Abstract
The mechanisms that regulate the transcription of the tumour suppressor genes p53 and IRF-1 are poorly understood. We have characterized a 68-kDa transcription factor, GAAP-1 (gatekeeper of apoptosis activating proteins), encoded by an alternative splice product of the PRDII-BF1 gene, that recognizes a novel regulatory element within the p53 and IRF-1 promoters. Transfection of U937 cells with GAAP-1 activates p53 and IRF-1 expression and leads to apoptosis, whereas over-expression of GAAP-1 in K562 cells that lack p53 and IRF-1 induces cell differentiation. Alterations in the 6p24 locus containing the GAAP-1 gene are frequent in acute myelogenous leukemia (AML), and AML-derived cell lines display reduced GAAP-1 mRNA levels. Together, these results suggest that GAAP-1 acts as a gatekeeper at a critical point in the tumour suppressor gene pathway.
INTRODUCTION
DNA damage results in enhanced p53 activity and transcriptional activation of genes involved in cell growth arrest, DNA repair or apoptosis (Vogelstein et al., 2000). A defective p53 response is caused by mutations in the coding region of the gene in >50% of human cancers, inactivation of the protein by viral oncoproteins or deregulated protein turnover (Soussi, 2000). The absence or reduced expression of the p53 gene is observed in certain tumour cell lines (Raman et al., 2000), suggesting that transcriptional dysregulation can also cause loss of p53 function.
Recent evidence suggests that interferon regulatory factor 1 (IRF-1), originally identified as a positive transcriptional regulator of the interferons, also controls susceptibility to tumours. Thus, IRF-1 is required for cell cycle arrest and activates at least two genes, p21waf1 and p27kip1, involved in cell cycle control. Furthermore, chromosomal deletion or inactivation of an IRF-1 allele occurs frequently in human leukemias or the preleukemic syndrome, and loss of functional IRF-1 mRNA by exon skipping is observed in 20% of patients with myelodysplastic syndrome. Loss of heterozygosity at the IRF-1 locus is also common in patients with gastric or esophageal cancers (Taniguchi et al., 2000).
We have identified a novel regulatory element, IPCS (IRF-1 p53 common sequence), within the promoter regions of the p53, IRF-1, Rb, Bax, Wt1, mm23H1 and p21WAF1 genes, that is required for the maintenance of basal or induced transcriptional activity of the p53 and IRF-1 genes (Lallemand et al., 1997). We have now cloned a cDNA encoding a polypeptide that binds specifically to the IPCS element, regulates p53 and IRF-1 dependent cell proliferation and apoptosis and induces differentiation in cells that lack p53 and IRF-1.
RESULTS AND DISCUSSION
Molecular cloning and sub-cellular localization of GAAP-1
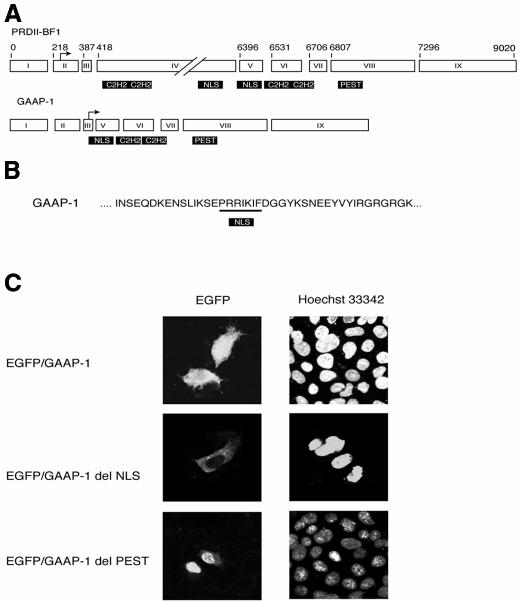
A yeast one-hybrid system was used to identify a cDNA encoding a polypeptide denominated GAAP-1, which binds specifically to the IPCS sequence. This cDNA is identical to the human PRDII-BF1 cDNA (Figure 1A) between nucleotides 6488 and 9020 (Baldwin et al., 1990) and corresponds to the 2.5-kb transcript described by Muchardt et al. (1992). The PRDII-BF1 cDNA was first described as encoding a 300-kDa zinc-finger protein that binds to the NF-κB recognition sequence and activates HIV-1 gene expression (Baldwin et al., 1990). Proteins of 200 and 68 kDa of previously unknown function, which do not activate HIV gene expression (Muchardt et al., 1992), are generated by alternative splicing of the PRDII-BF1 gene. GAAP-1, which is identical to the 68-kDa alternative splice product of the PRDII-BF1 gene, contains three principal motives (Figure 1A): a putative nuclear localization signal (NLS) (Figure 1B) distinct from the NLS identified previously, the two previously characterized C2-H2-type zinc fingers (Fan and Maniatis, 1990) and an acidic amino acid rich PEST-like domain.
Fig. 1. GAAP-1 is a differential splice product of PRDII-BF1 precursor mRNA. (A) Schematic representation of the exon composition of PRDII-BF1 and GAAP-1 transcripts. These data are inferred from the work of Xu et al. (1998) and Fan and Maniatis (1990). The nucleotide positions are as in Fan and Maniatis (1990). (B) Partial amino acid sequence of wild-type GAAP-1. The NLS is underlined. (C) Sub-cellular localization of EGFP/GAAP-1 variants. HuH7 cells were transiently transfected with the expression vector indicated. After 24 h, the nuclei were stained with Hoechst 33342 and examined by confocal microscopy.
Transient transfection of HuH7 cells with a plasmid encoding an EGFP/GAAP-1 fusion protein showed that the protein was localized in both the nucleus and the cytoplasm (Figure 1C), suggesting that the activity of GAAP-1 may be regulated by compartmentalization. In contrast, fluorescence was strictly cytoplasmic in cells transfected with the NLS deleted mutant (Figure 1C). More surprisingly, transfection of cells with the EGFP/GAAP-1 fusion protein with a deleted PEST-like sequence produced a strictly nuclear fluorescence, suggesting that post-translational mechanisms may be implicated in the regulation of GAAP-1 activity (Figure 1C).
GAAP-1 possesses a double binding specificity involving the same zinc-finger structure
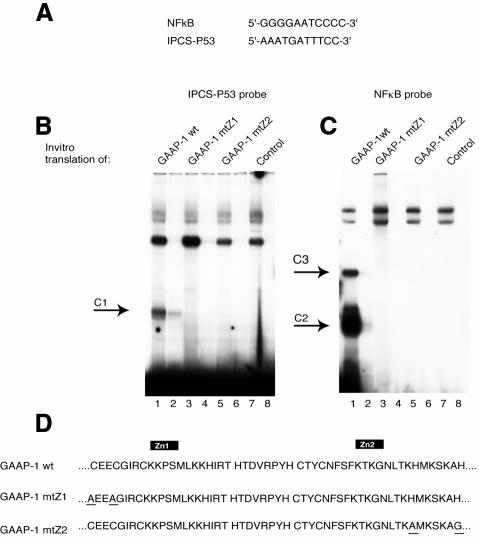
PRDII-BF1 was first described as a protein that bound to an NF-κB recognition sequence, a property conferred by the presence of two pairs of zinc fingers, one of which is contained within GAAP-1 (Fan and Maniatis, 1990). GAAP-1 was isolated, however, using a sequence unrelated to NF-κB, suggesting that GAAP-1 was able to bind to at least two unrelated DNA sequences (Figure 2A). To confirm the binding specificity of GAAP-1 for the IPCS target sequence, in vitro translated GAAP-1 was incubated with labelled oligonucleotides containing the IPCS or NF-κB recognition sequences and analysed by electrophoretic mobility shift assays (EMSAs). GAAP-1 formed a single complex, C1, with IPCS (Figure 2B, lane 1) and two complexes, C2 and C3, with the NF-κB oligonucleotide (Figure 2C, lane 1), demonstrating the dual specificity of GAAP-1 binding. The slower migrating complex, C3, observed when the NF-κB sequence was employed (Figure 2C, lane 1), was obtained using either wheatgerm extracts or reticulocyte lysates (data not shown), suggesting that this complex is a homodimeric form of GAAP-1 and does not result from the interaction of GAAP-1 and a component of the in vitro translation extract.
Fig. 2. GAAP-1 possesses a double binding specificity involving the same zinc-finger structure. (A) NF-κB and IPCS-p53 oligonucleotides. (B) EMSA using the IPCS-p53 probe and in vitro translated GAAP-1 (lane 1), GAAP-1 mtZ1 (lane 3), GAAP-1mt Z2 (lane 5) or reticulocyte lysate with the empty expression vector (lane 7). Even lanes: 50-fold molar excess of IPCS-p53 oligonucleotide was added to the reaction of the previous lane. The arrow indicates the specific complex. (C) The same as in (B), except that the IPCS-p53 probe was replaced by the NF-κB probe. Even lanes: 50-fold molar excess of NF-κB oligonucleotide. (D) Amino acid sequence of the two zinc-finger domains. The residues that are substituted in GAAP-1 mtZ1 or in GAAP-1 mtZ2 are underlined.
To determine whether each zinc finger of the GAAP-1 protein was responsible for binding to one of the two specific sequences, two mutants were constructed, each containing one zinc finger only, the other being destroyed by substitution of the cysteine or histidine, necessary for the tertiary structure of a finger, by an alanine or glycine (Figure 2D). The two in vitro translated mutants did not form any complex in EMSAs with either the IPCS-p53 (Figure 2B, lanes 3 and 5) or the NF-κB (Figure 2C, lane 3 and 5) probes, suggesting that both zinc fingers are required for the binding of GAAP-1 to either the IPCS or NF-κB sequences. Thus, to our knowledge, GAAP-1 is the first transcriptional activator to be described that binds specifically to two different recognition sequences with the same two zinc fingers.
Transcriptional properties of GAAP-1
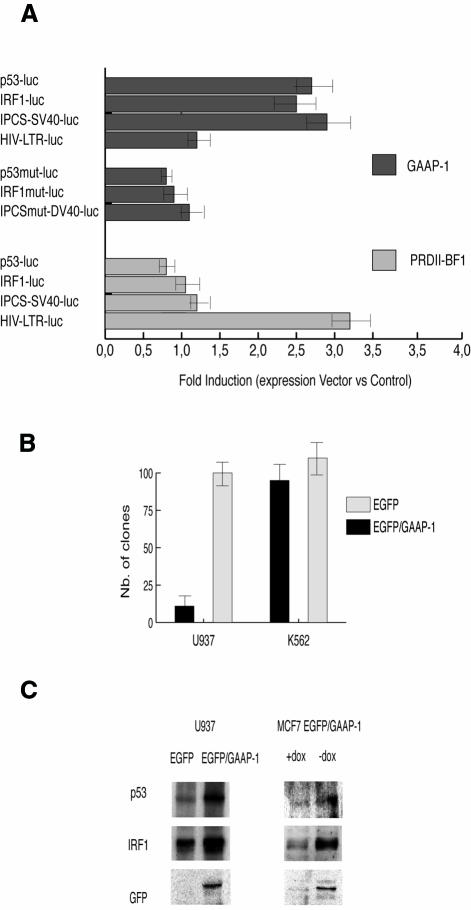
To determine the contribution of GAAP-1 to the transcriptional activity of the p53 and IRF-1 promoters, U937 cells were co-transfected with plasmids containing the luciferase reporter gene under the control of the p53 or IRF-1 wild-type promoters or the p53 and IRF-1 promoters mutated in the IPCS (Lallemand et al., 1997). Alternatively, cells were transfected with a chimeric promoter consisting of an IPCS minimum binding site or a mutated version of this binding site, cloned upstream of the simian virus 40 (SV40) minimal promoter directing the expression of the luciferase reporter gene. Co-transfection of cells with the GAAP-1 expression vector increased the p53-luc, IRF-1-luc or IPCS-SV40-luc activity ∼2.5-fold compared with cells transfected with the same reporter gene construct and the control expression vector (Figure 3A). In contrast, luciferase activity expressed by constructs containing the mutated IPCS sequence co-transfected with the GAAP-1 expression vector was comparable with the activity obtained by co-transfection of wild-type reporter vectors and an empty expression vector. These data show that GAAP-1 possesses positive transcriptional activity. The IPCS-mutated IRF-1 and p53 promoters contained functional NF-κB sites (Sims et al., 1993; Wu and Lozano, 1994), demonstrating that, although GAAP-1 can bind the NF-κB sequence, this sequence is not implicated in the transcriptional regulation of IRF-1 and p53 by GAAP-1. The failure of GAAP-1 to increase luciferase activity in cells co-transfected with a HIV-LTR-luc reporter gene construct, containing NF-κB binding sites, is in agreement with the results of Muchardt et al. (1992) and confirm that GAAP-1 was transcriptionally active only when bound to an IPCS sequence. In contrast, a similar experiment performed using a PRDII-BF1 expression vector resulted in increased HIV-LTR reporter gene activity, as reported previously (Seeler et al., 1994), while the reporter gene constructs containing an IPCS were not activated (Figure 3A).
Fig. 3. Transcriptional properties of GAAP-1. (A) Relative activity of the wild-type and mutated p53 and IRF-1 promoters, IPCS-SV40 chimeric promoter and the HIV-LTR in a co-transfection assay. U937 cells were transiently co-transfected with 3 µg of the reporter plasmids and 7 µg of the expression vectors indicated. The pcDNA3.1 vector was used as a control. The pRL-SV40 plasmid was used as an internal control for the normalization of transfection efficiency. The results shown in this figure represent the ratio between the luciferase activity obtained with each promoter construct in the presence of the co-transfected GAAP-1 or PRDII-BF1 plasmids and the activity obtained in their absence. The ratio between the luciferase activity obtained with the wild-type promoter constructs and the activity obtained with the mutant constructs in the absence of any co-transfected expression vector was ∼2- to 3-fold, reflecting the effect of endogenous GAAP-1 on the basal level of expression of the p53 and IRF-1 promoters. The transfected cells were harvested 8 h after transfection and assayed for luciferase activity. (B) U937 and K562 cells were stably transfected with GAAP-1 or EGFP expression vectors and selected using G418 in semi-solid methyl-cellulose medium. The number of clones was determined at 21 days. (C) Western blotting of 20 µg of cellular extracts from U937 cells or MCF7 Tet-Off cells stably transfected with either an EGFP or EGFP/GAAP-1 expression vector as indicated. In MCF7 Tet-Off cells, EGFP/GAAP-1 expression was induced by the removal of doxycycline from the culture medium. The blot was probed with anti-p53, anti-IRF-1 or anti-GFP antibodies.
Pro-apoptotic/apoptotic properties of GAAP-1
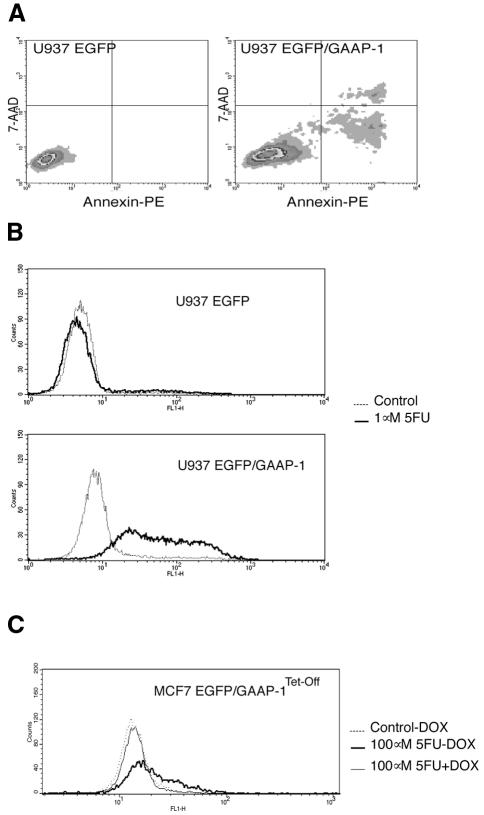
To determine whether GAAP-1 possesses growth-suppressive and/or apoptotic properties, U937 or MCF7 cells, which express both IRF-1 and p53 (Gabriele et al., 1999), were respectively transfected or retroviral transduced with the EGFP, GAAP-1 or EGFP/GAAP-1 expression vectors. The number of stable clones obtained in semi-solid medium from U937 cells transfected with GAAP-1 (data not shown) or EGFP/GAAP-1 was approximately one-tenth of that obtained with cells stably transfected with the EGFP control plasmid (Figure 3B). Moreover, sub-culturing these clones triggered a high level of spontaneous apoptosis (Figure 4A). The remaining viable clones, which were shown to express EGFP/GAAP-1, proliferated in a manner similar to parental cells. Western blotting of total cellular extracts from these cells showed that p53 and IRF-1 were induced 4- to 6-fold in U937-EGFP/GAAP-1 cells compared with U937-EGFP transfected cells (Figure 3C). Similar results were obtained using extracts of MCF7-EGFP/GAAP-1Tet-Off cells, indicating that GAAP-1 is implicated in the expression of endogenous p53 and IRF-1. Treatment of U937-EGFP/GAAP-1 cells with 5-fluoro-uracil (5FU) induced marked apoptosis determined by TdT-mediated dUTP nick end-labelling (TUNEL) analysis (Figure 4B) at a dose (1 µM) that did not induce apoptosis in control U937 cells. Similar results were also obtained using GAAP-1 transfected U937 cells (data not shown). Although MCF7-EGFP/GAAP-1Tet-Off cells did not exhibit spontaneous apoptosis in the absence of doxycycline, apoptosis was observed, however, after treatment of the cells with 100 µM 5FU (Figure 4C). Together, these results show that increased expression of p53 and IRF-1 occurs in GAAP-1 transfected cells and is correlated with enhanced susceptibility of the cells to apoptosis.
Fig. 4. Apoptotic properties of GAAP-1. (A) Spontaneous apoptosis of U937 cells stably transfected with EGFP or EGFP/GAAP-1 expression vectors was assayed by annexin V-PE/7-AAD double labelling. (B) U937 cells stably transfected with either the EGFP or EGFP/GAAP-1 fusion protein expression vectors were treated, or not, with 1 µM 5FU. (C) MCF7 Tet-Off cells stably transfected with the inducible EGFP/GAAP-1 fusion protein expression vector were treated with 100 µM 5FU, and the fusion protein was induced by the removal of doxycycline from the medium. Apoptosis was analysed by the TUNEL assay 72 h after treatment.
In contrast to the results obtained with U937 cells, the number of clones obtained following transfection of K562 cells with EGFP/GAAP-1, GAAP-1 or control EGFP vectors was similar (Figure 3B). Furthermore, K562 cells expressing GAAP-1 or EGFP/GAAP-1 did not exhibit increased apoptosis relative to control cells, following treatment with 5FU (data not shown). Thus, failure to trigger apoptosis by over-expression of GAAP-1 in K562 cells correlated with the lack of a functional p53 gene (Lubbert et al., 1988) and undetectable IRF-1 expression by western blotting (data not shown). Over-expression of either GAAP-1 or EGFP/GAAP-1, however, induced a 2.5-fold increase in the hemoglobin content of K562 cells (Figure 5A) relative to parental cells and was comparable to that observed in parental cells treated with Ara-C or hemin. Expression of GAAP-1 or EGFP/GAAP-1 also markedly enhanced the differentiation of K562 cells induced by Ara-C or hemin. These results suggest that GAAP-1 induces the expression of genes involved in erythroid differentiation independently of IRF-1 and p53 expression.
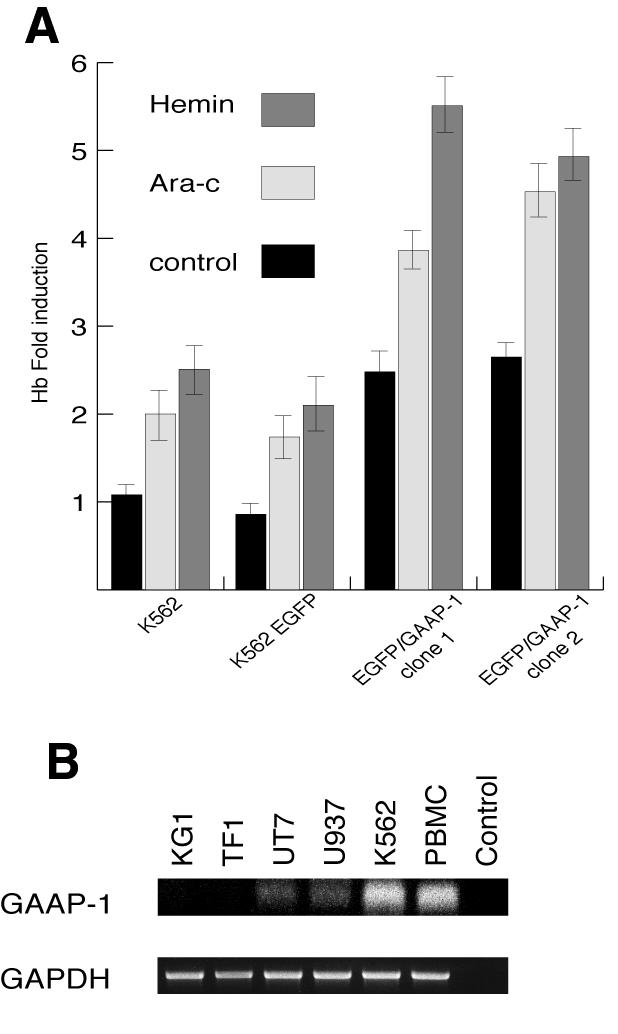
Fig. 5. Effect of GAAP-1 on the differentiation of K562 cells. (A) Parental K562 cells or clones stably transfected with either the EGFP or EGFP/GAAP-1 expression vectors were left untreated or treated for 4 days with Ara-C (1 µM) or hemin (5 µM) and then assayed for hemoglobin content. (B) Total RNA (1 µg) from the AML-derived cell lines KG1, TF1, UT7 and U937, the CML-derived cell line K562 and PBMC from a healthy donor were analysed by RT–PCR. Reverse transcription was carried out using an anti-sense primer localized in exon VI, and amplification was carried out using a pair of primers localized in exon III and exon V. The amplification of GAAP-1 mRNA produces a fragment of ∼300 bp, whereas mRNA from PRDII-BF1 is not amplified under these conditions due to its size of >6 kb. The identity of the 300-bp fragment was confirmed by direct sequencing.
GAAP-1 expression is altered in AML-derived cell lines
Although mutations in the p53 gene are common in certain human tumours, they are infrequent in acute myeloid leukemia (AML) (Nakano et al., 2000). In contrast, the 6p24 locus containing the PRDII-BF1 gene is frequently altered in AML (Chen et al., 2000), suggesting that alteration of GAAP-1 expression may be involved in the development of this malignancy. Thus, GAAP-1 mRNA was hardly detectable using RT–PCR in the AML-derived cell lines KG1, TF1, UT7 and U937, in contrast to the CML-derived cell line K562 and PBMC from healthy donors that expressed readily detectable levels of GAAP-1 mRNA (Figure 5B). Furthermore, GAAP-1 binding activity, as determined by EMSAs performed with nuclear extracts from U937 or K562 cells (data not shown), also appeared to be present at lower levels in U937 cells than in K562 cells.
Together, these results demonstrate the importance of GAAP-1 in the transcriptional control of p53 and IRF-1 activity, alone or in conjunction with other factors (Raman et al., 2000), and suggest that GAAP-1 targets are multiple and play an important role in the processes of cell growth control, apoptosis and differentiation. Thus, these results, together with the observation that the IPCS recognition sequence is present in numerous other human tumour suppressor genes, including Rb, Bax, Wt1, mm23H1 and p21WAF1, suggest that GAAP-1 may well act as a gatekeeper at a critical point in the tumour suppressor gene pathway and, as such, may represent a target for therapeutic intervention.
METHODS
Cloning of GAAP-1 using a yeast one-hybrid system. The reporter constructs were generated by inserting one copy of the double-stranded oligonucleotides IPCS-p53, IPCS-p53mut and IPCS-IRF-1 (Table I) in the pHISi vector (Clontech). Screening of a human leukocyte cDNA library (Clontech) encoding proteins fused with the GAL4 activation domain was performed by the lithium acetate method in Saccharomyces cerevisiae YM4271 (Clontech) carrying the HIS3 reporter gene under the control of one IPCS-p53 sequence.
Table I. Oligonucleotides used in this study.
| Oligonucleotide | Sequence 5′-3′ |
|---|---|
| IPCS-p53 |
AAAATGATTTCCAC |
| IPCS-p53mut |
AAAACGATTTCCAC |
| IPCS-IRF1 |
GAAATGACGGCACG |
| C1 s |
AGCATGGCATTAGGTAATCAAAAGTCCACAG |
| C1 as |
CCATCAGGTTGCTATCACAAGC |
| GFP s |
ACCATGGTGAGCAAGGGCGA |
| GFP/GAAP as |
GATTACCTAATGCTCTCTTGTACAGCTCG |
| GFP/GAAP s |
GGACGAGCTGTACAAGATGGGGCAGAAGTTTCAAAA |
| GAAP as |
CCATCAGGTTGCTATCACAAGC |
| NLS s |
CCTTAATCAAAAGTGAAGATGGAGGATATAAGTCA |
| NLS as |
ATCCTCCATCTTCACTTTTGATTAAGGAATT |
| Zn1 s |
GCAGAAGAAGCTGGAATACGTTGTAAGAAAC |
| Zn1 as |
AGCTTCTTCTGCAATGTATTTTCCTCTTCC |
| Zn2 s |
GCAATGAAGTCCAAGGCAGGAAGCAAGAAATGTGTGG |
| Zn2 as |
TCCTGCCTTGGACTTCATTGCTTTTGTCAGATTTCC |
| C4 as |
CCTACTGAGATGCCTAAATCCAC |
| C2 s |
GAACTTAATGGGGCAGAAGTTTC |
| C2 as |
GCTAGGTTTCTTACAACGTATTCG |
| PEST s |
TCAGTTATGAGCGAGCTGAATCAGTCCTGTC |
| PEST as | GACAGGACTGATTCAGCTCGCTCATAACTGA |
Cell culture, transfections and analysis. U937 and K562 cells were cultivated in RPMI 1640 medium with 10% fetal bovine serum (FBS) (Life Technologies) and HuH7 and MCF7 Tet-Off (Clontech) cells in DMEM medium and 10% FBS. Cells were transfected with 10 µg DNA per 106 cells in 0.8 ml of medium by electroporation as described previously (Lallemand et al., 1997). The generation of amphotropic retrovirus using transient transfection of the AmphoPack packaging line (Clontech) and infection of MCF7 Tet-Off cells was performed according to the manufacturer’s instructions (Clontech). Luciferase activity was determined using the Dual-Luciferase Reporter Assay System (Promega). 5FU, cytosine β-d-arabinofuranoside and hemin were purchased from Sigma-Aldrich. Apoptosis was analysed by flow cytometry using annexin V-PE/7-AAD double labelling (BD Pharmingen) or TUNEL assay (BD Pharmingen). Hemoglobin levels were determined using benzidine–HCl as described elsewhere (Ray et al., 1996).
Plasmids. The GAAP-1 expression vector was constructed by cloning the PCR product (nucleotides 6396–8601) from a human leukocyte cDNA library (Clontech) in the pcDNA3.1 expression vector using the C1 s and C1 as primers pairs. The plasmid expressing the EGFP/GAAP-1 fusion protein was obtained by fusion PCR (Higuchi, 1990), using the primer couples, GFP s and GFP/GAAP as, and GFP/GAAP s and GAAP as (Table I), to amplify, respectively, the EGFP and GAAP-1 coding region. The EGFP/GAAP-1 del NLS, the EGFP/GAAP-1 del PEST, the GAAP-1 mtZ1 and the GAAP-1 mtZ2 (Table I) were obtained by overlapping PCR using the primer couples, NLS s and NLS as, PEST s and PEST as, Zn1 s and Zn1 as, and Zn2 s and Zn2 as (Table I). All the coding regions were cloned in the expression vector pcDNA3.1 (Invitrogen) or in tetracycline controlled expression vector pRev-TRE (Clontech). The integrity of the constructs was verified by sequencing.
EMSAs and western blotting. In vitro transcription and translation of GAAP-1 proteins was carried out using the TNT coupled Reticulocyte Lysate Systems (Promega). EMSAs were carried out as described previously (Lallemand et al., 1997) using 4 µl of in vitro translated product and the oligonucleotides (and their complementary strands) described in Figure 2A. Western blotting was carried out as described previously (Lallemand et al., 1997). Typically, 20 µl of cellular extract was run on 10% SDS–PAGE, transferred to a PVDF membrane and incubated with anti-p53 (Pab 240) or anti-IRF-1(C-20) antibodies (Santa Cruz). Signals were detected with an ECL kit (Amersham).
RT–PCR. Reverse transcription was carried out using a Reverse Transcription System (Promega), 1 µg of RNA and a specific primer, C4 as (Table I). The cDNA was amplified by pfu DNA polymerase (Promega) using the C2 s and C2 as primers shown in Table I.
Acknowledgments
ACKNOWLEDGEMENTS
This work was supported by grants from ARC, Nouvelles Recherches Biomédicales, Fondation pour la Recherche Médicale and Ligue Nationale contre le Cancer.
REFERENCES
- Baldwin A.S. Jr, LeClair, K.P., Singh, H. and Sharp, P.A. (1990) A large protein containing zinc finger domains binds to related sequence element in the enhancer of the class I major histocompatibility complex and κ immunoglobulin genes. Mol. Cell. Biol., 10, 1406–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Issa, B., Brothman, L.J., Hendricksen, M., Button, D. and Brothman, A.R. (2000) Nonrandom rearrangements of 6p in malignant hematological disorders. Cancer Genet. Cytogenet., 121, 22–25. [DOI] [PubMed] [Google Scholar]
- Fan C.M. and Maniatis, T. (1990) A DNA-binding protein containing two widely separated zinc finger motifs that recognize the same DNA sequence. Genes Dev., 4, 29–42. [DOI] [PubMed] [Google Scholar]
- Gabriele L., Phung, J., Fukumoto, J., Segal, D., Wang, I.M., Giannakakou, P., Giese, N.A., Ozato, K. and Morse, H.C.,III (1999) Regulation of apoptosis in myeloid cells by interferon consensus sequence-binding protein. J. Exp. Med., 190, 411–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi R. (1990) Recombinant PCR. In Innis, M.A., Gelfand, D.H. and Sninsky, J.J. (eds), PCR Protocols: A Guide to Methods and Applications. Academic Press, New York, NY, pp. 177–183.
- Lallemand C., Bayat-Sarmadi, M., Blanchard, B. and Tovey, M.G. (1997) Identification of a novel transcriptional regulatory element common to the p53 and interferon regulatory factor 1 genes. J. Biol. Chem., 272, 29801–29809. [DOI] [PubMed] [Google Scholar]
- Lubbert M., Miller, C.W., Crawford, L. and Koeffler, H.P. (1988) p53 in chronic myelogenous leukemia: study of mechanisms of differential expression. J. Exp. Med., 167, 873–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchardt C., Seeler, J.S., Nirula, A., Shurland, D.L. and Gaynor, R.B. (1992) Regulation of human immunodeficiency virus enhancer function by PRDII-BF1 and c-rel gene products. J. Virol., 66, 244–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano Y., Naoe, T., Kiyoi, H., Kitamura, K. and Ohno, R. (2000) Prognostic value of p53 gene mutations and the product expression in de novo acute myeloid leukemia. Eur. J. Haematol., 65, 23–31. [DOI] [PubMed] [Google Scholar]
- Raman V., Martensen, S.A., Reisman, D., Evron, E., Odenwald, W.F., Jaffee, E., Marks, J. and Sukumar, S. (2000) Compromised HOXA5 function can limit p53 expression in human breast tumours. Nature, 405, 974–978. [DOI] [PubMed] [Google Scholar]
- Ray S., Bullock, G., Nunez, G., Tang, C. and Bhalla, K. (1996) Enforced expression of Bcl-XS induces differentiation and sensitizes K562 cells to 1-β-d-araC-mediated differentiation and apoptosis. Cell Growth Differ., 7, 1617–1623. [PubMed] [Google Scholar]
- Seeler J.S., Muchardt, C., Suessle, A. and Gaynor, R.B. (1994) Transcription factor PRDII-BF1 activates human immunodeficiency virus type 1 gene expression. J. Virol., 68, 1002–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims S.H., Cha, Y., Romine, M.F., Gao, P.Q., Gottlieb, K. and Deisseroth, A.B. (1993) A novel interferon-inducible domain: structural and functional analysis of the human interferon regulatory factor 1 gene promoter. Mol. Cell. Biol., 13, 690–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soussi T. (2000) The p53 tumor suppressor gene: from molecular biology to clinical investigation. Ann. NY Acad. Sci., 910, 121–137. [DOI] [PubMed] [Google Scholar]
- Taniguchi T., Ogasawara, K., Takaoka, A. and Tanaka, N. (2000) IRF family of transcription factors as regulators of host defense. Annu. Rev. Immunol., 19, 623–655. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Lane, D. and Levine, A.J. (2000) Surfing the p53 network. Nature, 408, 307–310. [DOI] [PubMed] [Google Scholar]
- Wu H. and Lozano, G. (1994) NF-κ B activation of p53: a potential mechanism for suppressing cell growth in response to stress. J. Biol. Chem., 269, 20067–20074. [PubMed] [Google Scholar]
- Xu G., Sze, S.H., Liu, C.P., Pevzner, P.A. and Arnheim, N. (1998) Gene hunting without sequencing genomic clones: finding exon boundaries in cDNAs. Genomics, 47, 171–179. [DOI] [PubMed] [Google Scholar]