Abstract
Three Campylobacter jejuni, biotype 2, serotype O:41 strains that were isolated from patients who developed Guillain-Barré syndrome (GBS) and one C. jejuni isolate from a patient who developed enteritis only were examined. The aim of the study was to determine the structure of the core oligosaccharide (OS) of the lipopolysaccharide (LPS) of C. jejuni serotype O:41, a serotype rarely associated with the development of GBS, and to determine if the LPS shares similar epitopes with any of the major human gangliosides. Electrophoretic analysis with silver staining or immunoblotting demonstrated that the strains had LPS profiles characteristic of low-molecular-weight LPS. Colorimetric analysis detected N-acetylneuraminic (sialic) acid in the core OSs of all the strains. Thin-layer chromatography with immunostaining showed that antisera raised against the GBS strains reacted with the GM1 ganglioside, suggesting that C. jejuni serotype O:41 LPSs and the GM1 ganglioside have similar epitopes. Furthermore, polyclonal anti-GM1 and anti-asialoGM1 antibodies cross-reacted with each C. jejuni O:41 LPS tested, suggesting that the serotype O:41 core OS has a GM1- and asialoGM1-like structure. LPSs extracted from C. jejuni serostrains O:2, O:3, and O:19 were also used in the study. Cholera toxin (a GM1 ligand) and peanut agglutinin (a Galβ1–3GalNAc ligand) recognized all serotype O:41 LPSs and the serostrain O:2 LPS. Immunoadsorption results confirmed GM1 relatedness. Moreover, the core OS was isolated from a GBS-associated C. jejuni O:41 LPS by gel permeation chromatography. An analysis by gas-liquid chromatography (GLC), GLC-mass spectrometry, and nuclear magnetic resonance showed the core OS of one of the C. jejuni O:41 GBS isolates to have a tetrasaccharide structure consistent with GM1 mimicry.
Guillain-Barré syndrome (GBS) is characterized as an acute, inflammatory polyneuropathy (48), and approximately two-thirds of GBS patients develop the syndrome following various infections of the respiratory or gastrointestinal tract (27). GBS is clinically very heterogeneous, and several variants of the disease occur and include both acute inflammatory demyelinating polyneuropathy (AIDP) and acute motor axonal neuropathy (AMAN). Campylobacter jejuni, a leading cause of acute gastroenteritis in humans, has been identified as the single most important predisposing factor associated with the development of GBS and occurs in up to 66% of patients (20, 27, 29, 47). Characteristically, 76% of the AMAN and 42% of the AIDP GBS patients have serologic evidence consistent with recent C. jejuni infection (17, 30).
C. jejuni can be serotyped based on differences in the saccharide structure (O side chain and core oligosaccharide [OS]) of the lipopolysaccharide (LPS; O antigen) of the bacterium (32, 45, 46). Some reports suggest that only specific C. jejuni serotypes are associated with GBS. A predominance of C. jejuni O:19, an uncommon serotype in gastroenteritis patients, has been found in Japanese GBS patients (23, 24). Similarly, Fujimoto et al. (11) described four C. jejuni isolates that belonged to serotype O:19. This same serotype has been isolated from GBS patients in the United States, where 33% of GBS isolates were of serotype O:19 (28). Other C. jejuni serotypes that have been identified in association with GBS include O:1, O:2, O:2/44, O:4/59, O:5, O:10, O:15, O:18, O:21, O:24, O:30, O:37, and O:64 (24, 37, 39, 43, 49). C. jejuni O:2, O:10, and O:23 (19, 52, 66) have been found in association with Miller-Fisher syndrome, a variant of GBS comprising areflexia, ataxia, and ophthalmoplegia without limb weakness (50).
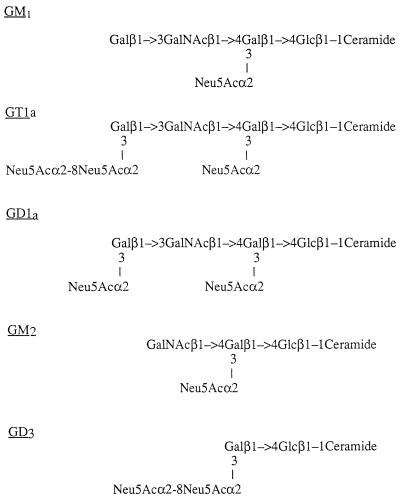
Serum antibodies against gangliosides have been observed in about 30% of GBS patients (27, 70). The structures of the major human gangliosides are shown in Fig. 1. Autoreactive antibodies to gangliosides, especially the GM1 ganglioside, occur in GBS patient sera after C. jejuni infection during the acute phase of the illness (14, 19, 41, 42, 54, 64, 69, 70). Conversely, antiganglioside antibodies, including those in sera from GBS patients, cross-react with LPSs of C. jejuni serotypes associated with GBS (19, 54). Antiganglioside antibodies may be involved in the pathogenesis of GBS because some individuals have developed GBS-like symptoms after the administration of gangliosides (10, 18, 59) and, moreover, because plasma exchange and administration of intravenous immunoglobulin (Ig) elicit a beneficial response (59).
FIG. 1.
Molecular structures of some of the major human gangliosides. Glc, glucose; Gal, galactose; GalNAc, N-acetylgalactosamine.
Chemical studies on LPS extracted from C. jejuni have shown that the structures of the terminal regions of the core OSs of specific serotypes mimic the structures of human gangliosides (2, 5, 6, 37), and research has focused on the view that molecular mimicry may be a factor in the pathogenesis of GBS (37). Furthermore, the core OSs of LPSs of C. jejuni O:19 isolates have been shown to mimic human gangliosides GM1, GD1a, GT1a, and GD3 (2, 3, 33, 64, 68). GM2-like OS structures occur in LPSs from serostrains O:1, O:23, and O:36 (6), whereas the core OS of C. jejuni serostrain O:4 mimics the GD1a ganglioside (6, 69). However, mimicry of C. jejuni O:2 is limited to that of a disaccharide which is present in a range of gangliosides including GD1a (4).
The present study describes the characterization of C. jejuni strains belonging to serotype O:41, three recovered from patients who developed GBS and one recovered from a patient who developed enteritis only. In particular, the presence of ganglioside-like epitopes in the LPSs of these strains was investigated and the chemical structure of the core OS of one strain was established.
MATERIALS AND METHODS
Patients.
The clinical details of patients at Groote Schuur Hospital (GSH) and Red Cross Hospital (RXH) in Cape Town, South Africa, from whom C. jejuni was isolated have been described previously (26). Briefly, a 26-year-old male (patient A) from whom C. jejuni 16971.94GSH was isolated developed GBS 10 days after an episode of diarrhea. A cerebrospinal fluid (CSF) study performed in the first 48 h of illness was normal, and electromyogram studies showed evidence of a severe predominately motor polyneuropathy with axonal loss. There was no sensory or bulbar involvement. A 22-month-old female (patient B) from whom C. jejuni 260.94RXH was isolated from a formed stool developed a more severe type of GBS. After ventilation and prolonged hospitalization, the patient progressed well with no relapse. A 29-year-old female (patient C), from whom C. jejuni 28134.94GSH was isolated, had suffered an episode of GBS at the age of 10 years. She had a history of upper respiratory tract infections. Her CSF characteristics were normal, and neurological tests showed evidence of a diffuse motor neuropathy with both demyelinating and axonal elements. C. jejuni 176.83 was isolated from a 9-year-old female (patient D) who developed enteritis but did not subsequently develop GBS.
Bacterial strains and growth conditions.
Isolation of Campylobacter strains involved filtration and incubation in an H2-enriched microaerobic atmosphere (GasPak BR38 [Oxoid Ltd., London, England] without a catalyst) according to an established protocol (26). Gram-negative, motile, spiral or curved rods were cultured and maintained on blood agar (tryptose agar base [Oxoid] with 10% unlysed horse blood) under microaerobic conditions as described previously (38). C. jejuni serostrains O:2 (ATCC 43430), O:3 (ATCC 43431), and O:19 (ATCC 43446) were obtained from the American Type Culture Collection (Manassas, Va.). Strains were routinely grown on blood agar in a manner identical to that described above. Bacterial biomass was harvested, and bulk extraction of LPS was performed by the phenol-water extraction procedure described previously (38, 61).
Biotyping and serotyping.
Bacterial identification was accomplished by established procedures (33, 46, 56). Isolates were biotyped by the scheme of Skirrow and Benjamin (56). Serotyping on the basis of thermostable somatic O antigens was performed with the 66 antisera of the Penner scheme (46) and an additional 30 antisera to new serotypes not included in the Penner scheme.
Hemolysis assay.
Hemolytic activity was screened by inoculating bacteria onto blood agar (tryptose agar base [Oxoid] with 2% horse blood) and incubating them at 42°C for 48 h (26).
Invasion assay.
The invasiveness of C. jejuni isolates was determined according to the method of Wassenaar et al. (60). Briefly, intestinal cells (INT-407; Flow Laboratories, Herts, England) were maintained in Dulbecco’s modified Eagle’s medium (DMEM; Gibco BRL, Grand Island, N.Y.), supplemented with 10% (vol/vol) fetal calf serum, penicillin (100 IU/ml), and streptomycin (100 μg/ml). The invasion assay was performed in 35-mm-diameter tissue culture dishes. Confluent monolayers (about 106 cells) were washed 30 min prior to infection with DMEM without supplements. A bacterial suspension in DMEM was added to each dish to give a multiplicity of infection of 103 bacteria per cell. Control experiments were performed with DMEM alone. Infected monolayers were incubated at 37°C for 2 h in an atmosphere of 95% air–5%CO2 to allow bacteria to adhere to the cells. After incubation, monolayers were washed five times with DMEM and reincubated for another 3 h with DMEM containing 250 μg of gentamicin per ml. Following this second incubation, the monolayers were washed three times with phosphate-buffered saline (PBS; pH 7.4; Oxoid) and lysed in 0.5% Triton X-100. The suspensions were diluted, and the numbers of CFU were calculated. Colonies were confirmed as being C. jejuni by standard CFU procedures (33, 46, 56).
Proteinase K digestion.
A procedure modified from that of Hitchcock and Brown (16) was used for the enzymatic digestion of whole-cell lysates. Bacteria were cultured on blood agar, harvested in PBS (pH 7.4), and diluted to an A600 of 0.3. Quantities of 1.5 ml were transferred to microtubes and centrifuged at 13,000 × g for 5 min. The resultant pellets were solubilized in 200 μl of lysing buffer (20% glycerol, 5% 2-β-mercaptoethanol, 4.6% sodium dodecyl sulfate [SDS], 0.125 M Tris-hydrochloride-buffered saline [pH 6.8], 0.004% bromophenol blue). The lysate was heated to 100°C for 5 min and cooled to room temperature, and 40 μl of a 2-mg/ml proteinase K solution (Sigma Chemical Co., St. Louis, Mo.) was added. The enzyme-treated lysates were incubated at 37°C for 1 h and subsequently heated at 100°C for 5 min prior to electrophoresis.
SDS-PAGE and immunoblotting.
The discontinuous buffer system of Laemmli (25) was used to fractionate the LPS prepared by proteinase K digestion of whole-cell lysates and also to examine purified LPS. SDS-polyacrylamide gel electrophoresis (SDS-PAGE) was performed with a stacking gel of 5% acrylamide and a separation gel of 15% acrylamide containing 3.2 M urea (BDH Laboratory Supplies, Poole, England). Samples were electrophoresed at a constant current of 35 mA until the tracking dye was within 0.5 cm of the bottom of the gel. After SDS-PAGE, the gels were fixed and the LPS was detected by silver staining, as described previously (58). Alternatively, LPS fractionated by SDS-PAGE was electrotransferred from gels to nitrocellulose membranes (pore size, 0.45 μm; Bio-Rad Laboratories, Hercules, Calif.) by using the buffer system described by Towbin et al. (57). Visualization of nitrocellulose blots was performed with rabbit antiserum as the first antibody and goat anti-rabbit IgG–horseradish peroxidase conjugate (Bio-Rad) as the second antibody (46).
TLC.
Gangliosides (Sigma) and LPS were analyzed by thin-layer chromatography (TLC) on precoated silica gel 60 glass plates (Merck, Darmstadt, Germany). Solvent systems consisting of chloroform–methanol–0.22% CaCl2 · 2H2O (50:45:10 [vol/vol/vol]) (51) and n-propanol–water–25% NH4OH (60/30/10 [vol/vol/vol]) (54, 65) were used as developers for gangliosides and LPS, respectively. Gangliosides and LPS were visualized by spraying plates with resorcinol-HCl reagent (55).
Immunostaining.
TLC with immunostaining was performed by the procedure of Saito et al. (51), as modified by Schwerer et al. (54). Briefly, developed TLC plates were dried for 30 min in a vacuum desiccator, fixed in 0.4% polyisobutylmethacrylate (Aldrich, Steinheim, Germany) in n-hexane (Merck) for 1.5 min, and dried as before. Lanes were overlaid with either rabbit antiserum to ganglioside GM1, GM2, or asialoGM1 (Matreya Inc., Pleasant Gap, Pa.) or rabbit antiserum to C. jejuni O:41 diluted 1:100 in a solution of PBS (Oxoid), pH 7.4, containing 0.3% gelatin (gelatin-PBS). The TLC plates were incubated at 4°C overnight in a humid chamber, washed three times with cold PBS, overlaid with peroxidase-conjugated anti-rabbit IgG (Sigma) diluted 1:500 in gelatin-PBS, and incubated in a humid chamber at room temperature for 1 h with gentle rocking. The plates were washed with cold PBS and immersed in a horseradish peroxidase color development solution (Bio-Rad) consisting of 60 mg of 4-chloro-1-naphthol dissolved in 20 ml of methanol and added to 100 ml of Tris-hydrochloride-buffered saline containing 60 μl of cold 30% aqueous H2O2 until the immunoreactants became visible. The substrate reaction was stopped by washing the plates with cold PBS.
Binding experiments with cholera toxin (CT)-peroxidase conjugate (Sigma) and peanut agglutinin (PNA)-peroxidase conjugate (Kem-En-Tec, Copenhagen, Denmark) were performed under the same conditions as those described for immunostaining. However, only one overlay step with CT-peroxidase conjugate at a dilution of 1:1,000 or PNA-peroxidase conjugate at a dilution of 1:50 in gelatin-PBS was used.
Inhibition experiments were performed by a modified TLC technique whereby the B subunit of CT (Sigma) was diluted 1:250 in gelatin-PBS from a stock solution (1 mg/ml) and overlaid onto TLC plates. The plates were incubated for 1 h at room temperature with gentle rocking. Antiserum was subsequently overlaid overnight at 4°C onto the plates, and detection of immunoreactants was performed with horseradish peroxidase color development solution.
Immunoadsorption.
Antiserum to C. jejuni O:41 was incubated with 200 μg of ganglioside GM1 or 200 μg of LPS for 2 h at 37°C with rotation of the test tubes (Cell Major Mixer, model CM200). Immunoprecipitates were removed by centrifugation (10,000 × g, 10 min), and supernatants were tested. The adsorbed antiserum was diluted 1:100 in gelatin-PBS before tests to measure residual IgG binding activity to GM1 and LPS. Similar experiments were performed with anti-GM1 antiserum by immunoadsorbance before being tested with 200 μg of LPS or 200 μg of GM1.
Determination of the chemical structure of C. jejuni O:41 LPS.
One portion of purified LPS was de-O-acetylated with anhydrous hydrazine; a second portion was acid hydrolyzed, and the water-soluble material was fractionated by gel permeation chromatography (3). Briefly, purified LPS was dissolved in 5 ml of aqueous 1% acetic acid and hydrolyzed by heating at 100°C for 90 min. Precipitated lipid A was removed by centrifugation at 3,500 × g for 1 h, the supernatant solution was freeze-dried, and the residue was redissolved in 2 ml of analytical-grade water for fractionation by gel permeation chromatography on a Bio-Gel P-6 column (1.0 by 100 cm) (Bio-Rad) with water as the eluent. Fractions were monitored by measuring A206. In addition, fractions were assayed by the phenol-sulfuric acid method for neutral carbohydrates (9) and the modified Ehrlich reaction assay for sialic acid (8). The relevant fractions were pooled and were freeze-dried. Methylation analysis, chemical modifications of OSs, and Smith degradations were performed on the resultant material. Structural studies on the core OS derivatives used chemical analyses, nuclear magnetic resonance (NMR) spectroscopy, and electron impact mass spectrometry as reported previously for LPS from C. jejuni O:19 (3, 33). Analysis using 1H- and 13C-NMR spectroscopy included recording one- and two-dimensional spectra.
RESULTS
Bacterial identification and classification.
Biochemical reactions of the three C. jejuni strains isolated from GBS patients and of one C. jejuni strain isolated from an enteritis patient were characteristic of the species (56). All four C. jejuni strains were biotype 2, according to the scheme of Skirrow and Benjamin (56). Thermostable antigenic preparations of the isolates reacted with O:41 antisera with titers of up to 1:2,560 but did not react with the other C. jejuni typing antisera. As previously described (26), the isolates were screened for hemolytic activity by incubation on tryptose blood agar, and invasiveness was measured by a tissue culture INT-407 invasion test. All the C. jejuni isolates were strongly hemolytic and strongly invasive (106 bacteria penetrated per 106 INT-407 cells). Furthermore, analysis of electrophoretic protein profiles and PCR-restriction fragment length polymorphisms of the GBS isolates has shown that these C. jejuni strains are completely different from other serotypes of C. jejuni biotype 2 and that they may be clonal (7, 26). Restriction fragment end labelling of the serotype O:41 strains confirmed this finding (36).
Yields of LPS.
LPS was extracted according to established procedures (38, 61), and for each strain, LPSs were obtained from both the water and phenol phases. The majority of LPS was isolated from the water phase, and yields of LPS from the phenol phase were minimal (up to 0.06%). Yields of purified LPS from the water phase differed slightly among strains. The greatest yield (dry weight) of LPS (6.8%) was obtained from C. jejuni 28134.94GSH. The other two GBS isolates yielded approximately 5.2% LPS. On the other hand, the enteritis isolate, C. jejuni 176.83, gave 4.8% LPS.
Electrophoretic patterns of C. jejuni serotype O:41 LPS.
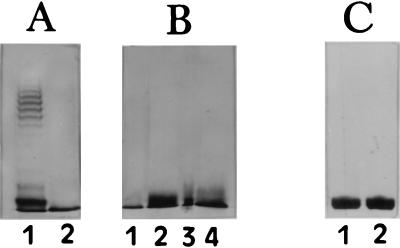
Silver-stained SDS-PAGE gels of water-phase LPS from C. jejuni O:41 exhibited a pattern of bands migrating near the bottom of the gel, corresponding to low-molecular-weight (low-Mr) rough-form LPS composed of core OS and lipid A, but bands characteristic of high-Mr LPS with O side chains were absent (Fig. 2). As C. jejuni high-Mr LPS is not visualized by the staining procedure of Tsai and Frasch (58), immunoblotting was performed with C. jejuni O:41 serotyping antiserum to visualize this high-Mr material (46). As with silver staining, no high-Mr LPS was visualized in any of the C. jejuni O:41 strains, and only low-Mr LPS was apparent (data not shown). Similar banding patterns were observed with low-Mr LPS of proteinase K-treated whole-cell lysates and purified LPS, thus indicating that the phenol-water extraction did not alter the structure of the low-Mr LPS. The LPSs from all of the C. jejuni O:41 bacterial isolates, including the enteritis isolate, had similar mobilities, each migrating as one distinct band at the bottom of the gel. The serotype O:41 LPSs migrated to the same region of the gel as serostrain O:19 LPS, indicating similar molecular weights of core OSs in C. jejuni O:41 and O:19 LPSs.
FIG. 2.
Silver-stained SDS-PAGE gels of proteinase K-treated whole-cell extracts of C. jejuni and of hot-phenol-water-extracted LPS from C. jejuni. (A) Lanes: 1, Escherichia coli (clinical isolate) LPS; 2, C. jejuni 176.83 LPS. (B) Lanes: 1, C. jejuni 28134.94GSH LPS; 2, proteinase K extract of C. jejuni 28134.94GSH; 3, C. jejuni 260.94RXH LPS; 4, proteinase K extract of C. jejuni 260.94RXH. (C) Lanes: 1, C. jejuni 16971.94GSH LPS; 2, proteinase K extract of C. jejuni 16971.94GSH. LPS samples of 1 μg were applied to the 15% acrylamide gel, and 10 μl of the proteinase K extracts was applied.
Assay for Neu5Ac.
Differing amounts of N-acetylneuraminic acid (Neu5Ac) were detected in the LPSs of the three serotype O:41 GBS isolates by using the Ehrlich reaction assay (8). In the LPS of C. jejuni 16971.94GSH, 200 nmol of Neu5Ac per mg was found, whereas a smaller amount of Neu5Ac was detected in the LPSs of the other two isolates (C. jejuni 260.94RXH, 132 nmol/mg; C. jejuni 28134.94GSH, 101 nmol/mg). The LPS of the enteritis isolate, C. jejuni 176.83, also contained Neu5Ac (114 nmol/mg). In addition, after methanolysis and peracetylation of the respective LPSs as described previously (38), the presence of Neu5Ac was confirmed in the LPSs by detection of its peracetylated methyl ketoside methyl ester derivative by gas-liquid chromatography. Furthermore, during this analysis, the levels of sialylation in the respective LPSs were the same as those by the colorimetric assay.
Binding of rabbit antiganglioside antisera to LPS.
As shown in Table 1, the specificity of rabbit antisera was demonstrated by the strong reaction of anti-GM1 antiserum with the GM1 ganglioside and the weak reaction with GD1b. Anti-asialoGM1 antibodies reacted strongly with the asialoGM1 ganglioside, and a weak reaction occurred with GM1. Anti-GM2 antisera reacted only with the GM2 ganglioside.
TABLE 1.
Reactions of antiganglioside, anti-C. jejuni antisera and ligands with gangliosides
| Antibody or ligand | Strength of reactiona with ganglioside:
|
||||||
|---|---|---|---|---|---|---|---|
| GM1 | GM2 | AsialoGM1 | GD1a | GD1b | GT1b | GQ1b | |
| Rabbit antibodies | |||||||
| Anti-GM1 | ++++ | + | ++ | — | + | (+) | ND |
| Anti-asialoGM1 | ++ | — | +++ | — | — | — | — |
| Anti-GM2 | — | ++ | (+) | (+) | — | — | — |
| Anti-C. jejuni 16971.94GSH | +++ | (+) | (+) | — | — | — | — |
| Anti-C. jejuni 260.94RXH | ++ | — | — | — | — | — | — |
| Ligands | |||||||
| CT | ++++ | — | + | — | +++ | — | — |
| PNA | ++ | — | +++ | — | — | — | — |
++++, very strong reaction; +++, strong reaction; ++, moderate reaction; +, weak reaction; (+), barely visible reaction; —, no reaction. ND, not done.
The reactions of antiganglioside antibodies with purified LPSs are shown in Table 2. Anti-GM1 antiserum bound to purified serotype O:41 LPSs from the three GBS-associated isolates but also bound to serotype O:41 LPS purified from the enteritis isolate, suggesting the presence of a GM1-like epitope in these LPSs. The anti-GM1 antibodies cross-reacted with serotype O:19 LPS and cross-reacted weakly with serostrain O:2 LPS. Anti-GM1 antiserum did not react with the LPS of C. jejuni O:3, a strain whose LPS is not sialylated and which is not associated with the development of GBS (1, 38).
TABLE 2.
Binding of antiganglioside, anti-C. jejuni antisera and ligands with C. jejuni LPS
| Antibody or ligand | Strength of reactiona with LPS of C. jejuni strain:
|
||||||
|---|---|---|---|---|---|---|---|
| O:41 16971.94GSH | O:41 260.94RXH | O:41 28134.94GSH | O:41 176.83 | O:2 | O:3 | O:19 | |
| Rabbit antibodies | |||||||
| Anti-GM1 | ++ | ++ | ++ | ++ | + | — | ++ |
| Anti-asialoGM1 | ++ | (+) | (+) | (+) | ++ | — | ++ |
| Anti-GM2 | — | — | — | — | — | — | — |
| Anti-C. jejuni O:41 16971.94GSH | +++ | +++ | ++ | ++ | (+) | — | + |
| Anti-C. jejuni O:41 260.94RXH | +++ | +++ | ++ | + | (+) | — | + |
| Ligands | |||||||
| PNA | ++ | ++ | +++ | ++ | +++ | — | + |
| CT | ++++ | ++++ | +++ | ++++ | ++ | — | ++++ |
++++, very strong reaction; +++, strong reaction; ++, moderate reaction; +, weak reaction; (+), barely visible reaction; —, no reaction.
Anti-asialoGM1 antiserum showed a strong reaction with C. jejuni 260.94RXH LPS and weaker binding with each of the other serotype O:41 LPSs, including that from the enteritis isolate (Table 2). A strong reaction of this antiserum was observed with serostrain O:2 LPS, and a weaker reaction occurred with serostrain O:19 LPS.
Anti-GM2 antiserum bound the GM2 ganglioside (Table 1) but did not bind to any serotype O:41 LPS, suggesting that this LPS does not have a GM2-like epitope (Table 2). This antiserum did not bind to serostrain O:2 or O:19 LPS, and antiganglioside antisera did not bind to serostrain O:3 LPS (Table 2). Control rabbit antiserum did not bind to LPS or gangliosides (data not shown).
Binding of rabbit anti-C. jejuni O:41 antisera to gangliosides and LPS.
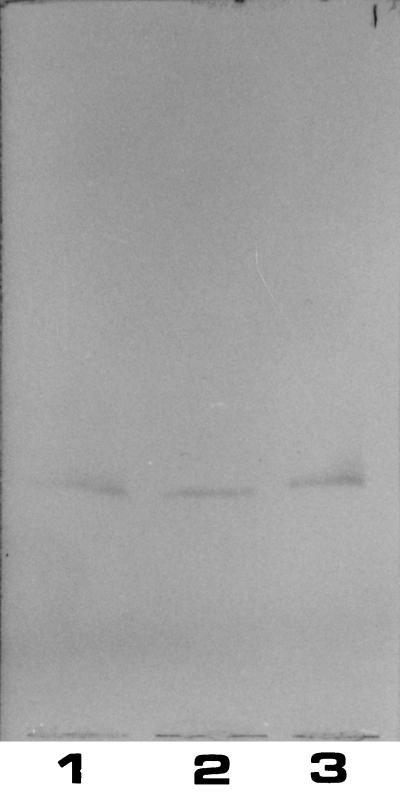
Rabbit antiserum against C. jejuni 16971.94GSH showed a strong reaction with GM1 and a much weaker reaction with asialo-GM1 (Table 1), suggesting the presence of cross-reactive epitopes in serotype O:41 LPSs and the GM1 ganglioside. As shown in Fig. 3, only one band of immunoreactive material was observed when anti-C. jejuni 16971.94GSH antiserum reacted with serotype O:41 LPSs from GBS patients. The antiserum also reacted with serostrain O:19 LPS and reacted weakly with serostrain O:2 LPS, but again no reaction was observed with serostrain O:3 LPS (Table 2). The antiserum against C. jejuni 260.94RXH yielded the same results. Results are summarized in the tables.
FIG. 3.
Binding of anti-C. jejuni 16971.94GSH rabbit antiserum to purified C. jejuni LPS. Lanes: 1, C. jejuni 16971.94GSH LPS; 2, C. jejuni 28134.94GSH LPS; 3, C. jejuni 260.94RXH LPS. The immunostained chromatogram was overlaid first with rabbit antiserum to C. jejuni 16971.94GSH and subsequently with anti-rabbit IgG. A sample of 1 μg of LPS was applied per lane.
Binding of CT and PNA to gangliosides and LPS.
CT, a ligand for the GM1 ganglioside, and PNA, a ligand for the disaccharide moiety Galβ1–3GalNAc, were tested for their abilities to react with a variety of gangliosides (Table 1) and C. jejuni LPS (Table 2). In addition to a reaction with GM1, CT showed a weaker binding to asialoGM1, GM2, and GD1b. On the other hand, PNA bound strongly to asialoGM1 as well as showing a weaker reaction with GM1. With respect to purified LPS, CT reacted strongly with serotype O:41 LPSs (those of strains 16971.94GSH, 260.94RXH, and 176.83) and strongly with serostrain O:19 LPS, and a weaker reaction was observed with LPS of C. jejuni 28134.94GSH. A weak reaction was observed with serostrain O:2 LPS, but no reaction was observed with serostrain O:3 LPS.
PNA showed strong binding to serostrain O:2 and O:19 LPSs and all the C. jejuni O:41 LPSs (Table 2), suggesting the presence of the Galβ1–3GalNAc disaccharide in the serotype O:41 LPS. However, PNA did not react with any of the other LPSs tested.
Immunoadsorption.
Antisera to C. jejuni 16971.94GSH and to C. jejuni 260.94RXH which had been preadsorbed with the respective LPSs were subsequently tested by TLC with immunostaining. Adsorption with serotype O:41 LPS removed antibody binding to the GM1 ganglioside and reduced the binding to serotype O:41 LPS. The lack of reaction of anti-GM1 antiserum subsequent to immunoadsorption with serotype O:41 LPS confirms our earlier findings that GM1 and O:41 LPS have similar epitopes.
Composition and structure of LPS from C. jejuni 16971.94GSH.
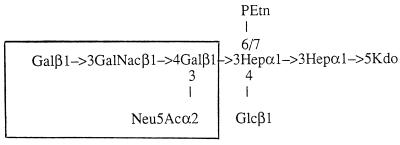
An analysis of de-O-acylated LPS and material derived from hydrolyzed LPS and subsequent gel chromatography revealed saccharide chains consistent with the core OS of LPS molecules. Structural analyses established the structure of the core OS as shown in Fig. 4. This core OS has a terminal tetrasaccharide mimicking that of ganglioside GM1 (Fig. 1); this mimicry resembles that observed in certain C. jejuni O:19 GBS isolates (3, 33, 68). The complete details of the chemical characterization of C. jejuni 16971.94GSH LPS will be published elsewhere (35).
FIG. 4.
Molecular structure of the OS of C. jejuni 16971.94GSH, from a patient who developed GBS. PEtn, phosphoethanolamine; Kdo, 3-deoxy-d-manno-octulosonic acid; Hep, 1-glycero-d-manno-heptose; Glc, glucose; Gal, galactose; GalNAc, N-acetylgalactosamine.
DISCUSSION
C. jejuni is a major cause of human gastroenteritis that is usually self-limiting, but in a small minority of cases GBS develops within 1 to 3 weeks after infection (20, 27). The association of GBS with the preceding infection has led to a search for candidate bacterial antigens which may precipitate autoimmune responses in the host (15, 47, 62, 63). Gangliosides have been extensively studied as possible host antigens for autoimmune disease since serum antibodies against gangliosides, especially GM1, are found during the acute phase of GBS when preceded by C. jejuni infection (14, 41, 48, 67, 70). Molecular mimicry between core OSs of certain C. jejuni serotypes associated with GBS (O:2, O:4, and O:19) and gangliosides has been established (3, 4, 6, 33, 37, 38, 62, 68). In Miller-Fisher syndrome studies, mimicry between a C. jejuni O:10 isolate and ganglioside GD3 has been established (52). Prompted by these previous findings, we undertook an investigation of the structure of the LPSs of C. jejuni isolates associated with GBS in South Africa. All three strains were strongly hemolytic and strongly invasive and may be clonal (26). We also examined a C. jejuni isolate of the same serotype that was isolated from a patient with enteritis alone. Serotyping (45) identified the isolates as serotype O:41, a serotype previously not associated with neurological disease. Hot phenol-water extraction resulted in high yields of LPS (5 to 6.8% of the dry weight), which are close to the theoretical LPS content of gram-negative bacteria (40). Although LPSs of C. jejuni strains were isolated from both the water and phenol phases of extracts, only minimal amounts of LPS were recovered from the phenol phase of C. jejuni serotype O:41 strains. Therefore, further studies were conducted only on the water phase LPS.
By SDS-PAGE analysis, C. jejuni serotype O:41 LPS from the three GBS isolates and the LPS from the enteritis isolate showed virtually identical rates of migration, reflecting core molecules with the same molecular weight. The serotype O:41 LPSs exhibited a profile characteristic of rough-form LPS, i.e., composed only of a lipid A region and core OS. LPS from the O:19 serostrain migrated to the same point in the gel as O:41 LPS, indicating that serostrain O:19 LPS and serotype O:41 LPS have core OSs with similar molecular weights. The high-Mr LPSs of C. jejuni strains are not visualized by silver staining (46), but bands corresponding to C. jejuni high-Mr LPSs with O side chains are visualized by immunoblotting with homologous antisera (44, 46). However, even by immunoblotting, no high-Mr bands were observed in immunoblots of any of the C. jejuni O:41 strains with O:41 typing antisera, consistent with previous observations (46). Preliminary chemical studies indicate the presence of high-Mr polysaccharides containing arabinose, fucose, altrose, and 6-deoxy-allo-heptose in the C. jejuni O:41 strains. However, to date, it remains unclear whether these polysaccharides are attached as O side chains to the core OS of C. jejuni O:41 LPS or are independent of LPS.
Sialic acid, i.e., Neu5Ac, is not commonly found in LPS but has been identified in C. jejuni LPS (6, 31, 38). When present in LPS, Neu5Ac is more commonly encountered as a constituent of the core OS (6, 21, 22, 34, 38) than the O side chain (12, 13). Neu5Ac was detected in C. jejuni O:41 LPS from the GBS patients in amounts ranging from 101 to 200 nmol/mg. The LPS from C. jejuni 28134.94GSH contained only half the amount of Neu5Ac than was present in C. jejuni 16971.94GSH LPS. Studies of the migration patterns of mutants of Neisseria species LPS in SDS-PAGE gels have shown that LPSs differing in the presence or absence of no more than one sialic acid residue can exhibit different mobilities in SDS-PAGE gels (53). However, no difference in migration pattern between the serotype O:41 LPSs was observed in the SDS-PAGE gel.
Antibodies against GM1 ganglioside reacted strongly with all serotype O:41 LPSs, including LPS from the enteritis isolate, suggesting the presence of a GM1-like epitope in serotype O:41 LPSs. Cross-reactivity was observed with serostrain O:19 and O:2 LPSs, and this indicates that the core OSs of the LPSs of serostrains O:19 and O:2 and that of serotype O:41 LPSs have similar structures. This concurs with the previously reported epitope mimicry between the LPS of the O:19 serostrain and the GM1 ganglioside (3, 24, 64, 65). Structural analyses have shown that C. jejuni O:19 LPS from the serostrain contains a 1:1 mixture of core OS mimicking GM1 and GD1a (3). However, the core OSs of LPSs of two GBS serotype O:19 isolates were examined and found to be not only different from each other but different also from that of the O:19 serostrain LPS (3). The GBS isolates, C. jejuni OH4384 and C. jejuni OH4382, had core OSs with terminal regions mimicking GT1a and GD3, respectively (3). This finding demonstrates the heterogeneity that can exist between LPSs within the same serotype. In addition to C. jejuni O:19 LPS, other serotypes mimic gangliosides. C. jejuni O:2 LPS shares a terminal disaccharide, Neu5Ac(α2-3)Gal, with many of the major gangliosides (4). Reports have shown that O:4 LPS shares a terminal pentasaccharide with the GD1a ganglioside (6). Yuki et al. (69) reported that O:4 LPS bears GM1-like and GD1a-like epitopes. The report observed that the fraction of O:4 LPS that bore the GM1-like epitope and that with the GD1a-like epitope had different mobilities in TLC, thus indicating the heterogeneity of structures in their LPS preparations.
Anti-asialoGM1 antibodies recognized serostrain O:2 LPS strongly and serostrain O:19 LPS weakly. These antibodies showed a strong reaction with C. jejuni 260.94RXH LPS and moderate binding to other serotype O:41 LPSs, including LPS from the enteritis isolate, suggesting the presence of an asialoGM1-like epitope in C. jejuni O:41 LPSs.
Antibodies against C. jejuni 16971.94GSH, raised in rabbits, were observed to react with all serotype O:41 LPSs and with serostrain O:19 LPS and to react weakly with serostrain O:2 LPS, again suggesting similar core OSs of these serostrains with serotype O:41 LPSs. No reaction was observed with C. jejuni O:3 LPS. This antiserum bound strongly to the GM1 ganglioside, supporting earlier results that O:41 LPS shares an epitope with the GM1 ganglioside. Similar results were obtained with anti-C. jejuni 260.94RXH antiserum.
CT, a ligand for GM1, was found to react with a variety of gangliosides. In addition to binding to GM1, it also bound asialoGM1, GD1b, and GM2, as these gangliosides share the GalNAcβ1–4Galβ1–3Glc trisaccharide. CT did not recognize GT1a, GD1a, and GQ1b, and thus the presence of a terminally sialylated galactose residue may prevent its binding. CT reacted with serostrain O:19 and O:2 LPSs and binds avidly to the serotype O:41 LPSs, again indicating a GM1-like structure. Yuki et al. (65) observed the binding of CT to serotype O:19 LPS and deduced that it had a GM1-related structure. In inhibition experiments, CT blocked the binding of anti-C. jejuni O:41 antibodies to GM1 and dramatically reduced the binding to serotype O:41 LPS, demonstrating the recognition of a similar epitope by CT and the anti-C. jejuni O:41 antibodies. In support of this, CT also blocked the binding of anti-GM1 antibodies to serotype O:41 LPS.
PNA, which recognizes the Galβ1–3GalNAc epitope, bound predominantly to asialoGM1 and reacted with all the serotype O:41 LPSs, suggesting that this disaccharide is present in the core OSs. PNA recognized serostrain O:19 LPS, which possesses the disaccharide, and recognized serostrain O:2 LPS, which does not but which possesses a Galβ1–3Gal disaccharide. PNA did not bind to serostrain O:3 LPS, which does not exhibit ganglioside mimicry.
Immunoadsorption results confirm that C. jejuni O:41 LPS bears a GM1-like epitope. Reactions of anti-GM1 antibodies with gangliosides and LPS after immunoadsorption with GM1 ganglioside were absent. No recognition of GM1 or serotype O:41 LPS occurs with anti-GM1 antibodies after immunoadsorption with GM1, and therefore the antibodies must recognize a similar epitope. In support of this, when anti-C. jejuni O:41 antisera are adsorbed in a similar way, all subsequent reactions of the adsorbed antisera are reduced or absent.
Consistent with the serological findings of our study, structural analyses showed the presence of GM1 mimicry in the form of a tetrasaccharide in the core OS of C. jejuni 16971.94GSH. Collectively, the results of the present study show that C. jejuni O:41 GBS isolates exhibit mimicry of the GM1 ganglioside as does a C. jejuni enteritis isolate. However, mimicry of the GM1 ganglioside by the core OS of C. jejuni O:41 is not limited to strains associated with GBS, since LPS from the enteritis strain reacted in a similar but not identical way to the GBS-associated LPS. This phenomenon has previously been observed by us with C. jejuni O:19 LPS (34), whereby an enteritis isolate mimics both the GM1 and GD1a gangliosides and a GBS isolate mimics the GM1 ganglioside. We suggested that a further attribute of the bacterium or the host contributed to the development of GBS. Mimicry by O side chains of C. jejuni O:19 LPS of hyaluronic acid was demonstrated, and an increased expression of O side chains was noted in strains associated with GBS (33). Although the mimicry of the GM1 ganglioside in the core OS of C. jejuni O:41 LPS is the same as that exhibited by other C. jejuni serotypes associated with GBS, the presence of similar mimicry in the core OSs of enteritis strains suggests that other bacterial and/or host attributes are involved in disease development. On the other hand, differences between the LPSs of C. jejuni O:41 strains in their reactions with antiganglioside antibodies (e.g., anti-asialoGM1 antibodies) may indicate the presence of slight differences in sugar substitution in the core OS or in the presentation of epitopes in the different LPSs. Whether these factors play a role in disease development requires further investigation.
ACKNOWLEDGMENTS
This study was supported by grants from the Irish Health Research Board (to A.P.M.) and the South African Research Council and the University of Cape Town (to A.J.L.).
We thank J. L. Penner (Toronto, Canada) for providing O:41 typing antisera for immunoblotting studies.
REFERENCES
- 1.Aspinall G O, Lynch C M, Pang H, Shaver R T, Moran A P. Chemical structures of the core region of Campylobacter jejuni O:3 lipopolysaccharide and an associated polysaccharide. Eur J Biochem. 1995;231:570–578. [PubMed] [Google Scholar]
- 2.Aspinall G O, Fujimoto S, McDonald A G, Pang H, Kurjanczyk L A, Penner J L. Lipopolysaccharides from Campylobacter jejuni associated with Guillain-Barré syndrome patients mimic human gangliosides in structure. Infect Immun. 1994;62:2122–2125. doi: 10.1128/iai.62.5.2122-2125.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aspinall G O, McDonald A G, Pang H, Kurjanczyk L A, Penner J L. Lipopolysaccharides of Campylobacter jejuni serotype O:19. Structures of the core oligosaccharide regions from the serostrain and two bacterial isolates from patients with the Guillain-Barré syndrome. Biochemistry. 1994;33:241–249. doi: 10.1021/bi00167a032. [DOI] [PubMed] [Google Scholar]
- 4.Aspinall G O, McDonald A G, Raju T S, Pang H, Kurjanczyk L A, Penner J L. Chemical structure of the core region of Campylobacter jejuni serotype O:2 lipopolysaccharide. Eur J Biochem. 1993;213:1029–1037. doi: 10.1111/j.1432-1033.1993.tb17850.x. [DOI] [PubMed] [Google Scholar]
- 5.Aspinall G O, McDonald A G, Raju T S, Pang H, Mills S D, Kurjanczyk L A, Penner J L. Serological diversity and chemical structures of Campylobacter jejuni low-molecular-weight lipopolysaccharides. J Bacteriol. 1992;174:1324–1332. doi: 10.1128/jb.174.4.1324-1332.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aspinall G O, McDonald A G, Raju T S, Pang H, Moran A P, Penner J L. Chemical structure of the core regions of Campylobacter jejuni serotypes O:1, O:4, O:23, and O:36 lipopolysaccharides. Eur J Biochem. 1993;213:1017–1027. doi: 10.1111/j.1432-1033.1993.tb17849.x. [DOI] [PubMed] [Google Scholar]
- 7.Ayling R D, Woodward M J, Evans S, Newell D G. Restriction fragment length polymorphism of polymerase chain reaction products applied to the differentiation of poultry Campylobacters for epidemiological investigations. Res Vet Sci. 1996;60:168–172. doi: 10.1016/s0034-5288(96)90013-2. [DOI] [PubMed] [Google Scholar]
- 8.Downs A, Pigman W. Qualitative and quantitative determination of sialic acids. Methods Carbohydr Chem. 1976;7:233–240. [Google Scholar]
- 9.Dubois M, Gilles K A, Hamilton J K, Rebers P A, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. [Google Scholar]
- 10.Figueras A, Morales-Olivas F J, Capella D, Palop V, Laporte T R. Bovine gangliosides and acute motor polyneuropathy. Br Med J. 1992;305:1330–1331. doi: 10.1136/bmj.305.6865.1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujimoto S, Yuki N, Itoh T, Amako K. Specific serotype of Campylobacter jejuni associated with Guillain-Barré syndrome. J Infect Dis. 1992;165:183. doi: 10.1093/infdis/165.1.183. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 12.Gamian A, Romanowska E, Dabrowski U, Dabrowski J. Structure of the O-specific, sialic acid-containing polysaccharide chain and its linkage to the core region in lipopolysaccharide from Hafnia alvei strain 2 as elucidated by chemical methods, gas liquid chromatography/mass spectroscopy, and 1H NMR. Biochemistry. 1991;30:5032–5038. doi: 10.1021/bi00234a027. [DOI] [PubMed] [Google Scholar]
- 13.Gamian A, Romanowska E, Ulrich J, Defaye J. The structure of the sialic acid-containing Escherichia coli O104 O-specific polysaccharide and its linkage to the core region in lipopolysaccharide. Carbohydr Res. 1992;236:195–208. doi: 10.1016/0008-6215(92)85016-s. [DOI] [PubMed] [Google Scholar]
- 14.Gregson N A, Koblar S, Hughes R A C. Antibodies to gangliosides in Guillain-Barré syndrome: specificity and relationship to clinical features. Q J Med. 1993;86:111–117. [PubMed] [Google Scholar]
- 15.Griffin J W, Ho T W H. The Guillain-Barré syndrome at 75: the Campylobacter connection. Ann Neurol. 1993;34:125–127. doi: 10.1002/ana.410340204. [DOI] [PubMed] [Google Scholar]
- 16.Hitchcock P J, Brown T M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983;154:269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ho T W, Mishu B, Li C Y, Gao C Y, Cornblath D R, Griffin G W, Asbury A K, Blaser M J, McKhann M J. Guillain-Barré syndrome in Northern China. Relationship to Campylobacter jejuni infection and anti-glycolipid antibodies. Brain. 1995;118:597–605. doi: 10.1093/brain/118.3.597. [DOI] [PubMed] [Google Scholar]
- 18.Illa I, Ortiz N, Gallard E, Juarez C, Grau J M, Dalakas M C. Acute axonal Guillain-Barré syndrome with IgG antibody against motor axons following parenteral gangliosides. Ann Neurol. 1995;38:218–224. doi: 10.1002/ana.410380214. [DOI] [PubMed] [Google Scholar]
- 19.Jacobs B C, Endtz H P, van der Meché F G A, Hazenberg M P, Achtereekte H A M, van Doorn P A. Serum anti-GQ1b IgG antibodies recognize surface epitopes on Campylobacter jejuni from patients with Miller Fisher syndrome. Ann Neurol. 1995;37:260–264. doi: 10.1002/ana.410370218. [DOI] [PubMed] [Google Scholar]
- 20.Kaldor J, Speed B R. Guillain-Barré syndrome and Campylobacter jejuni: a serological study. Br Med J. 1984;288:1867–1870. doi: 10.1136/bmj.288.6434.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krauss J H, Himmelspach K, Reuter G, Schauer R, Mayer H. Structural analysis of a novel sialic acid-containing trisaccharide from Rhodobacter capsulatus 37b4 lipopolysaccharide. Eur J Biochem. 1992;204:217–223. doi: 10.1111/j.1432-1033.1992.tb16627.x. [DOI] [PubMed] [Google Scholar]
- 22.Krauss J H, Reuter G, Schauer R, Weckesser J, Mayer H. Sialic acid-containing lipopolysaccharide in purple nonsulfur bacteria. Arch Microbiol. 1988;150:584–589. [Google Scholar]
- 23.Kuroki S, Haruta T, Yoshiota M, Kobayashi Y, Nukina M, Nakanishi H. Guillain-Barré syndrome associated with Campylobacter infection. Pediatr Infect Dis J. 1991;10:149–151. doi: 10.1097/00006454-199102000-00014. [DOI] [PubMed] [Google Scholar]
- 24.Kuroki S, Saida T, Nukina M, Haruta T, Yoshiota M, Kobayashi Y, Nakanishi H. Campylobacter jejuni strains from patients with Guillain-Barré syndrome belong mostly to Penner serogroup 19 and contain β-N-acetylglucosamine residues. Ann Neurol. 1993;33:243–247. doi: 10.1002/ana.410330304. [DOI] [PubMed] [Google Scholar]
- 25.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 26.Lastovica, A. J., E. A. Goddard, and A. C. Argent. 1997. Guillain-Barré syndrome in South Africa associated with Campylobacter jejuni O:41 strains. J. Infect. Dis. 176(Suppl. 2):S139–S143. [DOI] [PubMed]
- 27.Mishu B, Blaser M J. Role of infection due to Campylobacter jejuni in the initiation of Guillain-Barré syndrome. Clin Infect Dis. 1993;17:104–108. doi: 10.1093/clinids/17.1.104. [DOI] [PubMed] [Google Scholar]
- 28.Mishu, B., T. W. Ho, C. Y. Li, C. Y. Gao, J. W. Griffin, A. K. Asbury, D. R. Cornblath, C. M. Patton, G. M. McKhann, and M. J. Blaser. 1993. Guillain-Barré syndrome (GBS), acute motor axonal neuropathy and Campylobacter jejuni infection. Acta Gastro-Enterol. Belg. 56(Suppl.):S9.
- 29.Mishu B, IIyas A A, Koski C L, Vriesdorp F J, Cook S D, Milton F A, Blaser M J. Serologic evidence of previous Campylobacter jejuni infection in patients with the Guillain-Barré syndrome. Ann Intern Med. 1993;118:947–957. doi: 10.7326/0003-4819-118-12-199306150-00006. [DOI] [PubMed] [Google Scholar]
- 30.Monos, D. S., M. Papaioakim, T. W. Ho, C. Y. Li, and G. M. McKhann. 1997. Differential distribution of HLA alleles in two forms of Guillain-Barre syndrome. J. Infect. Dis. 176(Suppl. 2):S180–S182. [DOI] [PubMed]
- 31.Moran A P. Biological and serological characterization of Campylobacter jejuni lipopolysaccharides with deviating core and lipid A structures. FEMS Immunol Med Microbiol. 1995;11:121–130. doi: 10.1111/j.1574-695X.1995.tb00098.x. [DOI] [PubMed] [Google Scholar]
- 32.Moran A P, Kosunen T U. Serological analysis of the heat-stable antigens involved in serotyping Campylobacter jejuni and Campylobacter coli. APMIS. 1989;97:253–260. [PubMed] [Google Scholar]
- 33.Moran A P, O’Malley D T. Potential role of lipopolysaccharides of Campylobacter jejuni in the development of Guillain-Barré syndrome. J Endotoxin Res. 1995;2:233–235. [Google Scholar]
- 34.Moran A P, O’Malley D T, Kosunen T U, Helander I M. Biochemical characterization of Campylobacter fetus lipopolysaccharides. Infect Immun. 1994;62:3922–3929. doi: 10.1128/iai.62.9.3922-3929.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moran, A. P., and M. M. Prendergast. Unpublished data.
- 36.Moran, A. P., and C. V. Carroll. Unpublished data.
- 37.Moran A P, Prendergast M M, Appelmelk B J. Molecular mimicry of host structures by bacterial lipopolysaccharide and its contribution to disease. FEMS Immunol Med Microbiol. 1996;16:105–115. doi: 10.1111/j.1574-695X.1996.tb00127.x. [DOI] [PubMed] [Google Scholar]
- 38.Moran A P, Rietschel E T, Kosunen T U, Zahringer U. Chemical characterization of Campylobacter jejuni lipopolysaccharides containing N-acetylneuraminic acid and 2,3-diamino-2,3-dideoxy-d-glucose. J Bacteriol. 1991;173:618–626. doi: 10.1128/jb.173.2.618-626.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nachamkin, I. Unpublished data.
- 40.Nikaido H, Vaara M. Outer membrane. In: Neidhardt F C, Ingraham J L, Low K B, Magasanik B, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Vol. 1. Washington, D.C: American Society for Microbiology; 1987. pp. 7–22. [Google Scholar]
- 41.Nobile-Orazio E, Carpo M, Meucci N, Grassi M P, Capitani E, Sciacco M, Mangoni A, Scarlato G. Guillain-Barré syndrome associated with high titres of anti-GM1 antibodies. J Neurol Sci. 1991;109:200–206. doi: 10.1016/0022-510x(92)90169-l. [DOI] [PubMed] [Google Scholar]
- 42.Obayashi H, Saida T, Kuroki S, Nukina M, Nishitani Y. Guillain-Barré syndrome and Campylobacter jejuni infection. Shinkei Naika. 1993;38:431–438. [Google Scholar]
- 43.Oomes P G, Jacobs B C, Hazenberg M P H, Bänffer J R J, van der Meché F G A. Anti-GM1 IgG antibodies and Campylobacter bacteria in Guillain-Barré syndrome: evidence of molecular mimicry. Ann Neurol. 1995;38:170–175. doi: 10.1002/ana.410380208. [DOI] [PubMed] [Google Scholar]
- 44.Penner, J. L., and G. O. Aspinall. 1997. Diversity of lipopolysaccharide structures in Campylobacter jejuni. J. Infect. Dis. 176(Suppl. 2):S135–S138. [DOI] [PubMed]
- 45.Penner J L, Hennessy J N, Congi R V. Serotyping of Campylobacter jejuni and Campylobacter coli on the basis of thermostable antigens. Eur J Clin Microbiol. 1983;2:78–83. doi: 10.1007/BF02019474. [DOI] [PubMed] [Google Scholar]
- 46.Preston M A, Penner J L. Structural and antigenic properties of lipopolysaccharide from serotype reference strains of Campylobacter jejuni. Infect Immun. 1987;55:1806–1812. doi: 10.1128/iai.55.8.1806-1812.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rees J H, Gregson N A, Griffiths P L, Hughes R A C. Campylobacter jejuni and Guillain-Barré syndrome. Q J Med. 1993;86:623–634. doi: 10.1093/qjmed/86.10.623. [DOI] [PubMed] [Google Scholar]
- 48.Rees J H, Hughes R A C. Campylobacter jejuni and Guillain-Barré syndrome. Ann Neurol. 1994;35:248–249. doi: 10.1002/ana.410350228. [DOI] [PubMed] [Google Scholar]
- 49.Rees J H, Soudain S E, Gregson N A, Hughes R A C. Campylobacter jejuni infection and Guillain-Barré syndrome. N Engl J Med. 1995;333:1374–1379. doi: 10.1056/NEJM199511233332102. [DOI] [PubMed] [Google Scholar]
- 50.Ropper A H. The Guillain-Barré syndrome. N Engl J Med. 1992;326:1130–1136. doi: 10.1056/NEJM199204233261706. [DOI] [PubMed] [Google Scholar]
- 51.Saito M, Kasai N, Yu R K. In situ immunological determination of basic carbohydrate structures of gangliosides on thin-layer plates. Anal Biochem. 1985;148:54–58. doi: 10.1016/0003-2697(85)90627-x. [DOI] [PubMed] [Google Scholar]
- 52.Salloway S, Mermel L A, Seamans M, Aspinall G O, Nam Shin J E, Kurjanczyk L A, Penner J L. Miller-Fisher syndrome associated with Campylobacter jejuni bearing lipopolysaccharide molecules that mimic human ganglioside GD3. Infect Immun. 1996;64:2945–2949. doi: 10.1128/iai.64.8.2945-2949.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schneider H, Hale T L, Zollinger W D, Seid R C, Jr, Hammack C A, Griffiss J M. Heterogeneity of molecular size and antigenic expression within lipooligosaccharides of individual strains of Neisseria gonorrhoeae and Neisseria meningitidis. Infect Immun. 1984;45:544–549. doi: 10.1128/iai.45.3.544-549.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schwerer B, Neisser A, Polt R J, Bernheimer H, Moran A P. Antibody cross-reactivities between gangliosides and lipopolysaccharides of Campylobacter jejuni serotypes associated with Guillain-Barré syndrome. J Endotoxin Res. 1995;2:395–403. [Google Scholar]
- 55.Schwimmer S, Bevenue A. Reagent for differentiation of 1,4- and 1,6-linked glucosaccharides. Science. 1956;123:543–544. doi: 10.1126/science.123.3196.543. [DOI] [PubMed] [Google Scholar]
- 56.Skirrow M B, Benjamin J. Differentiation of enteropathogenic Campylobacter. J Clin Pathol. 1980;33:1122. doi: 10.1136/jcp.33.11.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Towbin H, Staehelin T, Gordon S. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tsai C M, Frasch C E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982;119:115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- 59.van der Meché F G A, Schmitz P I M the Dutch Guillain-Barré Study Group. A randomized trial comparing intravenous globulin and plasma exchange in Guillain-Barré syndrome. N Engl J Med. 1992;326:1123–1129. doi: 10.1056/NEJM199204233261705. [DOI] [PubMed] [Google Scholar]
- 60.Wassenaar T M, Bleumink-Pluym N M C, van der Zeijst B A M. Inactivation of Campylobacter jejuni flagellin genes by homologous recombination demonstrates that flaA but not flaB is required for invasion. EMBO J. 1991;10:2055–2061. doi: 10.1002/j.1460-2075.1991.tb07736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Westphal O, Jann K. Bacterial lipopolysaccharides: extraction with phenol water and further applications of the procedure. Methods Carbohydr Chem. 1965;5:83–92. [Google Scholar]
- 62.Willison H J, Kennedy P G E. Gangliosides and bacterial toxins in Guillain-Barré syndrome. J Neuroimmunol. 1993;46:105–112. doi: 10.1016/0165-5728(93)90239-u. [DOI] [PubMed] [Google Scholar]
- 63.Yuki N. Pathogenesis of axonal Guillain-Barré syndrome: hypothesis. Muscle Nerve. 1994;17:680–682. doi: 10.1002/mus.880170619. [DOI] [PubMed] [Google Scholar]
- 64.Yuki N, Handa S, Tai T, Takahashi M, Saito K, Tsujino Y, Taki T. Ganglioside-like epitopes of lipopolysaccharide from Campylobacter jejuni (Pen 19) in three isolates from patients with Guillain-Barré syndrome. J Neurol Sci. 1995;130:112–116. doi: 10.1016/0022-510x(95)00045-4. [DOI] [PubMed] [Google Scholar]
- 65.Yuki N, Handa S, Taki T, Kasama T, Takihashi M, Saito K, Miyatake T. Cross-reactive antigen between nervous tissue and a bacterium elicits Guillain-Barré syndrome: molecular mimicry between ganglioside GM1 and lipopolysaccharide from Penner’s serotype 19 of Campylobacter jejuni. Biomed Res. 1992;13:451–453. [Google Scholar]
- 66.Yuki N, Ichikawa H, Doi A. Fisher syndrome after Campylobacter jejuni enteritis: human leukocyte antigen and the bacterial serotype. J Pediatr. 1995;126:55–57. doi: 10.1016/s0022-3476(95)70500-7. [DOI] [PubMed] [Google Scholar]
- 67.Yuki N, Sato S, Itoh T, Miyatake T. HLA-B35 and acute axonal polyneuropathy following Campylobacter infection. Neurology. 1991;41:1561–1563. doi: 10.1212/wnl.41.10.1561. [DOI] [PubMed] [Google Scholar]
- 68.Yuki N, Taki T, Inagaki F, Kasama T, Takahashi T M, Saito M K, Handa S, Miyatake T. A bacterium lipopolysaccharide that elicits Guillain-Barré syndrome has a GM1 ganglioside-like structure. J Exp Med. 1993;178:1771–1775. doi: 10.1084/jem.178.5.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yuki N, Taki T, Takahashi M, Saito K, Tai T, Miyatake T, Handa S. Penner’s serogroup 4 of Campylobacter jejuni has a lipopolysaccharide that bears a GM1 ganglioside epitope as well as one that bears a GD1a epitope. Infect Immun. 1994;62:2101–2103. doi: 10.1128/iai.62.5.2101-2103.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yuki N, Yoshino H, Sato S, Miyatake T. Acute axonal polyneuropathy associated with anti-GM1 antibodies following Campylobacter enteritis. Neurology. 1990;40:1900–1902. doi: 10.1212/wnl.40.12.1900. [DOI] [PubMed] [Google Scholar]