for DISC isolation was obtained by immunoprecipitating Fas from unstimulated cells using 0.25 µg/ml Jo2 mAb plus 0.5 µg/ml streptavidin, considering that ∼10% of the anti-Fas antibodies bound to the cells in the stimulating conditions.
Cholesterol depletion treatment. Cells were incubated in serum-free medium, 10 mM HEPES, with MβCD or αCD (Sigma-Aldrich) at 37°C for 12 min, or with cholesterol oxidase (Calbiochem) at 37°C for 1 h. For Lovastatin treatment, cells were cultured for 24 h in serum-free DMEM containing Lovastatin (Calbiochem). The absence of serum in the culture did not reduce the raft-association of Fas. Following drug treatment, cells were washed once before the cell death assay was performed.
Cell death assay. To induce Fas-mediated cell death, thymocytes (2 × 106) and L12.10-Fas T (3 × 105) cells were placed in 5 ml polystyrene tubes and treated respectively with anti-Fas Jo2 (100 ng/ml) plus protein A (100 ng/ml), or with 50 ng/ml of the recombinant human Flag-Fas ligand (Alexis Corporation) plus 1 µg/ml of anti-Flag M2 (Sigma-Aldrich). To induce CD45/CD3-mediated cell death, thymocytes were incubated in the tubes pre-coated with anti-CD45 and anti-CD3 mAbs (Lesage et al., 1997). After an appropriate incubation time, cells were fixed in 70% ethanol and stained for 20 min at 37°C in 38 mM sodium citrate (pH 7.4) containing 69 µM propidium iodide (Sigma-Aldrich) and 5 µg/ml RNase A (Sigma-Aldrich). Twenty-thousand cells were analyzed in a flow cytometer (FACScalibur; Becton Dickinson) and the proportion of apoptotic cells represented by the sub-G1 peak (after exclusion of the objects with a fractional DNA content <10% of the intact G1 cells) determined.
Acknowledgments
ACKNOWLEDGEMENTS
We are very grateful to M. Chautan for participating in the initial phase of this work, P. Drevot, D. Marguet and A. Rouquette for technical advice, and J. Nunez and P. Chavrier for antibodies. We thank P. Golstein and E. Van Obberghen for critical reading of the manuscript and helpful discussions. This work was supported by institutional funds from Institut National de la Santé et de la Recherche Médicale (INSERM), the Centre National de la Recherche Scientifique (CNRS), and by grants from Association pour la Recherche contre le Cancer (ARC) and Ligue Nationale Française Contre le Cancer (LNFCC).
REFERENCES
- Ashkenazi A. and Dixit, V.M. (1998) Death receptors: signaling and modulation. Science, 281, 1305–1308. [DOI] [PubMed] [Google Scholar]
- Boldin M.P., Varfolomeev, E.E., Pancer, Z., Mett, I.L., Camonis, J.H. and Wallach, D. (1995) A novel protein that interacts with the death domain of Fas/APO1 contains a sequence motif related to the death domain. J. Biol. Chem., 270, 7795–7798. [DOI] [PubMed] [Google Scholar]
- Brown D.A. and London, E. (1998) Functions of lipid rafts in biological membranes. Annu. Rev. Cell. Biol., 14, 111–136. [DOI] [PubMed] [Google Scholar]
- Chinnaiyan A.M., O’Rourke, K., Tewari, M. and Dixit, V.M. (1995) FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell, 81, 505–512. [DOI] [PubMed] [Google Scholar]
- Garcia M., Mirre, C., Quaroni, A., Reggio, H. and Le Bivic, A. (1993) GPI-anchored proteins associate to form microdomains during their intracellular transport in Caco-2 cells. J. Cell Sci., 104, 1281–1290. [DOI] [PubMed] [Google Scholar]
- Grassmé H., Jekle, A., Riehle, A., Schwarz, H., Berger, J., Sandhoff, K., Kolesnick, R. and Gulbins, E. (2001) CD95 signaling via ceramide-rich membrane rafts. J. Biol. Chem., 276, 20589–20596. [DOI] [PubMed] [Google Scholar]
- Harder T., Scheiffele, P., Verkade, P. and Simons, K. (1998) Lipid domain structure of the plasma membrane revealed by patching of membrane components. J. Cell Biol., 141, 929–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes P.W., Ley, S.C. and Magee, A.I. (1999) Aggregation of lipid rafts accompanies signaling via the T cell antigen receptor. J. Cell Biol., 147, 447–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kischkel F.C., Hellbardt, S., Behrmann, I., Germer, M., Pawlita, M., Krammer, P.H. and Peter, M.E. (1995) Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J., 14, 5579–5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krammer P.H. (2000) CD95’s deadly mission in the immune system. Nature, 407, 789–795. [DOI] [PubMed] [Google Scholar]
- Langlet C., Bernard, A.M., Drevot, P. and He, H.T. (2000) Membrane rafts and signaling by the multichain immune recognition receptors. Curr. Opin. Immunol., 12, 250–255. [DOI] [PubMed] [Google Scholar]
- Lesage S., Steff, A.M., Philippoussis, F., Page, M., Trop, S., Mateo, V. and Hugo, P. (1997) CD4+ CD8+ thymocytes are preferentially induced to die following CD45 cross-linking, through a novel apoptotic pathway. J. Immunol., 159, 4762–4771. [PubMed] [Google Scholar]
- Locksley R.M., Killeen, N. and Lenardo, M.J. (2001) The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell, 104, 487–501. [DOI] [PubMed] [Google Scholar]
- Medema J.P., Scaffidi, C., Kischkel, F.C., Shevchenko, A., Mann, M., Krammer, P.H. and Peter, M.E. (1997) FLICE is activated by association with the CD95 death-inducing signaling complex (DISC). EMBO J., 16, 2794–2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melkonian K.A., Ostermeyer, A.G., Chen, J.Z., Roth, M.G. and Brown, D.A. (1999) Role of lipid modifications in targeting proteins to detergent-resistant membrane rafts. Many raft proteins are acylated, while few are prenylated. J. Biol. Chem., 274, 3910–3917. [DOI] [PubMed] [Google Scholar]
- Muzio M. et al. (1996) FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signaling complex. Cell, 85, 817–827. [DOI] [PubMed] [Google Scholar]
- Papoff G., Hausler, P., Eramo, A., Pagano, M.G., Di Leve, G., Signore, A. and Ruberti, G. (1999) Identification and characterization of a ligand-independent oligomerization domain in the extracellular region of the CD95 death receptor. J. Biol. Chem., 274, 38241–38250. [DOI] [PubMed] [Google Scholar]
- Rouvier E., Luciani, M.-F. and Golstein, P. (1993) Fas involvement in Ca++-independent T cell-mediated cytotoxicity. J. Exp. Med., 177, 195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R.M., Frederiksen, J.K., Zacharias, D.A., Chan, F.K., Johnson, M., Lynch, D., Tsien, R.Y. and Lenardo, M.J. (2000) Fas preassociation required for apoptosis signaling and dominant inhibition by pathogenic mutations. Science, 288, 2354–2357. [DOI] [PubMed] [Google Scholar]
- Simons K. and Ikonen, E. (1997) Functional rafts in cell membranes. Nature, 387, 569–572. [DOI] [PubMed] [Google Scholar]
- Simons K. and Toomre, D. (2000) Lipid rafts and signal transduction. Nature Rev. Mol. Cell. Biol., 1, 31–39. [DOI] [PubMed] [Google Scholar]
- Varfolomeev E.E. et al. (1998) Targeted disruption of the mouse Caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity, 9, 267–276. [DOI] [PubMed] [Google Scholar]
- Vidalain P.O., Azocar, O., Servet-Delprat, C., Rabourdin-Combe, C., Gerlier, D. and Manie, S. (2000) CD40 signaling in human dendritic cells is initiated within membrane rafts. EMBO J., 19, 3304–3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallach D., Varfolomeev, E.E., Malinin, N.L., Goltsev, Y.V., Kovalenko, A.V. and Boldin, M.P. (1999) Tumor necrosis factor receptor and Fas signaling mechanisms. Annu. Rev. Immunol., 17, 331–367. [DOI] [PubMed] [Google Scholar]
- Xu X., Bittman, R., Duportail, G., Heissler, D., Vilcheze, C. and London, E. (2001) Effect of the structure of natural sterols and sphingolipids on the formation of ordered sphingolipid/sterol domains (rafts): comparison of cholesterol to plant, fungal, and disease-associated sterols, and comparison of sphingomyelin, cerebrosides and ceramide. J. Biol. Chem., 276, 33540–33546. [DOI] [PubMed] [Google Scholar]
- Yeh W.C. et al. (1998) FADD: essential for embryo development and signaling from some, but not all, inducers of apoptosis. Science, 279, 1954–1958. [DOI] [PubMed] [Google Scholar]
- Zhang J., Cado, D., Chen, A., Kabra, N.H. and Winoto, A. (1998) Fas-mediated apoptosis and activation-induced T-cell proliferation are defective in mice lacking FADD/MORT1. Nature, 392, 296–300. [DOI] [PubMed] [Google Scholar]