Abstract
Spindle assembly and chromosome segregation require the concerted activities of a variety of microtubule-dependent motors. This review focuses on our current knowledge of the roles played by the chromosome-associated motors during mitosis. While some appear to function conventionally in moving chromosomes along microtubules others seem to act in different ways. For example, by docking microtubules to chromosome arms, chromatin-associated motors prevent chromosome loss and participate in spindle formation and stability. Kinetochore motors participate in the formation of stable kinetochore fibers or in the control of microtubule dynamics and are involved in spindle checkpoint activity. Chromosome-associated motors thus appear to be key molecules that function in complementary ways to ensure the accuracy of chromosome segregation.
Introduction
During each cell division, microtubules organize into a bipolar spindle (the minus ends being focused at the spindle poles and the plus ends projecting towards the spindle equator) that segregates the replicated chromosomes into the two daughter cells. The accuracy of chromosome segregation relies on the faithful attachment of all chromosomes to both spindle poles before anaphase can proceed. This attachment occurs through a ‘search and capture’ mechanism in which growing and shrinking microtubules emanating from the separated centrosomes start to interact with the condensed chromosomes. Some microtubules randomly hit a specialized proteinaceous region located on the centromere (the kinetochore) and are subsequently stabilized through their interactions with some of its components. Other microtubules are progressively recruited and a kinetochore fiber (K-fiber) forms, effectively anchoring the chromosome to the spindle pole. The establishment of a similar interaction between the sister kinetochore and the opposite pole leads to chromosome bi-orientation. Microtubules that do not participate in K-fiber formation interact either with chromosome arms or with microtubules emanating from the opposite pole. As all these interactions take place, each chromosome is simultaneously subjected to poleward forces acting at the sister kinetochores, and anti-poleward forces (the ‘polar ejection forces’) exerted on the arms. The combination of these forces results in movement of the chromosomes, contributes to their bi-orientation and leads to their alignment on the metaphase plate. In most cells, a highly conserved signal transduction pathway called the ‘spindle checkpoint’ monitors these events and prevents cells from entering anaphase, and thus chromosomes from segregating and migrating towards the opposite poles, prematurely.
Due to their intrinsic properties, molecular motors have been considered ideal candidates for generating the forces involved in chromosome movements during mitosis. Indeed, over the last few years, various molecular motors have been found to localize to chromosomes and to mediate the different classes of chromosome–microtubule interactions outlined above.
In light of recent results, we review the current knowledge of the roles played by chromosome-associated motors during mitosis. Interestingly, some of them do not function conventionally, i.e. by translocating cargo along microtubules, but play roles related to microtubule attachment, spindle assembly and spindle checkpoint activity.
Chromatin-associated motors lead to chromosome alignment at metaphase
The ‘polar ejection forces’ are generated by microtubules that interact with chromosome arms (Rieder and Salmon, 1994). The proportion of these microtubules is variable among species and correlates with the efficiency of chromosome alignment and stabilization on the metaphase plate (Figure 1). This observation supports the idea that polar ejection forces play an important role in chromosome alignment on the metaphase plate.
Fig. 1. Microtubules interacting with chromosome arms influence chromosome alignment on metaphase plate. Chromosomes, blue; microtubules, red; scale bar, 2 µm. (A) In budding yeast, microtubules do not interact with chromosome arms. Chromosomes do not align on a classical metaphase plate (by courtesy of F. Severin and A. Hyman). (B) In somatic cells (here Xenopus XL 177), microtubules interact both with chromosome arms and kinetochores. Chromosomes align at the spindle equator but keep oscillating with low amplitude around the metaphase plate. (C) In mouse oocytes, most microtubules interact with the chromatin itself and chromosomes remain tightly aligned on the metaphase plate during the metaphase II arrest.
The nature of the polar ejection forces has been speculative for some time. They are unlikely to be generated simply by the impact of growing microtubules on the chromosomes, since mitotic microtubules are highly unstable and mitotic chromatin is very elastic (Houchmandzadeh et al., 1997). Another possibility that has been considered is that molecular motors that localize along chromosome arms could generate these forces. During the last few years, this idea has been reinforced with the identification of several kinesin-like proteins (KLPs) that are associated with chromosome arms. Nonetheless, it was only recently that a good candidate for a polar ejection motor was identified. This Xenopus motor is a homolog of kid, a human DNA-binding KLP associated with chromosomes during mitosis (Tokai et al., 1996). In Xenopus egg extracts, Xkid is not required for spindle assembly but is essential for chromosome alignment (Antonio et al., 2000; Funabiki and Murray, 2000). It moves chromosome arms towards the spindle equator, starting in the early phases of mitosis and continuing until metaphase. Consistent with a role in chromosome alignment, Xkid must be degraded through a ubiquitin-dependent pathway to allow migration of chromatids to the poles during anaphase.
Although Xenopus Xklp1 and Drosophila Nod, two KLPs that localize to chromosome arms, were also initially proposed to generate polar ejection forces, further functional studies indicate that they actually function as ‘docking’ motors that set and maintain microtubule–chromatin interactions but do not directly generate movement. In the case of Xklp1, its activity is essential for spindle assembly and stability around the chromosomes (Vernos et al., 1995; Walczak et al., 1998). Nod, on the other hand, is required for maintaining the achiasmate chromosomes in the female meiotic spindle and for avoiding their loss (Theurkauf and Hawley, 1992; Afshar et al., 1995).
Therefore, among motors associated with chromosome arms, at least one of them functions as a conventional motor, moving chromosome arms along microtubules and generating the polar ejection forces that play a major role in chromosome alignment. Some seem to act as docking proteins, and by constraining chromosomes in the spindle provide a security mechanism to avoid chromosome loss. One caveat to this is that the functions of these motors have been largely characterized in systems in which microtubules interact extensively with chromosome arms. Although polar ejection forces also exist and participate in chromosome alignment in somatic cells (Rieder and Salmon, 1994), the precise role of these motors during mitosis remains to be clarified.
Kinetochore motors in chromosome attachment and motion
Kinetochores are responsible for chromosome bi-orientation and segregation in all eukaryotic cells. To fulfill these roles, they must establish and maintain stable attachments to dynamic microtubule plus ends that, prior to anaphase, alternately depolymerize and polymerize and, during anaphase, depolymerize at both kinetochores. Kinetochores are predominantly submitted to poleward forces that become continuous only at anaphase. Among the kinetochore proteins some must therefore function in microtubule attachment, in regulating microtubule dynamics and in generating movement. Motors are obvious candidates for generating the poleward forces exerted on the kinetochores. Indeed, three motors are currently known to localize to kinetochores and have been analyzed for such roles.
Dynein, the first motor to be found at kinetochores, was initially thought to be a prime candidate for generating poleward forces since it moves towards microtubule minus ends focused at the spindle poles. Indeed, Dynein can drive the fast poleward chromosome motion that occurs immediately after the first interaction between a microtubule and a kinetochore takes place (Rieder and Alexander, 1990). The importance of this event and a further role for this motor in chromosome alignment on the metaphase plate are, however, unclear. Indeed, neither the inhibition of Rough Deal (Rod) nor that of Zeste-white 10 (Zw10), conserved proteins that target Dynein to the kinetochore, impairs chromosome alignment (Karess and Glover, 1989; Williams et al., 1992). Dynein seems to be mainly active during anaphase, at which point it is actually required for chromosome movement to the poles (Savoian et al., 2000; Sharp et al., 2000).
In addition to Dynein, two KLPs have been found to localize to kinetochores. One of these, CENP-E, is required for chromosome alignment on the metaphase plate (Schaar et al., 1997; Wood et al., 1997). Recent results have shown that it moves towards microtubule plus ends and thus could not power ‘in a classical way’ the poleward movement of kinetochores (Wood et al., 1997). Furthermore, its inhibition does not suppress movements of bi-oriented chromosomes before metaphase (Schaar et al., 1997). CENP-E must therefore function in some other process that is essential for chromosome alignment. Indeed, it has been shown to participate in the formation and stabilization of K-fibers, and experiments performed in vitro suggest that it maintains the attachment of kinetochores to depolymerizing microtubule plus ends during poleward motion (Lombillo et al., 1995; Wood et al., 1997; Yao et al., 2000). This function, essential to chromosome alignment on the metaphase plate, may also contribute to poleward chromosome movements during anaphase.
The other KLP, Xkcm1/MCAK (Maney et al., 1998; Walczak et al., 1996), does not function as a conventional motor either. Instead, it promotes microtubule depolymerization (Walczak et al., 1996; Desai et al., 1999). This activity suggests that its normal role in the cell may be to promote the shortening of K-fibers during chromosome poleward movement. The finding that inhibition of MCAK function does not perturb chromosome alignment but does impair chromosome segregation at anaphase (Maney et al., 1998) suggests that, like Dynein, Xkcm1/MCAK may be essential only during anaphase to power chromosome migration towards the poles.
Based on the data reviewed here, with the exception of Dynein, the kinetochore motors identified so far seem mainly to power chromosome movements indirectly, acting in the formation and maintenance of stable connections between kinetochores and dynamic microtubules, or promoting microtubule depolymerization and thus shortening of the K-fibers. Most of them appear to be active mainly during anaphase to drive chromosome movements to the poles.
Kinetochore motors and spindle checkpoint activity
The spindle checkpoint monitors the fulfillment of specific requirements before the cell can enter into anaphase. These requirements include the attachment of microtubules to each kinetochore, the formation of the K-fibers and the establishment of tension between sister kinetochores (Li and Nicklas, 1995; Waters et al., 1998; Yu et al., 1999). Defects in any of these events prevent the spindle checkpoint from being inactivated and thus prevent cells from entering into anaphase.
Recent data suggest that some kinetochore motors may fulfill checkpoint functions. Cellular microinjection of antibodies directed against the non-motor domain of CENP-E does not impair chromosome alignment but inhibits anaphase transition (Yen et al., 1991), implying that, in addition to its function in stabilizing kinetochore–microtubule interactions, CENP-E is involved in the control of anaphase onset. This arrest requires BUBR1, a component of the checkpoint machinery. CENP-E interacts directly with BUBR1 (Chan et al., 1999) but is not required for the kinetochore localization of either BUBR1 or Mad2, another essential spindle checkpoint component (Yao et al., 2000). Thus, CENP-E is not required for spindle checkpoint activation and probably acts downstream of the spindle checkpoint machinery, under the control of BUBR1, and may be required to silence the spindle checkpoint. In contrast to the situation in somatic cells, in Xenopus egg extracts, where the spindle checkpoint can be activated after addition of very high densities of sperm nuclei, CENP-E is needed for Mad2 recruitment to kinetochores (Abrieu et al., 2000). It is possible that, in these extracts, CENP-E fulfills different checkpoint functions that are carried out by distinct motors in somatic cells. The recent identification of two CENP-E isoforms in Drosophila, CENP-E meta and ana, lends support to this idea (Yucel et al., 2000).
Dynein may also contribute to the spindle checkpoint activity. Its association with the kinetochore is regulated by Rod and Zw10, which have recently been shown to be required for spindle checkpoint activation (Basto et al., 2000; Chan et al., 2000). The Dynein–Zw10–Rod complex has been shown to move on the kinetochore fiber towards the spindle pole when chromosomes are subject to tension (Williams et al., 1996; Scaerou et al., 1999). These findings suggest that within the complex, the motor part, Dynein, acts as a sensor of the tension exerted at kinetochores and may activate the spindle checkpoint through Rod and Zw10. In the absence of Rod and Zw10 the association with kinetochores and probably the function of other checkpoint components (BUBR1, Mad2) are not impaired (Chan et al., 2000), suggesting that the whole Dynein–Zw10–Rod complex is part of a specific checkpoint pathway that monitors the tension exerted on kinetochores. However, in grasshopper spermatocytes, the association of Dynein with kinetochores has been shown to depend on K-fiber attachment and not on tension, indicating that Dynein may have a more complex role in the checkpoint (King et al., 2000).
The mechanical basis of spindle checkpoint activities appears to rely on kinetochore motors whose specific functions remain to be clarified. Motors like Dynein may act as sensors of the checkpoint that detect anomalies in either the tension between sister kinetochores or the maturation of K-fibers. Other motors such as CENP-E probably act downstream of the spindle checkpoint, allowing the correction of defects and, as a consequence, spindle checkpoint silencing.
Conclusions
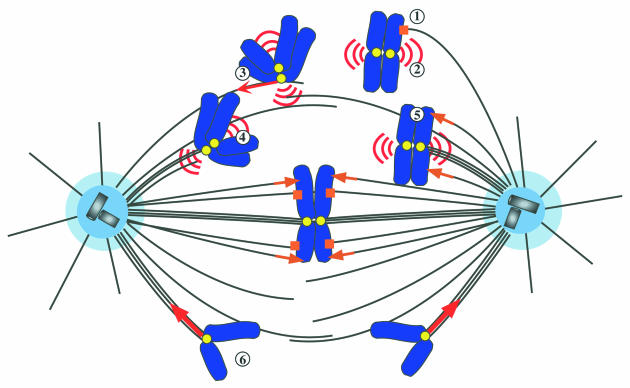
The accuracy of chromosome segregation relies on the functions of chromosome-associated motors. The forces exerted on the chromosomes and the key molecules discussed in the text are depicted in Figure 2.
Fig. 2. Roles of chromosome-associated motors during cell division. (1) Nod and Xklp1 (square) maintain chromatin–microtubule interactions that contribute to spindle stability and reduce the risk of chromosome loss. (2) Dynein and CENP-E are required for spindle checkpoint activity until metaphase. (3) Dynein promotes rapid sliding of the chromosome towards the spindle pole following the capture of a microtubule by a kinetochore. (4) CENP-E contributes to K-fiber formation. (5) Xkid (arrows) generates polar ejection forces towards the spindle equator. (6) Dynein and Xkcm1/MCAK promote chromosome poleward migration during anaphase.
Dynein and Xkid are currently the only motors with conventional activities, driving chromosomes as cargo along microtubules. Additional motors with similar activities may remain to be discovered. Alternatively, chromosome movements during mitosis may be mainly powered by microtubule dynamics, which appear to be regulated by some of the other motors that have been identified. At the kinetochore, CENP-E and Xkcm1/MCAK may function cooperatively, the latter inducing microtubule depolymerization and the former maintaining the attachment of the kinetochore to these depolymerizing microtubules. These combined activities may be sufficient to generate chromosome poleward motion during mitosis. Although speculative at this point, a similar mechanism could exist at the level of the chromosome arms. Docking motors coupled to factors promoting microtubule growth could contribute to polar ejection forces and chromosome migration towards the metaphase plate. Further work will be required to characterize all these motor-interacting partners and the modes of regulation that allow coordinated chromosome movements throughout the course of mitosis.
The recent finding that kinetochore motors provide a mechanical basis for the spindle checkpoint opens new lines of investigation in the field of molecular motors. The checkpoint signaling pathway components, the targets and activating stimuli are to some extent already identified. Molecular motors are emerging as the missing link between stimuli and the signaling pathway. However, the motors involved and their exact modes of action in activating and silencing the spindle checkpoint remain to be fully characterized. Moreover, many questions concerning the respective contributions of tension and K-fiber attachment in checkpoint activation are still unresolved. Kinetochore motors appear to be key molecules whose study will help to further dissect this complex process.
A second security mechanism contributing to faithful chromosome segregation involves chromatin-associated motors. Motors like Xkid, which push chromosomes towards the spindle equator, increase the probability that an unattached kinetochore of the pair will ‘capture’ microtubules emanating from the pole to which it must attach. This process contributes to chromosome bi-orientation and, consequently, reduces the possibility of chromosome segregation errors. In addition, docking motors such as Nod prevent the release of chromosomes into the cytoplasm. The efficiency of such security mechanisms is related to the spindle geometry, i.e. to the proportion of microtubules interacting with chromosome arms, and can even be dispensable, as is the case during the closed mitosis in budding yeast, where microtubules do not interact with chromosome arms (Winey et al., 1995; also, see Figure 1). In contrast, docking is crucial in situations where K-fiber formation is only a late event of prometaphase, as in female meiotic systems (Brunet et al., 1999; Figure 1). However, these theories about the importance of docking are based on work performed mostly in meiotic systems. The challenge is now to evaluate the relevance of such security mechanisms in mitotic systems where, for the moment at least, they still remain to be investigated.
Acknowledgments
Acknowledgements
We thank C. Gonzalez, E. Karsenti and D. Brunner for useful comments on the manuscript. We also acknowledge the contribution of anonymous reviewers and the editor to improve the quality of the manuscript. We apologize for not citing all primary works due to space limitations. S.B. is a former fellow from ‘la Ligue Nationale contre le Cancer’ and is currently supported by an EMBO long-term fellowship.
References
- Abrieu A., Kahana, J.A., Wood, K.W. and Cleveland, D.W. (2000) CENP-E as an essential component of the mitotic checkpoint in vitro. Cell, 102, 817–826. [DOI] [PubMed] [Google Scholar]
- Afshar K., Barton, N.R., Hawley, R.S. and Goldstein, L.S. (1995) DNA binding and meiotic chromosomal localization of the Drosophila nod kinesin-like protein. Cell, 81, 129–138. [DOI] [PubMed] [Google Scholar]
- Antonio C., Ferby, I., Wilhelm, H., Jones, M., Karsenti, E., Nebreda, A.R. and Vernos, I. (2000) Xkid, a chromokinesin required for chromosome alignment on the metaphase plate. Cell, 102, 425–435. [DOI] [PubMed] [Google Scholar]
- Basto R., Gomes, R. and Karess, R.E. (2000) Rough deal and Zw10 are required for the metaphase checkpoint in Drosophila. Nature Cell Biol., 2, 939–943. [DOI] [PubMed] [Google Scholar]
- Brunet S., Maria, A.S., Guillaud, P., Dujardin, D., Kubiak, J.Z. and Maro, B. (1999) Kinetochore fibers are not involved in the formation of the first meiotic spindle in mouse oocytes, but control the exit from the first meiotic M phase. J. Cell Biol., 146, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan G.K., Jablonski, S.A., Sudakin, V., Hittle, J.C. and Yen, T.J. (1999) Human BUBR1 is a mitotic checkpoint kinase that monitors CENP-E functions at kinetochores and binds the cyclosome/APC. J. Cell Biol., 146, 941–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan G.K., Jablonski, S.A., Starr, D.A., Goldberg, M.L. and Yen, T.J. (2000) Human Zw10 and ROD are mitotic checkpoint proteins that bind to kinetochores. Nature Cell Biol., 2, 944–947. [DOI] [PubMed] [Google Scholar]
- Desai A., Verma, S., Mitchison, T.J. and Walczak, C.E. (1999) Kin I kinesins are microtubule-destabilizing enzymes. Cell, 96, 69–78. [DOI] [PubMed] [Google Scholar]
- Funabiki H. and Murray, A.W. (2000) The Xenopus chromokinesin Xkid is essential for metaphase chromosome alignment and must be degraded to allow anaphase chromosome movement. Cell, 102, 411–424. [DOI] [PubMed] [Google Scholar]
- Houchmandzadeh B., Marko, J.F., Chatenay, D. and Libchaber, A. (1997) Elasticity and structure of eukaryote chromosomes studied by micromanipulation and micropipette aspiration. J. Cell Biol., 139, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karess R.E. and Glover, D.M. (1989) rough deal: a gene required for proper mitotic segregation in Drosophila. J. Cell Biol., 109, 2951–2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King J.M., Hays, T.S. and Nicklas, R.B. (2000) Dynein is a transient kinetochore component whose binding is regulated by microtubule attachment, not tension. J. Cell Biol., 151, 739–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X. and Nicklas, R.B. (1995) Mitotic forces control a cell-cycle checkpoint. Nature, 373, 630–632. [DOI] [PubMed] [Google Scholar]
- Lombillo V.A., Nislow, C., Yen, T.J., Gelfand, V.I. and McIntosh, J.R. (1995) Antibodies to the kinesin motor domain and CENP-E inhibit microtubule depolymerization-dependent motion of chromosomes in vitro. J. Cell. Biol., 128, 107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maney T., Hunter, A.W., Wagenbach, M. and Wordeman, L. (1998) Mitotic centromere-associated kinesin is important for anaphase chromosome segregation. J. Cell Biol., 142, 787–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder C.L. and Alexander, S.P. (1990) Kinetochores are transported poleward along a single astral microtubule during chromosome attachment to the spindle in newt lung cells. J. Cell Biol., 110, 81–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder C.L. and Salmon, E.D. (1994) Motile kinetochores and polar ejection forces dictate chromosome position on the vertebrate mitotic spindle. J. Cell Biol., 124, 223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savoian M.S., Goldberg, M.L. and Rieder, C.L. (2000) The rate of poleward chromosome motion is attenuated in Drosophila zw10 and rod mutants. Nature Cell Biol., 2, 948–952. [DOI] [PubMed] [Google Scholar]
- Scaerou F., Aguilera, I., Saunders, R., Kane, N., Blottiere, L. and Karess, R. (1999) The rough deal protein is a new kinetochore component required for accurate chromosome segregation in Drosophila. J. Cell Sci., 112, 3757–3768. [DOI] [PubMed] [Google Scholar]
- Schaar B.T., Chan, G.K., Maddox, P., Salmon, E.D. and Yen, T.J. (1997) CENP-E function at kinetochores is essential for chromosome alignment. J. Cell Biol., 139, 1373–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp D.J., Rogers, G.C. and Scholey, J.M. (2000) Cytoplasmic dynein is required for poleward chromosome movement during mitosis in Drosophila embryos. Nature Cell Biol., 2, 922–930. [DOI] [PubMed] [Google Scholar]
- Theurkauf W.E. and Hawley, R.S. (1992) Meiotic spindle assembly in Drosophila females: behavior of nonexchange chromosomes and the effects of mutations in the nod kinesin-like protein. J. Cell Biol., 116, 1167–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokai N., Fujimoto-Nishiyama, A., Toyoshima, Y., Yonemura, S., Tsukita, S., Inoue, J. and Yamamota, T. (1996) Kid, a novel kinesin-like DNA binding protein, is localized to chromosomes and the mitotic spindle. EMBO J., 15, 457–467. [PMC free article] [PubMed] [Google Scholar]
- Vernos I., Raats, J., Hirano, T., Heasman, J., Karsenti, E. and Wylie, C. (1995) Xklp1, a chromosomal Xenopus kinesin-like protein essential for spindle organization and chromosome positioning. Cell, 81, 117–127. [DOI] [PubMed] [Google Scholar]
- Walczak C.E., Mitchison, T.J. and Desai, A. (1996) XKCM1: a Xenopus kinesin-related protein that regulates microtubule dynamics during mitotic spindle assembly. Cell, 84, 37–47. [DOI] [PubMed] [Google Scholar]
- Walczak C.E., Vernos, I., Mitchison, T.J., Karsenti, E. and Heald, R. (1998) A model for the proposed roles of different microtubule-based motor proteins in establishing spindle bipolarity. Curr. Biol., 8, 903–913. [DOI] [PubMed] [Google Scholar]
- Waters J.C., Chen, R.H., Murray, A.W. and Salmon, E.D. (1998) Localization of Mad2 to kinetochores depends on microtubule attachment, not tension. J. Cell Biol., 141, 1181–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams B.C., Karr, T.L., Montgomery, J.M. and Goldberg, M.L. (1992) The Drosophila l(1)zw10 gene product, required for accurate mitotic chromosome segregation, is redistributed at anaphase onset. J. Cell Biol., 118, 759–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams B.C., Gatti, M. and Goldberg, M.L. (1996) Bipolar spindle attachments affect redistributions of ZW10, a Drosophila centromere/kinetochore component required for accurate chromosome segregation. J. Cell Biol., 134, 1127–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winey M., Mamay, C.L., O’Toole, E.T., Mastronarde, D.N., Giddings, T.H., Jr, McDonald, K.L. and McIntosh, J.R. (1995) Three-dimensional ultrastructural analysis of the Saccharomyces cerevisiae mitotic spindle. J. Cell Biol., 129, 1601–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood K.W., Sakowicz, R., Goldstein, L.S. and Cleveland, D.W. (1997) CENP-E is a plus end-directed kinetochore motor required for metaphase chromosome alignment. Cell, 91, 357–366. [DOI] [PubMed] [Google Scholar]
- Yao X., Abrieu, A., Zheng, Y., Sullivan, K.F. and Cleveland, D.W. (2000) CENP-E forms a link between attachment of spindle microtubules to kinetochores and the mitotic checkpoint. Nature Cell Biol., 2, 484–491. [DOI] [PubMed] [Google Scholar]
- Yen T.J., Compton, D.A., Wise, D., Zinkowski, R.P., Brinkley, B.R., Earnshaw, W.C. and Cleveland, D.W. (1991) CENP-E, a novel human centromere-associated protein required for progression from metaphase to anaphase. EMBO J., 10, 1245–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H.G., Muszynski, M.G. and Kelly Dawe, R. (1999) The maize homologue of the cell cycle checkpoint protein MAD2 reveals kinetochore substructure and contrasting mitotic and meiotic localization patterns. J. Cell Biol., 145, 425–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yucel J.K., Marszalek, J.D., McIntosh, J.R., Goldstein, L.S., Cleveland, D.W. and Philp, A.V. (2000) CENP-meta, an essential kinetochore kinesin required for the maintenance of metaphase chromosome alignment in Drosophila. J. Cell Biol., 150, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]