Abstract
The p8 protein is involved in the cellular stress response of many tissues. Because p8 is overexpressed in many cancers, we investigated whether its expression was required for tumour development. Mouse embryo fibroblasts (MEFs) from p8+/+ and p8–/– animals were transformed with the pBabe-rasV12/E1A retroviral vector, which expresses both the rasV12 mutated protein and the E1A oncogene. As expected, transformed p8+/+ MEFs could form colonies in soft agar. However, transformed p8–/– MEFs could not. In addition, subcutaneous or intraperitoneal injections of transformed p8+/+ MEFs always led to tumour formation in nude mice, but, again, no tumour was observed with transformed p8–/– MEFs. However, restoring p8 expression in transformed p8–/– MEFs before injection led to tumour formation. In the tumours, p8 expression was induced during tumour development. It was concluded that p8 expression in transformed MEFs is necessary for tumour formation, suggesting that the stress-response mechanisms governed by p8 are required for tumour establishment.
INTRODUCTION
Cancer progression occurs in several steps. During transformation, some cancer cells are positively selected within the tumour on the basis of their growth capacity, low response to apoptotic signals and ability to escape the immunological survey of the host. After leaving the primary tumour, transformed cells migrate through the body. However, metastases will not develop in all tissues (Yeatman and Nicolson, 1993). Capacity for invading the target organ is a first limitation. But once within the organ, metastasis will develop only if transformed cells can cope with their new microenvironment. In fact, the phenotype of the cells is adapted to their original environment but not necessarily to the environment generated by the invaded tissue. Therefore, invading cells are exposed to a stress, and their capacity to react by activating stress-associated genes should be determinant in metastasis formation. Several stress-associated genes have been found overexpressed in tumours (Jamora et al., 1996; Storm et al., 1996) and their expression level correlated with aggressiveness. Therefore, stress-associated genes might facilitate tumour progression and metastasis formation by helping cell adaptation to the microenvironment of the host tissue.
Previously, using a systematic approach, we identified a new gene, called p8, whose expression is strongly induced in pancreatic acinar cells during the acute phase of pancreatitis (Mallo et al., 1997). Further experiments have shown that p8 activation is not restricted to pancreatic cells. In vivo, p8 mRNA expression is activated in several tissues in response to systemic liposaccharides (Jiang et al., 1999), and in vitro studies showed that a variety of cell lines exhibited transient p8 mRNA expression in response to several stress agents (Mallo et al., 1997; Vasseur et al., 1999; Garcia-Montero et al., 2001a) through a p38-dependent pathway (Garcia-Montero et al., 2001a). The deduced protein sequence revealed that p8 was an 80-amino-acid polypeptide whose primary structure could not be aligned with any of the protein sequences present in public databases. In contrast, p8 contains a canonical bipartite signal for nuclear targeting, suggesting that p8 should be located in the nucleus. We detected p8 within the nucleus of Cos-7 cells transfected with a p8 expression plasmid, although it was also partly located to the cytoplasm (Vasseur et al., 1999). Furthermore, analysis of p8 primary structure suggested that it should be a DNA-binding protein (Mallo et al., 1997). More recently, we performed biochemical and biophysical studies showing that human recombinant p8 was in many structural aspects very similar to the high mobility group (HMG) proteins, although sharing with them only 35% amino-acid sequence homology (Encinar et al., 2001). In addition, p8 binding to DNA, rather weak with the native protein, was strongly enhanced after phosphorylation by PKA of serine/threonine residues (Encinar et al., 2001). HMG protein binding to DNA is also regulated by post-translational modifications (Bianchi and Beltrame, 2000). Thus, in spite of a low sequence homology, p8 can be considered as an HMG-like protein. We found recently that p8 is a co-transcription factor, since it can enhance the transcription activity of the Smad proteins in response to TGF-β (Garcia-Montero et al., 2001b). Therefore, p8 is a ubiquitous protein induced by cellular stress that, acting as a co-transcription factor, may participate in a general defence programme against cellular injury or cellular stress.
Concomitant studies by another laboratory (Ree et al., 1999) suggested that expression of the candidate of metastasis 1 (Com 1) protein, which is identical to the human p8 (Vasseur et al., 1999), could mediate the growth of tumour cells after metastatic establishment in a secondary organ, suggesting that activated expression of p8 in metastatic cells is required for tumour progression. Taken together, these results strongly suggest that p8 expression might regulate cellular functions required for tumour progression and metastasis. To explore the function of p8 in cancer progression, we used embryonic fibroblasts obtained from p8–/– mice and their p8+/+ counterparts. We transformed these fibroblasts and compared their growth properties in vitro and in vivo.
RESULTS AND DISCUSSION
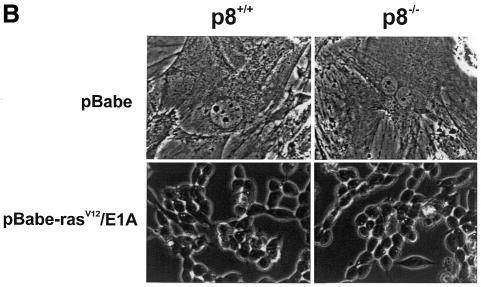
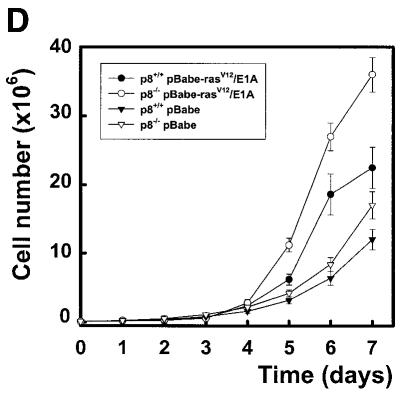
A conventional targeting vector was used to ablate p8 exon 2 in mouse embryonic stem cells. Details of this construction can be obtained from the authors upon request. Heterozygous mice for the mutated p8 allele were bred to generate mice homozygous for the disruption of p8. Primary embryo fibroblasts were isolated from 14.5-day-old mouse embryos following standard protocols (Harvey et al., 1993). p8 genotypes of cultured cells were confirmed by PCR on genomic DNA. Cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum (FCS). Expression of p8 mRNA in p8+/+ and p8–/– mouse embryo fibroblasts (MEFs) was evaluated by RT–PCR using specific primers (Figure 1A). Oncogenic ras transforms most immortal rodent cells to a tumorigenic state, whereas transformation of primary cells requires either a cooperating oncogene or the inactivation of a tumour suppressor. The adenovirus E1A oncogene cooperates with ras to transform primary rodent fibroblasts (Ruley, 1990) and abrogates ras-induced senescence (Serrano et al., 1997). Therefore, we transduced p8+/+ and p8–/– MEFs with the pBabe-rasV12/E1A retroviral vector, which expresses both the rasV12 mutated protein and the E1A oncogene, to obtain transformed MEFs with a defined p8 status. As control, we evaluated the ability of the retroviral vector to transduce p8+/+ and p8–/– MEFs by using a retroviral vector expressing the EGFP under the control of the retroviral promoter located in the long terminal repeat. More than 80% of p8-expressing and p8-deficient MEFs expressed high levels of EGFP fluorescence 48 h after transduction (data not shown), indicating that the retroviral vectors are well adapted tools to our experimental set-up. Transformation of p8+/+ and p8–/– MEFs by the pBabe-rasV12/E1A retroviral vector was evaluated by examining changes in their morphological aspect, by quantifying expression of the RAS protein by western blotting and by monitoring cell proliferation. The original pBabe retroviral vector that only contains and expresses the puromycin resistance gene was used as a control. As shown in Figure 1B, transduction of MEFs with the pBabe-rasV12/E1A retroviral vector induced important morphological changes typical of cell transformation (i.e. cells lost their flat and enlarged cytoplasm and became refractile with thin projections), whereas no change in morphology was observed in cells transduced with the pBabe retroviral vector; both transformed p8+/+ and p8–/– MEFs expressed high levels of RAS compared with control MEFs transduced with empty vector (Figure 1C); finally, transformed MEFs grew 2–3 times faster than control MEFs (Figure 1D). It is noteworthy that lack of p8 expression did not affect cell growth. On the contrary, p8–/– and transformed p8–/– MEFs grew more rapidly than p8+/+ and transformed p8+/+ MEFs, respectively. Based on these results, we assumed that p8+/+ and p8–/– MEFs were transformed by the pBabe-rasV12/E1A retroviral vector. The similar features acquired by p8+/+ and p8–/– MEFs upon transformation by rasV12/E1A co-expression suggested that p8 deficiency did not interfere with MEF transformation.
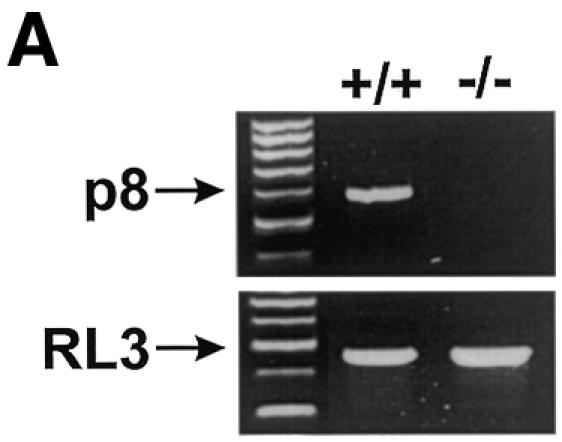
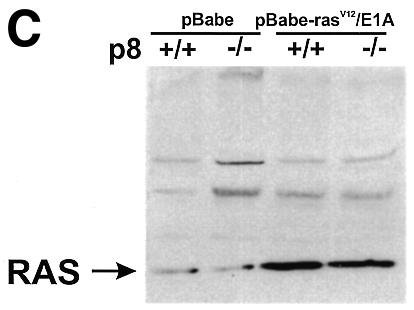
Fig. 1. To investigate the function of p8 in cancer progression, a null allele was generated in 129/Sv embryonic stem cells by replacing exon 2 of the p8 gene, which encodes 60% of the coding sequence, with a neomycin-resistance cassette. Germline transmission was obtained after injection of these embryonic stem cells into C57BL/6 blastocysts. Then, we intercrossed mice heterozygous for the targeted disruption to produce homozygous offsprings. These crosses provided litters of normal size, with living, apparently normal, p8–/– offspring occurring with a frequency consistent with Mendelian inheritance. p8 deficiency was verified by RT–PCR and/or Southern blotting and by immunostaining of the pancreas with acute pancreatitis using a p8 polyclonal antibody (data not shown). MEFs were isolated from 14.5-day-old p8+/+ and p8–/– mouse embryos, following standard protocols (Harvey et al., 1993). rasV12/E1A-transformed p8+/+ and p8–/– MEFs were obtained as described in Methods. (A) Expression of p8 mRNA in p8+/+ and p8–/– MEFs analysed by RT–PCR. (B) Representative photomicrographs of p8+/+ or p8–/– MEFs transduced with control pBabe or the pBabe-rasV12/E1A retroviruses. Photographs are at the same magnification (×200). (C) Expression of RAS was verified by immunoblotting in p8+/+ and p8–/– MEFs transduced with pBabe (control) or pBabe-rasV12/E1A (transformed) retroviruses. (D) Growth curves corresponding to p8+/+ and p8–/– MEFs transduced with pBabe or pBabe-rasV12/E1A retroviruses. Each value was determined in triplicate. Error bars represent the standard error of the mean.
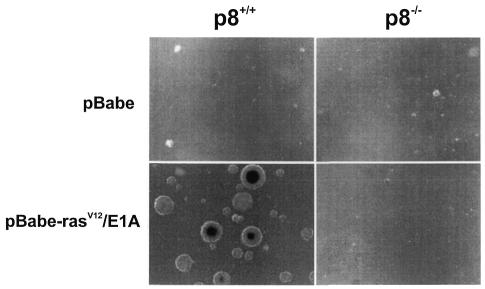
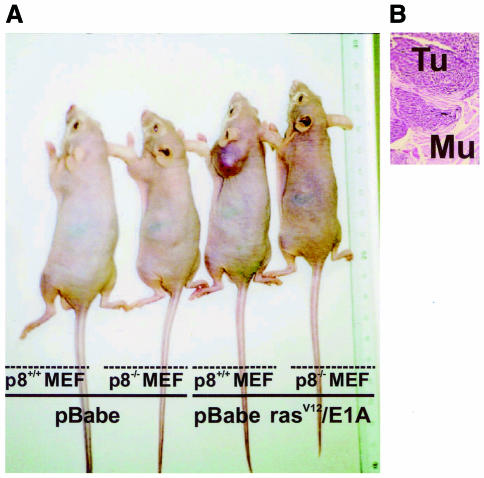
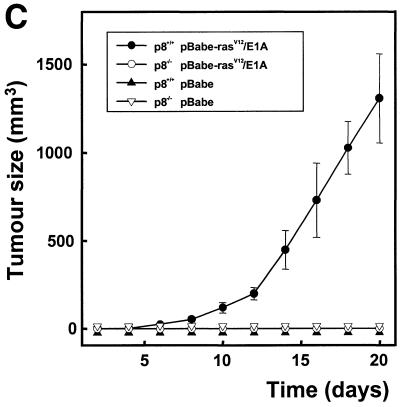
The tumorigenic properties of transformed p8+/+ and p8–/– MEFs were assessed by checking their ability to grow in vitro and to form tumours in immunocompromised mice. In soft-agar assays, transformed p8+/+ MEFs formed colonies at high frequency, as expected (Figure 2), but transformed p8–/– MEFs, like control p8+/+ or p8–/– MEFs transduced with the pBabe vector, were unable to form colonies (Figure 2). Similarly, transformed p8+/+ MEFs produced tumours in all athymic nude mice (6/6) when injected subcutaneously, whereas transformed p8-deficient MEFs, like control MEFs, did not (0/6) (Table I and Figure 3). As a control, we restored p8 expression in transformed p8–/– MEFs and found that they had recovered their ability to form tumours when injected into nude mice, as expected (Table I and Figure 4). Finally, we injected intraperitoneally the transformed p8+/+ and p8–/– MEFs to test their ability for dispersed growth. Remarkably, only transformed p8+/+ MEFs (Table I and Figure 5) and transformed p8–/– MEFs in which p8 expression was restored (Table I) showed intraperitoneal spreading. Again, transformed p8–/– MEFs, like non-transformed control MEFs, did not produce dispersed tumours (Table I). Therefore, although p8+/+ and p8–/– MEFs seem to be similarly transformed by the pBabe-rasV12/E1A retroviral vector as judged by their behaviour in standard culture conditions (Figure 1D), the inability of the transformed p8-deficient cells to form colonies in soft agar, form subcutaneous tumours or generate intraperitoneal spreading strongly suggest that expression of p8 is required for the organization and development of tumours.
Fig. 2. Analysis of anchorage-independent growth of the indicated MEF populations. Fifty thousand cells were plated in 0.3% agar in DMEM plus 10% FCS and overlaid on 0.6% agar in the same medium. Photomicrographs were taken 10 days after plating.
Table I. Ability of rasV12/E1A-transformed p8+/+ and p8–/– MEFs to form tumours and metastasis in nude mice.
| Cells injected | Route of inoculationa | Tumorigenicityb | Spreading abilityc |
|---|---|---|---|
| p8+/+ pBabe MEFs | Subcutaneous | 0/4 | |
| p8–/– pBabe MEFs | Subcutaneous | 0/4 | |
| p8+/+ pBabe-rasV12/E1A MEFs | Subcutaneous | 6/6 | |
| p8–/– pBabe-rasV12/E1A MEFs | Subcutaneous | 0/6 | |
| p8+/+ pBabe-rasV12/E1A MEFs | Intraperitoneal | 6/6d | 6/6 |
| p8–/– pBabe-rasV12/E1A MEFs | Intraperitoneal | 0/6 | 0/6 |
| p8–/– pBabe-rasV12/E1A MEFs + pLPChump8 | Subcutaneous | 6/6 | |
| p8–/– pBabe-rasV12/E1A MEFs + pLPC | Subcutaneous | 0/6 | |
| p8–/– pBabe-rasV12/E1A MEFs + pLPChump8 | Intraperitoneal | 3/3d | 3/3 |
| p8–/– pBabe-rasV12/E1A MEFs + pLPC | Intraperitoneal | 0/3 | 0/3 |
aEach mouse was inoculated with 106 cells in 200 µl of PBS.
bNumber of mice with tumours / number of injected mice.
cNumber of mice with metastasis / number of injected mice.
dInjection (intraperitoneal) led to metastasis formation without the presence of a primary tumour.
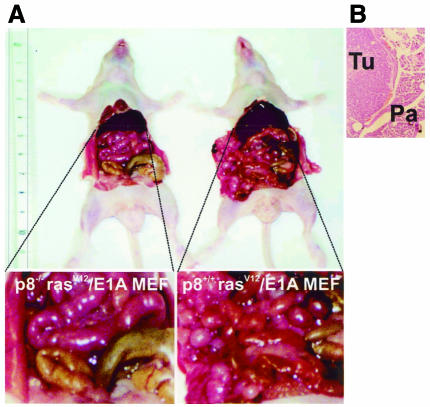
Fig. 3. p8 is required for tumour development. One million pBabe-rasV12/E1A-transformed p8+/+ or p8–/– MEFs or pBabe-transduced p8+/+ or p8–/– MEFs were injected in 200 µl PBS as xenografts into nude mice. Animals were killed after 20 days. (A) Representative mice at day 20. (B) Histological aspect of the tumour obtained with pBabe-rasV12/E1A-transformed p8+/+ MEFs: Tu, tumour; Mu, muscle. (C) Growth curves of pBabe-rasV12/E1A-transformed p8+/+ and p8–/– MEFs or pBabe-transduced p8+/+ and p8–/– MEFs. Each data point represents the average tumour size of six mice for pBabe-rasV12/E1A-transformed p8+/+ and p8–/– MEFs or three mice for pBabe-transduced p8+/+ and p8–/– MEFs. Error bars represent the SEM. Tumours that did not grow were assigned a volume of zero. Note that pBabe-rasV12/E1A-transformed p8–/– MEFs and control pBabe-transduced p8+/+ and p8–/– MEFs did not develop tumours at all.
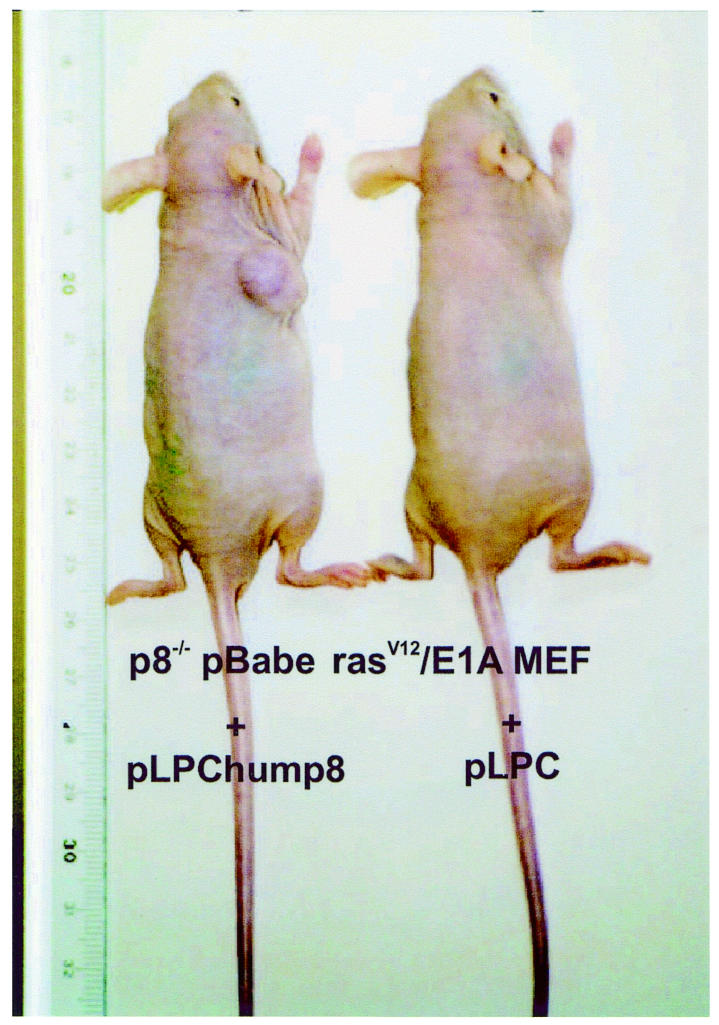
Fig. 4. Restoring p8 expression in transformed p8–/– MEFs allows tumour formation. One million pLPChump8 or pLPC empty retrovirus transduced pBabe-rasV12/E1A-transformed p8–/– MEFs were injected in 200 µl PBS as xenografts into nude mice. Animals were killed after 20 days. Representative mice at day 20 are shown. The experiment was repeated twice.
Fig. 5. Loss of spreading capacity in cells deficient in p8. One million pBabe-rasV12/E1A-transformed p8+/+ and p8–/– MEFs or pBabe-transduced p8+/+ and p8–/– MEFs were injected intraperitoneally in 200 µl PBS into athymic mice. Animals were killed after 20 days. Diffuse tumour growth tissue into the abdominal cavity was observed with pBabe-rasV12/E1A-transformed p8+/+ MEFs but not in mice injected with pBabe-rasV12/E1A-transformed p8-deficient cells nor in p8+/+ or p8–/– MEFs transduced with the control retrovirus, which were completely free of tumour formation 45 days after injection. (A) Photographs of representative mice injected with pBabe-rasV12/E1A-transformed p8+/+ and p8–/– MEFs. (B) Histological aspect of the paraffin-embedded intra-abdominal tumours formed with pBabe-rasV12/E1A-transformed p8+/+ MEFs: Tu, tumour; Pa, pancreas.
The molecular mechanisms by which p8 allows tumour progression are unknown. It might involve Ki-ras and PPARδ pathways, because their disruption and that of the p8 gene result in similar phenotypes (continued growth in vitro but poor growth in vivo) (Shirasawa et al., 1993; Park et al., 2001), but this has not been demonstrated. Nevertheless, in that context, it is conceivable that suppression of p8 expression in cancer cells would impede tumour progression. Consequently, p8 might be a new drug-targetable gene whose blockade would prevent cancer progression and metastasis development.
Speculation
So far, p8 induction has been associated with stress (Mallo et al., 1997; Jiang et al., 1999; Vasseur et al., 1999; Garcia-Montero et al., 2001a), suggesting that activation of p8 in transformed p8+/+ MEFs during subcutaneous or intraperitoneal tumour formation (Figure 6) is a consequence of the stress generated by the new microenvironment of MEFs. We propose that transformed p8-deficient cells fail to form tumours because they are unable to respond efficiently to such stress. This is supported by clinical data indicating that p8 expression in breast and pancreatic cancers correlates with aggressiveness (Ree et al., 2000; Su et al., 2001) and that metastatic cells express high levels of p8 (Ree et al., 1999; S. Garcia and J. Iovanna, unpublished data). Further studies are required to demonstrate that cells losing their capacity to cope with environmental stress are unable to develop tumours; if this is achieved, it will open up an original and promising area of therapeutic cancer research.
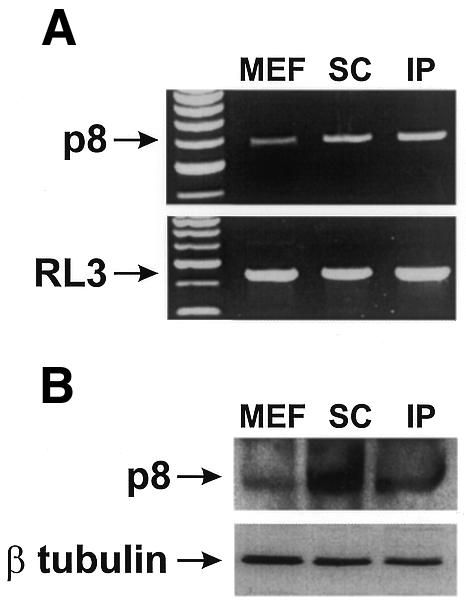
Fig. 6. Expression of p8 in tumour cells. p8 mRNA and protein expressions were estimated in pBabe-rasV12/E1A-transformed p8+/+ cells obtained from sub-confluent culture (MEF), in subcutaneous (SC) and intraperitoneal (IP) tumours. (A) RNA was extracted using the Trizol procedure and p8 and RL3 mRNA expressions were analysed by RT–PCR as described in Methods. (B) p8 protein concentration was estimated by western blotting using antibodies against human p8 and β tubulin with the monoclonal anti-β tubulin antibody (D-10) (Santa Cruz Biotechnology).
METHODS
Preparation of p8+/+ and p8–/– MEFs. Primary embryo fibroblasts were isolated from 14.5-day-old p8+/+ and p8–/– mouse embryos following standard protocols (Harvey et al., 1993). Cells were grown in DMEM supplemented with 10% FCS.
Retroviral vector and retroviral-mediated gene transfer. pBabe-rasV12/E1A and pBabe plasmids were obtained from M. Serrano (Spanish National Center of Biotechnology, Madrid, Spain) and S. Lowe (Cold Spring Harbor Laboratory, NY). Bosc 23 ecotropic packaging cells (106) were plated in a six-well plate, incubated for 24 h and then transfected with polyethylene imine with 5 µg of retroviral plasmid. After 48 h, the medium containing the virus was filtered (0.45 µm filter, Millipore) to obtain the first supernatant. Target MEFs were plated at 2 × 105 cells per 35-mm dish and incubated overnight. For infections, the culture medium was replaced by an appropriate mix of the first supernatant and culture medium (v/v), supplemented with 4 µg/ml polybrene (Sigma), and cells were incubated at 37°C. MEFs infected with the empty vector were used as control. To restore p8 expression in transformed p8–/– MEFs, we infected these cells with a retrovirus expressing human p8. The retroviral vector was constructed as follows: human p8 cDNA was subcloned in HindIII and XhoI restriction sites of the pLPC plasmid (obtained from S. Lowe). Ecotropic human p8-expressing retrovirus was then generated by transient transfection using Bosc 23 ecotropic packaging cells. Virus supernatant was used to infect transformed p8–/– MEFs, and the population of p8-expressing transformed p8–/– MEFs was isolated by selection in the presence of puromycin (0.7 µg/ml). As control, transformed p8–/– MEFs were infected with pLPC empty vector
Growth curves. One-hundred thousand cells per well were plated in a series of 35-mm culture dishes. The cell number was estimated daily in triplicate (from 1 to 7 days) in a hemocytometer. Within an experiment, each point was determined in triplicate and each curve was established at least two times.
RT–PCR analysis. RNA was extracted using Trizol (Life Technologies) procedures. Total RNA (1 µg) was analysed by RT–PCR with the SuperScript One-step RT–PCR System and the Platinum Taq kit (Life Technologies). RT–PCR was performed using different numbers of cycles to verify that the conditions chosen were within the linear range. The mRNA coding for p8 was specifically amplified with sense (5′-GGAGAGAGCAGACTAGGCATA-3′) and antisense (5′-GTTGCTGCCACCCAAGGGCAT-3′) primers, in positions 16 and 581 of the cDNA, respectively. As control, the transcript coding for the ribosomal protein RL3 was specifically amplified for 19 cycles with sense (5′-Gaaagaagtcgtggaggctg-3′) and antisense (5′-Atctcatcctgcccaaacac-3′) primers, in positions 216 and 637 of the cDNA, respectively.
Western blotting. One-hundred micrograms of total protein extracted from cells were separated with standard procedures on 12.5% SDS–PAGE using the Mini Protean System (Bio-Rad) and transferred to a nitrocellulose membrane (Sigma). The intracellular level of RAS was estimated by western blotting using the H-ras (C-20) polyclonal antibody (1:200) (Santa Cruz Biotechnology), and p8 was detected with a polyclonal antibody raised against human p8 (Vasseur et al., 1999).
Colony growth assays. To determine the ability of different cell lines to grow in soft agar, 5 × 104 cells were resuspended in 0.3% agar in DMEM plus 10% FCS and overlaid on 0.6% agar in the same medium in 12-well plates. Experiments were performed in triplicate and repeated two (pBabe-transduced cells) or three times (pBabe-rasV12/E1A-transduced cells).
In vivo tumour growth, spreading assays and histological analysis. Suspensions of the p8+/+ or p8–/– pBabe-rasV12/E1A-transformed MEFs or the p8+/+ or p8–/– pBabe-transduced MEFs [106/200 µl phosphate-buffered saline (PBS)] were injected subcutaneously into the flank of male athymic 7–8-week-old nu nu mice, and tumours were allowed to develop for 20 days. Tumours were measured externally with a caliper, in two dimensions, on the indicated days. Tumour volumes were determined from the equation V = (L × W 2) × 0.5, where L is length and W is width. For tumour spreading assays, 106 cells in 200 µl PBS were injected intraperitoneally into male nude mice, and animals were killed after 20 days. For histological examination, necropsies were performed on representative animals. Tumours were fixed in 10% buffered neutral formalin, processed in paraffin, sectioned at 5 µm and stained with hematoxylin and eosin.
Acknowledgments
ACKNOWLEDGEMENTS
We thank Drs M. Serrano and S. Lowe for pBabe-rasV12/E1A and pBabe plasmids and R. Feil and S. Kuhbandner for help in constructing the p8-deficient mice. We also thank Drs C. Mawas, I. Dikic, A. Ree, S. Moreno and M. Serrano for their help and critical comments. Production of retroviral vector was generously sponsored by the Institut Paoli-Calmettes (Marseille, France) and the GVPN (Gene Vector Production Network). This work was supported by grants from the Société de Secours des Amis des Sciences and an EMBO short-term fellowship (to S.V.), the Fondation pour la Recherche Médicale (to A.H.), the Ligue Nationale Contre le Cancer (LNCC) and the Association pour la Recherche sur le Cancer (ARC).
REFERENCES
- Bianchi M.E. and Beltrame, M. (2000) Upwardly mobile proteins. Workshop: the role of HMG proteins in chromatin structure, gene expression and neoplasia. EMBO rep., 1, 109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Encinar J.A. et al. (2001) Human p8 is a HMG-I/Y-like protein with DNA binding activity enhanced by phosphorylation. J. Biol. Chem., 276, 2742–2751. [DOI] [PubMed] [Google Scholar]
- Garcia-Montero A., Vasseur, S., Mallo, G.V., Soubeyran, P., Dagorn, J.C. and Iovanna, J.L. (2001a) Expression of the stress-induced p8 mRNA is transiently activated after culture medium change. Eur. J. Cell Biol., 80, 720–725. [DOI] [PubMed] [Google Scholar]
- Garcia-Montero A., Vasseur, S., Giono, L., Canepa, E., Moreno, S., Dagorn, J.C. and Iovanna, J.L. (2001b) TGFβ-1 enhances the Smads transcriptional activity through activation of p8 gene expression. Biochem. J., 357, 249–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey M. et al. (1993) In vitro growth characteristics of embryo fibroblasts isolated from p53-deficient mice. Oncogene, 8, 2457–2467. [PubMed] [Google Scholar]
- Jamora C., Dennert, G. and Lee, A.S. (1996) Inhibition of tumor progression by suppression of stress protein GRP78/BiP induction in fibrosarcoma B/C10ME. Proc. Natl Acad. Sci. USA, 93, 7690–7694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y.F., Vaccaro, M.I., Fiedler, F., Calvo, E.L. and Iovanna, J.L. (1999) Lipopolysaccharides induce p8 mRNA expression in vivo and in vitro. Biochem. Biophys. Res. Comm., 260, 686–690. [DOI] [PubMed] [Google Scholar]
- Mallo G.V., Fiedler, F., Calvo, E.L., Ortiz, E.M., Vasseur, S., Keim, V., Morisset, J. and Iovanna, J.L. (1997) Cloning and expression of the rat p8 cDNA, a new gene activated in pancreas during the acute phase of pancreatitis, pancreatic development, and regeneration, and which promotes cellular growth. J. Biol. Chem., 272, 32360–32369. [DOI] [PubMed] [Google Scholar]
- Park B.H., Vogelstein, B. and Kinzler, K.W. (2001) Genetic disruption of PPARδ decreases the tumorigenicity of human colon cancer cells. Proc. Natl Acad. Sci. USA, 98, 2598–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ree A.H., Tvermyr, M., Engebraaten, O., Rooman, M., Rosok, O., Hovig, E., Meza-Zepeda, L.A., Bruland, O.S. and Fodstad, O. (1999) Expression of a novel factor in human breast cancer cells with metastatic potential. Cancer Res., 59, 4675–4680. [PubMed] [Google Scholar]
- Ree A.H., Pacheco, M.M., Tvermyr, M., Fodstad, O. and Brentani, M.M. (2000) Expression of a novel factor, com1, in early tumor progression of breast cancer. Clin. Cancer Res., 6, 1778–1783. [PubMed] [Google Scholar]
- Ruley H.E. (1990) Transforming collaborations between ras and nuclear oncogenes. Cancer Cells, 2, 258–268. [PubMed] [Google Scholar]
- Serrano M., Lin, A.W., McCurrach, M.E., Beach, D. and Lowe, S.W. (1997) Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell, 88, 593–602. [DOI] [PubMed] [Google Scholar]
- Shirasawa S., Furuse, M., Yokoyama, N. and Sasazuki, T. (1993) Altered growth of human colon cancer cell lines disrupted at activated Ki-ras. Science, 260, 85–88. [DOI] [PubMed] [Google Scholar]
- Storm F.K., Mahvi, D.M. and Gilchrist, K.W. (1996) Heat shock protein 27 overexpression in breast cancer lymph node metastasis. Ann. Surg. Oncol., 3, 570–573. [DOI] [PubMed] [Google Scholar]
- Su S.B. et al. (2001) Expression of p8 in human pancreatic cancer. Clin. Cancer Res., 7, 309–313. [PubMed] [Google Scholar]
- Vasseur S., Mallo, G.V., Fiedler, F., Bodeker, H., Canepa, E., Moreno, S. and Iovanna, J.L. (1999) Cloning and expression of the human p8, a nuclear protein with mitogenic activity. Eur. J. Biochem., 259, 670–675. [DOI] [PubMed] [Google Scholar]
- Yeatman T.J. and Nicolson, G.L. (1993) Molecular basis of tumor progression: mechanisms of organ-specific tumor metastasis. Semin. Surg. Oncol., 9, 256–263. [PubMed] [Google Scholar]