Abstract
gastrulation defective (gd) encodes a serine protease required for specification of dorsal-ventral cell fates during Drosophila embryogenesis. Using RNA microinjection, I show that wild-type gd RNA can restore ventrolateral pattern elements with correct polarity with respect to egg shape in embryos lacking gd function. While low RNA concentrations restore ventrolateral pattern elements, higher concentrations ventralize the embryo. Gastrulation defective concentration has a rate-limiting effect on the domain of high Dorsal concentration but little effect upon the slope of the gradient. In embryos from pipe-null females, much higher RNA concentrations generate an ectopic axis oriented with respect to the site of injection. The data suggest that the Dorsal gradient is not directly determined by asymmetric cues in the eggshell but arises de novo within the perivitelline space as a consequence of self-regulatory properties of the protease cascade. A homology to the mammalian complement factors C2 and B is also described.
INTRODUCTION
Cell fates along the dorsal-ventral (d-v) axis of the Drosophila embryo are established by a signal transduction pathway directing the production of the Dorsal morphogen gradient, reviewed by (Morisato and Anderson, 1995). Dorsal, an NF-κB family transcription factor, translocates from the cytoplasm and accumulates in cellular blastoderm nuclei. Nuclei on the ventral side acquire high concentrations while dorsolateral nuclei acquire lower amounts (Steward et al., 1988; Roth et al., 1989; Rushlow et al., 1989). The polarity and shape of the Dorsal gradient is determined by an extracellular signal originating on the ventral side of the perivitelline space (PVS). Generation of this signal has been proposed to involve a protease cascade leading to the processing and activation of a ‘ventralizing’ or ventral specifying ligand (DeLotto and Spierer, 1986; Chasen and Anderson, 1989; Morisato and Anderson, 1994; DeLotto and DeLotto, 1998). How this process is spatially and temporally regulated and precisely where and when a gradient of any component or activity first appears is presently unknown.
Asymmetric positional cues for embryonic dorsal-ventral polarity are laid down during oogenesis and require the genes gurken, torpedo and fs(1)K10 (Ray and Schupbach, 1996). The products of these genes function on the dorsal side of the developing oocyte to suppress ventral cell fate in the somatic follicle cells. The genes nudel, pipe and windbeutel are required in the follicle cells that secrete the egg shell and the vitelline membrane.
Maternal effect genes including snake (snk), easter (ea), gastrulation defective (gd) and spaetzle (spz), are required in the germ line and their products are secreted into the PVS (Roth, 1994). gd, snk and ea encode serine proteases, which function in a protease cascade, and spz encodes a precursor of an NGF-like molecule, which is generally thought to be the ventralizing ligand (DeLotto and Spierer, 1986; Chasen and Anderson, 1989; Morisato and Anderson, 1994; DeLotto and DeLotto, 1998; Konrad et al., 1998; Han et al., 2000; Dissing et al., 2001). GD functions before Snake, making GD the earliest known germline component and therefore a candidate for a protease which could directly interpret the ventral prepattern (Smith and DeLotto, 1994; Dissing et al., 2001). Here, I address the role of GD in orienting and shaping the Dorsal gradient.
RESULTS
Gastrulation defective has structural similarities to the mammalian complement factors C2/B
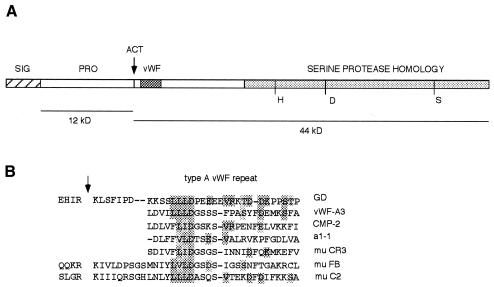
The predicted protein encoded by gd was compared with members of the serine protease superfamily and it lacks sequence conservation within the activation peptide cleavage site. Examination of the protein data bank revealed two serine proteases, which also deviate from the concensus within the activation peptide region. The complement factors C2 and B are activated by cleavage between an arginine and lysine residue and ∼12 amino acids after the cleavage site in factor C2 and B, a type A von Willebrand repeat motif is found (reviewed in Arlaud et al., 1998). The gd amino acid sequence was compared with factors C2 and B and an alignment revealed that GD possesses an arginine lysine pair as well as a type A von Willebrand motif in a conserved geometry (Figure 1A). These features suggest that gd encodes a serine protease zymogen, which is structurally similar to factors C2 and B, and is therefore activated by cleavage between Arg128 and Lys129. Site-directed mutagenesis studies and the analysis of interallelic complementation at gd are consistent with this model (Ponomareff et al., 2001).
Fig. 1. (A) A GD structural model. The serine protease homology domain is lightly stippled and the positions of the active site residues (H, D, S) are indicated. The arrow indicates the position of the RK residue pair and the von Willebrand type A homology motif is in heavily stippled, while the predicted signal peptide is hatched. Expected molecular weights of cleaved fragments are indicated below. (B) A protein sequence comparison of GD and type A von Willibrand factor motifs. The arrow indicates the position of the activation site in murine complement factors C2 and B.
Phenotypic rescue of gastrulation defective by microinjection of gd SP6 RNA transcripts
To determine whether wild-type (wt) gd transcripts can rescue embryos from gd– mothers, gd RNA at different concentrations was microinjected into embryos lacking gd function. As shown in Table I, injection of wt gd RNA restores ventrolateral pattern elements such as filzkorper or ventral denticles, in a concentration-dependent manner. While no ventrolateral pattern elements were observed in uninjected embryos, when injected with 1 µg/ml RNA, 19% of embryos exhibited filzkorper. At 100 µg/ml RNA, 2% of embryos exhibited filzkorper and 44% exhibited ventral denticles. At 1 mg/ml, 31% of the embryos exhibited ventral denticles and the number of embryos with no cuticle increased to 68%. The increase in the no-cuticle phenotype is consistent with high concentrations of gd RNA specifying mesodermal cell fate, which does not make cuticle.
Table I. Phenotypic rescue of embryos from gd9/gd9 females by microinjection with wt gd RNA.
| RNA concentration (µg/ml) | #emb | No cuticle (%) | Dorsalized (%) | filzkorper (%) | Ventral denticles (%) | Hatching (%) |
|---|---|---|---|---|---|---|
| Uninjected | 100 | 3 | 97 | 0 | 0 | 0 |
| 1 | 105 | 49 | 23 | 19 | 9 | 0 |
| 10 | 139 | 38 | 6 | 16 | 40 | 0 |
| 100 | 127 | 52 | 2 | 2 | 44 | 0 |
| 1000 | 81 | 68 | 0 | 1 | 31 | 0 |
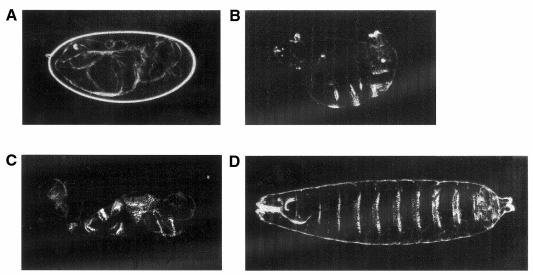
The phenotype of gd rescued embryos differs from snk RNA and ea RNA rescued embryos. Microinjection of snk and ea RNA can rescue completely the phenotype of embryos from snk– and ea– mothers and normalize d-v cell fates, producing hatching larvae (Figure 2D). However, in no case has ventralization been observed for high concentrations of wt snake or ea RNA (Chasen and Anderson, 1989; Smith et al., 1994). While gd RNA restores ventrolateral pattern elements in a concentration-dependent manner the pattern was never normalized (N >2000). Furthermore, while snk and ea RNA rescue shows no position dependence, the rescue observed with gd RNA is largely localized to the injection site (Figure 2B). Embryos injected with moderate RNA concentrations (n = 127) produce ventrally open cuticles with split ventral denticles near the site of injection (Figure 2C).
Fig. 2. (A) A dorsalized cuticule from a gd9/gd9 mother. (B) An embryo from a gd9/gd9 mother that was injected in the posterior pole with wt gd SP6 RNA at 10 µg/ml. (C) An embryo injected as in (B) but showing split ventral denticles. (D) Complete phenotypic rescue of an embryo from a snk229/snk229 mother by injection of wt snake SP6 RNA at the posterior pole.
gd RNA ventralizes by expanding mesodermal cell fate without altering the slope of the Dorsal gradient
To determine whether microinjection of gd RNA transcripts could alter d-v cell fates in wt embryos, gd RNA was injected into OregonR embryos and the pattern was analyzed. After injection both the gastrulation pattern and larval cuticles were locally ventralized and all injected embryos failed to hatch. To determine how gd RNA microinjection alters the fate map, embryos were injected with gd RNA, and then either probed with antibodies against Twist, a mesodermal cell fate marker, or hybridized in situ for rhomboid, a ventrolateral cell fate marker (Thisse et al., 1988; Arlaud et al., 1998). In wt embryos, Twist is expressed as ventral stripe, 16–18 cells wide along the anterior-posterior length of the blastoderm in the future mesoderm. The first domain of rhomboid expression in wt embryos is two ventrolateral stripes between 6 and 8 cells wide, within the neuroectodermal primordium. Since early lateral rhomboid expression is limited to a defined intermediate concentration of Dorsal, it has been used as a sensitive indicator of the slope and position of the Dorsal gradient (Roth, 1993).
To determine how gd RNA concentration affects the Dorsal gradient, RNA was injected at 50 µg/ml at the posterior of embryos from gd9/gd9 or wt females. Embryos were allowed to develop and in situ hybridized with rhomboid. Injected wt embryos (n = 145) exhibit two ventrolateral stripes, however they are shifted dorsally near the site of injection (Figure 3B). The width of the rho stripe is identical to that of wt embryos. Figure 4 illustrates a similar injection analyzed by expression of Twist. Near the site of injection, Twist expression is expanded dorsally whereas at the anterior it is closer to normal (Figure 4B). Thus, increasing GD dosage in wt embryos locally expands the mesodermal anlagen without altering the slope of the Dorsal gradient.
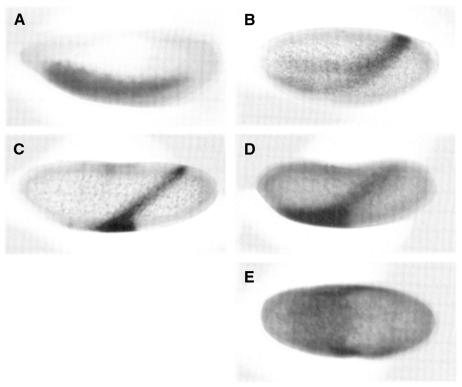
Fig. 3. Effects of gd RNA injection upon rhomboid expression. (A) In situ expression of rhomboid in a wt embryo. (B) rhomboid in a wt embryo injected at the posterior pole with wt gd SP6 RNA. (C) rhomboid expression in an embryo from a gd9/gd9 mother, similarly injected (D) and (E). Lateral and ventral views of rhomboid in an embryo from a gd9/gd9 female injected with gd RNA showing expansion of rhomboid expression in a ventral zone. Orientation: anterior left, dorsal up.
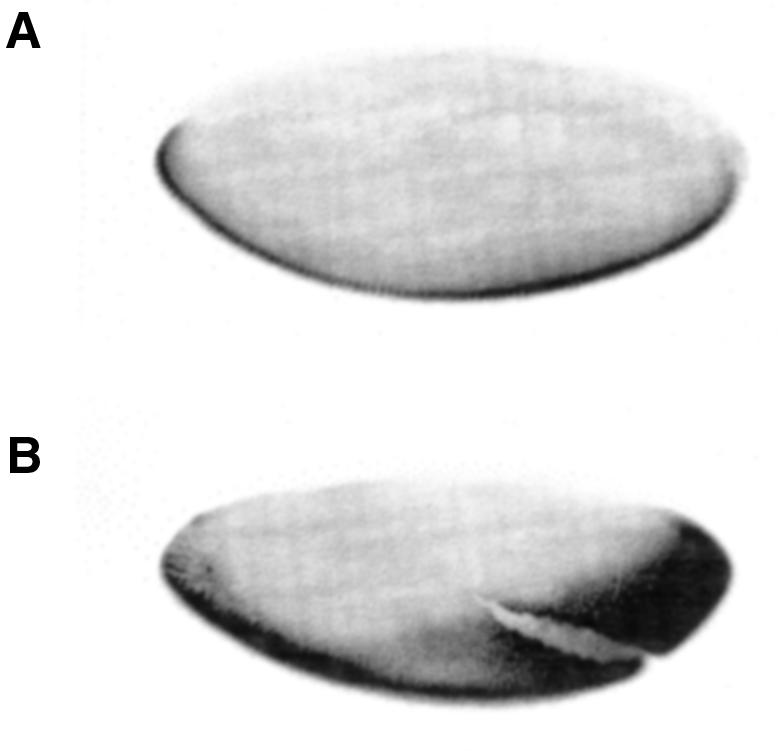
Fig. 4. (A) Anti-Twist staining of a wt OregonR embryo. (B) Anti-Twist staining of an OregonR embryo injected at the posterior pole with wt gd SP6 RNA. Orientation: anterior left, dorsal up.
gd RNA transcripts were injected into an embryo from a gd9/gd9 mother (Figure 3C–E). Uninjected gd9/gd9 embryos show no lateral rhomboid expression at cellular blastoderm. Over the dorsal and ventrolateral regions of the gd injected embryo, rho is expressed as a uniform stripe approximately six cells wide, shifted dorsally near the site of injection. However, away from the injection site on the ventral side, the stripe broadens to cover a large part of the ventral region. Figure 3C and D illustrate the degree of variation seen in one injection series (n = 122). As before, the primary consequence of increasing gd concentration is to shift the position of the boundary between mesoderm and ventral neurogenic ectoderm dorsally, while the width of the ventral neurogenic ectodermal primordium remains constant. However, the ventral part of the egg responds more to an equivalent gd RNA concentration than dorsal or ventrolateral parts of the embryo as rho expands. In all injected embyros, the transition from stripe to broad expression is sharp and at the same d-v position. This increased response on the ventral side defines a previously unidentified stripe-like ventral zone.
High gd RNA concentrations produce an ectopic dorsal-ventral axis in embryos from pipe null females
Pipe is a candidate for defining an asymmetric cue directing d-v pattern during embryogenesis (Sen et al., 1998). To test whether gd RNA ventralization requires pipe, gd RNA was injected into embryos from pip664/pip664 females (Figure 5). At low gd RNA concentrations, no effect was seen. However, at 100 µg/ml, small ectopic patches of ventral denticles were observed (data not shown). Between 100–500 µg/ml localized expression of Twist appeared close to the injection site (Figure 5A). When hybridized in situ for rho, either one or two radial stripes spanning the d-v axis were generated (Figure 5B). In wt embryos a third rho stripe appears in the future amnioserosa very shortly after the two lateral stripes appear (Arlaud et al., 1998; Roth, 1993). When two rho stripes were observed in embyros from pipe-null mothers, the distance between them was the same as the distance between the lateral rho stripes and the amnioserosal rho stripe. All injected embryos stained for Twist (n > 100) show only one patch of Twist expression near the injection site. When it appears, the second rho stripe correlates with amnioserosal rho expression. I conclude that high gd RNA concentrations in a pipe-null background induces an ectopic axis with the ventral pole defined by the point of injection. Embryos from, snk229-, ea1- and spz197-null females were microinjected with 100 µg/ml gd RNA and remained dorsalized indicating that this effect requires their activities (data not shown).
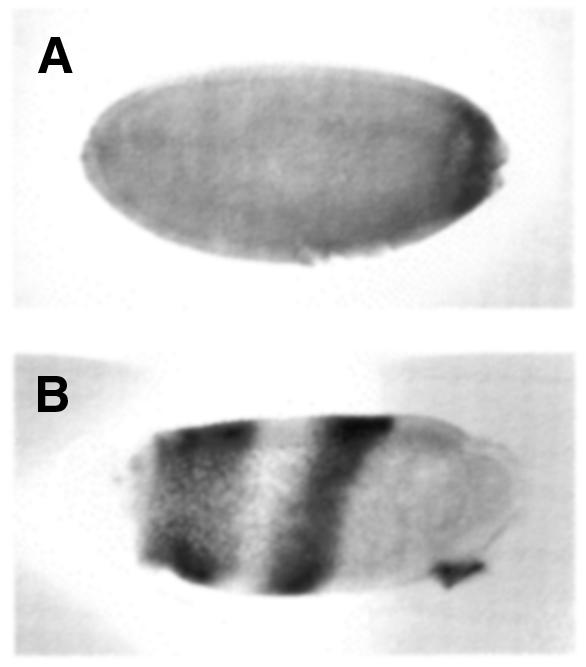
Fig. 5. Orientation of the d-v axis by gd RNA in embryos from pip664/pip664 mothers. (A) anti-Twist antibody staining. (B) rhomboid in situ hybridization. RNA was injected at the posterior pole in both embryos.
DISCUSSION
The data presented here suggest that the shape of the Dorsal gradient is not directly determined by asymmetric cues in the eggshell but rather arises within the perivitelline space as a consequence of self-regulatory properties of the protease cascade triggered by GD (Dissing et al., 2001). Localized phenotypic rescue is consistent with the idea that GD is membrane bound (Stein and Nüsslein-Volhard, 1992). Since GD can produce an ectopic axis in a pipe-null background, pipe activity is not required for binding. Binding of GD to a surface within the PVS is therefore independent of the spatial control of activation which is normally ventrally restricted and requires Pipe.
The spatial activation of GD must be regulated subsequently to and independently of binding. This could be explained via interaction of GD with a Pipe-modified proteoglycan. Since pipe is an heparan sulfate 2-O-sulfotransferase and its expression is restricted to the ventral third of the somatic follicle cell, it presumably modifies a somatically expressed proteoglycan (Sen et al., 1998). Ventrally restricted modification by Pipe of this proteoglycan could control GD activation. It is known that complement and coagulation pathways can be both positively and negatively regulated by interaction with heparan sulfate proteoglycans (Selleck, 2000).
Since direct quantitative measurement of the Dorsal gradient is difficult, I chose to use one of the most sensitive markers for the position and slope of the Dorsal gradient, rho expression. The data suggest that a self-regulating patterning mechanism exists, which is independent of GD concentration and can determine the slope of the gradient. The injection phenotype is not exactly like that of embryos from females carrying mutations in gurken and torpedo (DER) (Roth and Schupbach, 1994; Roth et al., 1995). In these mutations, while rho stripes are shifted dorsally, the mesoderm splits into two ventral furrows. This splitting has not been observed in any gd RNA injections.
An intriguing observation from these injections is that of a discontinuity along the width of the embryo. A ventral region appears to be more sensitive than lateral and dorsal parts of the embryo are to GD activity. While over most of the ventrolateral and dorsal surfaces a uniform width rho stripe was generated, away from the point of injection, rho was expressed in a broad ventral patch. The transition between stripe and broad zone is sharp, bilaterally symmetrical and always at the same relative position in injected embryos. These results suggest the existence of a ventral prepattern in the form of a broad ‘stripe-like’ zone with distinct boundaries.
Han et al. (2000), while also reporting ventralization by gd microinjection, indicated that microinjection of gd RNA into embryos from gd7/gd7 mothers can also normalize and rescue some embryos to hatching. While this differs from my observations in gd4 and gd9, it is possible that the ability to rescue gd7 is an allele-specific effect. gd7, while an amorphic allele, results from a single amino acid change of Gly44 to Asp in contrast to gd4 and gd9, which are deletions and truncations, respectively. Allele-specific effects are observed at gd (Ponomareff et al., 2001).
The RNA concentration required to produce an ectopic ventralizing signal in pipe-null embryos is significantly greater than that necessary to ‘rescue’ an embryo from a gd-null mother. In the pipe– injections, I propose that high local concentrations of GD become proteolytically active at the ectopic site either by ‘titrating out’ regulatory serpins that normally control the cascade. Serpins specifically regulating this pathway have not yet been identified, but there is evidence for a serpin which binds Easter and evidence for a serpin regulating Spaetzle processing in innate immunity (Misra et al., 1998; Levashina et al., 1999). While the Dorsal gradient formed in injected pipe nulls is somewhat broader than in wt, it is still formed independently of Pipe. Therefore, the part of the pathway responsible for shaping the Dorsal gradient must have self-regulating properties which are independent of the ventral prepattern.
Speculation
The data suggest a model in which the Dorsal gradient is shaped dynamically via a proteolytic activation cascade during embryogenesis starting with a ventral stripe prepattern. The prepattern can be visualized as initially consisting of: (i) a broad ventral zone or stripe in which GD activity is potentiated, and (ii) the remainder of the PVS in which GD or the other proteases are inhibited. An interesting possibility is that the ventral stripe zone results from the modification of a uniformly distributed inhibitory substance through pipe post-translational modification. The sharp boundaries of the zone identified in these experiments suggests the ventral prepattern is not already graded. The fine-tuning of the slope of the Dorsal gradient may involve complex interactions within the proteolytic cascade downstream of GD or via feedback to GD. The results presented here are consistent with GD being a protease which has a pivotal role in interpreting the d-v prepattern as well as initiating and shaping the Dorsal gradient during embryogenesis.
METHODS
Isolation of full-length gd cDNA. A 0–2 h embryonic cDNA library (N. Brown, Cambridge) containing cDNA inserted after a Xenopus β-globin untranslated leader and an SP6 promoter was screened using a [γ-32P] end-labeled oligonucleotide (5′-CCATTGTCGGCAATGACG-3′) derived from published gd cDNA sequence using standard methods. A full-length clone (gdcD7) was isolated and its primary DNA sequence was found to correspond to that described previously (Konrad et al., 1998).
Synthesis of RNA transcripts and microinjection. Phenotypic rescue assays were conducted with eggs from homozygous females derived from the following stocks: OregonR (wt), gd9/FM7 and ru st e pip664 ca/TM3;Sb. gd cD7 was linearized at the 3′ end by digestion with NotI and RNA was synthesized as described previously (Smith and DeLotto, 1994). RNA was injectected into stage 2 embryos, which developed for 2 days at 20°C in a humid chamber, and cuticles prepared as described (Wieschaus and Nüsslein-Volhard, 1986).
Antibody staining and in situ hybridization. Rabbit anti-Twist antisera was a generous gift of Dr Maria Leptin. Stainings were conducted as described previously (Smith and DeLotto, 1994). cDNA for the synthesis of rhomboid probes (Arlaud et al., 1998) was provided by S. Roth and in situ hybridizations were carried out as described (Roth, 1993).
Acknowledgments
ACKNOWLEDGEMENTS
Special thanks to Y. DeLotto for superb technical assistance and S. Brown, G. Thon and M. Dissing for helpful comments on the manuscript. I would like to thank the US National Science Foundation, the Danish Research Council, the Danish Cancer Fund and the Vera and Carl Johan Michaelsens Legacy for support.
REFERENCES
- Arlaud G.J., Volonakis, J.E., Thielens, N.M., Rossi, V. and Xu, Y. (1998) The atypical serine proteases of the complement system. Adv. Immunol., 69, 249–307. [PubMed] [Google Scholar]
- Bier E., Jan, L. and Jan, Y.-N. (1990) rhomboid, a gene required for dorsoventral axis establishment and peripheral nervous system development in Drosophila melanogaster. Genes Dev., 4, 190–203. [DOI] [PubMed] [Google Scholar]
- Chasen R. and Anderson, K.V. (1989) The role of easter, an apparent serine protease, in organizing the dorsal-ventral axis of the Drosophila embryo. Cell, 56, 391–400. [DOI] [PubMed] [Google Scholar]
- DeLotto R. and Spierer, P. (1986) A gene required for the specification of dorsal-ventral pattern in Drosophila appears to encode a serine protease. Nature, 323, 688–692. [DOI] [PubMed] [Google Scholar]
- DeLotto Y. and DeLotto, R. (1998) Proteolytic processsing of the Drosophila Spaetzle protein by Easter generates a dimeric NGF-like molecule with ventralizing activity. Mech. Dev., 72, 141–148. [DOI] [PubMed] [Google Scholar]
- Dissing M., Giordano, H. and DeLotto, R. (2001) Autoproteolysis and feedback in a protease cascade directing Drosophila dorsal-ventral cell fate. EMBO J., 20, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J.H., Lee, S.H., Tan, Y.Q., LeMosy, E. and Hashimoto, C. (2000) Gastrulation Defective is a serine protease involved in activating the receptor Toll to polarize the Drosophila embryo. Proc. Natl Acad. Sci. USA, 97, 9093–9097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad K.D., Goralski, T.J., Mahowald, A.P. and Marsh, J.L. (1998) The gastrulation defective gene of Drosophila melanogaster is a member of the serine protease superfamily. Proc. Natl Acad. Sci. USA, 95, 6819–6824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levashina E.A., Langley, E., Green, C., Gubb, D., Ashburner, M., Hoffmann, J.A. and Reichhart, J.M. (1999) Constitutive activation of toll-mediated antifungal defense in serpin-deficient Drosophila. Science, 285, 1917–1919. [DOI] [PubMed] [Google Scholar]
- Misra S., Hecht, P., Maeda, R. and Anderson, K.V. (1998) Positive and negative regulation of Easter, a member of the serine protease family that controls dorsal-ventral patterning in the Drosophila embryo. Development, 125, 1261–1267. [DOI] [PubMed] [Google Scholar]
- Morisato D. and Anderson, K. (1994) The spaetzle gene encodes a component of the extracellular signalling pathway establishing the dorsal-ventral pattern of the Drosophila embryo. Cell, 76, 677–688. [DOI] [PubMed] [Google Scholar]
- Morisato D. and Anderson, K.V. (1995) Signaling pathways that establish the dorsal-ventral pattern of the Drosophila embryo. Annu. Rev. Genet., 29, 371–399. [DOI] [PubMed] [Google Scholar]
- Ponomareff G., Giordano, H., DeLotto, Y. and DeLotto,R. (2001) Interallelic complementation at the Drosophila melanogaster gastrulation defective locus defines discrete functional domains of the protein. Genetics, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray R.P. and Schupbach, T. (1996) Intercellular signaling and the polarization of body axes during Drosophila oogenesis. Genes Dev., 10, 1711–1723. [DOI] [PubMed] [Google Scholar]
- Roth S. (1993) Mechanisms of dorsal-ventral axis determination in Drosophila embryos revealed by cytoplasmic transplatations. Development, 117, 1385–1396. [DOI] [PubMed] [Google Scholar]
- Roth S. (1994) Proteolytic generation of a morphogen. Curr. Biol., 4, 755–757. [DOI] [PubMed] [Google Scholar]
- Roth S., Neuman-Silberberg, F.S., Barcelo, G. and Schupbach, T. (1995) Cornichon and the EGF receptor signaling process are necessary for both anterior-posterior and dorsal-ventral pattern formation in Drosophila. Cell, 81, 967–978. [DOI] [PubMed] [Google Scholar]
- Roth S. and Schupbach, T. (1994) The relationship between ovarian and embryonic dorsoventral patterning in Drosophila. Development, 120, 2245–2257. [DOI] [PubMed] [Google Scholar]
- Roth S., Stein, D. and Nüsslein-Volhard, C. (1989) A gradient of nuclear localization of the dorsal protein determines dorsoventral pattern in the Drosophila embryo. Cell, 59, 1189–1202. [DOI] [PubMed] [Google Scholar]
- Rushlow C., Han, K., Manley, J. and Levine, M. (1989) The graded distribution of the dorsal morphogen is initaited by selective nuclear transport in Drosophila. Cell, 59, 1165–1177. [DOI] [PubMed] [Google Scholar]
- Selleck S.B. (2000) Proteoglycans and pattern formation: sugar biochemistry meets developmental genetics. Trends Genet., 16, 206–212. [DOI] [PubMed] [Google Scholar]
- Sen J., Goltz, J.S., Stevens, L. and Stein, D. (1998) Spatially restricted expression of pipe in the Drosophila egg chamber defines embryonic dorsal-ventral polarity. Cell, 95, 471–481. [DOI] [PubMed] [Google Scholar]
- Smith C. and DeLotto, R. (1994) Ventralizing signal determined by protease activation in Drosophila embryogenesis. Nature, 368, 548–551. [DOI] [PubMed] [Google Scholar]
- Smith C., Giordano, H. and DeLotto, R. (1994) Mutational analysis of the Drosophila snake protease: an essential role for domains within the proenzyme polypeptide chain. Genetics, 136, 1355–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein D. and Nüsslein-Volhard, C. (1992) Multiple extracellular activities in Drosophila egg perivitelline fluid are required for establishment of embryonic dorsal-ventral polarity. Cell, 68, 429–440. [DOI] [PubMed] [Google Scholar]
- Steward R., Zusman, S., Huang, L. and Schedl, P. (1988) The dorsal protein is distributed in a gradient in early Drosophila embryos. Cell, 55, 487–495. [DOI] [PubMed] [Google Scholar]
- Thisse B., Stoetzel, C., Gorostiza-Thisse, C. and Perrin-Schmidt, F. (1988) Sequence of the twist gene and nuclear localization of its protein in endomesodermal cells of early Drosophila embryos. EMBO J., 7, 2175–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieschaus E. and Nüsslein-Volhard, C. (1986) Looking at embryos. In Roberts, D.B. (ed.), Drosophila, A Practical Approach. IRL Press, Oxford, UK, pp. 199–228.