Abstract
Epithelial cells are refractory to extracellular lipopolysaccharide (LPS), yet when presented inside the cell, it is capable of initiating an inflammatory response. Using invasive Shigella flexneri to deliver LPS into the cytosol, we examined how this factor, once intracellular, activates both NF-κB and c-Jun N-terminal kinase (JNK). Surprisingly, the mode of activation is distinct from that induced by toll-like receptors (TLRs), which mediate LPS responsiveness from the outside-in. Instead, our findings demonstrate that this response is mediated by a cytosolic, plant disease resistance-like protein called CARD4/Nod1. Biochemical studies reveal enhanced oligomerization of CARD4 upon S. flexneri infection, an event necessary for NF-κB induction. Dominant-negative versions of CARD4 block activation of NF-κB and JNK by S. flexneri as well as microinjected LPS. Finally, we showed that invasive S. flexneri triggers the formation of a transient complex involving CARD4, RICK and the IKK complex. This study demonstrates that in addition to the extracellular LPS sensing system mediated by TLRs, mammalian cells also possess a cytoplasmic means of LPS detection via a molecule that is related to plant disease-resistance proteins.
INTRODUCTION
In contrast to what is known about Toll-like receptors (TLRs) in mediating responsiveness to bacteria and bacterial products in cells of the myeloid lineage, the role played by TLRs in pathogen recognition in epithelial cells remains poorly defined since these cells are largely unresponsive to extracellular lipopolysaccharide (LPS) and to non-pathogenic bacteria (Cario et al., 2000; Philpott et al., 2000). Since most epithelial surfaces are constantly exposed to extracellular bacteria and bacterial products, lack of responsiveness of epithelial cells likely prevents the induction of immune defense mechanisms against the normal microbial flora. However, invasion of epithelial cells by pathogenic bacteria initiates inflammatory responses. Infection of epithelial cells with invasive pathogens, including Shigella flexneri (Philpott et al., 2000) can induce the innate induction of NF-κB leading to the production of the pro-inflammatory cytokine, IL-8. Therefore, epithelial cells can initiate defensive responses to invasive bacterial pathogens indicating that these cells might possess an alternate means of pathogen detection.
Entry of S. flexneri into epithelial cells is essential for virulence and also necessary for the induction of inflammatory responses as assessed by activation of NF-κB and the production of IL-8 (Philpott et al., 2000). Bacterial internalization allows presentation of LPS to the intracellular compartment, an event sufficient to initiate the inflammatory response since microinjection of this bacterial product directly into epithelial cells induces NF-κB activation (Philpott et al., 2000). The mechanism by which intracellular LPS activates this response, however, has not yet been determined. In this study, we sought to identify the mechanism through which intracellular LPS, delivered by invasive S. flexneri, activates cell signaling pathways leading to NF-κB and c-Jun N-terminal kinase (JNK) activation. Here we show that CARD4/Nod1, a cytosolic protein that resembles a plant disease resistance protein, is involved in both NF-κB and JNK activation by intracellular LPS. These findings suggest the existence of an evolutionarily conserved system of intracellular pathogen recognition and signal transduction in epithelial cells that is dependent on CARD4.
RESULTS AND DISCUSSION
Activation of the JNK pathway by invasive S. flexneri is dependent upon intracellular presentation of LPS
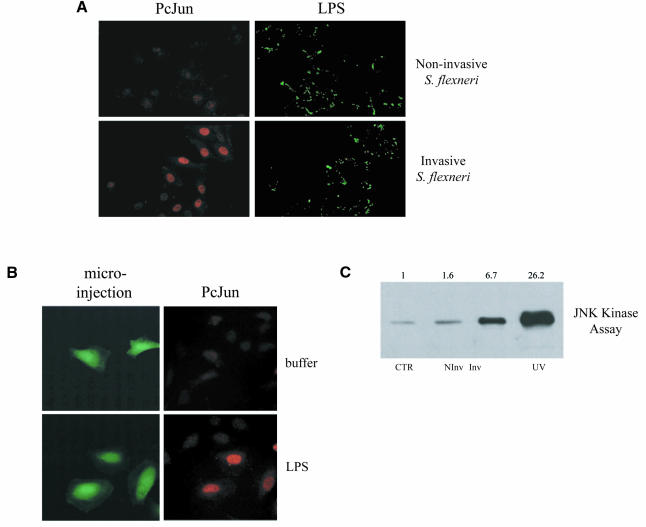
Our previous work had demonstrated that S. flexneri activated NF-κB in epithelial cells (including the intestinal cell line Caco-2) through a pathway dependent upon bacterial entry and the intracellular presentation of LPS. Next, we investigated whether cellular responses to invasive S. flexneri included the activation of JNK, a kinase important in the stress response to numerous stimuli. The major target of JNK, c-Jun, is phosphorylated by this kinase, which is an event that increases its transcriptional activity. c-Jun is a component of the transcription factor AP-1, another important regulator in the inflammatory response (Foletta et al., 1998). Infection of HeLa epithelial cells with invasive S. flexneri, but not non-invasive strains, led to the accumulation of phospho-c-Jun in the nucleus of infected cells (Figure 1A). This response could be mimicked by the direct microinjection of LPS (Figure 1B). Furthermore, invasive S. flexneri led to increased JNK kinase activity compared to uninfected cells or cells infected with non-invasive strains (Figure 1C). Therefore, we could demonstrate for the first time that S. flexneri, as well as intracellular LPS when directly microinjected into cells, are both potent inducers of the JNK signaling pathway.
Fig. 1. (A) Shigella flexneri infection leads to the phosphorylation of c-Jun. HeLa cells were infected with wild-type invasive S. flexneri or the plasmid-cured, non-invasive strain for 2 h. Infected cells were then stained for both phospho-c-Jun using a monoclonal phospho-specific antibody to this protein and LPS to label the infecting bacteria using a rabbit polyclonal anti-LPS antibody. Stained cells were viewed using conventional fluorescence microscopy. (B) Microinjection of LPS activates c-Jun phosphorylation. HeLa cells were microinjected with FITC-dextran to identify microinjected cells plus buffer alone or purified Escherichia coli LPS O111:B4. Cells were then stained for phospho-c-Jun and examined by fluorescence microscopy. (C) Infection with S. flexneri increases JNK kinase activity. HeLa cells were transfected with Flag-JNK1 for 40 h and then left either uninfected (CTR) or infected for 20 min with invasive (Inv) or non-invasive (NInv) S. flexneri. Cells were treated for 20 min with 80 J/m2 UVC as a positive control. Cells were collected and protein extracts were then used for a JNK kinase assay (see Methods). Fold activation compared to uninfected cells is presented.
CARD4/Nod1, a cytosolic protein capable of mediating LPS responsiveness, self-associates following S. flexneri infection
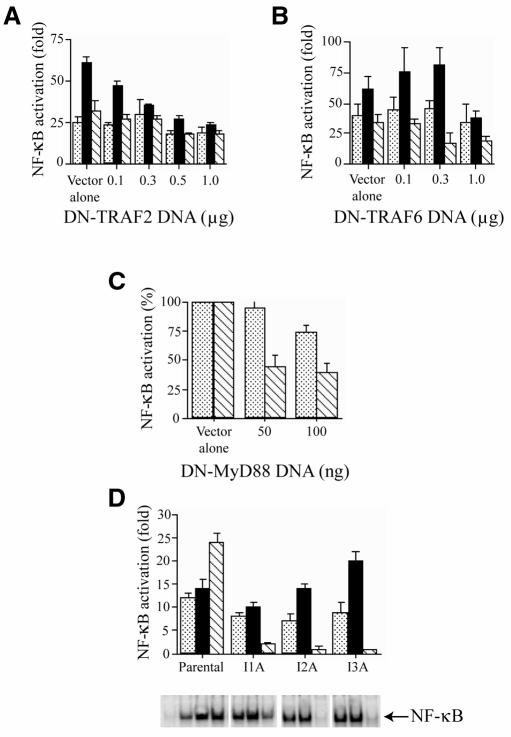
Our focus was then to identify the signal transduction pathway leading from intracellular LPS detection to NF-κB and JNK activation. We found that invasive S. flexneri activates NF-κB and JNK via a signal transduction pathway that is distinct from the TLR/IL-1 pathway (reviewed in O’Neill and Greene, 1998). Through transient transfection of dominant-negative molecules, TRAF2 (Song et al., 1997), TRAF6 (Cao et al., 1996) and MyD88 (Muzio et al., 1997) were shown not to play a role in NF-κB activation by invasive S. flexneri (Figure 2A–C). Moreover, S. flexneri infection activated NF-κB (Figure 2D) and JNK (data not shown) in three cell lines deficient in IL-1 signaling components upstream of TRAF6 (Li et al., 1999), including one deficient in IRAK. Therefore, an alternate LPS detection system, independent of the one involving the TLRs, is likely to exist in mammalian cells in order to respond to intracellular LPS.
Fig. 2. Shigella flexneri induces NF-κB through a signaling pathway distinct from the TLR/IL-1 pathway. Dominant-negative forms of TRAF2 (A) and TRAF6 (B) do not inhibit the induction of NF-κB by invasive S. flexneri (stippled bars), whereas these dominant-negative proteins inhibit NF-κB activation by TNFα (filled bars) or IL-1 (hatched bars), respectively. HEK293 cells were transfected with vector alone or increasing amounts of DNA encoding for the dominant-negative forms of TRAF2 (DN-TRAF2) or TRAF6 (DN-TRAF6) along with a NF-κB luciferase reporter plasmid and a β-galactosidase plasmid. After 30 h, cells were infected with wild-type S. flexneri or treated with TNFα (100 ng/ml) or IL-1 (10 ng/ml) for 4 h and assayed for luciferase activity. (C) Dominant-negative MyD88 (DN-MyD88) does not inhibit NF-κB activation by invasive S. flexneri (stippled bars) but affects IL-1- (hatched bars) induced activation of the reporter gene. Increasing amounts of DNA encoding for dominant-negative MyD88 were transfected into HEK293 cells and assayed for NF-κB luciferase activity as described in (A). (D) Shigella flexneri induces NF-κB in HEK293 deficient in IL-1 specific signaling components (see Methods; Li et al., 1999). NF-κB activation after S. flexneri infection (stippled bars), TNFα (filled bars) or IL-1 (hatched bars) treatment was compared in parental HEK293 cells or three IL-1 signaling mutant cell lines, I1A (IRAK-negative), I2A and I3A. NF-κB activity was assessed following infection or cytokine treatment by the NF-κB reporter assay (refer to above) or EMSA. NF-κB reporter assays were performed in duplicate at least three times and display mean values ± SEM.
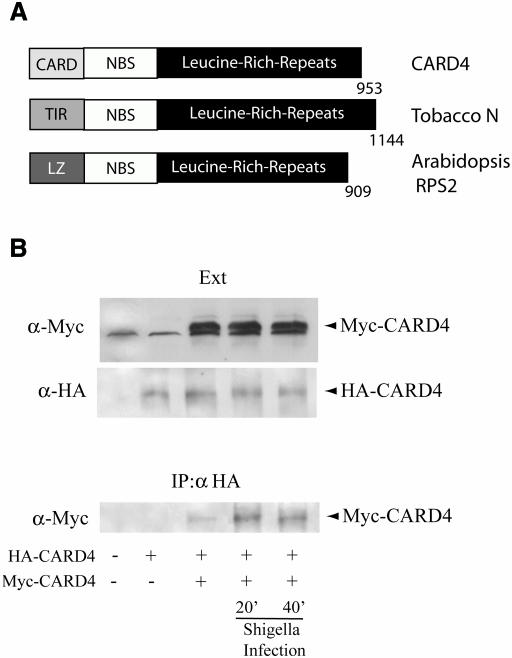
Due to the evolutionarily conserved function of TLRs as pathogen recognition molecules, the possible existence of a cytosolic TLR-like molecule was pursued. A candidate protein was found called CARD4 (Bertin et al., 1999) also known as Nod1 (Inohara et al., 1999). Because of its similarity to plant R proteins (Figure 3A), its cytosolic location (data not shown) and its ability to activate NF-κB when overexpressed (Bertin et al., 1999; Inohara et al., 1999), we hypothesized that CARD4 may play a role in the detection of intracellular LPS in epithelial cells infected with invasive pathogens such as S. flexneri. Additionally, a recent study demonstrated the ability of overexpressed CARD4 to mediate responsiveness to extracellular LPS when co-incubated with cells for 16 h (Inohara et al., 2001). This approach contrasts with our experimental model where infection with S. flexneri results in a cytoplasmic presentation of LPS, followed by a much faster activation of downstream signaling pathways, within 20 min for IKK (Philpott et al., 2000) and JNK activation (Figure 1C).
Fig. 3. CARD4 oligomerization following S. flexneri infection. (A) Domain structure of CARD4 compared with plant disease resistance proteins: Tobacco N protein and Arabidopsis RPS2 protein. (B) Infection with S. flexneri induces self-association of CARD4. HEK293 cells were transfected with empty vector or expression vectors encoding either HA-CARD4 or Myc-CARD4 for 24 h and were left either uninfected or S. flexneri-infected for 20 or 40 min. Cells were collected and protein extracts (Ext) were subjected to western blotting with rabbit polyclonal antibodies to Myc or HA to identify the expression levels of the overexpressed proteins. Another fraction of the protein extracts was used for immunoprecipitation using a polyclonal anti-HA antibody. Oligomerized CARD4 was revealed in the immunoprecipitates by western blotting using antibodies to the Myc-tagged CARD4.
Recently, it was shown that enforced oligomerization of CARD4 via the NBS domain is an event that is sufficient for the induction of NF-κB (Inohara et al., 2000). We, therefore, investigated the possibility that S. flexneri infection could induce the activation of CARD4 by enhancing its self-oligomerization. A full-length myc-tagged CARD4 was co-expressed with a full-length hemagglutinin (HA)-tagged CARD4 in order to perform co-immunoprecipitation experiments following S. flexneri infection. Enhanced self-association of CARD4 was observed as early as 20 min post-infection with invasive S. flexneri (Figure 3B). In contrast, infection with the non-invasive mutant of S. flexneri did not lead to enhanced oligomerization of CARD4 (data not shown). These findings provide the first evidence that infection with invasive S. flexneri is a (patho-) physiological signal inducing CARD4 self-association in infected epithelial cells.
ΔCARD CARD4 and the LRR domain of CARD4 are dominant-negative inhibitors of NF-κB and JNK induction by invasive S. flexneri
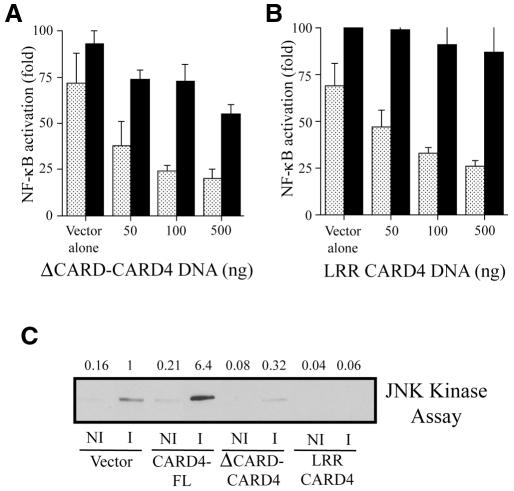
We then investigated whether CARD4 is involved in NF-κB and JNK activation following S. flexneri infection. Since the CARD domain is necessary for NF-κB activation (Bertin et al., 1999; Inohara et al., 1999), we hypothesized that a CARD4 molecule lacking this domain may act as a dominant-negative inhibitor of NF-κB and JNK induction by S. flexneri. Indeed, overexpression of a ΔCARD CARD4 molecule acted in a dose-dependent manner to inhibit S. flexneri-induced NF-κB activation (Figure 4A). Induction of the NF-κB reporter construct by ΤΝFα was much less affected by overexpression of ΔCARD CARD4 testifying to the specificity of this response. Furthermore, overexpression of the ΔCARD CARD4 molecule also blocked JNK induction by S. flexneri as assessed by an in vitro kinase assay (Figure 4C). The dominant-negative effect induced by ΔCARD CARD4 overexpression after S. flexneri infection is likely to be due to either an interference in the propagation of the signal following oligomerization of CARD4 through the NBS domain, or titration of the LPS-induced signaling pathway upstream of CARD4 through the LRR domain. Therefore, we also overexpressed the LRR domain of CARD4 alone and showed that it inhibited both NF-κB and JNK activation by S. flexneri (Figure 4B and C), thus reinforcing the hypothesis that LRR domain overexpression interferes with upstream signaling pathways initiated by infection. Whereas the CARD domain appears necessary for the activation of downstream signaling components, the LRR domain of CARD4 is likely to be responsible for sensing intracellular LPS released from invasive S. flexneri. Accordingly, in vitro studies recently demonstrated the presence of LPS in CARD4-containing complexes (Inohara et al., 2001). However, whether LPS directly binds to CARD4 or interacts with this protein via other intracellular factors has yet to be determined. We also observed that activation of JNK in cells infected with S. flexneri was enhanced following overexpression of the full-length molecule (Figure 4C) implying that low endogenous levels of this protein may restrict activation.
Fig. 4. Constructs expressing either ΔCARD CARD4 or the LRR domain of CARD4 act as dominant-negative inhibitors in S. flexneri-induced activation of NF-κB and JNK. Increasing amounts of DNA encoding either (A) ΔCARD CARD4 or (B) the LRR domain inhibits NF-κB induction by S. flexneri (stippled bars) in a DNA concentration-dependent manner but only marginally affects NF-κB activation by TNFα (filled bars) at the higher concentrations of DNA. Plasmids encoding the truncated forms of CARD4 were transfected into HEK293 cells along with NF-κB and β-galactosidase reporter plasmids. Luciferase activity was assayed as described in the Methods. (C) Effect of the overexpression of full length and truncated forms of CARD4 on JNK activation. HeLa cells were transfected with Flag-JNK1 and either empty vector or with expression vectors encoding for CARD4 full length (CARD4-FL), ΔCARD CARD4 or the LRR of CARD4 for 40 h followed by 20 min infection by invasive (i) or non-invasive (ni) S. flexneri. JNK kinase assays were performed as described in the Methods. Fold activation compared to the S. flexneri-induced activation in vector alone expressing cells is presented.
Overexpression of the LRR domain of CARD4 also inhibited signal transduction induced by microinjected LPS (see Supplementary Table I). Therefore, taken together, these findings implicate CARD4 as a component of an intracellular LPS detection system capable of inducing innate immune responses mediated through the activation of NF-κB and JNK.
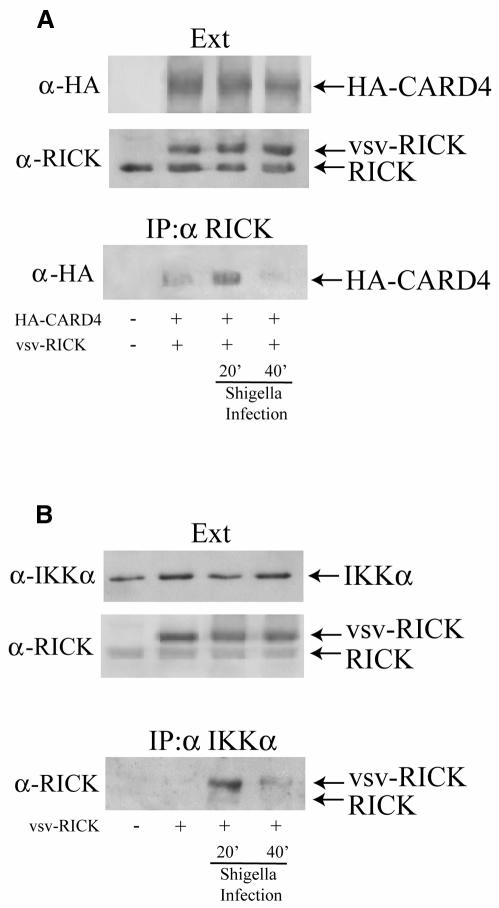
Formation of a transient complex containing CARD4, RICK and the IKK complex following S. flexneri infection
An induced proximity model for activation of NF-κB by CARD4 demonstrates that oligomerization of CARD4 leads to the recruitment of RICK, another CARD-containing molecule (also known as CARDIAK or RIP2; Thome et al., 1998). In turn, RICK has been shown to recruit the members of the IKK complex through NEMO, which leads to the activation of NF-κB (Inohara et al., 2000). We investigated whether infection with the invasive strain of S. flexneri could also result in the formation of such complexes. Co-immunoprecipitation studies revealed that, while a basal interaction between overexpressed CARD4 and RICK could be detected in uninfected conditions, infection for 20 min with invasive S. flexneri resulted in an enhanced interaction between CARD4 and RICK (Figure 5A). These results are in agreement with our findings that demonstrate oligomerization of CARD4 following infection (see Figure 3B), since CARD4 self-oligomerization is the critical step for induction of the CARD4–RICK interaction (Inohara et al., 2000). Interestingly, this increased association was transient since most of the CARD4–RICK interaction was lost 40 min after infection. As the oligomerization of CARD4 was not inhibited at this time following infection (see Figure 3B), the observed downregulation of the CARD4–RICK complex suggests that this interaction might be modulated at this level through a mechanism yet uncharacterized. This transient interaction between RICK and CARD4 may be responsible for Shigella-induced activation of the JNK pathway since we observed that oligomerization of RICK is sufficient to activate this pathway (data not shown).
Fig. 5. Interaction between CARD4 and RICK and RICK and IKKα of the IKK complex folowing S. flexneri infection. (A) Immunoprecipitation using anti-RICK antibodies demonstrates that RICK and CARD4 interact transiently following S. flexneri infection. (B) Immunoprecipitation of the IKK complex using anti-IKKα antibodies showed that RICK (both endogenous and overexpressed vsv-tagged forms of RICK) associates transiently with the IKK complex following S. flexneri infection. Experiments were carried out using HEK293 cells as in Figure 3.
A potential interaction between RICK and the IKK complex following infection was also investigated. RICK was overexpressed and antibodies to endogenous IKKα were used to immunoprecipitate the IKK complex following invasive S. flexneri infection to investigate whether the IKK complex interacted with RICK. Similar to the observed interaction between RICK and CARD4, RICK was observed to transiently associate with IKKα; complex formation was observed 20 min post-infection, while by 40 min post-infection the complex appeared to be downregulated (Figure 5B). These results provide the first evidence for the induced proximity model of NF-κB activation through CARD4–RICK–IKK complex formation initiated by a physiological stimulus, infection with S. flexneri.
We have used S. flexneri as a paradigm of an invasive Gram-negative pathogen to define a signaling pathway that is involved in the initiation of the inflammatory response following bacterial infection. CARD4 or other CARD4-like molecules may represent a common mode of bacterial detection involved in initiating defensive responses to a number of Gram-negative pathogens since LPS is a conserved component of this group of bacteria. Moreover, many of these infections lead to inflammation driven by the induction of NF-κB and/or AP-1. Further characterization of the signaling pathways induced by other pathogens may implicate CARD4 as a common mediator of inflammatory processes initiated upon infection.
CARD4 is a member of a new family of proteins that possess a C-terminal LRR and a NBS (Bertin and Distefano, 2000). This study on CARD4 suggests the possibility that this family of proteins represents human homologues of plant disease-resistance proteins. These cytosolic proteins may be involved in mediating defensive responses to distinct intracellular pathogens or pathogen products. As it has been shown for the TLRs, different CARD4-like proteins may exist that are involved in the recognition of distinct bacterial products. The findings provided here present the first indication that one of these family members, CARD4, mediates intracellular pathogen recognition and signal transduction.
METHODS
Infection of HeLa, HEK293 cells or IL-1 signaling mutant cell lines.
HeLa and HEK293 cells were grown as described presiously (Philpott et al., 2000). Infection with S. flexneri activates downstream signaling pathways in both of these cells lines (Philpott et al., 2000). The three HEK293 cell lines deficient in IL-1 signaling components have been described previously (Li et al., 1999). The I1A cell line is deficient in IRAK whereas the defect in the I2A and I3A cell lines have not been fully characterized, yet the defect likely lies between the IL-1 receptor complex and IRAK (Li et al., 1999).
Wild-type S. flexneri (M90T, serotype 5A) or a plasmid-cured derivative of the parental strain (BS176) were used to infect cells at a multiplicity of infection (MOI) of 50. Previous studies (Philpott et al., 2000) and preliminary evidence had indicated that this MOI was sufficient to generate a strong NF-κB or phospho-c-Jun response in 30 min or 1 h, respectively. Infections were carried out as described previously (Philpott et al., 2000). For phospho-c-Jun staining, a specific c-Jun antibody recognizing phosphorylation of c-Jun at serine 63 (Lallemand et al., 1998) was used followed by anti-mouse secondary antibodies coupled to Cy3 (Jackson). Staining of LPS using an anti-LPS antibody (from Dr Armelle Phalipon) followed by FITC-conjugated secondary antibodies was used to visualize infecting bacteria.
Expression vectors and transient transfections.
Vector constructs expressing dominant-negative MyD88 have been described previously (Muzio et al., 1997). The LRR domain and the myc-tagged CARD4 plasmids were constructed by PCR from the HA-tagged CARD4 plasmid and inserted into pcDNA3 (Invitrogen). The HA-tagged CARD4 and ΔCARD CARD4 plasmids have been described previously (Bertin et al., 1999).
For NF-κB reporter gene assays, HEK293 cells were transfected with FuGene using 300 ng of the NF-κB luciferase reporter was added along with the indicated amounts of effector plasmid and a β-galactosidase reporter in order to normalize transfection efficiencies (Philpott et al., 2000).
Microinjection and fluorescence microscopy.
HeLa cells were plated onto coverslips, transfected and microinjected the following day with purified Escherichia coli LPS (O111:B4; Sigma) or bacteria-free supernatants from wild-type S. flexneri as described previously (Philpott et al., 2000). Staining for NF-κB and phospho-c-Jun was carried out as described above.
Immunoprecipitation experiments and JNK assays.
For immunoprecipitation experiments, HEK293 cells were plated in 10 cm dishes and transfected with FuGene the following day using 500 ng of Myc-CARD4, HA-CARD4 and/or VSV-RICK expression plasmids. Following S. flexneri infection for the indicated times, immunoprecipitation of HA-CARD4, RICK or IKKα was performed as previously described (Bertin et al., 1999) using polyclonal anti-HA (Santa Cruz), polyclonal anti-RICK (Cayman Chemical, Ann Arbor, MI) or monoclonal IKKα (Transduction Laboratories). Immunoprecipitates and total protein extracts were resolved by SDS–PAGE, transferred to nitrocellulose and western blotting was performed using polyclonal antibodies against Myc or HA (Santa Cruz Biotechnology).
JNK activity was analyzed by a quantitative JNK kinase assay (Girardin and Yaniv, 2001). Briefly, HeLa cells were transfected with 500 ng of Flag-JNK1 expression vector plus 2 µg of empty vector, HA-tagged CARD full length (FL), ΔCARD CARD4 or LRR CARD4 vectors. Forty hours post-transfection, cells were left uninfected or infected with either the plasmid-cured, non-invasive (ni) strain or wild-type, invasive (i) S. flexneri for 20 min. Flag-JNK1 was immunoprecipitated using antibodies to the Flag epitope. JNK1 activity of the immunoprecipitates was determined by dividing the level of c-Jun phosphorylation by the amount of overexpressed Flag-JNK1 levels for each sample as described (Girardin and Yaniv, 2001). The level of c-Jun phosphorylation and Flag-JNK1 expression were determined by densitometry following STORM and analyses using ImageQuant software (Molecular Dynamics).
Supplementary data.
Supplementary data are available at EMBO reports Online.
Supplementary Material
Acknowledgments
ACKNOWLEDGEMENTS
We thank Dr Frédéric Relaix, Institut Pasteur, for providing the dominant-negative construct of TRAF2, Dr Simon Whiteside, Institut Pasteur, for dominant-negative TRAF6 and VSV-RICK, and Dr Marta Muzio, Mario Negri Institute, Milan, for the dominant-negative MyD88 construct. We also thank Dr Dominique Lallemand for the preparation of anti phospho-c-Jun antibody. S.E.G. was supported by a Pasteur-Weitzman fellowship from the Institut Pasteur. P.J.S. is a Howard Hughes International Research Scholar. D.J.P. was supported by a fellowship from the Canadian Institutes of Health Research. Research in the Yaniv and Sansonetti laboratories is supported by grants from the ARC and EEC.
REFERENCES
- Bertin J. and Distefano, P.S. (2000) The PYRIN domain: A novel motif found in apoptosis and inflammation proteins. Cell Death Differ., 7, 1273–1274. [DOI] [PubMed] [Google Scholar]
- Bertin J. et al. (1999) Human CARD4 protein is a novel CED-4/Apaf-1 cell death family member that activates NF-κB. J. Biol. Chem., 274, 12955–12958. [DOI] [PubMed] [Google Scholar]
- Cao Z., Xiong, J., Takeuchi, M., Kurama, T. and Goeddel, D.V. (1996) TRAF6 is a signal transducer for interleukin-1. Nature, 383, 443–446. [DOI] [PubMed] [Google Scholar]
- Cario E., Rosenberg, I.M., Brandwein, S.L., Beck, P.L., Reinecker, H.C. and Podolsky, D.K. (2000) Lipopolysaccharide activates distinct signaling pathways in intestinal epithelial cell lines expressing Toll-like receptors. J. Immunol., 164, 966–972. [DOI] [PubMed] [Google Scholar]
- Foletta V.C., Segal, D.H. and Cohen, D.R. (1998) Transcriptional regulation in the immune system: all roads lead to AP-1. J. Leukoc. Biol., 63, 139–152. [DOI] [PubMed] [Google Scholar]
- Girardin S.E. and Yaniv, M. (2001) A direct interaction between JNK1 and CrkII is critical for Rac1- and EGF-induced JNK activation. EMBO J., 20, 3437–3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inohara N. et al. (1999) Nod1, an Apaf-1-like activator of caspase-9 and nuclear factor-κB. J. Biol. Chem., 274, 14560–14567. [DOI] [PubMed] [Google Scholar]
- Inohara N., Koseki, T., Lin, J., del Peso, L., Lucas, P.C., Chen, F.F., Ogura, Y. and Nunez, G. (2000) An induced proximity model for NF-κ B activation in the Nod1/RICK and RIP signaling pathways. J. Biol. Chem., 275, 27823–27831. [DOI] [PubMed] [Google Scholar]
- Inohara N., Ogura, Y., Chen, F.F., Muto, A. and Nunez, G. (2001) Human Nod1 confers responsiveness to bacterial lipopolysaccharides. J. Biol. Chem., 276, 2551–2554. [DOI] [PubMed] [Google Scholar]
- Lallemand D., Ham, J., Garbay, S., Bakiri, L., Traincard, F., Jeannequin, O., Pfarr, C.M. and Yaniv, M. (1998) Stress-activated protein kinases are negatively regulated by cell density. EMBO J., 17, 5615–5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Commane, M., Burns, C., Vithalani, K., Cao, Z. and Stark, G.R. (1999) Mutant cells that do not respond to interleukin-1 (IL-1) reveal a novel role for IL-1 receptor-associated kinase. Mol. Cell. Biol., 19, 4643–4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzio M., Ni, J., Feng, P. and Dixit, V.M. (1997) IRAK (Pelle) family member IRAK-2 and MyD88 as proximal mediators of IL- 1 signaling. Science, 278, 1612–1615. [DOI] [PubMed] [Google Scholar]
- O’Neill L.A.J. and Greene, C. (1998) Signal transduction pathways activated by the IL-1 receptor family: ancient signaling machinery in mammals, insects and plants. J. Leukoc. Biol., 63, 650–657. [PubMed] [Google Scholar]
- Philpott D.J., Yamaoka, S., Israel, A. and Sansonetti, P.J. (2000) Invasive Shigella flexneri activates NF-κB through a lipopolysaccharide-dependent innate intracellular response and leads to IL-8 expression in epithelial cells. J. Immunol., 165, 903–914. [DOI] [PubMed] [Google Scholar]
- Song H.Y., Regnier, C.H., Kirschning, C.J., Goeddel, D.V. and Rothe, M. (1997) Tumor-necrosis factor (TNF)-mediated kinase cascades: bifurcation of nuclear factor-κB and c-jun N-terminal kinase (JNK/SAPK) pathways at TNF receptor-associated factor 2. Proc. Natl Acad. Sci. USA, 94, 9792–9796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thome M., Hofmann, K., Burns, K., Martinon, F., Bodmer, J.L., Mattmann, C. and Tschopp, J. (1998) Identification of CARDIAK, a RIP-like kinase that associates with caspase-1. Curr. Biol., 8, 885–888. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.