Abstract
FRS2 has been identified in mammalian cells as a protein that is tyrosine phosphorylated and binds to Grb2 and Shp2 in response to fibroblast growth factor (FGF) or nerve growth factor (NGF) stimulation. But neither its existence in other vertebrate classes or invertebrates nor its function during embryonic development has been defined. Here we have identified and characterized a Xenopus homolog of FRS2 (xFRS2). xFRS2 is tyrosine phosphorylated in early embryos, and overexpression of an unphosphorylatable form of xFRS2 interferes with FGF-dependent mesoderm formation. The Src family kinase Laloo, which was shown to function in FGF signaling during early Xenopus development, binds to xFRS2 and promotes tyrosine phosphorylation of xFRS2. Moreover, xFRS2 and Laloo are shown to bind to Xenopus FGF receptor 1. These results suggest that xFRS2 plays an important role in FGF signaling in cooperation with Laloo during embryonic development.
INTRODUCTION
Fibroblast growth factor (FGF) signaling has important roles in cell proliferation, cell differentiation and many developmental processes (Basilico and Moscatelli, 1992). FRS2 (FGF receptor substrate 2) was identified in mammalian cultured cells as a tyrosine-phosphorylated protein that binds to Grb2 in response to FGF but not epidermal growth factor (EGF) (Kouhara et al., 1997). FRS2 is also called SNT (suc-associated neurotrophic factor target) as it binds to p13suc1 (Rabin et al., 1993). FRS2 functions as a docking protein linking the activation of FGF receptor and nerve growth factor (NGF) receptor with their downstream components (Kouhara et al., 1997; Hadari et al., 1998). It possesses a myristylation site and a phosphotyrosine-binding domain (PTB domain) (Kouhara et al., 1997). Deletion of the PTB domain abolishes both its association with FGF receptor and its tyrosine phosphorylation in response to FGF (Xu et al., 1998). FRS2 has multiple tyrosine residues, which become phosphorylated upon FGF or NGF stimulation and serve as binding sites for Grb2 and the tyrosine phosphatase Shp2 (Ong et al., 1996, 1997; Wang et al., 1996; Kouhara et al., 1997; Hadari et al., 1998). So far, FRS2 has been identified only in mammals, and whether it exists in other vertebrate classes or invertebrates has not been determined. Moreover, all the experiments have been performed in mammalian cultured cells.
In Xenopus, FGFs play an essential role in mesoderm patterning (Slack et al., 1996; Isaacs, 1997). Downstream signal transduction components such as Shp2, Ras, Raf and MAPK have also been demonstrated to be required for early development (Whitman and Melton, 1992; MacNicol et al., 1993; Gotoh et al., 1995; LaBonne et al., 1995; Tang et al., 1995; Umbhauer et al., 1995). Furthermore, the Src family kinase Laloo was identified as a novel factor that is involved in FGF-mediated mesoderm patterning (Weinstein et al., 1998; Weinstein and Hemmati-Brivanlou, 2001). However, its molecular relationship with other signaling components has not been defined.
In this study, we have identified and characterized a Xenopus homolog of FRS2 (xFRS2). Ectopic expression of xFRS2 is able to induce mesodermal markers in animal cap explants. Moreover, expression of an unphosphorylatable form of xFRS2 abolishes FGF-induced mesoderm differentiation in animal cap explants and interferes with mesoderm patterning in whole embryos. We have further found that Laloo enhances tyrosine phosphorylation of xFRS2. Furthermore, xFRS2, Laloo and Xenopus FGF receptor 1 (xFGFR1) form a functional complex. These results suggest that the signaling complex composed of xFRS2, Laloo and xFGFR1 plays an essential role in FGF signaling during early Xenopus development.
RESULTS AND DISCUSSION
Cloning and expression of xFRS2
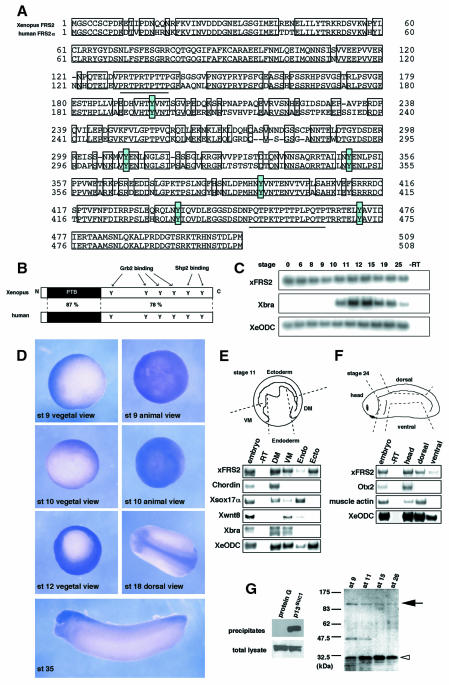
We isolated a Xenopus homolog of FRS2 (xFRS2) whose cDNA sequence revealed an open reading frame of 509 amino acids with 81 and 47% identity to human FRS2α and β, respectively (Figure 1A and B, and data not shown). xFRS2 contains six potential sites of tyrosine phosphorylation. These tyrosines include four potential binding sites for Grb2 at Tyr195, Tyr308, Tyr350 and Tyr393 and two potential binding sites for Shp2 at Tyr437 and Tyr472, and are completely conserved in xFRS2 and human FRS2α (Figure 1A and B). Putative binding sites for SH3 (Pro-X-X-Pro) are also conserved in xFRS2 (Figure 1A, underlined).
Fig. 1. Cloning and expression of Xenopus FRS2. (A) Alignment of the deduced amino acid sequences of Xenopus FRS2 and human FRS2α. Residues that are identical are boxed. Dashes denote gaps. Underlines indicate P-X-X-P sequences. Six conserved tyrosines are shaded blue. (B) The schematic structures of xFRS2 and human FRS2α. (C) xFRS2 mRNA is present during Xenopus early embryogenesis. The total RNA isolated from indicated stages was subjected to RT–PCR. Xenopus embryonic ornithine decarboxylase (XeODC) is a loading control. Xbra was also examined. No signal was observed in the absence of reverse transcriptase (–RT). (D) Whole-mount in situ hybridization for xFRS2. (E) xFRS2 is expressed in the mesoderm and ectoderm regions at stage 11. Dorsal mesoderm (DM), ventral mesoderm (VM), endoderm (Endo) and ectoderm (Ecto) regions were dissected from ten embryos (stage 11) as shown. Each was processed for RT–PCR. The dorsal mesoderm marker Chordin, the endoderm marker Xsox17α, the ventral mesoderm marker Xwnt8 and the pan-mesodermal marker Xbra were also analyzed. (F) xFRS2 is expressed in the head and dorsal structures at stage 24. Head, dorsal and ventral regions were dissected from five embryos as shown. Each was processed for RT–PCR. The forebrain marker Otx2 and the somitic muscle marker muscle actin were also analyzed. (G) Endogenous xFRS2 is tyrosine phosphorylated. Myc-tagged xFRS2 mRNA was injected and the injected embryos were extracted at stage 11. The extract then was subjected to precipitation with p13suc1-agarose beads. Protein G beads were used as a control. The precipitates were detected by anti-myc antibody (left). Extracts of embryos from stage 9 to 26 were subjected to precipitation with p13suc1-agarose beads and the precipitates were analyzed with anti-phosphotyrosine antibody (right). A black arrow indicates xFRS2 and a white arrowhead Cdc2. The DDBJ/EMBL/GenBank accession number for xFRS2 is AB064525.
xFRS2 mRNA was expressed maternally and throughout early embryogenesis (Figure 1C). Expression of xFRS2 was mainly seen in presumptive mesoderm and ectoderm during blastula (stage 9) and gastrula stages (stage 10, 12) (Figure 1D). The strong staining was detected in head and dorsal structures during neurula (stage 18) and tailbud stages (stage 35). An RT–PCR with microsectioning gave essentially the same results (Figure 1E and F).
Mammalian FRS2 has been shown to bind to p13suc1 (Rabin et al., 1993; Kouhara et al., 1997). The result in Figure 1G, left, showed that xFRS2 is also able to bind to p13suc1. Then, extracts of early embryos (from stage 9 to 26) were subjected to precipitation with the p13suc1 beads and the precipitates were immunoblotted with anti-phosphotyrosine antibody. Three protein bands were detected as tyrosine-phosphorylated proteins in the precipitates, whose apparent molecular masses were 100, 45 and 32-kDa, respectively (Figure 1G, right). The 100 and 32-kDa proteins are presumably xFRS2 and Cdc2, respectively, but the 45-kDa band has not been identified. These results suggest that xFRS2 is tyrosine phosphorylated during early embryogenesis.
Role of xFRS2 in early embryogenesis
We injected xFRS2 mRNA into animal poles of two-cell stage embryos. RT–PCR analysis showed that expression of xFRS2 stimulated the expression of pan-mesodermal markers, Xbra and Xcad3, in 3 h-cultured animal cap explants (Figure 2A, upper), although the extent of the stimulation was lower than that induced by FGF treatment (Figure 2A, upper). When cultured for 24 h, a posterior mesodermal marker Xhox3, but not muscle actin, was stimulated also by xFRS2 (Figure 2A, lower). A dorsal mesodermal marker goosecoid was not induced by xFRS2 (data not shown). Thus, a profile of mesodermal markers whose expression is stimulated by xFRS2 is very similar to that of genes induced by FGF.
Fig. 2. Involvement of xFRS2 in mesoderm patterning. (A) xFRS2 induces mesodermal markers in animal caps. Wild-type xFRS2 mRNA (100 pg, upper panel; 1 ng, lower panel) was injected into animal poles of two-cell stage embryos. At stage 8, the animal caps were dissected and harvested at midgastrula (upper) or tailbud stages (lower). Total RNA of caps was analyzed by RT–PCR. EF-1α is a loading control. RNA from whole embryos (indicated as embryo) provides a positive control. The concentration of FGF is 50 ng/ml. (B) xFRS2-6F inhibits the induction of mesodermal markers by FGF in animal caps. Indicated amounts of WT (wild type) xFRS2 mRNA or xFRS2-6F mRNA were injected. Animal caps were dissected and cultured without or with FGF (50 ng/ml). Caps were harvested at the midgastrula stage and subjected to RT–PCR. (C) An inhibitory effect of xFRS2-6F on FGF-dependent induction of mesoderm markers is rescued by WT xFRS2. xFRS2-6F mRNA (400 pg) was co-injected with or without WT xFRS2 mRNA (100 pg). Animal caps were cultured in the presence or absence of FGF (50 ng/ml) and subjected to RT–PCR. (D) Expression of mesodermal markers in whole embryos is inhibited by xFRS2-6F. WT xFRS2 mRNA or xFRS2-6F mRNA (400 pg or 2 ng) was injected into the marginal zones of four-cell stage embryos. Injected embryos were collected at stage 11 and subjected to RT–PCR. (E) xFRS2-6F inhibits mesodermal patterning in whole embryos. Indicated amounts of mRNAs were injected into the marginal zones of four-cell stage embryos and cultured until the tadpole stage. Top panel, control embryos; middle, embryos injected with 400 pg of xFRS-6F mRNA (61% with trunk and tail truncations; n = 93) and embryos injected with 2 ng of xFRS2-6F mRNA (73% with trunk and tail truncations; n = 55); bottom, embryos injected with 400 pg of xFRS2-6F mRNA and 4 pg of active Ras (human H-Ras V12) mRNA (17% with trunk and tail truncations; n = 57).
It was shown that a mutant form of mammalian FRS2, FRS2-5F, in which five out of the six tyrosine residues serving as binding sites for Grb2 and Shp2 are replaced by phenylalanines, is a loss-of-function mutant and may act as a weak dominant-negative mutant in PC12 cells (Hadari et al., 1998). Then, we constructed a mutant of xFRS2, xFRS2-6F, in which all six tyrosine residues are replaced by phenylalanines. In the animal cap assay, xFRS2-6F, unlike wild-type (WT) xFRS2, did not induce Xbra or Xcad3 (Figure 2B). Furthermore, xFRS2-6F significantly suppressed the FGF-dependent induction of Xbra and Xcad3 (Figure 2B). This suppression was rescued by co-expression of WT xFRS2 (Figure 2C). These results suggest that xFRS2-6F can act in a dominant-negative fashion. It is possible that the 6F mutant (xFRS2-6F) may be stronger than the 5F mutant (FRS2-5F) with respect to its dominant-negative action. Both mammalian FRS2 (Xu et al., 1998; Dhalluin et al., 2000; Ong et al., 2000) and xFRS2 (Figure 4) bind to the juxtamembrane (JM) region of FGF receptor 1 (FGFR1) in their PTB domain, so these YF mutants of FRS2 may compete with endogenous FRS2 for FGFR and thus act in a dominant-negative way.
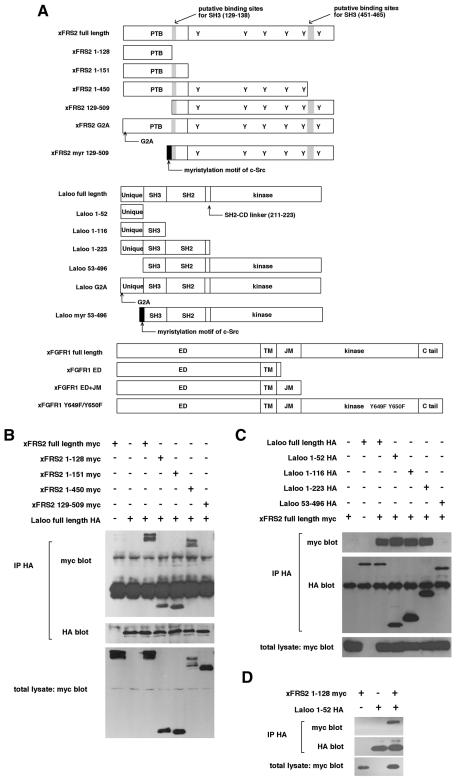
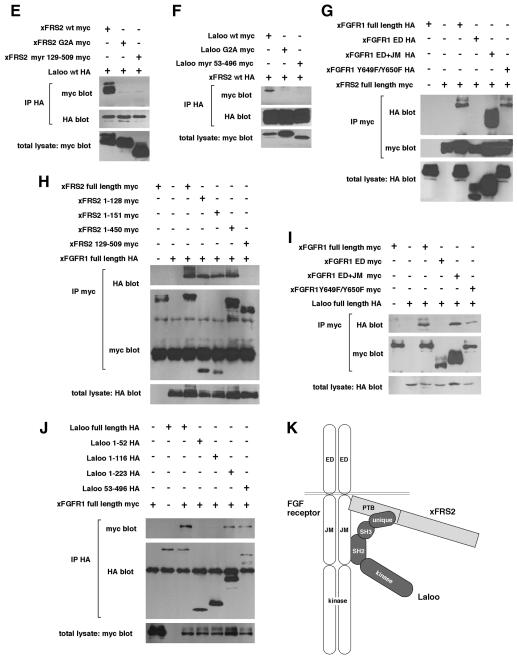
Fig. 4. Physical interactions of xFRS2, Laloo and xFGFR1. (A) Schematic representation of full-length and truncated forms of xFRS2, Laloo and xFGFR1. ED, extracellular domain; TM, transmembrane domain; JM, juxtamembrane domain. (B) HA-tagged full-length Laloo was transiently co-expressed with myc-tagged full-length or truncated xFRS2 in C2C12 cells. Lysates of cells were immunoprecipitated (IP) with anti-HA antibody and co-immunoprecipitated full-length or truncated xFRS2 was detected by immunoblotting with anti-myc antibody (top). Comparable amounts of Laloo were immunoprecipitated in each lane (middle). The expression level of full-length or truncated xFRS2 was similar (bottom). (C) Binding of myc-tagged full-length xFRS2 to HA-tagged full-length or truncated Laloo. (D) Binding of myc-tagged xFRS2 (1–128) to HA-tagged Laloo (1–52). (E) Binding of HA-tagged Laloo to myc-tagged full-length or mutated xFRS2. (F) Binding of HA-tagged xFRS2 to myc-tagged full-length or mutated Laloo. (G) Binding of myc-tagged full-length xFRS2 to HA-tagged full-length or truncated xFGFR1. (H) Binding of HA-tagged full-length xFGFR1 to myc-tagged full-length or truncated xFRS2. (I) Binding of HA-tagged full-length Laloo to myc-tagged full-length or truncated xFGFR1. (J) Binding of myc-tagged full-length xFGFR1 to HA-tagged full-length or truncated Laloo. (K) A hypothetical model for the interactions among xFRS2, Laloo and xFGFR1.
Next, we examined the effect of expression of xFRS2-6F on embryonic development. xFRS2-6F mRNA or WT FRS2 mRNA was injected into the marginal zones of four-cell stage embryos. In xFRS2-6F-injected embryos at stage 11, expression of pan-mesodermal markers, Xbra and Xcad3, was significantly reduced while expression of a dorsal mesodermal marker, Chordin, was little affected (Figure 2D). In WT xFRS2-injected embryos, however, expression of all of these mesodermal markers was mostly unaffected (Figure 2D). At the tailbud stage, the xFRS2-6F-injected embryo, but not the WT xFRS2-injected embryo (data not shown), showed a dorsal bending or a dorsal open phenotype, and trunk and tail truncation occurred in the embryo (Figure 2E). This apparent phenotype of the tailbud embryo resembled that induced by expression of dominant-negative FGFR (Amaya et al., 1991). Coexpression of constitutively active Ras (Ras V12) partially rescued the xFRS2-6F-induced phenotype (Figure 2E), suggesting that xFRS2 functions upstream of Ras in mesoderm patterning. We could not rescue the xFRS2-6F-induced phenotype by co-injection of WT xFRS2 mRNA because the co-injected embryos showed embryonic death between gastrula and neurula stages (data not shown). Injection of a high dose of WT xFRS2 mRNA alone caused embryonic death (data not shown). Overexpression of xFRS2 in the marginal zone appeared to have some toxic effect on the embryo. In any case, all these results suggest that xFRS2 plays an important role in early mesoderm patterning downstream of FGFR.
Interaction of xFRS2 with the Src family kinase Laloo
Since the Src family kinase Laloo is involved in FGF-mediated mesoderm formation (Weinstein et al., 1998), it is possible that xFRS2 interacts functionally with Laloo. To test this, we first examined tyrosine phosphorylation of xFRS2. When Xenopus FGFR1 (xFGFR1) was co-expressed with xFRS2 in animal caps, xFRS2 became tyrosine phosphorylated (Figure 3A). This tyrosine phosphorylation was not detected in the absence of the co-expressed xFGFR1 (Figure 3A) and occurred on either of the six tyrosine residues because xFRS2-6F was not phosphorylated at all under the same conditions (Figure 3A). When an active form of Laloo, Laloo Y492F (Weinstein et al., 1998), was co-expressed instead of xFGFR1, xFRS2 also became tyrosine phosphorylated (Figure 3A). xFRS2-6F was not phosphorylated at all (Figure 3A). These data indicate that Laloo, like xFGFR1, is able to induce tyrosine phosphorylation of xFRS2. Then, we examined whether tyrosine phosphorylation of xFRS2 by xFGFR1 depends on Laloo or vice versa. Phosphorylation of xFRS2 induced by xFGFR1 was decreased significantly by co-expression of a kinase-inactive form of Laloo, Laloo K259E (Figure 3B), suggesting that xFGFR1-induced phosphorylation of xFRS2 is partially dependent on the kinase activity of Laloo. Phosphorylation of xFRS2 induced by Laloo Y492F was not reduced by co-expression of xFGFR1 Y649F/Y650F, a non-activatable mutant which is mutated at two autophosphorylation sites that are essential for activation of the kinase activity (Mohammadi et al., 1996). However, phosphorylation by Laloo Y492F was inhibited markedly by co-expression of XFD (Amaya et al., 1991), a mutant of xFGFR1 that lacks an entire region of intracellular domain (Figure 3B). These results suggest that tyrosine phosphorylation of xFRS2 by Laloo is independent of the activated kinase activity of xFGFR1 but requires the intracellular domain of xFGFR1.
Fig. 3. Interaction of xFRS2 with the Src family kinase Laloo. (A) Laloo enhances tyrosine phosphorylation of xFRS2. Effect of co-injection of xFGFR1 or Laloo Y492F (an active form) on tyrosine phosphorylation of xFRS2 was analyzed. Indicated sets of mRNA were injected at animal poles of 2-cell stage embryos and animal caps were dissected. The lysates of caps were immunoprecipitated (IP) with anti-myc antibody and immunoblotted with anti-phosphotyrosine antibody (upper) or anti-myc antibody (lower). (B) xFGFR1 and Laloo Y492F cooperate to induce tyrosine phosphorylation of xFRS2. The experiments were performed as described in (A). (C) xFRS2 binds to Laloo. Lysates of caps co-injected with the indicated combinations of mRNA encoding myc-tagged xFRS2 or HA-tagged Laloo were immunoprecipitated with anti-HA antibody. Co-immunoprecipitated xFRS2 was detected by blotting with anti-myc antibody (top). The amounts of the precipitated Laloo (middle) and the expression level of xFRS2 (bottom) were also determined. (D) Xbra induction by Laloo Y492F is dependent on xFRS2. Indicated combinations of mRNA encoding Laloo Y492F, WT xFRS2, or xFRS2-6F were co-injected into animal poles of 2-cell stage embryos. The animal cap assay and the RT–PCR analysis were performed as in Figure 2.
Next, we examined a possible physical interaction of xFRS2 with Laloo. xFRS2 was co-immunoprecipitated with Laloo (Figure 3C), indicating the physical interaction of xFRS2 with Laloo. We then performed the animal cap assay to investigate a functional relationship of xFRS2 with Laloo. The induction of Xbra by Laloo Y492F was significantly inhibited by expression of xFRS2-6F (Figure 3D). It was previously reported that Laloo K259E inhibits Xbra induction by FGF (Weinstein et al., 1998). Thus, xFGFR1 and Laloo cooperate to induce tyrosine phosphorylation of xFRS2, which is important for FGF-dependent mesoderm patterning.
To characterize further the binding between xFRS2 and Laloo, we expressed various truncated mutants of xFRS2 and Laloo (Figure 4A) in C2C12 cells. Their binding ability was determined by co-immunoprecipitation assay. First, a series of truncation constructs of xFRS2 was tested against Laloo (full-length). xFRS2 (full-length), xFRS2 (1–128), xFRS2 (1–151) and xFRS2 (1–450) were able to bind to Laloo (full-length), while xFRS2 (129–509), which contains two putative binding sites for SH3 (the PXXP sequences, residues 129–138 and residues 451–465) (Figure 4A), was not (Figure 4B), indicating that the N-terminal region (1–128) of xFRS2 lacking the PXXP sequence (Figure 4A) is sufficient for binding to Laloo. Next, a series of truncation constructs of Laloo was tested against xFRS2 (full-length). Among the four truncation constructs of Laloo, only Laloo (53–496) was unable to bind to xFRS2 (Figure 4C), indicating that the N-terminal region (1–52) of Laloo including the unique domain (Figure 4A) interacts with xFRS2. The interaction of xFRS2 (1–128) with Laloo (1–52) was also observed (Figure 4D). Thus, the interaction of Laloo with xFRS2 does not seem to be mediated through the binding between the SH3 domain and the PXXP sequence.
We then examined the possible role of myristylation in the interaction between xFRS2 and Laloo. We generated myristylation-deficient mutants, xFRS2 G2A and Laloo G2A, in which a key glycine residue in the consensus myristylation sequence (MGXXXS/T) was replaced by alanine (Figure 4A). xFRS2 G2A could not bind to wild-type Laloo (Figure 4E) and Laloo G2A could not bind to wild-type xFRS2 (Figure 4F), suggesting that the optimal interaction of xFRS2 with Laloo may require myristylation. To examine whether or not the interaction of xFRS2 with Laloo might be a non-specific one that is due to myristylation, we constructed xFRS2 myr (129–509) and Laloo myr (53–496), which were fusion proteins in which the myristylation motif of c-Src (Cross et al., 1984) was fused to the N-terminus of xFRS2 (129–509) or Laloo (53–496), respectively (Figure 4A). xFRS2 myr (129–509) was unable to bind to wild-type Laloo (Figure 4E) and Laloo myr (53–496) was unable to bind to wild-type xFRS2 (Figure 4F). These data suggest that the myristylation alone, if xFRS2 and Laloo lack their binding domain (the N-terminal domain in xFRS2 and the N-terminal domain in Laloo), cannot induce their interaction. Therefore, the interaction of xFRS2 with Laloo may not be a non-specific one caused by myristylation. We speculate that membrane recruitment of both proteins may increase their local concentrations and thus enhance their interaction.
Interaction of xFGFR1 with xFRS2 and Laloo
Since the PTB domain of mammalian FRS2 was shown to bind to the JM domain of FGFR1 (Xu et al., 1998; Ong et al., 2000), the binding of xFRS2 to xFGFR1 was also analyzed. xFGFR1 (full-length), xFGFR1 (ED+JM) and xFGFR1 (Y649F/Y650F) bound to xFRS2 (full-length), whereas xFGFR1 (ED) which lacks the JM domain did not (Figure 4G). xFRS2 (full-length), xFRS2 (1–128), xFRS2 (1–151) and xFRS2 (1–450) were able to bind to xFGFR1 (full-length), while xFRS2 (129–509) was not (Figure 4H). Therefore, the PTB domain of xFRS2 binds to the JM domain of xFGFR1.
Our co-immunoprecipitation assays also showed that Laloo (full-length) binds to xFGFR1 (full-length) (Figure 4I and J). Then, their binding regions were determined by using their truncation constructs. xFGFR1 (ED+JM) and xFGFR1 (Y649F/Y650F) were able to bind to Laloo, while xFGFR1(ED) was not (Figure 4I), indicating that the JM domain of xFGFR1 is the binding site. Whereas, Laloo (full-length), Laloo(1–223) and Laloo(53–496) co-immunoprecipitated with xFGFR1 (full-length), Laloo(1–52) or Laloo(1–116) did not (Figure 4J), indicating that the region of residues 117–223 of Laloo is the binding site. This region includes the SH2 domain (residues 117–210) and the SH2–CD (catalytic domain) linker region (residues 211–223) (Figure 4A). Further analyses are required to determine which site in this region is important for the binding to xFGFR1. It is possible, however, that the binding may not be mediated via phosphorylated tyrosines on xFGFR1, since the mutant xFGFR1 (Y649F/Y650F) was also able to bind to Laloo (Figure 4I).
Our domain analyses concerning physical interactions among xFRS2, Laloo and xFGFR1 have shown that these three molecules interact specifically with one another. Although whether or not a stable ternary complex forms could not be determined here, our analyses suggest that Laloo and xFGFR1 functionally cooperate to induce tyrosine phosphorylation of xFRS2. We may hypothesize that xFGFR1, Laloo and xFRS2 form a dynamic, functional complex (Figure 4K), which may be important for FGF-dependent mesoderm patterning during early embryonic development. There are several signaling proteins recruited to receptor tyrosine kinase complexes that are phosphorylated by the Src family kinases (Thomas and Brugge, 1997). For example, the transcription factor Stat is associated with ErbB receptors and phosphorylated by c-Src (Olayioye et al., 1999). In summary, our results have identified xFRS2 and defined its role in early development. This is the first study to identify non-mammalian FRS2 and characterize its role in developmental processes. Moreover, our results have demonstrated novel interactions of the Src family kinase Laloo with xFGFR1 and xFRS2, which are important for FGF signaling.
METHODS
Molecular cloning and plasmid construction. A Xenopus oocyte cDNA library (Clontech) was screened using the human FRS2α coding region as a probe, and a full-length cDNA clone of xFRS2 was obtained. The entire coding region of xFRS2 was amplified by PCR. The cDNAs of Laloo and xFGFR1 were also isolated from a Xenopus oocyte cDNA library. All constructs were prepared by PCR. Laloo Y492F and Laloo K259E were constructed as described (Weinstein et al., 1998). Expression plasmids were prepared as described (Moriguchi et al., 1999). HA or myc tag was added to the C-terminus of xFRS2, Laloo and xFGFR1.
Xenopus embryo manipulation, in situ hybridization and RT–PCR. In vitro fertilization, injection, whole-mount in situ hybridization, animal cap assays and RT–PCR were performed as described (Masuyama et al., 1999; Hanafusa et al., 2000). Primers for Xbra, EF1α, muscle actin, otx2, Xsox17α, Chordin, Xwnt8, Xhox3 and XeODC have been described elsewhere (Hudson et al., 1997; Shibuya et al., 1998; Weinstein et al., 1998; Masuyama et al., 1999). The sequences of other primer pairs used were as follows: xFRS2 [forward (f), 5′-AGATGACTCGCTAGGACCAA; reverse (r), 5′- TCTCGTAGGTGTTTGTGGGA]; Xcad3 (f, 5′-ATAACCACACAGCGCAGAAC; r, 5′-CAGTTTTCCCCATCCATTCG). [α-32P]dCTP-incorporated PCR products were detected by BAS2500 (FUJI film).
Anti-phosphotyrosine blot. Dissected animal caps or embryos were lysed in a buffer consisting of 50 mM Tris–HCl pH 7.5, 25 mM 2-β glycerophosphate, 10 mM EDTA, 10 mM sodium pyrophosphate, 10 mM NaF, 1 mM vanadate, 1 mM PMSF, 0.5% aprotinin, 5 µg/ml leupeptin and 1% NP-40. Myc-tagged xFRS2 was immunoprecipitated from the lysates by incubation with 1 µg of anti-myc antibody (9E10; Santa Cruz) and protein G–Sepharose beads (10 µl; Amersham Pharmacia Biotech) for 3 h at 4°C. For p13suc1 agarose precipitation, 20 µl of p13suc1-agarose beads (Upstate) were added to the extracts of 30 whole embryos from each stage and incubated for 2 h at 4°C. Each precipitate was washed three times with a lysis buffer containing 0.1% NP-40 and analyzed by immunoblotting using anti-phosphotyrosine antibody (4G10; Upstate), anti-myc antibody (A-14; Santacruz) and anti-HA antibody (Y-11; Santacruz).
Cell culture, transfection and co-immunoprecipitation. Cell culture and transfection were performed as described (Masuyama et al., 1999). Injected animal caps or transfected cells were lysed in a buffer consisting of 20 mM HEPES pH 7.5, 100 mM NaCl, 1.5 mM MgCl2, 1 mM EGTA, 10 mM sodium pyrophosphate, 10% glycerol, 10 mM NaF, 1 mM vanadate, 1 mM PMSF, 0.5% aprotinin, 5 µg/ml leupeptin and 1% NP-40. Anti-myc antibody (9E10) or anti-HA antibody (12CA5) was used for immunoprecipitation. Precipitates were washed three times with the same buffer containing no detergent.
Acknowledgments
ACKNOWLEDGEMENTS
We thank K. Kawachi for technical comments and members of our laboratory for helpful discussions. This work was supported by grants from the Ministry of Education, Science, and Culture of Japan (to E.N.).
REFERENCES
- Amaya E., Musci, T.J. and Kirschner, M.W. (1991) Expression of a dominant negative mutant of the FGF receptor disrupts mesoderm formation in Xenopus embryos. Cell, 66, 257–270. [DOI] [PubMed] [Google Scholar]
- Basilico C. and Moscatelli, D. (1992) The FGF family of growth factors and oncogenes. Adv. Cancer. Res., 59, 115–165. [DOI] [PubMed] [Google Scholar]
- Cross F.R., Garber, E.A., Pellman, D. and Hanafusa, H. (1984) A short sequence in the p60src N terminus is required for p60src myristylation and membrane association and for cell transformation. Mol. Cell Biol., 4, 1834–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhalluin C., Yan, S.K., Plotnikova, O., Lee, W.K., Zeng, L., Kuti, M., Mujtaba, S., Goldfarb, P.M. and Zhou, M.M. (2000) Structural basis of SNT PTB domain interactions with distinct neurotrophic receptors. Mol. Cell, 6, 921–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh Y., Masuyama, N., Suzuki, A., Ueno, N. and Nishida, E. (1995) Involvement of the MAP kinase cascade in Xenopus mesoderm induction. EMBO J., 14, 2491–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadari Y.R., Kouhara, H., Lax, I. and Schlessinger, J. (1998) Binding of Shp2 tyrosine phosphatase to FRS2 is essential for fibroblast growth factor-induced PC12 cell differentiation. Mol. Cell. Biol., 18, 3966–3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafusa H., Masuyama, N., Kusakabe, M., Shibuya, H. and Nishida, E. (2000) The TGF-β family member derriere is involved in regulation of the establishment of left right asymmetry. EMBO Rep., 1, 32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson C., Clements, D., Friday, R.V., Stott, D. and Woodland, H.R. (1997) Xsox17α and -β mediate endoderm formation in Xenopus. Cell, 91, 397–405 [DOI] [PubMed] [Google Scholar]
- Isaacs H.V. (1997) New perspectives on the role of the fibroblast growth factor family in amphibian development. Cell Mol. Life Sci., 53, 350–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouhara H., Hadari, Y.R., Spivak-Kroizman, T., Schilling, J., Bar-Sagi, D., Lax, I. and Schlessinger, J. (1997) A lipid-anchored Grb2-binding protein that links FGF-receptor activation to the Ras/MAPK signaling pathway. Cell, 89, 693–702. [DOI] [PubMed] [Google Scholar]
- LaBonne C., Burke, B. and Whitman, M. (1995) Role of MAP kinase in mesoderm induction and axial patterning during Xenopus development. Development, 121, 1475–1486. [DOI] [PubMed] [Google Scholar]
- MacNicol A.M., Muslin, A.J. and Williams, L.T. (1993) Raf-1 kinase is essential for early Xenopus development and mediates the induction of mesoderm by FGF. Cell, 73, 571–583. [DOI] [PubMed] [Google Scholar]
- Masuyama N., Hanafusa, H., Kusakabe, M., Shibuya, H. and Nishida, E. (1999) Identification of two Smad4 proteins in Xenopus. Their common and distinct properties. J. Biol. Chem., 274, 12163–12170. [DOI] [PubMed] [Google Scholar]
- Mohammadi M., Dikic, I., Sorokin. A., Burgess, W.H., Jaye, M. and Schlessinger, J. (1996) Identification of six novel autophosphorylation sites on fibroblast growth factor receptor 1 and elucidation of their importance in receptor activation and signal transduction. Mol. Cell. Biol., 16, 977–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi T., Kawachi, K., Kamakura, S., Masuyama, N., Yamanaka, H., Matsumoto, K., Kikuchi, A. and Nishida, E. (1999) Distinct domains of mouse dishevelled are responsible for the c-Jun N-terminal kinase/stress-activated protein kinase activation and the axis formation in vertebrates. J. Biol. Chem., 274, 30957–30962. [DOI] [PubMed] [Google Scholar]
- Olayioye M.A., Beuvink, I., Horsch, K., Daly, J.M., and Hynes, N.E. (1999) ErbB receptor-induced activation of stat transcription factors is mediated by Src tyrosine kinases. J. Biol. Chem., 274, 17209–17218. [DOI] [PubMed] [Google Scholar]
- Ong S.H., Goh, K.C., Lim, Y.P., Low, B.C., Klint, P., Claesson-Welsh, L., Cao, X., Tan, Y.H. and Guy, G.R. (1996) Suc1-associated neurotrophic factor target (SNT) protein is a major FGF-stimulated tyrosine phosphorylated 90-kDa protein which binds to the SH2 domain of GRB2. Biochem. Biophys. Res. Commun., 225, 1021–1026. [DOI] [PubMed] [Google Scholar]
- Ong S.H., Lim, Y.P., Low, B.C. and Guy, G.R. (1997) SHP2 associates directly with tyrosine phosphorylated p90 (SNT) protein in FGF-stimulated cells. Biochem. Biophys. Res. Commun., 238, 261–266. [DOI] [PubMed] [Google Scholar]
- Ong S.H., Guy, G.R., Hadari, Y.R, Laks, S., Gotoh, N., Schlessinger, J. and Lax, I. (2000) FRS2 proteins recruit intracellular signaling pathways by binding to diverse targets on fibroblast growth factor and nerve growth factor receptors. Mol. Cell. Biol., 20, 979–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin S.J., Cleghon, V. and Kaplan, D.R. (1993) SNT, a differentiation-specific target of neurotrophic factor-induced tyrosine kinase activity in neurons and PC12 cells. Mol. Cell. Biol., 13, 2203–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya H., Iwata, H., Masuyama, N., Gotoh, Y., Yamaguchi, K., Irie, K., Matsumoto, K., Nishida, E. and Ueno, N. (1998) Role of TAK1 and TAB1 in BMP signaling in early Xenopus development. EMBO J., 17, 1019–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack J.M., Isaacs, H.V., Song, J., Durbin, L. and Pownall, M.E. (1996) The role of fibroblast growth factors in early Xenopus development. Biochem. Soc. Symp., 62, 1–12. [PubMed] [Google Scholar]
- Tang T.L., Freeman, R.M. Jr., O’Reilly, A.M., Neel, B.G. and Sokol, S.Y. (1995) The SH2-containing protein-tyrosine phosphatase SH-PTP2 is required upstream of MAP kinase for early Xenopus development. Cell, 80, 473–483. [DOI] [PubMed] [Google Scholar]
- Thomas S.M. and Brugge, J.S. (1997) Cellular functions regulated by Src family kinases. Annu. Rev. Cell. Dev. Biol. 13, 513–609. [DOI] [PubMed] [Google Scholar]
- Umbhauer M., Marshall, C.J., Mason, C.S., Old, R.W. and Smith, J.C. (1995) Mesoderm induction in Xenopus caused by activation of MAP kinase. Nature, 376, 58–62. [DOI] [PubMed] [Google Scholar]
- Wang J.K., Xu, H., Li, H.C. and Goldfarb, M. (1996) Broadly expressed SNT-like proteins link FGF receptor stimulation to activators of Ras. Oncogene, 13, 721–729. [PubMed] [Google Scholar]
- Weinstein D.C. and Hemmati-Brivanlou, A.A. (2001) Src family kinase function during early Xenopus development. Dev. Dyn., 220, 163–168. [DOI] [PubMed] [Google Scholar]
- Weinstein D.C., Marden, J., Carnevali, F. and Hemmati-Brivanlou, A. (1998) FGF-mediated mesoderm induction involves the Src-family kinase Laloo. Nature, 394, 904–908. [DOI] [PubMed] [Google Scholar]
- Whitman M. and Melton, D.A. (1992) Involvement of p21ras in Xenopus mesoderm induction. Nature, 357, 252–254. [DOI] [PubMed] [Google Scholar]
- Xu H., Lee, K.W. and Goldfarb, M. (1998) Novel recognition motif on fibroblast growth factor receptor mediates direct association and activation of SNT adapter proteins. J. Biol. Chem., 273, 17987–17990. [DOI] [PubMed] [Google Scholar]