Abstract
Rationale & Objective
Patients with kidney failure have poor physical performance, but its trajectory is less clear. We examined physical function over the course of kidney disease, including the transition to dialysis.
Study Design
Observational cohort.
Setting & Participants
Community-dwelling adults aged ≥45 years in the Brain in Kidney Disease (BRINK) cohort study.
Predictors
Estimated glomerular filtration rate (eGFR) and urine albumin to creatinine ratio (UACR).
Outcomes
Change in physical performance using the Short Physical Performance Battery (SPPB) (primary) and gait speed (secondary).
Analytical Approach
Linear mixed effects regression models.
Results
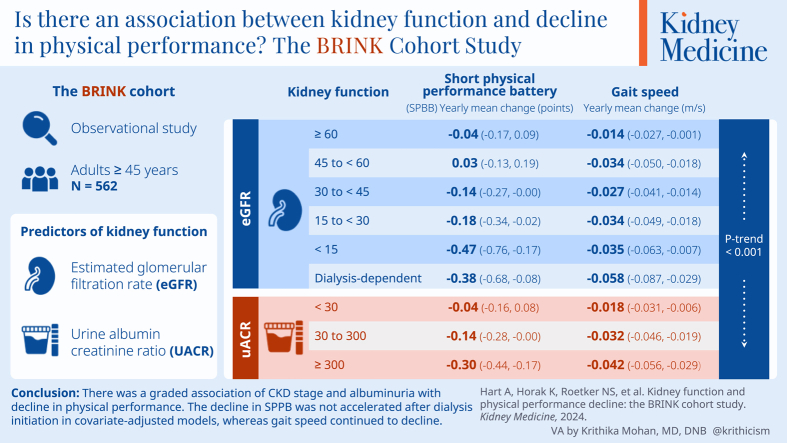
The analytical cohort included 562 participants with mean age of 69.3 (SD, 9.8) years followed for up to 63 months. In total, 49.8% were women. In addition, 79.9% self-identified as White, and 15.3% self-identified as Black. In total, 48.8% had diabetes. Mean eGFR at baseline was 48.1 (SD, 24.3) mL/min/1.73 m2. In unadjusted analysis, lower eGFR was associated with greater decline in SPPB score (P trend < 0.001). The decline in SPPB score was larger among participants with lower eGFR, with a gradient from -0.15 (95% CI, -0.23 to -0.07) points per year for participants with eGFR ≥60 mL/min/1.73 m2 to -0.56 (95% CI, -0.84 to -0.27) for participants with eGFR <15 mL/min/1.73 m2 and -0.61 (95% CI, -0.90 to -0.33) after dialysis initiation. In covariate-adjusted models, SPPB did not continue to decline after dialysis initiation. In secondary analyses evaluating change in gait speed, gait speed continued to decline after dialysis initiation. Higher UACR was also associated with a greater decline in SPPB score and gait speed in unadjusted and adjusted models.
Limitations
Small number of participants started dialysis.
Conclusions
We found a graded association of chronic kidney disease stage and albuminuria with decline in physical performance. The decline in SPPB was not accelerated after dialysis initiation in covariate-adjusted models, whereas gait speed continued to decline.
Index Words: Chronic kidney disease, dialysis, kidney disease progression, physical function, physical performance
Plain-Language Summary
Physical function is an important patient-centered outcome in chronic kidney disease (CKD), but whether physical performance changes as kidney disease progresses or when patients start dialysis is not well understood. We found that measures of physical performance, like strength and walking speed, worsened as kidney disease worsened. However, 1 combination of physical performance tests appeared stable (rather than getting worse) after starting dialysis compared to those with very advanced CKD who had not yet started dialysis, while gait speed continued to get worse. This information may help counsel patients who are learning about CKD and considering treatment options. It may also help guide research on interventions to improve physical function in patients with CKD.
Visual Abstract
Editorial, • • •
Physical performance is considerably worse among patients with kidney failure than among age-matched individuals without kidney failure.1 These physical limitations have been linked to difficulty performing activities of daily living and low quality of life.2 However, the “trajectory” of decline in physical function during the course of chronic kidney disease (CKD) is less clear. Most, but not all, cross-sectional studies have reported worse physical function among individuals with lower estimated kidney function.3, 4, 5, 6 However, these studies do not provide information about the rate of decline at different stages of kidney disease, which is important because decline may not be constant across stages of kidney disease. Such a nonlinear trajectory was recently reported for body weight and lean body mass, which are likely related to physical function.7 Body composition remained relatively stable among participants in the Chronic Renal Insufficiency Cohort until estimated glomerular filtration rate (eGFR) reached approximately 35 mL/min/1.73 m2, after which body weight and lean body mass decreased linearly with further eGFR decline. Understanding how physical function changes among individuals with CKD could help elucidate the pathophysiology of physical function decline and direct interventions to improve function or prevent decline.
The effect of dialysis initiation on physical function is also not clear. A seminal study among older nursing home residents initiating dialysis showed a large decrease in functional status around the time of dialysis initiation followed by continued decline thereafter, but few studies have focused on community-dwelling individuals with CKD.8 In cross-sectional analysis of the Brain in Kidney Disease (BRINK) study cohort at baseline, eGFR was not associated with physical performance, whereas higher urine albumin to creatinine ratio (UACR) was associated with decreased physical performance.6 Recently, investigators in the Canadian Frailty Observation and Interventions Trial (CanFIT) reported that transition to dialysis was associated with accelerated decline in physical function, but this study was based on only a single assessment before dialysis initiation and a single follow-up assessment, separated by a median of 1.7 years.9 Thus, it was not possible to determine how much of the decline occurred before and how much after dialysis initiation. These results are compatible with an acute decline around the time of dialysis initiation, a more rapid decline after dialysis initiation, or both.
The BRINK study is a multicenter observational cohort study designed to examine the association between CKD and cognitive function and other geriatric outcomes.10 We leveraged longitudinal assessment of physical function using the Short Physical Performance Battery (which includes tests of gait speed, chair standing, and balance) at annual BRINK study visits to examine the trajectory of physical performance over the course of kidney disease, including the transition to dialysis. We hypothesized that physical function would decline more rapidly among individuals with more advanced kidney disease than among those with less severe disease and that the decline would continue after initiation of dialysis.
Methods
Study Participants
The BRINK study included 574 community-dwelling adults 45 years of age or older in Minnesota.10 To ensure that participants with a wide range of eGFR were included, participants were enrolled in groups based on eGFR: <45 mL/min/1.73 m2, 45 to 59 mL/min/1.73 m2, and ≥60 mL/min/1.73 m2 (“controls”). At the time of enrollment, no participants had received a transplant or were on any form of kidney replacement therapy. The baseline study visit included serum creatinine and UACR measurement as well as physical performance measures. Participants attended annual comprehensive (same as baseline) follow-up assessments and phone call visits every 6 months for up to 8 years. For those that initiated kidney replacement therapy with dialysis during follow-up, a postdialysis in-person visit was scheduled within 1-3 months after dialysis initiation and every 6 months thereafter. Physical performance was assessed at annual and biannual follow-up visits before and after starting kidney replacement therapy, respectively; therefore, these are the visits included in this analysis. The institutional review boards of each institution approved the study, and all participants provided written informed consent (Veterans Administration IRB #4364-B, Hennepin Healthcare Research Institute IRB #11-3393, University of Minnesota IRB #1203M11122, and Health Partners IRB #A12-282).
Sociodemographic, Laboratory, and Clinical Measures
Primary Predictors
The primary predictor was eGFR. In this analysis, we used the creatinine-based 2021 Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation, which does not include a race adjustment factor, to estimate GFR.11,12 eGFR was then categorized by CKD stage as follows: ≥60, 45-59, 30-44, 15-29, and <15 mL/min/1.73 m2 as well as dialysis dependent. The secondary predictor, UACR, was categorized into 3 categories: <30, 30-299, and ≥300 mg/g.
Covariates
Sociodemographic covariates included age, sex, race, and years of education. Race was self-reported and categorized as White, Black, and other. Laboratory values obtained at the baseline and annual or biannual follow-up assessments included serum creatinine (calibrated to isotope-dilution mass spectrometry), hemoglobin A1c, and urine albumin and creatinine concentrations. Nonfasting blood samples were obtained and stored at -80° within 2 hours. All analytes were processed and measured centrally by a Clinical Laboratory Improvement Amendments-certified laboratory.
Comorbid conditions, including diabetes and heart failure, were identified using laboratory measurements, medical history questionnaires, and participants’ electronic medical record. Diabetes was defined as a nonfasting glucose ≥200 mg/dL, hemoglobin A1c ≥6.5%, self-reported diabetes, or antidiabetic drugs on the medication list. Body mass index was calculated from measured height and weight. Cognitive impairment was measured using the global cognitive impairment score, as defined by having a T-score <35 for any of the 3 domains (memory, executive function, or language).
Physical Performance
Physical performance was assessed using the Short Physical Performance Battery (SPPB), which includes tests of balance, chair standing, and gait speed.13 Balance was assessed while participants stood with their feet in a side-by-side position, a semi-tandem position, and a tandem position. Participants were asked to stand from a chair 5 times as quickly as they were able without using their arms, and the time was recorded in seconds. Gait speed (meters/sec) was measured over 4 meters at a participant’s usual speed. Each test was scored on a scale of 0-4 (with 4 indicating best performance) and summed for a maximum score of 12.
Statistical Analysis
We excluded participants with missing baseline eGFR or UACR and those with baseline SPPB scores of 0 (meaning that they were unable to stand at baseline). Participant characteristics at baseline were reported as mean ± standard deviation (SD) or percentage and were stratified by the primary exposure (eGFR category) and, separately, by the secondary exposure (UACR category). The primary outcome was SPPB score, and the secondary outcome was gait speed; each were analyzed continuously. In the primary analysis, we used linear mixed effects regression to model the association of eGFR category with change in SPPB score over time. Mixed linear effects modeling is useful for repeated longitudinal measures, where these repeated measures in 1 individual are not independent.14 We used an unbalanced longitudinal design, with time modeled continuously. Each model included terms for time, eGFR, each covariate, the product of time and eGFR, and the products of time and each covariate. After initially exploring model fit based on the Akaike and Bayesian information criteria (Table S1), our final models included a random intercept to allow for individual variation in baseline physical performance and used a first-order autoregressive covariance structure to account for correlation due to repeated measures across study visits. Analogously, we also constructed separate models to examine the associations of eGFR category with change in gait speed, UACR category with change in SPPB score, and UACR category with change in gait speed. To test for linear trends across the eGFR and UACR categories, we modeled the exposure (eGFR or UACR category) ordinally. For participants who initiated dialysis during the study, UACR data from study visits conducted after dialysis initiation were not included in data analyses.
A series of models was iteratively adjusted for covariates. Model 1 was unadjusted. In Model 2, we adjusted for age, sex, race, and years of education to assess how the addition of demographic variables impacted the crude association. Model 3 was additionally adjusted for medical and comorbid conditions, including body mass index, diabetes, heart failure, and cognitive impairment. Comorbid conditions were selected a priori based on prior analyses and likely association with physical performance to limit the number of covariables relative to the sample size.6 We updated the exposure, outcome, and nonsociodemographic covariates at follow-up visits. In cases when a participant attended a follow-up visit but had some missing variables, data were imputed using the last observation carried forward method. Except in the case of body mass index, imputed data were never carried forward, meaning that a sequential visit with missing data was excluded from the analysis. Data not imputed for visits that were completely missed. The most common reason for missed visits was illness or hospitalization.
To assess the combination of eGFR and UACR, we conducted a sensitivity analysis using Kidney Disease: Improving Global Outcomes (KDIGO) risk category (which encompasses both eGFR and UACR) as a predictor variable, using the same analytic approach described above.15
Results
Of the 574 participants in the BRINK cohort, 5 had missing baseline SPPB score, eGFR, or UACR. In addition, 2 had other missing covariates, and 5 had a baseline SPPB score of 0, resulting in an analytical cohort of 562 participants included in the analysis, for a total of 1,902 person-visits. For the primary SPPB analysis, participants were followed for up to 63 months, with a mean follow-up time of 31.1 months. Of these 562 participants, 39 initiated dialysis and had at least 1 follow-up visit after dialysis initiation, with a mean follow-up time of 33.5 (SD, 15.5) months and 2.3 (SD, 1.7) follow-up visits.
Participant Characteristics
Baseline characteristics of the cohort overall and by eGFR category and UACR category are shown in Tables 1 and 2. The mean age of the cohort was 69.3 (SD, 9.8) years, and 49.8% were women. In total, 79.9% self-identified as White, 15.3% as Black, and 4.8% as another race. Approximately half of the cohort (48.8%) had diabetes, and the mean eGFR of the cohort at baseline was 48.1 (SD 24.3) mL/min/1.73 m2. The UACR median was 25.5 mg/g (25th and 75th percentiles, 0 and 218.8). Participants with the lowest kidney function (eGFR <15 mL/min/1.73 m2) tended to be younger (mean age 61.9 [SD 10.6] years). Black race, male sex, and diabetes were also more common among participants with worse kidney function. Similar patterns in the distributions of these covariates were also observed for participants with elevated albuminuria. The mean SPPB score at baseline was 9.5 (SD, 2.5) points, and the mean gait speed was 0.95 (SD, 0.27) m/s. The overlap between UACR and eGFR at each person visit is shown in Table S2. In total, 724 (40.2%) participants were in the very high-risk category, 435 (24.2%) were low risk, 266 (14.8%) were moderately high risk, and 376 (20.9%) were high risk.
Table 1.
Baseline Characteristics, Overall and by eGFR Category
| Characteristic | Overall (N = 562) |
eGFR ≥60 mL/min/1.73 m2 (N = 145) | eGFR 45-<60 mL/min/1.73 m2 (N = 96) | eGFR 30-<45 mL/min/1.73 m2 (N = 191) | eGFR 15-<30 mL/min/1.73 m2 (N = 106) | eGFR <15 mL/min/1.73 m2 (N = 24) |
|---|---|---|---|---|---|---|
| SPPB, mean (SD) | 9.5 (2.5) | 10.2 (2.3) | 9.2 (2.8) | 9.5 (2.5) | 9.0 (2.6) | 9.0 (2.7) |
| Gait speed (m/s), mean (SD) | 0.95 (0.27) | 1.02 (0.25) | 0.94 (0.29) | 0.93 (0.26) | 0.91 (0.27) | 0.98 (0.25) |
| eGFR (mL/min/1.73 m2), mean (SD) | 48.1 (24.3) | 83.5 (12.9) | 51.6 (4.2) | 37.6 (4.1) | 23.6 (4.2) | 11.9 (2.7) |
| UACR (mg/g), mean (SD) | 367.0 (947.9) | 41.8 (162.0) | 151.4 (571.0) | 299.0 (667.6) | 826.7 (1282.7) | 1703.4 (2339.6) |
| Age (years) mean (SD) | 69.3 (9.8) | 67.9 (9.8) | 70.5 (9.2) | 70.4 (9.6) | 69.7 (9.5) | 61.9 (10.6) |
| Female sex, N (%) | 280 (49.8) | 82 (56.6) | 48 (50.0) | 101 (52.9) | 44 (41.5) | 5 (20.8) |
| Race, N (%) | ||||||
| White | 449 (79.9) | 117 (80.7) | 78 (81.3) | 161 (84.3) | 80 (75.5) | 13 (54.2) |
| Black | 86 (15.3) | 20 (13.8) | 12 (12.5) | 21 (11.0) | 23 (21.7) | 10 (41.7) |
| Other | 27 (4.8) | 8 (5.5) | 6 (6.3) | 9 (4.7) | 3 (2.8) | 1 (4.2) |
| Education (years), mean (SD) | 14.3 (2.7) | 15.1 (2.6) | 14.4 (3.0) | 14.3 (2.6) | 13.5 (2.6) | 13.1 (2.5) |
| BMI (kg/m2), mean (SD) | 31.4 (7.2) | 29.6 (6.9) | 32.4 (7.8) | 32.1 (7.3) | 31.5 (6.4) | 31.2 (8.9) |
| DM, N (%) | 274 (48.8) | 61 (42.1) | 46 (47.9) | 90 (47.1) | 63 (59.4) | 14 (58.3) |
| Moderate to severe CI, N (%) | 159 (28.3) | 37 (25.5) | 22 (22.9) | 47 (24.6) | 41 (38.7) | 12 (50.0) |
| CHF, N (%) | 79 (14.1) | 7 (4.8) | 15 (15.6) | 34 (17.8) | 18 (17.0) | 5 (20.8) |
| Hemoglobin (g/dL), mean (SD) | 13.0 (1.7) | 13.8 (1.2) | 13.3 (1.3) | 12.9 (1.6) | 12.0 (1.9) | 11.3 (1.7) |
| Systolic BP (mm Hg), mean (SD) | 132 (18.5) | 129 (17.3) | 130 (14.6) | 133 (17.9) | 136 (19.8) | 142 (28.8) |
Abbreviations: BMI, body mass index; BP, blood pressure; CHF, congestive heart failure; CI, cognitive impairment; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate using creatinine; SD, standard deviation; SPPB, short physical performance battery; UACR, urine albumin to creatinine ratio.
Table 2.
Baseline Characteristics by UACR Category
| Characteristic | UACR <30 mg/g (N = 294) |
UACR 30-<300 mg/g (N = 150) | UACR ≥300 mg/g (N = 118) |
|---|---|---|---|
| SPPB, mean (SD) | 10.0 (2.3) | 8.9 (2.6) | 9.0 (2.7) |
| Gait speed (m/s), mean (SD) | 0.99 (0.25) | 0.90 (0.28) | 0.93 (0.29) |
| eGFR (mL/min/1.73 m2), mean (SD) | 58.3 (23.9) | 41.6 (20.6) | 30.9 (15.2) |
| UACR (mg/g), mean (SD) | 4.5 (8.6) | 104.8 (70.6) | 1,603.4 (1,530.1) |
| Age (years), mean (SD) | 69.9 (9.6) | 70.5 (9.8) | 66.1 (9.7) |
| Female sex, N (%) | 179 (60.9) | 61 (40.7) | 40 (33.9) |
| Race, N (%) | |||
| White | 250 (85.0) | 114 (76.0) | 85 (72.0) |
| Black | 30 (10.2) | 29 (19.3) | 27 (22.9) |
| Other | 14 (4.8) | 7 (4.7) | 6 (5.1) |
| Education (years), mean (SD) | 14.7 (2.7) | 14.1 (2.6) | 13.7 (2.8) |
| BMI (kg/m2), mean (SD) | 30.9 (7.2) | 31.2 (7.4) | 32.7 (7.0) |
| DM, N (%) | 107 (36.4) | 85 (56.7) | 82 (69.5) |
| Moderate to severe CI, N (%) | 68 (23.1) | 51 (34.0) | 40 (33.9) |
| CHF, N (%) | 23 (7.8) | 32 (21.3) | 24 (20.3) |
| Hemoglobin (g/dL), mean (SD) | 13.4 (1.4) | 12.8 (1.8) | 12.2 (1.8) |
| Systolic BP (mm Hg), mean (SD) | 128 (17.2) | 134 (16.9) | 141 (20.1) |
Abbreviations: BMI, body mass index; BP, blood pressure; CHF, congestive heart failure; CI, cognitive impairment; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate using creatinine; SD, standard deviation; SPPB, short physical performance battery; UACR, urine albumin to creatinine ratio.
Association Between eGFR and Change in Physical Performance
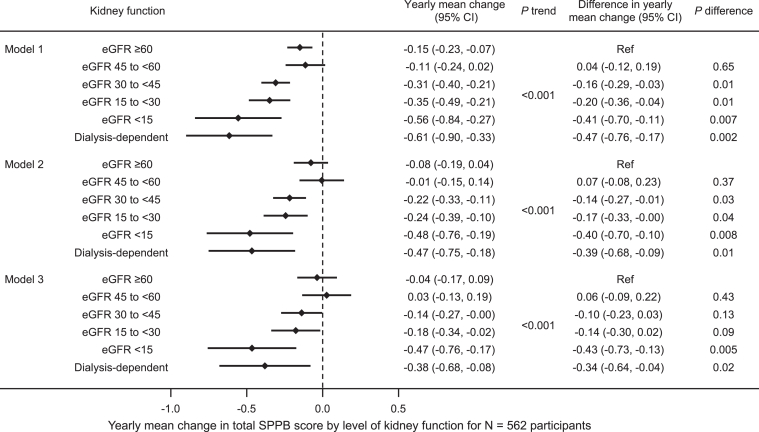
In the unadjusted model, lower eGFR was associated with greater yearly mean decline in SPPB score (P trend <0.001) (Fig 1, Model 1). The mean decline in SPPB score was larger among participants with lower eGFR, with a gradient from -0.15 (95% confidence interval [CI], -0.23 to -0.07) points per year for participants with eGFR ≥60 mL/min/1.73 m2 to -0.56 (95% CI, -0.84 to -0.27) for participants with eGFR <15 mL/min/1.73 m2 and -0.61 (95% CI, -0.90 to -0.33) after dialysis initiation. This pattern persisted, but was attenuated, in covariate-adjusted models. In the fully adjusted model that included age, sex, race, education, body mass index, diabetes, heart failure, and cognitive impairment, the yearly mean change in SPPB score was -0.04 (95% CI, -0.17 to 0.09) for participants with eGFR ≥60 mL/min/1.73 m2, -0.47 (95% CI, -0.76 to -0.17) for participants with eGFR <15 mL/min/1.73 m2, but only -0.38 (95% CI, -0.68 to -0.08) for participants requiring dialysis (Fig 1, Model 3).
Figure 1.
Yearly mean change in short physical performance battery (SPPB) score by level of kidney function using eGFRCr among 562 participants. Model 1 is unadjusted. Model 2 is adjusted for age, sex, race, and years of education. Model 3 is also adjusted for body mass index, diabetes, heart failure, and cognitive impairment.
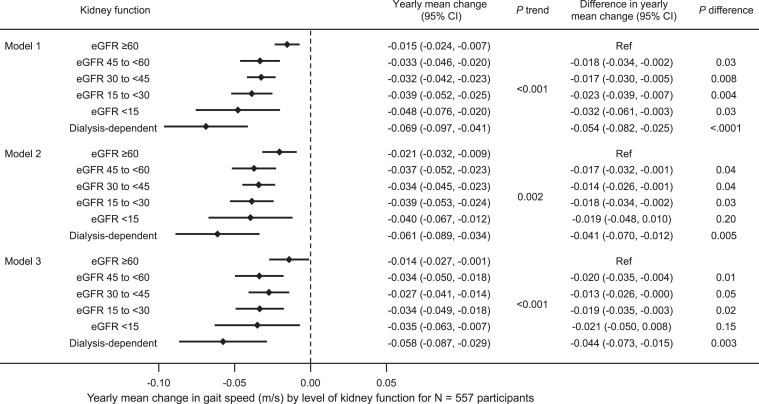
In secondary analyses evaluating the association between eGFR and change in gait speed over time, results were similar. Lower eGFR category was associated with greater annual decline in gait speed in the unadjusted model with a gradient from -0.015 (95% CI, -0.024 to -0.007) m/s/year for participants with eGFR ≥60 mL/min/1.73 m2 to -0.048 (95% CI, -0.076 to -0.020) for participants with eGFR <15 mL/min/1.73 m2. However, the mean decline in gait speed was also greater after dialysis initiation (-0.069 [95% CI, -0.097 to -0.041]) than when eGFR was <15 mL/min/1.73 m2 (Fig 2, Model 1). The relationship remained consistent with iterative adjustment for sociodemographic factors (P trend = 0.002, Model 2) and comorbid conditions (P trend < 0.001) (Fig 2, Model 3). In the fully adjusted model, mean gait speed decline was -0.035 (95% CI, -0.063 to -0.007) m/s/year among participants with eGFR <15 mL/min/1.73 m2 and -0.058 (95% CI, -0.087 to -0.029) m/s/year among participants on dialysis.
Figure 2.
Yearly mean change in gait speed (m/s) by level of kidney function using eGFRCr among 557 participants. Model 1 is unadjusted. Model 2 is adjusted for age, sex, race, and years of education. Model 3 is also adjusted for body mass index, diabetes, heart failure, and cognitive impairment.
Association Between UACR and Change in Physical Performance
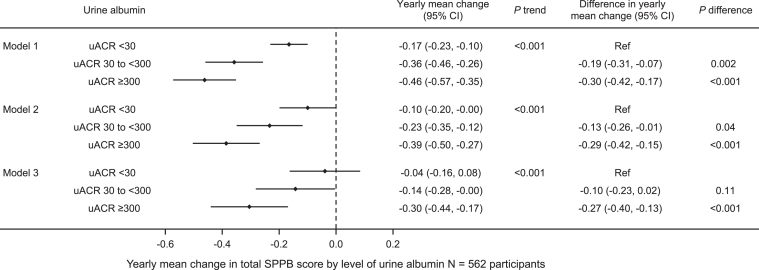
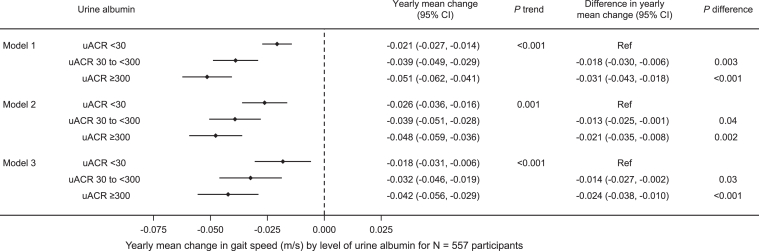
Higher UACR category was associated with a greater decline in SPPB score in the unadjusted model as well as in models additionally adjusted for sociodemographic factors and comorbid conditions. Adjusted mean annual decline in SPPB score was -0.04 (95% CI, -0.16 to 0.08) points for participants with UACR <30 mg/g and -0.30 (95% CI, -0.44 to -0.17) points for participants with UACR ≥300 mg/g (Fig 3, Model 3). Similarly, higher UACR category was associated with greater annual mean gait speed decline after adjustment for sociodemographic factors and comorbidities (Fig 4).
Figure 3.
Yearly mean change in short physical performance battery (SPPB) score by urine albumin to creatinine ratio (UACR) among 562 participants. Model 1 is unadjusted. Model 2 is adjusted for age, sex, race, and years of education. Model 3 is also adjusted for body mass index, diabetes, heart failure, and cognitive impairment.
Figure 4.
Yearly mean change in gait speed (m/s) by urine albumin to creatinine ratio (UACR) among 557 participants. Model 1 is unadjusted. Model 2 is adjusted for age, sex, race, and years of education. Model 3 is also adjusted for body mass index, diabetes, heart failure, and cognitive impairment.
Association Between KDIGO Risk Category and Physical Performance Measures
In sensitivity analyses using the KDIGO risk score incorporating both UACR and eGFR, results were similar. KDIGO risk category was associated with a graded association between KDIGO risk score and both for both SPPB and gait speed (Table S3).
Discussion
In this longitudinal analysis of the association between kidney function and physical performance, we found that more advanced CKD stage was associated with a greater decline in SPPB score even after adjusting for demographic factors and comorbid conditions. Findings were similar in analyses of the association between CKD stage and gait speed. Similarly, in secondary analyses of the association between albuminuria and physical performance measures, higher levels of albuminuria were associated with greater declines in both SPPB and gait speed. Notably, in the analysis of CKD stage, the estimated decline in physical performance measures appeared to worsen markedly between stage 4 and stage 5 CKD. However, the estimated SPPB decline was similar among participants with stage 5 CKD and those on dialysis, suggesting that some decline in physical performance may stabilize after dialysis initiation in this population of community-dwelling older adults. However, in the secondary analysis evaluating only gait speed, the decline in gait speed persisted after dialysis initiation.
Counseling and treatment methods for individuals with CKD are increasingly focused on patient-centered metrics of health, including physical function, which is linked to physical performance measures. Patients learning about and considering treatment options for advanced CKD may want to know that physical performance declines more as patients progress from stage 4 to stage 5 CKD. In addition, the landmark study showing worsening physical function in older adult residing in care facilities increased awareness of the fact that some older adults may not functionally benefit from dialysis initiation.8 It is notable that in this study of community-dwelling older adults, the decline in 1 physical performance measure did not worsen between stage 5 CKD and dialysis-dependent end-stage kidney disease, while gait speed did continue to decline.
Our findings produced both similar and contrasting results with the CanFIT study of 386 individuals with eGFR <30 mL/min/1.73 m2, which assessed physical function at the study visit before and the study visit after dialysis initiation compared with physical function changes among those who did not start dialysis.9 That study reported a larger increase in chair stand time among the 162 individuals who progressed to dialysis. Unlike our study, they found no differences in change in gait speed between the advanced CKD and dialysis initiation groups, nor did they find a difference in grip strength. These mixed results are difficult to interpret and contrast somewhat with our findings. However, we did not assess chair stand independently but rather included it as part of the SPPB score. Taken together, these analyses suggest that community-dwelling older adults may experience accelerated decline in physical performance in the late stages of CKD but may or may not experience further accelerated decline after dialysis initiation. This study is not able to determine the reason for this finding. It may be that dialysis initiation mitigates some of the adverse effects of uremia on physical performance. It is also possible that patients have already exhausted their muscle and functional ‘reserve’ before they initiate dialysis.
The interplay between eGFR and UACR and their associations with physical performance measures are complex. In a cross-sectional analysis of the baseline BRINK cohort, we found that UACR but not eGFR was associated with SPPB after adjusting for covariates, suggesting that inflammation and epithelial cell function may be a more important contributor than filtration rate alone.6 Conversely, in this longitudinal analysis, there was a dose-response noted between CKD stage by eGFR and declining physical performance, wherein those with stage 5 CKD and on dialysis declined more rapidly than those with eGFR ≥60 mL/min/1.73 m2. In our sensitivity analysis using KDIGO risk category (which encompasses both eGFR and UACR) as a predictor, results were similar, suggesting that both eGFR and UACR are important factors in physical performance measures.
Studies evaluating the impact of progressive CKD are critical to counseling patients but are often challenged by the number of years required to detect a measurable change in outcomes. Indeed, the clinically significant decrease in SPPB of 3 points among patients with CKD stage 5 would occur over the course of approximately 6 years according to the annual decline noted in our analysis. In addition, longitudinal cohorts of CKD patients that continue to follow outcomes after dialysis initiation are extremely rare, making it difficult to counsel patients about what to expect after dialysis initiation.
Although this analysis represents one of the few prospective cohorts of community-dwelling patients with CKD including progression to dialysis, it does have some important limitations. The number of participants in the cohort who started dialysis was small, making adjusted analyses difficult. It is possible that the small sample size resulted in a lack of statistical significance between the physical function estimates for CKD stage 5 and the patients on dialysis, although the point estimates were also similar. Similarly, the total follow-up time after dialysis initiation was short, which may have limited detection of a change in physical performance trajectory; however, assessments were made more frequently after dialysis initiation. Second, this study uses estimated, rather than measured GFR. Most participants were of White race, and approximately 16% of the participants were Black. Given that kidney disease disproportionately affects non-White patients, additional studies inclusive of more non-White participants are needed. The models evaluate the association between kidney disease and physical performance. However, the association may be bidirectional, and these models cannot infer causality. Survival bias may also have impacted the results; a sensitivity analysis was attempted using inverse probability of attrition weighting in an attempt to address this issue, but the data collection schedule in the BRINK study was not ideal for this analysis. However, the point estimates were very similar, suggesting that survivor bias was not a strong factor in the results. Finally, although we adjusted for a range of sociodemographic and medical confounders, unmeasured confounders may exist. Larger studies might allow for the evaluation of models with more covariables, including more comorbid conditions.
In conclusion, we found a graded association of both CKD stage and albuminuria with decline in physical performance measures as assessed by SPPB and gait speed, but the decline in SPPB did not appear to accelerate after dialysis initiation. Additional studies of community-dwelling adults who initiate dialysis with longer follow-up time will assist in counseling patients with CKD who are considering treatment options for kidney failure. These data would also inform the development and evaluation of interventions targeted at improving physical function in patients with CKD.
Article Information
Authors’ Full Names and Academic Degrees
Allyson Hart, MD, MS, Kayla Horak, MS; Nicholas S. Roetker, PhD, Ashley Farnum, BA, Anne Murray, MD, MSc, and Kirsten L. Johansen, MD.
Authors’ Contributions
Study design: AH, KJ, and AM; BRINK cohort study design: AM; data acquisition: AF; statistical analysis: KM and NSR; data interpretation: AH, KJ, AM, AF, KH, and NSR. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
This research was supported by the National Institute of Aging (R01AG037551 and RF1AG058729) and by the National Institute of Digestive and Diabetes and Kidney Disease (R01DK107269-01A1).
Financial Disclosure
The authors declare that they have no relevant financial interests.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Peer Review
Received June 13, 2023. Evaluated by 3 external peer reviewers, with direct editorial input by the Statistical Editor and the Editor-in-Chief. Accepted in revised form September 24, 2023.
Footnotes
Complete author and article information provided before references.
Table S1: AIC and BIC for Competing Correlation Structures.
Table S2: Number of Participants in KDIGO Risk categories, Including eGFR and UACR at Nondialysis Person-Visits.
Table S3: Yearly Mean Change in Total SPPB Score and Gait Speed (m/s) by KDIGO CKD Risk Group.
Descriptive Text for Online Delivery
Tables S1-S3.
References
- 1.Johansen K.L., Chertow G.M., da Silva M., Carey S., Painter P. Determinants of physical performance in ambulatory patients on hemodialysis. Kidney Int. 2001;60(4):1586–1591. doi: 10.1046/j.1523-1755.2001.00972.x. [DOI] [PubMed] [Google Scholar]
- 2.Fusco O., Ferrini A., Santoro M., Lo Monaco M.R., Gambassi G., Cesari M. Physical function and perceived quality of life in older persons. Aging Clin Exp Res. 2012;24(1):68–73. doi: 10.1007/BF03325356. [DOI] [PubMed] [Google Scholar]
- 3.Hiraki K., Yasuda T., Hotta C., et al. Decreased physical function in pre-dialysis patients with chronic kidney disease. Clin Exp Nephrol. 2013;17(2):225–231. doi: 10.1007/s10157-012-0681-8. [DOI] [PubMed] [Google Scholar]
- 4.Fried L.F., Lee J.S., Shlipak M., et al. Chronic kidney disease and functional limitation in older people: health, aging and body composition study. J Am Geriatr Soc. 2006;54(5):750–756. doi: 10.1111/j.1532-5415.2006.00727.x. [DOI] [PubMed] [Google Scholar]
- 5.Roshanravan B., Patel K.V., Robinson-Cohen C., et al. Creatinine clearance, walking speed, and muscle atrophy: a cohort study. Am J Kidney Dis. 2015;65(5):737–747. doi: 10.1053/j.ajkd.2014.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mello R., Johansen K.L., Murray A., Davey C., Hart A. Estimated GFR, albuminuria, and physical function: the brain in kidney disease (BRINK) cohort study. Kidney Med. 2022;4(10) doi: 10.1016/j.xkme.2022.100531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ku E., Kopple J.D., Johansen K.L., et al. Longitudinal weight change during CKD progression and its association with subsequent mortality. Am J Kidney Dis. 2018;71(5):657–665. doi: 10.1053/j.ajkd.2017.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurella Tamura M., Covinsky K.E., Chertow G.M., Yaffe K., Landefeld C.S., McCulloch C.E. Functional status of elderly adults before and after initiation of dialysis. N Engl J Med. 2009;361(16):1539–1547. doi: 10.1056/NEJMoa0904655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rampersad C., Darcel J., Harasemiw O., et al. Change in physical activity and function in patients with baseline advanced nondialysis CKD. Clin J Am Soc Nephrol. 2021;16(12):1805–1812. doi: 10.2215/CJN.07050521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murray A.M., Bell E.J., Tupper D.E., et al. The brain in kidney disease (BRINK) cohort study: design and baseline cognitive function. Am J Kidney Dis. 2016;67(4):593–600. doi: 10.1053/j.ajkd.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inker L.A., Eneanya N.D., Coresh J., et al. New creatinine-and cystatin C–based equations to estimate GFR without race. N Engl J Med. 2021;385(19):1737–1749. doi: 10.1056/NEJMoa2102953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller W.G., Kaufman H.W., Levey A.S., et al. National Kidney Foundation Laboratory Engagement Working Group recommendations for implementing the CKD-EPI 2021 race-free equations for estimated glomerular filtration rate: practical guidance for clinical laboratories. Clinic Chem. 2022;68(4):511–520. doi: 10.1093/clinchem/hvab278. [DOI] [PubMed] [Google Scholar]
- 13.Guralnik J.M., Simonsick E.M., Ferrucci L., et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 14.Detry M.A., Ma Y. Analyzing repeated measurements using mixed models. JAMA. 2016;315(4):407–408. doi: 10.1001/jama.2015.19394. [DOI] [PubMed] [Google Scholar]
- 15.Levey A.S., Eckardt K.U., Dorman N.M., et al. Nomenclature for kidney function and disease: report of a Kidney Disease: Improving Global Outcomes (KDIGO) Consensus Conference. Kidney Int. 2020;97(6):1117–1129. doi: 10.1016/j.kint.2020.02.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1-S3.