Abstract
The lactoferrin receptor genes from two strains of Moraxella catarrhalis have been cloned and sequenced. The lfr genes are arranged as lbpB followed by lbpA, a gene arrangement found in lactoferrin and transferrin receptor operons from several bacterial species. In addition, a third open reading frame, orf3, is located one nucleotide downstream of lbpA. The deduced lactoferrin binding protein A (LbpA) sequences from the two strains were found to be 99% identical, the LbpB sequences were 92% identical, and the ORF3 proteins were 98% identical. The lbpB gene was PCR amplified and sequenced from a third strain of M. catarrhalis, and the encoded protein was found to be 77% identical and 84% similar to the other LbpB proteins. Recombinant LbpA and LbpB proteins were expressed from Escherichia coli, and antisera raised to the purified proteins were used to assess antigenic conservation in a panel of M. catarrhalis strains. The recombinant proteins were tested for the ability to bind human lactoferrin following gel electrophoresis and electroblotting, and rLbpB, but not rLbpA, was found to bind lactoferrin. Bactericidal antibody activity was measured, and while the anti-rLbpA antiserum was not bactericidal, the anti-rLbpB antisera were found to be weakly bactericidal. Thus, LbpB may have potential as a vaccine candidate.
Moraxella (Branhamella) catarrhalis is a human pathogen that has only recently been recognized as a significant health problem (5). It is a commensal organism colonizing the respiratory tract in children and adults, with the highest incidence in young children and in adults of >60 years of age (10). It is the third most common cause of otitis media and sinusitis in children, after Streptococcus pneumoniae and Haemophilus influenzae, and is responsible for an estimated 15 to 20% of disease. In adults, M. catarrhalis infection can lead to exacerbation of chronic bronchitis or development of pneumonia in patients with pre-existing pulmonary disease. More rarely, it also causes bacteremia and meningitis (10, 17, 23).
Otitis media affects approximately 70% of all children by the age of three, with many children experiencing recurrent disease (2). Chronic otitis media can lead to hearing, speech, and cognitive impairment in children, since it tends to occur at a time when language is developing. The incidence of M. catarrhalis-induced otitis media is variable depending upon the population being studied, ranging from 7 to 20% in the United States, (21), 0 to 10% in Greenland (16), and about 1% in Spain (7). Antibiotic resistance, especially penicillin resistance due to the expression of β-lactamase, is very common in clinical isolates of M. catarrhalis, reaching 80 to 85% in United States and European isolates (24). A vaccine against otitis media caused by M. catarrhalis is clearly needed.
Iron restriction is a general host defense mechanism against microbial pathogens, and in the human host, iron is sequestered by transferrin, lactoferrin, hemoglobin, and other complex molecules. A number of bacterial species, including Bordetella pertussis (22), Helicobacter pylori (9), M. catarrhalis (33), Neisseria gonorrhoeae (1), Neisseria meningitidis (29, 33), Prevotella nigrescens (8), and Treponema spp. (34), have been shown to express outer membrane proteins which specifically bind human lactoferrin. M. catarrhalis, N. gonorrhoeae, and N. meningitidis utilize both transferrin and lactoferrin binding complexes, and a single lactoferrin binding protein of ∼105 kDa was originally identified in these organisms (33). The lbpA genes from N. gonorrhoeae and N. meningitidis have been cloned and sequenced (1, 27), but until recently there was no evidence for the existence of an lbpB gene (3, 13, 25, 28).
We report here the cloning and sequencing of the M. catarrhalis lactoferrin binding protein genes lbpA and lbpB. The recombinant proteins were expressed in Escherichia coli, and high-titer antibodies were raised. Anti-rLbpA antiserum was not bactericidal, but anti-rLbpB antisera were weakly bactericidal against the autologous and heterologous strains.
MATERIALS AND METHODS
Recombinant DNA techniques.
Restriction endonucleases were purchased from Boehringer Mannheim (Laval, Quebec, Canada), New England Biolabs, Bethesda Research Laboratories, or Pharmacia and were used according to the manufacturers’ specifications. Oligonucleotides were synthesized on an Applied Biosystems, Inc. (ABI), model 380B DNA synthesizer and purified by chromatography (Oligonucleotide Purification Cartridge; Perkin-Elmer, Culver City, Calif.). Other recombinant DNA methods were performed according to Sambrook et al. (32).
Bacterial strains and media.
M. catarrhalis otitis media clinical isolates 4223 and 3 were kindly provided by T. Murphy (State University of New York, Buffalo, N.Y.), strain Q8 was a gift from M. Bergeron (University of Laval, Laval, Quebec, Canada), strain VH19 was provided by V. Howie (University of Texas, Galveston, Tex.), strain H-04 was from G. D. Campbell (Louisiana State University, Shreveport, La.), and strain LES-1 was obtained from L. E. Stenfors (University of Tromso, Tromso, Norway). M. catarrhalis strains were maintained on Mueller-Hinton agar (Becton Dickinson, Cockeysville, Md.) or grown in brain heart infusion (BHI) medium (Difco, Detroit, Mich.) with or without the addition of ethylenediamine-di(o-hydroxyphenylacetic acid) (EDDA) (Sigma, St. Louis, Mo.), as described previously (15). E. coli strains were grown in YT medium supplemented with 50 μg of ampicillin ml−1 as required.
Purification of LbpA and protein sequence determination.
Native LbpA was purified by affinity chromatography under high-stringency conditions with immobilized lactoferrin (3). The purified LbpA protein was digested overnight with cyanogen bromide; then, fragments were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and submitted for sequence analysis on an ABI model 477A protein sequencer. A 13-kDa protein fragment was found to have the N-terminal sequence MVQYTYRKGKENKAH.
Generation of a probe for screening libraries.
A degenerate oligonucleotide primer was prepared based upon the internal LbpA sequence QYTRKGENKA 5′ CAA TAT ACI CGT/C AAA GGT/C GAA AAT/C AAA GC 3′
There is a conserved C-terminal pentapeptide, LEMKF, found in all known LbpA and TbpA protein sequences. An oligonucleotide primer was prepared based upon the complementary DNA sequence encoding this pentapeptide: LEMKF* 5′ CTT GAA ATG AAG TTT TAA 3′ 3′ GAA CTT TAC TTC AAA ATT 5′
Chromosomal DNA was prepared from M. catarrhalis 4223 and Q8. PCR amplification was performed in buffer containing 10 mM Tris-HCl (pH 8.3), 50 mM potassium chloride, and 1.5 mM magnesium chloride. Each 100-μl reaction mixture contained 1 μg of chromosomal DNA, 0.1 μg of each primer, 2.5 units of Amplitaq DNA polymerase (Perkin-Elmer Cetus, Foster City, Calif.), and 10 mM (each) deoxynucleoside triphosphate (Perkin-Elmer Cetus). The cycling conditions were 24 cycles at 94°C for 1 min, 47°C for 30 s, and 72°C for 1 min. A specific band of ∼2.2 kb was amplified, and partial sequence analysis was done to ensure that the gene product was related to lbpA and was not tbpA (manuscript submitted). This 2.2-kb fragment was labelled with [α-32P]dCTP (random-primed DNA labelling kit; Boehringer Mannheim) and used to screen genomic libraries.
Construction and screening of genomic libraries.
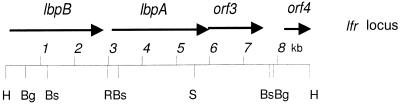
M. catarrhalis 4223 and Q8 EMBL3 libraries were prepared as described previously (20). Briefly, chromosomal DNA was partially digested with Sau3AI, and DNA fragments of 15 to 23 kb were purified. The DNA was cloned into BamHI-digested EMBL3 arms (Promega, Madison, Wis.) and packaged according to the manufacturer’s instructions. The libraries in E. coli LE392 cells were plated, and plaques were lifted onto nitrocellulose membranes for hybridization with the labelled 2.2-kb lbpA PCR fragment. Several putative phage clones were obtained from each library, and phage DNA was prepared for further analysis. Restriction enzyme and Southern blot analyses indicated that at least a portion of lbpA was localized to a ∼9-kb HindIII fragment from each phage clone. The Q8 HindIII fragment was subcloned into pBluescript, generating plasmid pLDW1, and the 4223 HindIII fragment was subcloned into pUC18, generating plasmid pLD1-8. Figure 1 illustrates the restriction map and gene placement within the M. catarrhalis lfr locus.
FIG. 1.
Partial restriction map of the M. catarrhalis lactoferrin receptor locus. Restriction enzyme sites: Bg, BglI; Bs, BstEII; H, HindIII; R, EcoRI; S, SphI.
Sequencing of the lfr genes.
Plasmid DNA was prepared from 50-ml overnight cultures by using the Qiagen Plasmid Midi kit (Qiagen Inc., Chatsworth, Calif.). DNA samples were sequenced on an ABI model 373A DNA sequencer using dye terminator chemistry. Oligonucleotide primers of 17 to 25 bases in length were used to sequence both strands of the DNA.
PCR amplification of the VH19 lbpB gene.
Chromosomal DNA was prepared from M. catarrhalis VH19. Oligonucleotide primers were designed based upon the flanking sequence of the 4223 lbpB gene. The sense primer was 5′ AAGCTTAGCATGATGGCATCGGCT 3′, and the antisense primer was 5′ TTAGCCCAAGGCAAATCTGGTGCA 3′. Two independent PCR amplifications were performed, as above, and specific 2.9-kb fragments were amplified and subcloned into pCR II (Invitrogen, Carlsbad, Calif.), generating plasmids pVH19pcr1 and pVH19pcr2 for sequence analysis. A third PCR amplification was performed without subcloning of the resultant DNA. PCR-amplified DNA was purified for direct sequencing with a Qiagen PCR purification kit.
Construction of clones expressing recombinant LbpA and LbpB.
In order to express lbpA, 5′ and 3′ fragments of lbpA were generated by PCR amplification and were ligated to an internal 2.3-kb fragment to recreate a full-length gene. The primers used to amplify an ∼200-bp 5′ fragment to a BstEII site were MSKSIT 5′ GGAATTCCAT ATG TCA AAA TCT ATC ACA AA 3′
(where an NdeI site is underlined) and LDAITVTAA 5′ T TTA GAT GCC ATC ACG GTA ACC GCC GCC CC 3′ 3′ A AAT CTA CGG TAG TGC CAT TGG CGG CGG GG 5′
(where the BstEII site is underlined). The primers used to amplify a 515-bp 3′ fragment from an SphI site were GKLDLHAMTS 5′ GGC AAA CTG GAT TTG CAT GCC ATG ACA TCA 3′
(where the SphI site is underlined) and SLEMKF* 5′ AGT CTT GAA ATG AAG TTT TAA 3′ 3′ TCA GAA CTT TAC TTC AAA ATT GCCCTAGGGC 5′
(where a BamHI site is underlined). An NdeI site encompassing the ATG start codon and a BamHI site following the termination codon were added for cloning purposes. The PCR fragments were ligated with an internal 2.3 kb-BstEII-SphI fragment of lbpA and cloned into pT7-7, which had been digested with NdeI and BamHI. The resulting pT7-lbpA expression clones were designated pRD1A and pQW1A for 4223 and Q8, respectively. BL21(DE3) cells were transformed by electroporation for expression studies.
By analogy with TbpB proteins, LbpB was assumed to be a lipoprotein, and constructs were designed for expression of LbpB with or without a lipopeptide signal sequence. There is a unique BglI site in lbpB. To express the full-length LbpB protein with leader sequence (construct A), an ∼429-bp 5′ fragment from the Met1 start codon to the BglI site was PCR amplified; to express the mature protein (construct B), an ∼329-bp 5′ fragment from the putative Cys32 start codon to the BglI site was PCR amplified. The sense primers were MSTVKTPH 5′ GGAATTCCAT ATG AGT ACT GTC AAA ACC CCC CAC A 3′
(where an NdeI site is underlined) for construct A and MCRSDDISVN 5′ GGAATTCCAT ATG TGC CGC TCT GAT GAC ATC AGC GTC AAT 3′
(where an NdeI site is underlined) for construct B, and the antisense primer was GKNLRGPI 5′ GGT AAA AAC TTG CGT CAG CCC ATC 3′ 3′ CCA TTT TTG AAC GCA GTC GGG TAG 5′
(where the BglI site is underlined). The Q8 lfr-containing plasmid pLDW1 was digested with BglI and EcoRI to release a 2.3-kb lbpB fragment, which was ligated with the NdeI-BglI PCR fragment and cloned into pT7-7, which had been digested with NdeI and EcoRI. The resulting plasmids, pQW2A and pQW2B, thus contained the Q8 lbpB gene encoding the full-length or mature LbpB proteins under control of the T7 promoter. The plasmids expressing the 4223 full-length or mature LbpB proteins were constructed in a similar manner and designated pRD2A and pRD2B. Plasmids were introduced into E. coli BL21(DE3) cells by electroporation.
Purification of recombinant proteins.
The strain Q8 rLbpA protein was expressed at about 10% of total protein as inclusion bodies, but the strain 4223 rLbpA protein was expressed at substantially lower levels. E. coli cells from a 500-ml culture were resuspended in 40 ml of 50 mM Tris-HCl, pH 8.0, containing 5 mM 4-(2-aminoethyl)-benzenesulfonylfluoride protease inhibitor (Calbiochem, La Jolla, Calif.) and 0.1 M NaCl and disrupted by sonication (three times for 10 min each; 70% duty circle). The extract was centrifuged at 20,000 × g for 30 min, and the resultant supernatant, which contained >95% of the soluble proteins from E. coli, was discarded. The remaining pellet was further extracted in 40 ml of 50 mM Tris-HCl, pH 8.0, containing 0.5% Triton X-100 and 10 mM EDTA. The mixture was stirred at 4°C for at least 1 h and then centrifuged at 20,000 × g for 30 min, and the supernatant containing residual soluble proteins and the majority of the membrane proteins was discarded. The resultant pellet was further extracted in 40 ml of 50 mM Tris-HCl, pH 8.0, containing 1% octylglucoside. The mixture was stirred at 4°C for at least 1 h and then centrifuged at 20,000 × g for 30 min. The supernatant containing residual contaminating proteins was discarded. The resultant pellet obtained after the above extractions contained the inclusion bodies. The recombinant LbpA protein (rLbpA) was solubilized in 50 mM Tris-HCl, pH 8.0, containing 6 M guanidine and 5 mM dithiothreitol (DTT). After centrifugation, the resultant supernatant was further purified on a Superdex 200 gel filtration column equilibrated in 50 mM Tris-HCl, pH 8.0, containing 2 M guanidine and 5 mM DTT. The fractions were analyzed by SDS-PAGE, and those containing purified rLbpA were pooled. Triton X-100 was added to the pooled rLbpA fraction to a final concentration of 0.1%. The fraction was dialyzed overnight at 4°C against phosphate-buffered saline (PBS) and then centrifuged at 20,000 × g for 30 min. The purified rLbpA was stored at −20°C.
There was no measurable expression of rLbpB from constructs containing the signal sequence; however, the mature rLbpB proteins were expressed at 5 to 10% of total proteins as inclusion bodies. Recombinant LbpB proteins were purified by the same process as that described for rLbpA.
Lactoferrin binding and transferrin binding assays.
Human lactoferrin (Sigma) was conjugated to horseradish peroxidase (HRP) by using an EZ-Link maleimide-activated HRP kit (Pierce, Rockford, Ill.) according to the manufacturer’s instructions. Briefly, 1 mg of human lactoferrin, resuspended in 1 ml of PBS, was mixed with 20 μl of SATA solution (Pierce) to form SATA derivative. The solution was incubated for 30 min and then deacetylated for 2 h at room temperature. Separation of the deacetylated human lactoferrin derivative from hydroxylamine-HCl and by-products was achieved with a desalting column (1 by 10 cm). Fractions (500 μl) were collected; those containing deacetylated human lactoferrin were pooled, and the protein concentration (about 0.5 mg ml−1) was confirmed by measuring at A280. The protein pool (1 ml) was added to 1 mg of EZ-Link maleimide-activated HRP and incubated for 1 h at room temperature. The resulting conjugate was used for the lactoferrin binding assay.
The lactoferrin binding and transferrin binding activities of rLbpA and rLbpB were assessed by a modification of the procedure of Schryvers and Lee (33). Briefly, purified rLbpA, rLbpB, or rTbpB (as control [manuscript submitted]) was subjected to discontinuous electrophoresis through an SDS–12.5% polyacrylamide gel (18). The proteins were electrophoretically transferred to a polyvinylidene difluoride membrane (Millipore, Bedford, Mass.) and incubated with HRP-conjugated human lactoferrin (1:50 dilution) or HRP-conjugated human transferrin (1:50 dilution) (Jackson ImmunoResearch Labs Inc., Mississauga, Ontario, Canada) at 4°C overnight. LumiGLO substrate (Kirkegaard and Perry Laboratories, Inc., Gaithersburg, Md.) was used for chemiluminescent detection of HRP activity, according to the manufacturer’s instructions.
Immunization of animals and immunoassays.
Groups of two guinea pigs (Hartley outbred; Charles River, LaSalle, Quebec) were immunized intramuscularly on day 1 with 5 μg of purified rLbpA or rLbpB protein emulsified in complete Freund’s adjuvant. Animals were boosted on days 14 and 29 with the same doses of protein emulsified in incomplete Freund’s adjuvant. Serum samples were collected on day 42 for determination of bactericidal activity. Anti-Lbp antibody titers in guinea pig immune sera were determined by antigen-specific enzyme-linked immunosorbent assays (ELISAs), as previously described (40). Microtiter wells (Nunc-MAXISORB; Nunc, Roskilde, Denmark) were coated with 50 μl of protein (0.5 μg ml−1). The reactive titer of an antiserum was defined as the reciprocal of the highest dilution consistently showing a twofold increase in absorbance at 450 nm over that obtained with the preimmune serum samples.
Whole-cell ELISAs.
M. catarrhalis 4223 was grown in the presence of EDDA, as described above. Cell pellets were collected by centrifugation, washed with PBS, and resuspended in 50 mM carbonate-bicarbonate buffer, pH 9.6. The optical density of the suspension was adjusted to 0.5 at 490 nm, and 200 μl of a 1:100 dilution of whole bacteria was used to coat microtiter wells. The plates were air-dried at 37°C overnight and then blocked with PBS–0.1% bovine serum albumin (BSA) at 37°C for 1 h (250 μl per well). After three washes with PBS–0.1% Tween 20, 200 μl of antisera at an appropriate dilution (in PBS–0.1% gelatin) was added to the wells and further incubated at 37°C for 2 h. Affinity-purified F(ab′)2 fragment of donkey anti-guinea pig immunoglobulin G (H+L) antibodies conjugated to HRP (Jackson ImmunoResearch Laboratories) was used as reporter. The reactions were developed with tetramethylbenzidine-H2O2 (ADI), and absorbancies were measured at 450 nm (with 540 nm as a reference wavelength) in a Flow Multiskan MCC microplate reader (ICN Biomedicals).
Antigenic conservation of LbpA and LbpB in M. catarrhalis strains.
To demonstrate the iron-dependent expression of the lbpA and lbpB genes, representative M. catarrhalis strains were grown in BHI with or without 25 μM EDDA. Whole-cell lysates were separated by SDS-PAGE and electrophoretically transferred to a nitrocellulose membrane. Guinea pig anti-Q8 rLbpA, anti-Q8 rLbpB, and anti-4223 rLbpB antisera were used as first antibodies, and HRP-conjugated protein G (Zymed Laboratories, San Francisco, Calif.) was used as reporter.
To assess antigenic conservation, approximately 90 M. catarrhalis strains obtained from North America or Finland were grown in BHI plus 25 μM EDDA, and immunoblots were probed with guinea pig anti-4223 rLbpB antibody, as described above.
Bactericidal antibody assay.
The bactericidal antibody assay was performed as previously described (39). Briefly, the M. catarrhalis strains were grown to an optical density at 578 nm of 0.5 in BHI medium containing 25 μM EDDA. The bacteria were diluted so that 150 to 450 CFU were added to each reaction. Guinea pig anti-rLbpA or anti-rLbpB antisera and prebleed controls were heated to 56°C for 30 min to inactivate endogenous complement and were diluted with veronal buffer containing 0.1% BSA (VBS). Guinea pig complement (BioWhittaker, Walkersville, Md.) was diluted 1:10 in VBS. Twenty-five microliters each of diluted antiserum, bacteria, and complement was added to duplicate wells of a 96-well microtiter plate (Nunc). The plates were incubated at 37°C for 60 min with gentle shaking at 70 rpm on a rotary platform. Fifty microliters of each reaction mixture was plated onto Mueller-Hinton agar plates (Becton Dickinson) which were incubated at 37°C for 24 h, and then at room temperature for 24 h, before the bacteria were counted. Antisera were determined to be bactericidal if ≥50% of bacteria were killed compared with preimmune serum controls. Assays were performed at least twice, and two different guinea pig antisera were tested against the autologous strains.
In order to assess the potential bactericidal activity of preimmune sera, samples containing bacteria, complement, and preimmune sera were compared with those containing only bacteria and complement. No differences between the two groups were detected, indicating that preimmune sera were not bactericidal for the strains tested. An additional control included bacteria with antisera but no complement. No bactericidal activity was observed, indicating that there was no antibody-mediated clumping of bacteria resulting in false activity.
Nucleotide sequence accession numbers.
The sequence data in this report have been submitted to the GenBank database under accession no. AF043131 through AF043133.
RESULTS
Cloning of the M. catarrhalis lfr genes.
In order to clone the M. catarrhalis lactoferrin receptor genes, native LbpA protein was purified from strain 4223 by affinity chromatography under high-stringency conditions with immobilized lactoferrin (3) and submitted for N-terminal sequence analysis. The N terminus was found to be blocked, so the protein was digested with cyanogen bromide and the N-terminal sequence was obtained from an internal 13-kDa fragment. The sequence, MVQYTYRKGKENKAH, was not found in M. catarrhalis TbpA (manuscript submitted) or in other known TbpA or LbpA proteins. Based upon this unique LbpA sequence, a degenerate sense primer was designed for PCR amplification of part of the lbpA gene. An antisense PCR primer was designed based upon the sequence LEMKF, which has been found at the carboxyl terminus of all known LbpA and the related TbpA proteins. By using these primers, specific 2.2-kb fragments were PCR amplified from strain 4223 and Q8 chromosomal DNA. Partial sequence analysis determined that the fragments were not from the M. catarrhalis tbpA gene (manuscript submitted). The 2.2-kb fragment was used as a probe to screen M. catarrhalis 4223 and Q8 chromosomal libraries in EMBL3. Putative phage clones containing approximately 16-kb inserts were identified, and the lbpA gene was localized to a ∼9-kb HindIII fragment by restriction enzyme and Southern blot analyses. The 9-kb HindIII fragments from the strain 4223 and Q8 libraries were subcloned, generating plasmids pLD1-8 and pLDW1, respectively.
Analysis of the nucleotide sequence of the lfr genes.
The inserts from pLD1-8 and pLDW1 were sequenced, and three complete open reading frames (ORFs) and one partial ORF were identified. The gene arrangement was lbpB–lbpA–orf3, with a fourth partial ORF located downstream (Fig. 1). The coding sequence of the lbpB gene was approximately 2.7 kb, and putative promoter elements were identified upstream of lbpB (Fig. 2A). The potential −10 and ribosome binding site (RBS) sequences were more widely spaced than those found in E. coli consensus sequences. The separation of these two elements is greater in the Q8 lbpB sequence than in the 4223 lbpB sequence due to the presence of an extra 11 nucleotides (italicized in Fig. 2A). A putative Fur binding site was identified overlapping the −10 region of the lbpB promoter (Fig. 2B). The intergenic distance between lbpB and lbpA was 184 bp, and there was a second possible promoter region upstream of lbpA, which more closely resembles consensus E. coli promoters. The coding sequence of the lbpA gene was approximately 3.0 kb, and the intergenic distance between lbpA and the orf3 gene was only a single nucleotide. Possible consensus sequences for promoter elements were identified upstream of orf3, within the coding sequence of lbpA. The coding sequence of orf3 was 1.6 kb, and the intergenic sequence between orf3 and orf4 was 583 bp. Promoter elements were identified upstream of orf4.
FIG. 2.
Nucleotide sequences in the potential promoter regions of the strain Q8 lfr locus. (A) Potential promoter elements for the lbpB, lbpA, and orf3 genes. Potential −35, −10, and RBS sequences are underlined. The stop codon for lbpA is indicated by an asterisk. The italicized sequence in the promoter region of Q8 lbpB designates the 11 nucleotides missing from the 4223 lbpB sequence. (B) Comparison of Fur binding sequences in E. coli (14), N. meningitidis tbpB (19), H. influenzae tbpB (12), and M. catarrhalis lbpB. Dots indicate nucleotides identical to the E. coli consensus sequence. Underlining indicates dyad symmetry. The italicized sequence in the Fur binding region of Q8 lbpB is the 11 nucleotides missing from the 4223 lbpB sequence.
Analysis of the deduced amino acid sequences of the lactoferrin binding proteins.
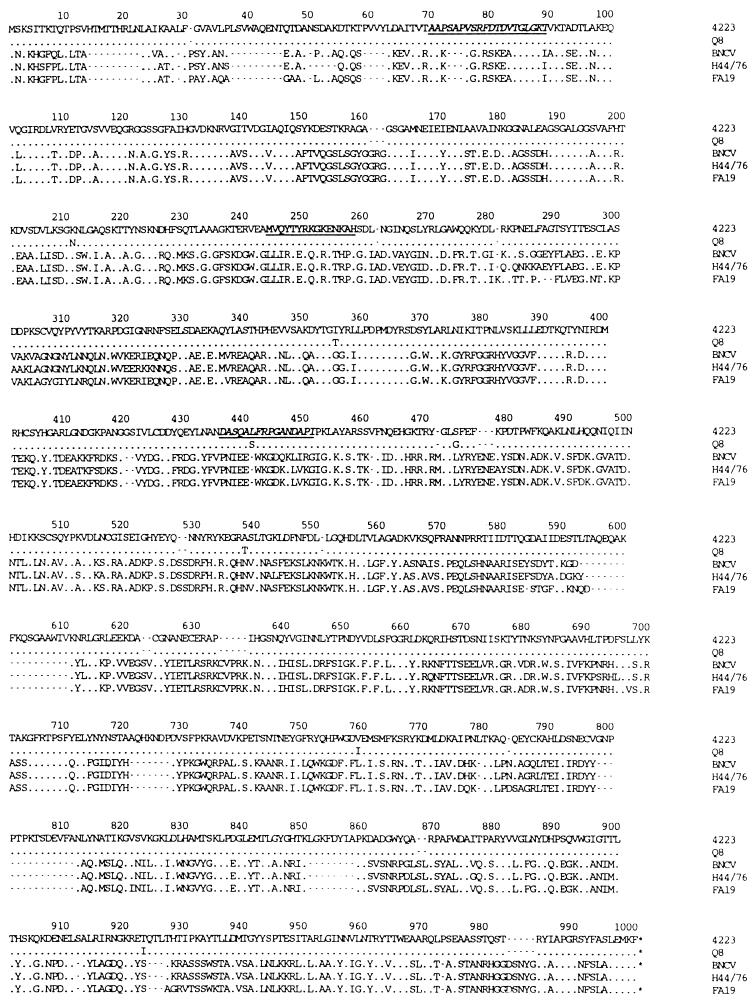
The M. catarrhalis Q8 and 4223 lbpA genes encode proteins of molecular mass 110.8 kDa that are 99% identical, with only seven different residues between them. Compared with known LbpA sequences from N. meningitidis (27, 28) and N. gonorrhoeae (1), there is about 32% sequence identity and 50% sequence similarity between the M. catarrhalis and the neisserial LbpA proteins (Fig. 3). The main differences between the M. catarrhalis and neisserial LbpA proteins are several small inserts, particularly in the N-terminal 80 amino acids and between residues 593 and 858. The deduced sequences of the LbpA proteins can be aligned with peptide sequences derived from purified native M. catarrhalis 141 LbpA (4). For the first peptide, 19 of 20 residues are identical, and for the second peptide, 16 of 16 residues are identical (Fig. 3).
FIG. 3.
Alignment of the amino acid sequences of LbpA from M. catarrhalis Q8 and 4223, N. meningitidis BNCV and H44/76 (27, 28), and N. gonorrhoeae FA19 (1). Dots indicate identical residues, and gaps have been introduced to maximize sequence alignments. The internal cyanogen bromide fragment used to design the oligonucleotide probe for cloning of the lbpA gene is underlined. The locations of the homologous peptide sequences obtained from M. catarrhalis 141 LbpA (4) are italicized and underlined.
The Q8 and 4223 LbpB proteins have molecular masses of 98.9 and 98.6 kDa, respectively, and are 92% identical and 95% similar to each other. Because of the unexpectedly high level of sequence similarity of the LbpB proteins from these two strains, the lbpB gene was PCR amplified and sequenced from a third strain, VH19. The VH19 LbpB protein is 100.2 kDa and exhibits 77% sequence identity and 84% similarity with the other M. catarrhalis LbpB proteins. The deduced LbpB protein sequences from M. catarrhalis Q8, 4223, and VH19 are compared in Fig. 4. A cysteine residue at position 32 is preceded by a consensus sequence for lipoproteins (38), suggesting that LbpB, like TbpB, is a lipoprotein. The mature LbpB proteins from strains Q8, 4223, and VH19 would have molecular masses of 95.2, 95.6, and 96.8 kDa, respectively. There is very little sequence similarity between the M. catarrhalis LbpB protein and the published TbpB proteins; however, there are a few scattered conserved residues (underlined in Fig. 4), the most notable being NRFVG at positions 390 to 394 and LEGGFYG at positions 430 to 436. An unusual feature of the LbpB proteins is the high combined aspartic acid and asparagine content, which is nearly 20%. In addition, the LbpB amino acid composition from residues 699 to 748 is approximately 54% aspartic acid. There is an Arg-Gly-Asp (RGD) sequence near the carboxyl terminus of the three LbpB proteins. As illustrated in Fig. 4, there are some regions of homology between the M. catarrhalis LbpB sequences and the partial sequences of the putative LbpB proteins from N. meningitidis (28) and N. gonorrhoeae (translated from the sequence of Biswas and Sparling [1]).
FIG. 4.
Alignment of the amino acid sequences of LbpB from M. catarrhalis Q8, 4223, and VH19 and the partial carboxyl-terminal sequences of LbpB from N. meningitidis BNCV and H44/76 (27, 28) and N. gonorrhoeae FA19 (translated from Biswas and Sparling [1]). Dots indicate identical residues, and gaps have been introduced to maximize sequence alignments. The residues conserved with TbpB proteins (26) are underlined, and the RGD sequence is italicized.
The 4223 and Q8 orf3 genes encode 98% identical proteins of molecular masses of 60.7 and 60.4 kDa, respectively (Fig. 5). Following a database search, it was found that the ORF3 proteins have no significant homology to other known proteins. Notable features of the ORF3 protein are a potential signal sequence, a terminal phenylalanine, which is often associated with membrane anchored proteins (36), and an internal repeat sequence of DGLG.
FIG. 5.
Alignment of the amino acid sequences of the ORF3 protein from M. catarrhalis Q8 and 4223. Dots indicate identical residues, and gaps have been introduced to achieve maximum sequence alignment. The DGLG repeat sequence is underlined.
Expression of rLbpA and rLbpB from E. coli and protein purification.
The lbpA and lbpB genes were cloned into plasmid pT7-7 (37), and the recombinant proteins were expressed in E. coli BL21(DE3) cells. Strain Q8 rLbpA was expressed at about 10% of total proteins as inclusion bodies, but the strain 4223 rLbpA protein was expressed at much lower yield and was not studied further. Two rLbpB proteins were expressed: one from the ATG start codon, which included the signal sequence, and the other as the mature protein starting from the cysteine residue. The mature LbpB proteins from strains Q8 and 4223 were made at 5 to 10% of total proteins as inclusion bodies, but the rLbpB proteins with the leader sequence were made in very low yield and were not studied further. The recombinant ORF3 protein could not be expressed in E. coli.
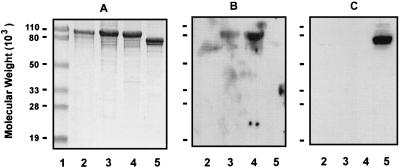
The Q8 rLbpA, Q8 rLbpB, and 4223 rLbpB proteins were purified by the same procedure. Briefly, the cell pellet from an induced bacterial culture was lysed by sonication and enriched for inclusion bodies, and the recombinant proteins were purified by gel filtration (Fig. 6). The rLbpB proteins were found to bind human lactoferrin (Fig. 7B) but not human transferrin (Fig. 7C). Under the same conditions, rLbpA and rTbpB did not bind human lactoferrin (Fig. 7B).
FIG. 6.
Purification of rLbpA and rLbpB proteins. (A) SDS-PAGE of the purification of Q8 rLbpA. Panels B and C show the purification of Q8 rLbpB and 4223 rLbpB, respectively. Lane 1, molecular weight markers; lane 2, whole-cell lysates; lane 3, inclusion bodies; lane 4, purified protein.
FIG. 7.
Lactoferrin binding of recombinant proteins. (A) SDS-PAGE of purified recombinant proteins. (B) Binding of recombinant proteins to human lactoferrin. (C) Binding of recombinant proteins to human transferrin. Lane 1, molecular weight markers; lane 2, Q8 rLbpA; lane 3, Q8 rLbpB; lane 4, 4223 rLbpB; lane 5, 4223 rTbpB.
Immunogenicity and antigenic conservation of LbpA and LbpB.
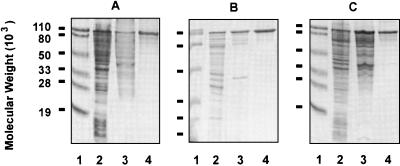
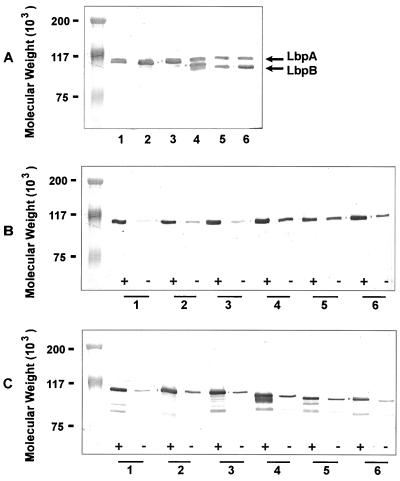
Both rLbpA and rLbpB were found to be immunogenic. Immunoblot analysis of M. catarrhalis isolates showed that eight of eight strains examined expressed an approximately 105-kDa protein recognized by anti-rLbpA antibody, and all of the approximately 90 strains tested expressed a protein recognized by anti-rLbpB antibodies. Representative immunoblots are shown in Fig. 8. The M. catarrhalis LbpB proteins were surprisingly homogenous in molecular mass, with about 57% of strains expressing an LbpB protein that comigrated with LbpA and the remainder of the strains expressing a slightly smaller protein (Fig. 8A). Both the LbpA and LbpB proteins appeared to be expressed constitutively in M. catarrhalis, although an increase in expression was observed with iron restriction (Fig. 8B and C). There was also weak recognition, by anti-LbpB antibody, of approximately 85- to 90-kDa protein bands in some strains grown under iron-reduced conditions (Fig. 8C). The anti-rLbpA and anti-rLbpB antibody titers were measured by ELISA, and the anti-rLbpB titers were found to be very high (Table 1).
FIG. 8.
Immunoblot of M. catarrhalis strains reacted with anti-rLbpA and anti-LbpB antibodies. (A) Whole-cell lysates probed with anti-Q8 rLbpA plus anti-Q8 rLbpB antisera. All cells were grown in the presence of EDDA. (B) Whole-cell lysates probed with anti-Q8 rLbpA antibody. (C) Whole-cell lysates probed with anti-Q8 rLbpB antibody. Lane 1, strain Q8; lane 2, strain 4223; lane 3, strain VH19; lane 4, strain LES-1; lane 5, strain H-04; lane 6, strain 3. +, with EDDA; −, without EDDA.
TABLE 1.
ELISA titers for guinea pig anti-Lbp antibodies raised against recombinant lactoferrin binding proteins
| Coated antigen | Titera
|
||
|---|---|---|---|
| Anti-Q8 rLbpA | Anti-Q8 rLbpB | Anti-4223 rLbpB | |
| Q8 rLbpA | 3,200 | ND | ND |
| 25,600 | |||
| Q8 rLbpB | ND | 1,638,400b | 409,600b |
| 4223 rLbpB | ND | 409,600b | 819,200b |
Anti-rLbp antibody titers in sera collected after three immunizations were determined by ELISA. The reactive titer was defined as the reciprocal of the dilution consistently showing a twofold increase in absorbance over that obtained with the prebleed sample. ND, not determined.
Identical titers from two guinea pigs.
Bactericidal antibody activity.
Bactericidal antibody assays were performed with guinea pig antisera. Two guinea pig antisera were tested against the autologous strain, and in each case they were found to be equivalent. Neither of the two guinea pig anti-Q8 rLbpA antisera killed strain Q8, even at antibody dilutions of only 1:8. When bactericidal activity was defined as ≥50% killing, a 1:64 dilution of anti-4223 rLbpB antiserum and a 1:16 dilution of anti-Q8 rLbpB antiserum were bactericidal against their autologous strains. A 1:32 dilution of anti-4223 rLbpB antiserum also killed strain Q8, and a 1:16 dilution of anti-Q8 rLbpB killed strain 4223. Anti-4223 rLbpB antiserum at a dilution of 1:64 was used to screen for bactericidal activity against four additional heterologous strains, VH-19, LES-1, H-04, and 3, and was found to kill three of them (Table 2).
TABLE 2.
Bactericidal antibody activity of guinea pig anti-rLbpB antibodies
| Strain | Localea | Sourceb | LbpB size (kDa) | Bactericidal antibody activityc |
|---|---|---|---|---|
| 4223 | New York | MEF | 105 | ++ |
| Q8 | Quebec, Canada | Sputum | 105 | ± |
| VH19 | Texas | MEF | 105 | + |
| LES-1 | Finland | MEF | 102 | − |
| H-04 | Nova Scotia | MEF | 100 | + |
| 3 | New York | Sputum | 100 | ++ |
Geographic locale where strain was isolated.
Anatomical source of clinical isolate. MEF, middle ear fluid from otitis media patient.
Killing by anti-4223 rLbpB antiserum diluted 1:64, compared to negative controls: −, 0 to 25% killing; ±, 26 to 49% killing; +, 50 to 75% killing; ++, 76 to 100% killing.
DISCUSSION
Bacterial transferrin and lactoferrin receptors are heterodimeric complexes of proteins, TbpA-TbpB and LbpA-LbpB, known to be functionally and genetically related (13). In order to clone the M. catarrhalis lfr genes and not the tfr genes, a specific lbpA probe was generated. PCR primers were designed based upon an internal cyanogen bromide fragment of affinity-purified M. catarrhalis LbpA and the conserved carboxyl-terminal sequence LEMKF, thus far identified in all TbpA and LbpA proteins. By this approach, specific 2.2-kb lbpA gene fragments were amplified from strains 4223 and Q8 and these were used to probe the gene libraries and clone the complete lfr loci. The sense primer used to PCR amplify the 4223 and Q8 lbpA fragments had two codons missing (Y at position 6 and K at position 10) due to an error in transcribing the N-terminal sequence analysis report. The fact that a fragment of the lbpA gene could still be cloned was probably due to the two-step process of first generating a PCR fragment and then using that as the probe for the libraries.
The N. meningitidis and H. influenzae tfr operons are comprised of the tbpB and tbpA genes arranged in tandem with a single promoter region upstream of tbpB. The intergenic distance between the tbpB and tbpA genes ranges from 13 to 87 bp. Pettersson et al. (28) proposed that the N. meningitidis lactoferrin binding proteins may also be encoded on an operon having the gene arrangement lbpB–lbpA, especially since the lbpB and lbpA genes overlap. In M. catarrhalis, the lfr genes are arranged as tandem genes, with lbpB followed by lbpA at an intergenic distance of 184 bp. The putative lbpB promoter sequences have an unusually large separation between the putative −10 and RBS sequences, especially in the Q8 locus, which contains an extra 11 nucleotides in this region. Promoter elements can be readily identified upstream of M. catarrhalis lbpA, suggesting that lbpB and lbpA may be independently transcribed. Of particular interest in the cloned lfr locus is the presence of a third gene immediately downstream of lbpA, which is apparently unique to M. catarrhalis. Since the orf3 gene was cloned from two independent libraries, it is unlikely to be an experimental artifact. Potential promoter elements for orf3 can be identified within the lbpA gene. What role ORF3 may have, if any, in the lactoferrin receptor protein complex is unknown.
Expression of the tfr and lfr genes has been shown to be inducible under iron repression in vitro, a process thought to mimic the iron-restricted environment in the human host. From our data, there is a basal level of expression of the M. catarrhalis lfr genes observed in iron-sufficient medium, with an enhanced expression evident upon iron restriction. These data confirm the dot blot experiments of Schryvers and Lee, who showed that M. catarrhalis expressed low levels of transferrin and lactoferrin binding proteins under iron-sufficient growth conditions (33). The product of the ferric uptake regulation (fur) gene is thought to be responsible for this regulation of gene expression, and Fur binding sequences have been identified in the −10 region of the promoters for both the N. meningitidis and H. influenzae tbpB genes (12, 19). A potential Fur binding sequence was identified upstream of N. meningitidis lbpA; however, Pettersson et al. (28) were unable to demonstrate its functionality. In the case of N. meningitidis lfr, which is probably an operon, it seems likely that the Fur binding sequence is located upstream of lbpB, rather than lbpA, and will be identified once the complete N. meningitidis lbpB sequence is known. Compared with the consensus sequence for Fur binding sites (14), a homologous sequence can be identified in the −10 region of the M. catarrhalis strain Q8 lbpB promoter, but there is no obvious consensus sequence in the −10 region of the lbpA promoter. There are 11 nucleotides missing in the 4223 lbpB promoter region which are located within the putative Fur binding site of the Q8 lbpB promoter. The loss of these nucleotides in 4223 lbpB results in the loss of the Fur binding site and suggests another iron regulation mechanism for 4223 LbpB.
When the lactoferrin binding proteins from N. meningitidis, N. gonorrhoeae, and M. catarrhalis were first described, a single Lbp protein of an approximate molecular mass 105 kDa was identified (33). Subsequently, an 84-kDa protein isolated by low-stringency binding to lactoferrin was identified as LbpB (3). However, Bonnah et al. have recently demonstrated that the 84-kDa protein is CopB, and a 95-kDa protein has been identified as LbpB (4). Our data demonstrate clearly that, in some strains, the LbpA and LbpB proteins comigrate (Fig. 8A). The LbpA protein is quite homogeneous at about 105 kDa (Fig. 8B), and in 51 of the 90 strains examined, the LbpB protein comigrates with LbpA. In the remainder of the strains, the LbpB protein is apparently slightly smaller, but overall there is very little size heterogeneity for the M. catarrhalis LbpB proteins. This is in contrast to the TbpB proteins, which have been shown to be quite variable in size, ranging from about 68 to 88 kDa for N. meningitidis (30) and from about 60 to 90 kDa for H. influenzae (20), although less size heterogeneity was observed for the N. gonorrhoeae TbpB proteins, at 78 to 86 kDa (6).
The M. catarrhalis LbpA proteins were found to be 99% identical to each other. The N. meningitidis LbpA proteins from strains BNCV and H44/76 have been shown to be 95% identical to each other (27, 28) and 94% identical to the N. gonorrhoeae LbpA protein (1). Thus, as was previously found for the transferrin binding proteins, in which TbpA was highly conserved within a species, the LbpA proteins are also highly conserved. When compared with the neisserial LbpA proteins, there are several small inserts found in the M. catarrhalis LbpA proteins. Compared to the TbpA-LbpA topology model described by Gray-Owen and Schryvers (13), these inserts occur within the N-terminal periplasmic tail and extracellular loops 7, 9, 10, and 11.
Based upon the sequence variability of known TbpB proteins, it was expected that the LbpB proteins would show significant sequence variability; however, the three M. catarrhalis LbpB proteins showed surprising similarity, with strains Q8 and 4223 having 92% identical LbpB proteins. Strain Q8 was originally isolated from patient sputum in Montreal, Quebec, Canada; strain 4223 was isolated from middle ear fluid from a patient in Buffalo, N.Y.; and strain VH19 was isolated from middle ear fluid from a patient in Galveston, Tex. Strains Q8 and 4223 are also phenotypically distinct (39). The M. catarrhalis LbpB proteins show limited homology with the partial sequences of the putative N. meningitidis LbpB proteins. There is also very little homology with the known TbpB proteins, with the exception of short scattered sequences, the most notable being NRFVG at positions 390 to 394 and LEGGFYG at positions 430 to 436 (25). The conservation of these scattered residues (underlined) in bacterial TbpB proteins and in M. catarrhalis LbpB suggests that they may play a functional role in these iron-binding molecules. There is an unusually high content of Asp and Asn residues in LbpB, with a region of 50 residues that is ∼54% Asp and another region of 26 residues that is ∼42% Asn. The purpose of such concentrations of identical residues is unknown, but the fact that they are conserved among the three encoded LbpB proteins in this study suggests that they serve some function. Another unique feature of the M. catarrhalis LbpB proteins is the presence of a conserved RGD motif. This sequence is well established as a site for attachment of bacteria to eukaryotic cells (31), suggesting that the M. catarrhalis LbpB protein may act as an adhesin. An RGD motif has not been identified in any of the published TbpB sequences, and it will be interesting to see whether it is present in LbpB proteins from other species.
When the H. influenzae Rd genome was sequenced, it was found that there were several copies of transferrin or lactoferrin binding-like proteins (11). To demonstrate that we had indeed cloned the M. catarrhalis lfr genes and not a variant of the tfr genes, we tested the binding of the recombinant Lbp proteins to human lactoferrin. As demonstrated for transferrin binding proteins where only the TbpB protein binds to human transferrin after gel electrophoresis and electroblotting, only the LbpB, not the LbpA protein, specifically bound human lactoferrin. Since lactoferrin is known to be a sticky molecule, we also demonstrated that rTbpB did not bind to human lactoferrin under the same conditions. In addition, a corollary experiment was performed in which it was shown that only rTbpB, not rLbpA or rLbpB, bound human transferrin. Finally, the internal peptide sequences identified by Bonnah et al. (4) from M. catarrhalis 141 LbpA can be found in our sequences (Fig. 3). These data clearly demonstrate that we have cloned the lbpA and lbpB genes of M. catarrhalis.
The most unique finding in the M. catarrhalis lfr locus is the presence of the third gene, orf3. The putative ORF 3 protein has no homology to known proteins in the databases, and it contains an internal repeat of the tetramer DGLG. Such repeats sometimes represent phenotypic switches used to regulate virulence factors (35). We had hoped to be able to generate anti-rORF3 antibodies in order to determine whether ORF3 is expressed in M. catarrhalis, but we were unable to express the recombinant protein.
Guinea pigs immunized with purified rLbpA or rLbpB proteins elicited high-titer antibodies. There is no animal model for otitis media caused by M. catarrhalis, but a bactericidal antibody assay has been established (39). The clumping nature of M. catarrhalis strains makes this assay difficult to perform, so the data is only qualitative, not quantitative. The anti-Q8 rLbpA antibody was not bactericidal against its autologous strain. Since native LbpA protein is a transmembrane protein, it is possible that antibody raised to inclusion body-derived rLbpA protein would not recognize the native protein in intact organisms. However, in whole cell ELISAs, it was demonstrated that both anti-rLbpA and anti-rLbpB antisera recognized intact cells at titers ranging from 400 to 1,600 (data not shown). The anti-rLbpB antisera were weakly bactericidal, although the anti-4223 rLbpB antiserum appeared to be slightly more potent than the anti-Q8 rLbpB antiserum against their autologous strains. Heterologous strains were screened with a 1:64 dilution of anti-4223 rLbpB antiserum, and an arbitrary cutoff of ≥50% killing was defined as bactericidal activity. The heterologous strains that were tested were chosen based upon the diversity of their geographic origins and the inclusion of a mixture of molecular masses and anatomical sources. The data in Table 2 show that anti-4223 rLbpB antiserum was able to kill three of five heterologous strains by this stringent definition. There does not appear to be any correlation between the antibacterial activity of anti-4223 rLbpB and any other factor.
In this study, we have characterized the genes of the M. catarrhalis lfr locus and found that there are three closely spaced genes encoding conserved proteins. The lbpA and lbpB genes show some homology to other bacterial lbpA, tbpA, and tbpB genes, but the nature and function of the third gene is unknown. Recombinant LbpA and LbpB proteins were produced as inclusion bodies, and the purified proteins were used to generate high-titer antibodies. The anti-rLbpA antibody was not bactericidal, but the anti-rLbpB antibodies were bactericidal for autologous and heterologous strains of M. catarrhalis. Thus, rLbpB proteins may represent candidate vaccine antigens to protect against M. catarrhalis disease.
ACKNOWLEDGMENTS
We thank Bill Bradley for oligonucleotide synthesis, Diane England for DNA sequencing, and Manjit Haer, Wayne Williams, and Wan Xu-Li for excellent technical assistance.
REFERENCES
- 1.Biswas G D, Sparling P F. Characterization of lbpA, the structural gene for a lactoferrin receptor in Neisseria gonorrhoeae. Infect Immun. 1995;63:2958–2967. doi: 10.1128/iai.63.8.2958-2967.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bluestone C D. Modern management of otitis media. Pediatr Clin North Am. 1989;36:1371–1377. doi: 10.1016/s0031-3955(16)36794-3. [DOI] [PubMed] [Google Scholar]
- 3.Bonnah R A, Yu R H, Schryvers A B. Biochemical analysis of lactoferrin receptors in the Neisseriaceae: identification of a second bacterial lactoferrin receptor protein. Microb Pathog. 1995;19:285–297. doi: 10.1016/s0882-4010(96)80002-7. [DOI] [PubMed] [Google Scholar]
- 4.Bonnah R A, Yu R-H, Wong H, Schryvers A B. Biochemical and immunological properties of lactoferrin binding proteins from Moraxella (Branhamella) catarrhalis. Microb Pathog. 1998;24:89–100. doi: 10.1006/mpat.1997.0173. [DOI] [PubMed] [Google Scholar]
- 5.Catlin B W. Branhamella catarrhalis: an organism gaining respect as a pathogen. Clin Microbiol Rev. 1990;3:293–320. doi: 10.1128/cmr.3.4.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cornelissen C N, Biswas G D, Tsai J, Paruchuri D K, Thompson S A, Sparling P F. Gonococcal transferrin binding protein 1 causes Escherichia coli to bind to human transferrin. J Bacteriol. 1992;174:5788–5797. doi: 10.1128/jb.174.18.5788-5797.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.del Castillo F, Garcia-Perea A, Baquero-Artigao F. Bacteriology of acute otitis media in Spain: a prospective study based on tympanocentesis. Pediatr Infect Dis J. 1996;15:541–543. doi: 10.1097/00006454-199606000-00014. [DOI] [PubMed] [Google Scholar]
- 8.de Lillo A, Fierro J F. Identification of a lactoferrin-binding protein in Prevotella nigrescens. FEMS Microbiol Lett. 1997;150:61–64. doi: 10.1111/j.1574-6968.1997.tb10350.x. [DOI] [PubMed] [Google Scholar]
- 9.Dhaenens L, Szczebara F, Husson M O. Identification, characterization, and immunogenicity of the lactoferrin-binding protein from Helicobacter pylori. Infect Immun. 1997;65:514–518. doi: 10.1128/iai.65.2.514-518.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Enright M C, McKenzie H. Moraxella (Branhamella) catarrhalis—clinical and molecular aspects of a rediscovered pathogen. J Med Microbiol. 1997;46:360–371. doi: 10.1099/00222615-46-5-360. [DOI] [PubMed] [Google Scholar]
- 11.Fleischmann R D, Adams M D, White O, Clayton R A, Kirkness E F, Kerlavage A R, Bult C J, Tomb J-F, Dougherty B A, Merrick J M, McKenney K, Sutton G, FitzHugh W, Fields C, Gocayne J D, Scott J, Shirley R, Liu L-I, Glodek A, Kelley J M, Weidman J F, Phillips C A, Spriggs T, Hedblom E, Cotton M D, Utterback T R, Hanna M C, Nguyen D T, Saudek D M, Brandon R C, Fine L D, Fritchman J L, Fuhrmann J L, Geoghagen N S M, Gnehm C L, McDonald L A, Small K V, Fraser C M, Smith H O, Venter J C. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science. 1995;269:496–512. doi: 10.1126/science.7542800. [DOI] [PubMed] [Google Scholar]
- 12.Gray-Owen S D, Loosmore S, Schryvers A B. Identification and characterization of genes encoding the human transferrin-binding proteins from Haemophilus influenzae. Infect Immun. 1995;63:1201–1210. doi: 10.1128/iai.63.4.1201-1210.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gray-Owen S D, Schryvers A B. Bacterial transferrin and lactoferrin receptors. Trends Microbiol. 1996;4:185–191. doi: 10.1016/0966-842x(96)10025-1. [DOI] [PubMed] [Google Scholar]
- 14.Griggs D W, Konisky J. Mechanism for iron-regulated transcription of the Escherichia coli cir gene: metal-dependent binding of Fur protein to the promoters. J Bacteriol. 1989;171:1048–1054. doi: 10.1128/jb.171.2.1048-1054.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harkness R E, Guimond M-J, McBey B-A, Klein M H, Percy D H, Croy B A. Branhamella catarrhalis pathogenesis in SCID and SCID/beige mice. APMIS. 1993;101:805–810. [PubMed] [Google Scholar]
- 16.Homoe P, Prag J, Farholt S, Henrichsen J, Hornsleth A, Kilian M, Jensen J S. High rate of nasopharyngeal carriage of potential pathogens among children in Greenland: results of a clinical survey of middle-ear disease. Clin Infect Dis. 1996;23:1081–1090. doi: 10.1093/clinids/23.5.1081. [DOI] [PubMed] [Google Scholar]
- 17.Ioannidis J P A, Worthington M, Griffiths J K, Snydman D R. Spectrum and significance of bacteremia due to Moraxella catarrhalis. Clin Infect Dis. 1995;21:390–397. doi: 10.1093/clinids/21.2.390. [DOI] [PubMed] [Google Scholar]
- 18.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 19.Legrain M, Mazarin V, Irwin S W, Bouchon B, Quentin-Millet M-J, Jacobs E, Schryvers A B. Cloning and characterization of Neisseria meningitidis genes encoding the transferrin-binding proteins Tbp1 and Tbp2. Gene. 1993;130:73–80. doi: 10.1016/0378-1119(93)90348-7. [DOI] [PubMed] [Google Scholar]
- 20.Loosmore S M, Yang Y-P, Coleman D C, Shortreed J M, England D M, Harkness R E, Chong P S-C, Klein M H. Cloning and expression of the Haemophilus influenzae transferrin receptor genes. Mol Microbiol. 1996;19:575–586. doi: 10.1046/j.1365-2958.1996.406943.x. [DOI] [PubMed] [Google Scholar]
- 21.McCarty J M. Bacterial susceptibility and tympanocentesis in acute otitis media. Pediatr Infect Dis J. 1995;14:S45–S50. [Google Scholar]
- 22.Menozzi F D, Gantiez C, Locht C. Identification and purification of transferrin- and lactoferrin-binding proteins of Bordetella pertussis and Bordetella bronchiseptica. Infect Immun. 1991;59:3982–3988. doi: 10.1128/iai.59.11.3982-3988.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meyer G A, Shope T R, Waeker N J, Jr, Lanningham F H. Moraxella (Branhamella) catarrhalis bacteremia in children. Clin Pediatr. 1995;34:146–150. doi: 10.1177/000992289503400305. [DOI] [PubMed] [Google Scholar]
- 24.Nissinen A, Gronroos P, Huovinen P, Herva E, Katila M-L, Klaukka T, Kontiainen S, Liimatainen O, Oinonen S, Makela P H. Development of β-lactamase-mediated resistance to penicillin in middle-ear isolates of Moraxella catarrhalis in Finnish children, 1978–1993. Clin Infect Dis. 1995;21:1193–1196. doi: 10.1093/clinids/21.5.1193. [DOI] [PubMed] [Google Scholar]
- 25.Ogunnariwo J A, Schryvers A B. Rapid identification and cloning of bacterial transferrin and lactoferrin receptor protein genes. J Bacteriol. 1996;178:7326–7328. doi: 10.1128/jb.178.24.7326-7328.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogunnariwo J A, Woo T K W, Lo R Y C, Gonzalez G C, Schryvers A B. Characterization of the Pasteurella haemolytica transferrin receptor genes and the recombinant receptor proteins. Microb Pathog. 1997;23:273–284. doi: 10.1006/mpat.1997.0156. [DOI] [PubMed] [Google Scholar]
- 27.Pettersson A, van der Ley P, Poolman J T, Tommassen J. Molecular characterization of the 98-kilodalton iron-regulated outer membrane protein of Neisseria meningitidis. Infect Immun. 1993;61:4724–4733. doi: 10.1128/iai.61.11.4724-4733.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pettersson A, Klarenbeek V, van Deurzen J, Poolman J T, Tommassen J. Molecular characterization of the structural gene for the lactoferrin receptor of the meningococcal strain H44/76. Microb Pathog. 1994;17:395–408. doi: 10.1006/mpat.1994.1085. [DOI] [PubMed] [Google Scholar]
- 29.Pettersson A, Maas A, Tommassen J. Identification of the iroA gene product of Neisseria meningitidis as a lactoferrin receptor. J Bacteriol. 1994;176:1764–1766. doi: 10.1128/jb.176.6.1764-1766.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rokbi B, Maitre-Wilmotte G, Mazarin V, Fourrichon L, Lissolo L, Quentin-Millet M J. Variable sequences in a mosaic-like domain of meningococcal tbp2 encode immunoreactive epitopes. FEMS Microbiol Lett. 1995;132:277–283. doi: 10.1016/0378-1097(95)00326-z. [DOI] [PubMed] [Google Scholar]
- 31.Ruoslahti E, Pierschbacher M D. Arg-Gly-Asp: a versatile cell recognition signal. Cell. 1986;44:517–518. doi: 10.1016/0092-8674(86)90259-x. [DOI] [PubMed] [Google Scholar]
- 32.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 33.Schryvers A B, Lee B C. Comparative analysis of the transferrin and lactoferrin binding proteins in the family Neisseriaceae. Can J Microbiol. 1989;35:409–415. doi: 10.1139/m89-063. [DOI] [PubMed] [Google Scholar]
- 34.Staggs T M, Greer M K, Baseman J B, Holt S C, Tryon V V. Identification of lactoferrin-binding proteins from Treponema pallidum subspecies pallidum and Treponema denticola. Mol Microbiol. 1994;12:613–619. doi: 10.1111/j.1365-2958.1994.tb01048.x. [DOI] [PubMed] [Google Scholar]
- 35.Stern A, Brown M, Nickel P, Meyer T F. Opacity genes in Neisseria gonorrhoeae: control of phase and antigenic variation. Cell. 1986;47:61–71. doi: 10.1016/0092-8674(86)90366-1. [DOI] [PubMed] [Google Scholar]
- 36.Struye M, Moons M, Tommassen J. Carboxy-terminal phenylalanine is essential for the correct assembly of a bacterial outer membrane protein. J Mol Biol. 1991;218:141–148. doi: 10.1016/0022-2836(91)90880-f. [DOI] [PubMed] [Google Scholar]
- 37.Tabor S, Richardson S S. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci USA. 1985;82:1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu H C, Tokunaga M. Biogenesis of lipoproteins in bacteria. Curr Top Microbiol Immunol. 1986;125:127–157. doi: 10.1007/978-3-642-71251-7_9. [DOI] [PubMed] [Google Scholar]
- 39.Yang Y P, Myers L E, McGuinness U, Chong P, Kwok Y, Klein M H, Harkness R E. The outer membrane protein, CD, extracted from Moraxella (Branhamella) catarrhalis is a potential vaccine antigen that induces bactericidal antibodies. FEMS Immunol Med Microbiol. 1997;17:187–199. doi: 10.1111/j.1574-695X.1997.tb01012.x. [DOI] [PubMed] [Google Scholar]
- 40.Yang Y P, Munson R S, Jr, Gross S, Chong P, Harkness R E, Gisonni L, James O, Kwok Y, Klein M H. Effect of lipid modification on the physicochemical, structural, antigenic and immunoprotective properties of Haemophilus influenzae outer membrane protein P6. Vaccine. 1997;15:976–987. doi: 10.1016/s0264-410x(96)00296-4. [DOI] [PubMed] [Google Scholar]