Abstract
Loss of the retinoblastoma protein (pRb) induces a cell-nonautonomous defect in both erythroid and neuronal differentiation. It has previously been thought that this reflects a requirement for pRb function in cells that normally support erythropoiesis and neurogenesis, rather than in the erythrocytes or neurons themselves. However, recent studies have challenged this interpretation, and it appears that erythrocytes and neurons themselves have the intrinsic requirement for pRb function. This requirement can be bypassed by signals supplied by wild-type erythroid or neuronal cells. The existence of such a signalling mechanism has implications not only in understanding pRb function but also in the interpretation of other cell-nonautonomous phenotypes.
The pRb protein
The retinoblastoma protein (pRb) is the founding member of the family of so-called pocket proteins, which also includes the proteins p107 and p130 (Lipinski and Jacks, 1999). Although pRb plays a pivotal role in controlling gene expression to regulate cellular proliferation, apoptosis and differentiation (Lipinski and Jacks, 1999; Vooijs and Berns, 1999; Nevins, 2001; Zhang and Dean, 2001), it lacks a DNA-binding domain and is tethered to promoters through its interaction with other transcription factors (Zhang and Dean, 2001). The interaction of pRb with such factors is inhibited by its phosphorylation (Sherr, 1996), a process that is mediated by cyclin-dependent kinases (CDKs), which are themselves regulated by the CDK inhibitors (Sherr and Roberts, 1999). The E2F family of transcription factors is among those bound and regulated by hypophosphorylated pRb (Nevins, 2001). The latter acts both by directly inhibiting E2F transactivating activity and by recruiting transcriptional repression complexes to promoters containing E2F sites (Lipinski and Jacks, 1999). In fact, pRb phosphorylation results in disruption of pRb/E2F complexes and is thought to be a crucial event in the G1 to S phase transition (Sherr, 1996; Sherr and Roberts, 1999). Hypophosphorylated pRb also binds to and regulates the function of other proteins, including the helix–loop–helix protein Id2 (Lasorella et al., 2000), CAAT/enhancer binding proteins (Chen et al., 1996) the HMG family member HBP1 (Shih et al., 1998) and, possibly, basic-helix–loop–helix proteins such as MyoD (Gu et al., 1993). pRb also appears to regulate gene transcription by recruiting chromatin remodelling factors such as histone deacetylases, the SWI/SNF complex, DNA methyltransferases and histone methyltransferases (Ferreira et al., 2001; Nielsen et al., 2001; Zhang and Dean, 2001).
Loss of pRb in mice
Recent work on the role of pRb in erythropoiesis has revealed a novel link between this cell cycle regulatory protein and intercellular signalling. pRb regulates the development and differentiation of various tissues, including central and peripheral nervous system, muscle, retina, lens and blood (Lipinski and Jacks, 1999; Vooijs and Berns, 1999). However, pRb does not induce a simple cell-autonomous defect in some of these tissues, i.e. the phenotype of the cell does not directly correlate with its genotype. This was demonstrated by the finding that, in pRb null chimeric animals, the mutant cells contributed signficantly to both the erythroid and neuronal lineages (Maandag et al., 1994; Williams et al., 1994). It is important to understand how pRb functions in this cell-nonautonomous manner in these lineages.
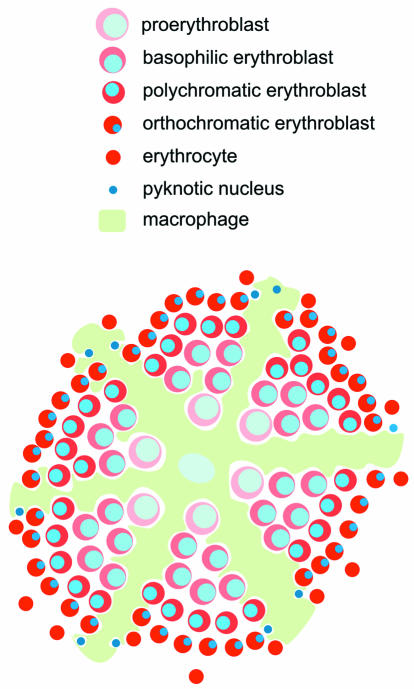
The requirement for pRb function was demonstrated by gene disruption experiments in mice. Complete loss of pRb resulted in lethality during gestation at around 14 days post coitum (d.p.c.). pRb null embryos displayed excessive neuronal cell death and were anemic. The latter effect appears to have been due to the disruption of fetal liver erythropoiesis. Normally, at this stage in development, definitive (or adult) enucleated erythrocytes replace primitive (or embryonic) nucleated yolk-sac derived erythrocytes in the circulation. The first and major site of definitive erythropoiesis at this stage is the fetal liver. Definitive erythropoiesis in the fetal liver (and later in the spleen and bone marrow) occurs in erythroblastic islands (Bessis et al., 1983). These contain a central macrophage surrounded by erythroid cells at different stages of maturation. The latter are arranged in a characteristic pattern, with immature cells close to the centre and mature cells near the edge (Figure 1) (Bessis et al., 1983; Bernard, 1991). In pRb null embryos, the number of the definitive erythrocytes was dramatically reduced. Instead, immature (nucleated) fetal liver derived cells were present in the circulation in high numbers (Clarke et al., 1992; Jacks et al., 1992; Lee et al., 1992). Furthermore, erythroid colonies grown from pRb null fetal livers were defective in differentiation, i.e. the colonies were more numerous and the cells were paler and failed to enucleate efficiently (Jacks et al., 1992).
Fig. 1. A simplified model of the erythroblastic island. The position of the central macrophage and the arrangement of the erythroid precursors within the macrophage cytoplasmic extensions are shown (as indicated by the key, in order of maturation). The processes of enucleation and phagocytosis of expelled pyknotic nuclei are represented.
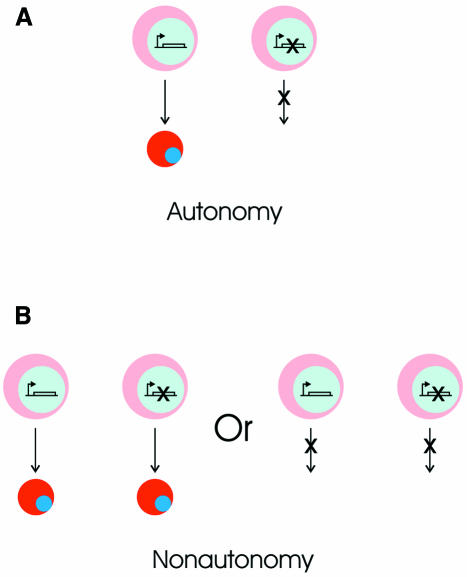
From these experiments, it is unclear whether pRb is intrinsically required in the erythroid cells for their normal differentiation or in a cell type that supports erythropoiesis, such as a growth factor producing stromal cell. Chimeric mice are usually generated to determine whether a gene functions autonomously or nonautonomously in a particular lineage. Gene function is considered cell autonomous when a cell displays a phenotype corresponding to its genotype, regardless of the genotype of surrounding cells (Figure 2A). In the case of the pRb null chimeric mice (Maandag et al., 1994; Williams et al., 1994), the function of pRb in the erythroid lineage would be considered cell autonomous if pRb null cells failed to differentiate and the surrounding wild-type erythroid cells differentiated normally. Gene function is defined as cell nonautonomous when a cell displays a phenotype that does not correspond to its genotype (Figure 2B) (Apfeld and Kenyon, 1998): in other words, where wild-type cells display the phenotype of mutant cells or mutant cells display the phenotype of wild-type cells. In the chimeric animals, pRb null cells contributed highly to the erythroid compartment (Maandag et al., 1994; Williams et al., 1994). Importantly, the differentiation of pRb null erythroid cells was indistinguishable from that of wild-type cells. The only phenotypic change in a few mice was a transient persistence of nucleated cells (both wild-type and pRb null) in the circulation of chimeras with a relatively higher contribution of pRb null cells (Maandag et al., 1994). Since genotypically pRb null erythroid cells display a wild-type phenotype, the function of pRb in erythropoiesis is, by definition, cell nonautonomous.
Fig. 2. Cell autonomy and cell nonautonomy in heterocellularly mutant cell populations. (A) Gene function is considered cell autonomous when a cell displays a phenotype corresponding to its genotype, regardless of the genotype of surrounding cells. The pink cell on the left, with a wild-type genome, becoming a red cell represents normal differentiation. The cell on the right has a mutation (shown as a crossed gene) that prevents differentiation. (B) In cell nonautonomy, mutant cells display the phenotype of wild-type cells (left panel) or wild-type cells display the phenotype of mutant cells (right panel; this situation is not discussed in this paper).
The cell that requires pRb
These observations beg the question: in which cell type is the function of the pRb gene required for normal erythropoiesis? If the defect had been cell autonomous, the interpretation would have been that the pRb gene is required in the erythroid cells themselves for their normal development and/or differentiation. Conversely, when cell nonautonomy occurs, as is the case in the pRb chimeras, the gene is thought to function in cells other than those that exhibit the original phenotype (Rossant and Spence, 1998). In principle, if a cell-nonautonomous phenotype is displayed by cell A, then the function of gene X is required in cell B (Figure 3). In such a model, cell B could be supplying a signal that is required for the wild-type phenotype of cell A. Cell A then responds to this signal by displaying a wild-type phenotype, regardless of whether or not gene X is present in its own genome. In the case of the erythroid pRb phenotype, it was proposed that pRb is required in a stromal cell (cell B), which supports erythroid differentiation, and that pRb is not intrinsically required by the erythroid cells themselves (cell A) (Figure 3A) (Maandag et al., 1994; Williams et al., 1994; Vooijs and Berns, 1999). This interpretation is a heterotypic signalling model, where cell A is of a different lineage to cell B. However, subsequent experiments cast doubt on this interpretation. When lethally irradiated wild-type animals (all hematopoietic cells essentially eliminated) were repopulated with pRb null hematopoietic cells, all hematopoietic lineages examined appeared normal, with the exception of the erythroid lineage. In this case, the erythoid cells failed to differentiate normally, showing increased proliferation and a block in differentiation, manifest as a stable persistence of nucleated erythroid cells in the circulation and extensive erythropoiesis outside the bone marrow (Hu et al., 1997). There are two possible outcomes of the irradiation relevant to this experiment, i.e. the stromal cells in the recipient animal survive or not. If the host stromal cells did not survive the irradiation, all donor derived hematopoietic cell lineages requiring stromal cells would be affected, regardless of their pRb requirement (i.e. the same would be observed with wild-type donor tissue). In contrast, if the host stromal cells (pRb positive) persisted after irradiation, the heterotypic model described above predicts that all hematopoietic lineages (pRb negative) would be normal. Neither of these possibilities occurred. This indicates that pRb function must be required in a critical transplantable cell that is lost in the recipient during the lethal irradiation. This cell, in turn, expresses a signal that is required for the normal differentiation of pRb null erythroid cells. This may be a non-erythroid cell, consistent with a heterotypic signalling model. However, such a critical non-erythroid cell has not been identified, making this model unsubstantiated.

Fig. 3. Two possible interpretations of cell nonautonomy. (A) In a heterotypic signalling model, gene function is not required in cell A. The function of the gene is required by cell B, which is of a different lineage. Cell B supplies a signal (red arrows) that is required for the differentiation of cell A. (B) In a homotypic signalling model, the function of the gene is required for the differentiation from cell A to cell B. Cell B supplies a signal that activates the differentiation of wild-type cell A and is required for the differentiation of mutant cell A.
In contrast to a heterotypic model, one can propose a homotypic signalling model (Figure 3B). In this case, cell B, which supplies the signal required by cell A, has differentiated further along the same lineage. According to this model, in the pRb null chimeric mice, the surrounding wild-type erythroid cells would supply the signal that activates the differentiation of less mature pRb null erythroid cells. This is distinct from a heterotypic model, as it is based on the premise that pRb functions in the erythroid cell itself. In a homotypic model, loss of pRb in erythroid cells disrupts the expression of the signal required for the differentiation of pRb null cells. If the gene disrupted is, for example, a paracrine growth factor expressed and required by cells of a particular lineage, such a homotypic model is intuitively obvious. However, pRb is not a paracrine growth factor and it is unlikely that it would regulate only the expression of such factors by erythroid cells. Nevertheless, pRb may indirectly affect the expression of paracrine factors by regulating the differentiation process itself. Indeed, many studies suggest that pRb plays an intrinsic role in regulating the proliferation and differentiation of erythroid cells. During hematopoiesis, pRb is expressed in the erythroid lineage and down-regulated in other lineages (Condorelli et al., 1995). Accumulation of hypophosphorylated pRb is characteristically observed during erythroid differentiation (Sehy et al., 1992; Kiyokawa et al., 1994; Zhuo et al., 1995; Hseih et al., 2000). pRb-mediated G1 arrest is thought to be important in chemically induced differentiation of virally transformed murine erythroleukemia (MEL) cells (Zhuo et al., 1995) and in activin-A induced differentiation of human erythroleukemia cells (Sehy et al., 1992). Inhibition of MEL cell differentiation by the overexpression of the erythroid transcription factor GATA-1 is also associated with reduced levels of hypophosphorylated pRb and the inhibition of differentiation-associated G1 arrest (Whyatt et al., 1997). Antisense experiments suppressing pRb activity have suggested that pRb regulates erythropoiesis (Condorelli et al., 1995; Bergh et al., 1997; Konishi et al., 1999). Various studies have proposed functional cross-regulation between pRb and factors such as Fli-1, PU.1, PML and Tal-1 during erythropoiesis (Konishi et al., 1999; Labbaye et al., 1999; Tamir et al., 1999; Vitelli et al., 2000). When considered alongside the transplantation experiment (Hu et al., 1997), these studies argue that erythroid cells have an intrinsic requirement for pRb activity. A homotypic signalling model reconciling this with the cell nonautonomy found in the chimeras is shown in Figure 3B. In this model, when an erythroid cell is pRb null, the requirement for pRb activity can be bypassed by signals supplied by cells that have already undergone the maturation process.
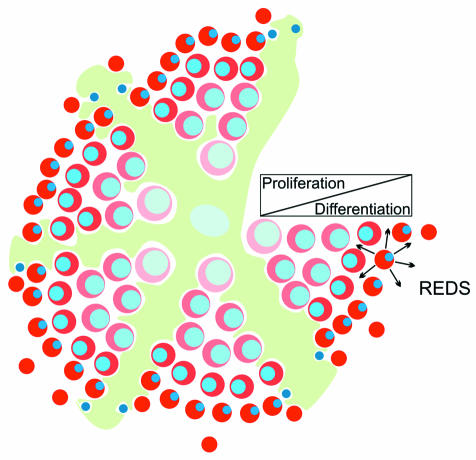
In support of the above, a similar model has already been proposed that is based on an experiment in which the transcription factor GATA-1 was overexpressed in erythroid cells (Whyatt et al., 2000). GATA-1 is a zinc-finger-like transcription factor required for erythroid differentiation, and loss of this factor induced cell-autonomous apoptosis in immature erythroid cells (Pevny et al., 1991; Weiss and Orkin, 1995). Interestingly, mice overexpressing GATA-1 under erythroid-specific activating sequences had an erythroid phenotype similar to that of the pRb null mice (Whyatt et al., 2000). The mice were anemic due to a failure in fetal liver derived erythropoiesis and died at around 13.5 d.p.c. Erythroid cells overexpressing GATA-1 were intrinsically defective and failed to differentiate in mice overexpressing GATA-1 in all erythroid cells. However, this defect was also cell nonautonomous. It is postulated that GATA-1 overexpressing erythroid cells differentiate in response to a signal (designated REDS, for red cell differentiation signal) that is supplied by relatively mature erythroid cells (Whyatt et al., 2000) in the erythroblastic island (Figure 4). In mosaic animals, immature erythroid cells overexpressing GATA-1 would be juxtaposed with wild-type ‘mature’ cells in the same erythroblastic island and/or the neighbouring island, making REDS signalling possible and thereby reversing the defects induced by high GATA-1 levels. In mice overexpressing GATA-1 in all cells there would be no such juxtaposition, explaining the failure of erythroid differentiation (Whyatt et al., 2000).
Fig. 4. Homotypic signalling within the erythroblastic island. Arrows indicate the signal REDS being produced by relatively mature erythroid cells. The bar represents the switch from predominantly proliferation to differentiation during erythropoiesis.
Nonautonomous pRb function in the nervous system
Although pRb null neurons in chimeric mice autonomously display ectopic S-phase entry, the neuronal apoptosis that otherwise occurs in the absence of pRb is suppressed (Lipinski et al., 2001). Thus, the effect of pRb loss is partially cell nonautonomous in neuronal tissues. Theoretically, pRb null cells could secrete an apoptosis-inducing factor that is diluted in chimeras (Lipinski et al., 2001). However, it is unlikely that pRb would specifically regulate the expression of such a secreted factor by neurons. In fact, loss of pRb in the neuronal cells themselves is likely to disrupt their differentiation and thereby lead to apoptosis (Lee et al., 1994; Macleod et al., 1996; Tsai et al., 1998). It has been suggested that a signal supplied by wild-type cells might inhibit the apoptosis of pRb null neurons (Lipinski et al., 2001). If this were the case and the cells supplying the signal were wild-type neurons, homotypic signalling would again be the factor that overcomes the loss of pRb in neurogenesis.
Homotypic signalling in other systems
Homotypic signalling mechanisms also appear to explain cell-nonautonomous phenomena in other systems. One precedent for the homotypic model presented here exists in the nematode Caenorhabditis elegans. In this organism, the insulin/IGF receptor homologue DAF-2 regulates adult development and ageing. A mild decrease in daf-2 activity can lengthen lifespan, and a more severe decrease in activity can cause larvae to enter a state of diapause (dauer formation) rather than progressing to adulthood. Mosaic analysis demonstrated that daf-2 functions cell nonautonomously in both processes. For example, during development, larvae null for daf-2 become dauers. In contrast, larvae mosaic for daf-2 can become adults and all cells, regardless of genotype, display an adult phenotype. Importantly, an adult phenotype was not associated with a requirement for daf-2 activity in any particular cell. It is thought that secondary signals operate to ensure that all cells adopt the same developmental fate (Apfeld and Kenyon, 1998). Similarly, in mice, homotypic signalling between neural crest cells has been proposed to explain the cell-nonautonomous defect in colonization of the lower gut by endothelin-receptor B null enteric neuroblasts (Kapur et al., 1995).
Perspectives
Homotypic signalling potentially activates pathways that allow defects, such as pRb loss or GATA-1 overexpression, to be tolerated. However, what would be the function of such a signalling mechanism during erythropoiesis when such defects are not present? Mature erythroid cells regularly leave the erythroblastic island and must be replaced. Signals, such as the postulated REDS, may normally regulate the balance of proliferating and differentiating cells within the island. REDS produced by mature cells may activate the maturation and differentiation (at the expense of proliferation) of more immature cells. This would lead to the reinforcement or acceleration of maturation among all responsive cells in the island. This, in turn, would create a feedback mechanism in which the departure of mature cells would allow immature cells to proliferate and expand, whereas the accumulation of mature cells would inhibit the proliferation and activate the differentiation of immature cells. In addition to a normal role in regulating the balance between differentiation and proliferation, REDS could also provide a mechanism to activate differentiation during periods of rapid proliferation, e.g. during Epo-induced erythroid expansion following hypoxia (Krantz, 1991). As immature cells moved towards the source of REDS, i.e. relatively mature cells on the periphery of the island, differentiation would become synchronized, maintaining the distribution of mature cells on the periphery. This could explain the observation that the process of erythroid differentiation within islands, including the timing of cell division, can be highly synchronous (Allen and Testa, 1991). Such synchronization may be functionally important in the efficient release of mature cells into the circulation, as it would result in a large proportion of cells being at the same late stage of maturation (Allen and Testa, 1991).
In conclusion, it appears that the intrinsic requirement for pRb function in the differentiation of some cell types can be bypassed by signals supplied by wild-type cells, particularly in systems in which mature and immature cells are often in contact with one another. This phenomenon has implications in cancer, as loss of signal responsiveness could contribute to the tumorigenecity of cells that have lost pRb function. Furthermore, the existence of such a signalling mechanism indicates that previous interpretations of cell-nonautonomous defects may, in fact, attribute gene function to incorrect cell types.
Acknowledgments
Acknowledgements
We would like to thank D. Meijer, S. Philipsen, G. Zafarana and L. Gutierrez for critically reviewing the manuscript. The authors are supported by the Dutch Cancer Society.
REFERENCES
- Allen T.D. and Testa, N.G. (1991) Cellular interactions in erythroblastic islands in long-term bone marrow cultures, as studied by time-lapse video. Blood Cells, 17, 29–43. [PubMed] [Google Scholar]
- Apfeld J. and Kenyon, C. (1998) Cell nonautonomy of C. elegans daf-2 function in the regulation of diapause and life span. Cell, 95, 199–210. [DOI] [PubMed] [Google Scholar]
- Bergh G., Ehinger, M., Olofsson, T., Baldetorp, B., Johnsson, E., Brycke, H., Lindgren, G., Olsson, I. and Gullberg, U. (1997) Altered expression of the retinoblastoma tumor-suppressor gene in leukemic cell lines inhibits induction of differentiation but not G1-accumulation. Blood, 89, 2938–2950. [PubMed] [Google Scholar]
- Bernard J. (1991) The erythroblastic island: past and future. Blood Cells, 17, 5–14. [PubMed] [Google Scholar]
- Bessis M., Lessin, L.S. and Beutler, E. (1983) Morphology of the erythron. In Williams, W.J., Beutler, E., Erslev, A.J. and Lichtman, M.A. (eds), Hematology. McGraw-Hill, New York, NY, pp. 257–279.
- Chen P.-L., Riley, D.J., Chen, Y. and Lee, W.-H. (1996) Retinoblastoma protein positively regulates terminal adipocyte differentiation through direct interaction with C/EBPs. Genes Dev., 10, 2794–2804. [DOI] [PubMed] [Google Scholar]
- Clarke A.R., Maandag, E.R., van Roon, M., van der Lugt, N.M., van der Valk, M., Hooper, M.L., Berns, A. and te Riele, H. (1992) Requirement for a functional Rb-1 gene in murine development. Nature, 359, 328–330. [DOI] [PubMed] [Google Scholar]
- Condorelli G.L. et al. (1995) Modulation of retinoblastoma gene in normal adult hematopoiesis: peak expression and functional role in advanced erythroid differentiation. Proc. Natl Acad. Sci. USA, 92, 4808–4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira R., Naguibneva, I., Pritchard, L.L., Ait-Si-Ali, S. and Harel-Bellan, A. (2001) The Rb/chromatin connection and epigentic control: opinion. Oncogene, 28, 3128–3133. [DOI] [PubMed] [Google Scholar]
- Gu W., Schneider, J.W., Condorelli, G., Kaushal, S., Mahdavi, V. and Nadal-Ginard, B. (1993) Interaction of myogenic factors and the retinoblastoma protein mediates muscle cell commitment and differentiation. Cell, 72, 309–324. [DOI] [PubMed] [Google Scholar]
- Hseih F.F., Barnett, L.A., Green, W.F., Freedman, K., Matushansky, I., Skoultchi, A.I. and Kelley, L.L. (2000) Cell cycle exit during terminal erythroid differentiation is associated with accumulation of p27Kip1 and inactivation of cdk2 kinase. Hematopoiesis, 96, 2746–2754. [PubMed] [Google Scholar]
- Hu N., Gulley, M.L., Kung, J.T. and Lee, E.Y.-H. (1997) Retinoblastoma gene deficiency has mitogenic but not tumorigenic effects on erythropoiesis. Cancer Res., 57, 4123–4129. [PubMed] [Google Scholar]
- Jacks T., Fazeli, A., Schmitt, E.M., Bronson, R.T., Goodell, M.A. and Weinberg, R.A. (1992) Effects of an Rb mutation in the mouse. Nature, 359, 295–300. [DOI] [PubMed] [Google Scholar]
- Kapur R.P., Sweetser, D.A., Doggett, B., Siebert, J.R. and Palmiter, R.P. (1995) Intercellular signals downstream of the endothelin-B receptor mediate colonization of the large intestine by enteric neuroblasts. Development, 121, 3787–3795. [DOI] [PubMed] [Google Scholar]
- Kiyokawa H., Richon, V.M., Rifkind, R.A. and Marks, P.A. (1994) Suppression of cyclin-dependent kinase 4 during induced differentiation of erythroleukaemia cells. Mol. Cell. Biol., 14, 7195–7203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi Y., Tominaga, M., Watanabe, Y., Imamura, F., Goldfarb, A., Maki, R., Blum, M., De Robertis, E.M. and Tominaga, A. (1999) GOOSECOID inhibits erythrocyte differentiation by competing with Rb for PU.1 binding in murine cells. Oncogene, 18, 6795–6805. [DOI] [PubMed] [Google Scholar]
- Krantz S.B. (1991) Erythropoietin. Blood, 77, 419–434. [PubMed] [Google Scholar]
- Labbaye C., Valtieri, M., Grignani, F., Puglisi, R., Luchetti, L., Masella, B., Alcalay, M., Testa, U. and Peschle, C. (1999) Expression and role of PML gene in normal adult hematopoiesis: functional interaction between PML and Rb proteins in erythropoiesis. Oncogene, 18, 3529–3540. [DOI] [PubMed] [Google Scholar]
- Lasorella A., Noseda, M., Beyna, M., Yokota, Y. and Iavarone, A. (2000) Id2 is a retinoblastoma protein target and mediates signalling by Myc oncoproteins. Nature, 407, 592–598. [DOI] [PubMed] [Google Scholar]
- Lee E.Y., Chang, C.Y., Hu, N., Wang, Y.C., Lai, C.C., Herrup, K., Lee, W.H. and Bradley, A. (1992) Mice deficient for Rb are nonviable and show defects in neurogenesis and hematopoiesis. Nature, 359, 288–294. [DOI] [PubMed] [Google Scholar]
- Lee E., Hu, N., Yuan, S.-S., Cox, L., Bradley, A., Lee, W.-H. and Herrup, K. (1994) Dual roles of the retinoblastoma protein in cell cycle regulation and neuron differentiation. Genes Dev., 8, 2008–2021. [DOI] [PubMed] [Google Scholar]
- Lipinski M.M. and Jacks, T. (1999) The retinoblastoma gene family in differentiation and development. Oncogene, 18, 7873–7882. [DOI] [PubMed] [Google Scholar]
- Lipinski M.M., Macleod, K.F., Williams, B.O., Mullaney, T.L., Crowley, D. and Jacks, T. (2001) Cell-autonomous and non-cell-autonomous functions of the Rb tumor suppressor in developing nervous system. EMBO J., 20, 3402–3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maandag E.C., van der Valk, M., Vlaar, M., Feltkamp, C., O’Brien, J., van Roon, M., van der Lugt, N., Berns, A. and te Riele, H. (1994) Developmental rescue of an embryonic-lethal mutation in the retinoblastoma gene in chimeric mice. EMBO J., 13, 4260–4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macleod K.F., Hu, Y. and Jacks, T. (1996) Loss of Rb activates both p53-dependent and independent cell death pathways in the developing mouse nervous system. EMBO J., 15, 6178–6188. [PMC free article] [PubMed] [Google Scholar]
- Nevins J.R. (2001) The Rb/E2F pathway and cancer. Hum. Mol. Genet., 10, 699–703. [DOI] [PubMed] [Google Scholar]
- Nielsen S.J. et al. (2001) Rb targets histone H3 methylation and HP1 to promoters. Nature, 412, 561–565. [DOI] [PubMed] [Google Scholar]
- Pevny L., Simon, M.C., Robertson, E., Klein, W.H., Tsai, S.F., D’Agati, V., Orkin, S.H. and Costantini, F. (1991) Erythroid differentiation in chimaeric mice blocked by a targeted mutation in the gene for transcription factor GATA-1. Nature, 349, 257–260. [DOI] [PubMed] [Google Scholar]
- Rossant J. and Spence, A. (1998) Chimera and mosaics in mouse mutant analysis. Trends Genet., 14, 358–363. [DOI] [PubMed] [Google Scholar]
- Sehy D.W., Shao, L.-E., Yu, A.L., Tsai, W.-M. and Yu, J. (1992) Activin A-induced differentiation in K562 cells is associated with a transient hypophosphorylation of RB protein and the concomitant block of cell cycle at G1 phase. J. Cell. Biochem., 50, 255–265. [DOI] [PubMed] [Google Scholar]
- Sherr C.J. (1996) Cancer cell cycles. Science, 274, 1672–1677. [DOI] [PubMed] [Google Scholar]
- Sherr C.J. and Roberts, J.M. (1999) CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev., 13, 1501–1512. [DOI] [PubMed] [Google Scholar]
- Shih H.H., Tevosian, S.G. and Yee, A.S. (1998) Regulation of differentiation by HBP1, a target of the retinoblastoma protein. Mol. Cell. Biol., 18, 4732–4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamir A., Howard, J., Higgins, R.R., Li, Y.-J., Berger, L., Zacksenhaus, E., Reis, M. and Ben-David, Y. (1999) Fli-1, an ets-related transcription factor, regulates erythropoietin-induced erythroid proliferation and differentiation: evidence for direct transcriptional repression of the Rb gene during differentiation. Mol. Cell. Biol., 19, 4452–4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai K.Y., Hu, Y., Macleod, K.F., Crowley, D., Yamasaki, L. and Jacks, T. (1998) Mutation of E2F-1 suppresses apoptosis and inappropriate S phase entry and extends survival of Rb-deficient mouse embryos. Mol. Cell, 2, 293–304. [DOI] [PubMed] [Google Scholar]
- Vitelli L., Condorelli, G., Lulli, V., Hoang, T., Luchetti, L., Croce, C.M. and Peschle, C. (2000) A pentamer transcriptional complex including tal-1 and retinoblastoma protein downmodulates c-kit expression in normal erythroblasts. Mol. Cell. Biol., 20, 5330–5342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vooijs M. and Berns, A. (1999) Developmental defects and tumor predisposition in Rb mutant mice. Oncogene, 18, 5293–5303. [DOI] [PubMed] [Google Scholar]
- Weiss M.J. and Orkin, S.H. (1995) Transcription factor GATA-1 permits survival and maturation of erythroid precursors by preventing apoptosis. Proc. Natl Acad. Sci. USA, 92, 9623–9627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyatt D.J. et al. (1997) The level of the tissue-specific factor GATA-1 affects the cell-cycle machinery. Genes Funct., 1, 11–24. [DOI] [PubMed] [Google Scholar]
- Whyatt D. et al. (2000) An intrinsic but cell-nonautonomous defect in GATA-1 overexpressing mouse erythroid cells. Nature, 406, 519–524 [Erratum, Nature, 408, 498]. [DOI] [PubMed] [Google Scholar]
- Williams B.O., Schmitt, E.M., Remington, L., Bronson, R.T., Albert, D.M., Weinberg, R.A. and Jacks, T. (1994) Extensive contribution of Rb-deficient cells to adult chimeric mice with limited histopathological consequences. EMBO J., 13, 4251–4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H.S. and Dean, D.C. (2001) Rb-mediated chromatin structure regulation and transcriptional repression. Oncogene, 20, 3134–3138. [DOI] [PubMed] [Google Scholar]
- Zhuo S., Fan, S., Huang, S. and Kaufman, S. (1995) Study of the role of retinoblastoma protein in terminal differentiation of murine erythroleukemia cells. Proc. Natl Acad. Sci. USA, 92, 4234–4238. [DOI] [PMC free article] [PubMed] [Google Scholar]